Abstract
OBJECTIVE—To study insulin sensitivity and perfusion in skeletal muscle together with the β-cell function in subjects with the m.3243A>G mutation in mitochondrial DNA, the most common cause of mitochondrial diabetes.
RESEARCH DESIGN AND METHODS—We measured skeletal muscle glucose uptake and perfusion using positron emission tomography and 2-[18F]fluoro-2-deoxyglucose and [15O]H2O during euglycemic hyperinsulinemia in 15 patients with m.3243A>G. These patients included five subjects with no diabetes as defined by the oral glucose tolerance test (OGTT) (group 1), three with GHb <6.1% and newly found diabetes by OGTT (group 2), and seven with a previously diagnosed diabetes (group 3). Control subjects consisted of 13 healthy individuals who were similar to the carriers of m.3243A>G with respect to age and physical activity. β-Cell function was assessed using the OGTT and subsequent mathematical modeling.
RESULTS—Skeletal muscle glucose uptake was significantly lower in groups 1, 2, and 3 than in the control subjects. The glucose sensitivity of β-cells in group 1 patients was similar to that of the control subjects, whereas in group 2 and 3 patients, the glucose sensitivity was significantly lower. The insulin secretion parameters correlated strongly with the proportion of m.3243A>G mutation in muscle.
CONCLUSIONS—Our findings show that subjects with m.3243A>G are insulin resistant in skeletal muscle even when β-cell function is not markedly impaired or glucose control compromised. We suggest that both the skeletal muscle insulin sensitivity and the β-cell function are affected before the onset of the mitochondrial diabetes caused by the m.3243A>G mutation.
Impaired insulin sensitivity characterizes adult-onset diabetes and has been attributed to decreased insulin-stimulated glucose uptake in major metabolic tissues such as skeletal muscle, liver, and adipose tissue (1,2). It predicts diabetes strongly in subjects with high hereditary risk (3). Decreased glucose uptake in skeletal muscle is the major determinant of impaired insulin sensitivity, because skeletal muscle is the tissue that accounts for the majority of insulin-stimulated glucose uptake in diabetes and in nondiabetic subjects (4). Impaired insulin sensitivity has been correlated with decreased mitochondrial function and with decreased expression of genes involved in mitochondrial oxidative phosphorylation in skeletal muscle (5,6). Interestingly, similar findings in oxidative phosphorylation have recently been made in healthy insulin-resistant subjects with high hereditary predisposition for diabetes (7).
In addition to the impaired insulin sensitivity, a gradual β-cell failure is pivotal to the onset of diabetes in adulthood (8,9). It is noteworthy that most gene variants associated with adult-onset diabetes influence the β-cell insulin secretion (10). Genes that contribute to mitochondrial oxidative phosphorylation are located both in the nuclear DNA and in the maternally inherited mitochondrial DNA (mtDNA) (11). Intrinsic or acquired causes that could impair oxidative phosphorylation in the mitochondrion have been proposed to impair both skeletal muscle insulin sensitivity and β-cell function (12,13).
The involvement of mtDNA mutations in the hereditary forms of diabetes is evident (14,15). The mtDNA m.3243A>G mutation accounts for 1–2% of adult-onset diabetes, and it has been estimated that most carriers of this mutation develop diabetes during their adulthood (16). This renders the m.3243A>G mutation an interesting pathogenic model for decreased mitochondrial function in adult-onset diabetes. The m.3243A>G mutation is heteroplasmic, i.e., the mutant allele and the wild-type allele co-occur in mitochondria, and the proportion of mutated mtDNA varies across patients and tissues (11). The hetero-plasmy is known to modify the phenotype in patients with m.3243A>G (17), but previous studies on glucose metabolism have not included mutation heteroplasmy as a variable. Furthermore, such studies have been small, so that more than four subjects with m.3243A>G have been examined in only a few of them (18–24). Most of these studies have revealed defects in insulin secretion (18–21,24). Hyperinsulinemic clamp technique has been applied in only two previous studies on m.3243A>G subjects, but these studies could not identify peripheral insulin resistance as the primary pathogenic factor (18,19).
The aims of this study were 1) to characterize insulin secretion and sensitivity in patients with a substantial m.3243A>G mutation load and in age-matched healthy subjects, and 2) by using this disease model, to further enlighten the role of mitochondria in the pathogenesis of diabetes. We assessed whole-body glucose uptake by using the hyperinsulinemic clamp technique together with regional measurements of muscle perfusion and glucose uptake by positron emission tomography (PET). Model-based analysis of β-cell function was carried out, and measurements were correlated with mutation heteroplasmy.
RESEARCH DESIGN AND METHODS
Fifteen patients with the m.3243A>G mutation, most ascertained in a previous epidemiological study (25), and 13 healthy control subjects were recruited. It was required that the m.3243A>G mutation heteroplasmy was >10%. Eight patients had previously normal fasting glucose (group 1 and 2), and seven patients had overt diabetes (group 3). The control subjects had normal glucose tolerance. In the case of the control subjects, it was also required that no diabetes was present in the first-degree relatives before the age of 55 years. All subjects were Finnish and in stable weight for the last 3 months before the study. Written informed consent was obtained after the purpose, nature, and potential risks were explained to the subjects. The study was approved by the Ethics Committee of the Turku University Hospital.
Each subject underwent an oral glucose tolerance test (OGTT) on the first study day and indirect calorimetry and hyperinsulinemic-euglycemic clamp on the second study day. Skeletal muscle glucose uptake and perfusion measurements using PET and 2-[18F]fluoro-2-deoxyglucose and [15O]H2O were performed during the hyperinsulinemic-euglycemic clamp. All subjects were instructed to avoid caffeine, smoking, alcohol, homeopathic preparations, and changes in diet or in physical activity for 2 days before the study. All studies were conducted after a 10-h fast and insulin cease. Metabolically active substances except thyroid hormone were discontinued at least 24 h before the studies (Table 1). Physical activity was assessed by a physical activity questionnaire (International Physical Activity Questionnaire [IPAQ]) (26).
TABLE 1.
Subject characteristics
| Control subjects | m.3243A>G mutation
|
|||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||
| n | 13 | 5 | 3 | 7 |
| Clinical features | ||||
| Men (n) | 2 | 0 | 0 | 4 |
| Age (years) | 47 ± 3.0 | 48 ± 4.0 | 46 ± 5.1 | 46 ± 4.6 |
| BMI (kg/m2) | 24.1 ± 0.8 | 25.9 ± 1.2 | 23.9 ± 2.2 | 20.6 ± 1.2* |
| Physical activity (daily metabolic equivalent minutes) | 442 ± 79 | 655 ± 115 | 548 ± 157 | 351 ± 58 |
| Epithelial heteroplasmy (%) | 0 | 33 ± 10 | 47 ± 3 | 53 ± 4* |
| Muscle heteroplasmy (%) | — | 46 ± 8 | 74 ± 1 | 80 ± 5* |
| Hearing aid (n) | 0 | 0 | 1 | 4 |
| Duration of diabetes (years) | — | — | 0 | 9.7 ± 1.2 |
| Medication | ||||
| Insulin | — | — | — | 6/7 |
| Metformin and nateglinide | — | — | — | 1/7 |
| Statins | — | 1/5 | — | 4/7 |
| β-Blockers | — | 1/5 | — | 1/7 |
| Thyroid hormone | 1/13 | 2/5 | — | — |
| ACE inhibitors | — | 1/5 | — | 1/7 |
| Valsartan | — | — | — | 2/7 |
| Smokers | 1/13 | — | 1/3 | 2/7 |
| Metabolic characteristics | ||||
| Fasting glucose (mmol/l) | 5.2 ± 0.1 | 5.4 ± 0.1 | 6.0 ± 0.3 | 9.2 ± 0.8†‡§ |
| Glycated hemoglobin (%) | 5.4 ± 0.1 | 5.4 ± 0.2 | 6.0 ± 0.1 | 7.7 ± 0.4†* |
| Fasting insulin (pmol/l) | 39 ± 4 | 40 ± 7 | 60 ± 16 | 31 ± 4¶* |
| Fasting C-peptide (nmol/l) | 0.55 ± 0.04 | 0.60 ± 0.05 | 0.72 ± 0.12 | 0.44 ± 0.07 |
| Fasting free fatty acids (mmol/l) | 0.45 ± 0.1 | 0.46 ± 0.1 | 0.66 ± 0.2 | 0.66 ± 0.1 |
| Uric acid (μmol/l) | 252 ± 13 | 285 ± 29 | 297 ± 83 | 328 ± 50 |
| Creatine kinase (units/l) | 91 ± 8 | 116 ± 48 | 164 ± 83 | 155 ± 32 |
| Resting energy expenditure (kcal/min) | 1.07 ± 0.02 | 1.05 ± 0.08 | 1.00 ± 0.11 | 0.93 ± 0.07 |
| Resting energy expenditure (kcal · kg−1 · min−1) | 16 ± 0.8 | 16 ± 0.9 | 16 ± 1.1 | 18 ± 1.1 |
Data are means ± SE. Comparisons between groups were made when overall P < 0.05. A P value for significant pairwise differences is given.
P < 0.05 vs. group 1.
P < 0.001 vs. healthy subjects.
P < 0.01 vs. group 1.
P < 0.05 vs. group 2.
P < 0.05 vs. healthy subjects.
OGTT and assessment of β-cell function.
Subjects ingested 75 g glucose, and blood samples were collected for glucose, insulin, and C-peptide at 0, 15, 30, 60, 90, and 120 min. The insulinogenic index (IGI), the ratio of the rise of the insulin and glucose concentrations over basal level at 30 min, and the ratio of insulin and glucose areas under the curve (AUCs) using trapezoidal integration (AUCI/AUCG) were calculated. Mathematical modeling based on C-peptide deconvolution (27) was used to describe insulin secretion as the sum of two components as previously stated (28). The first component, Sg(t), represents the dependence on insulin secretion in absolute glucose concentration (G) at any time point and is characterized by a dose-response function, f(G). The slope of f(G) in the observed glucose range is denoted as β-cell glucose sensitivity. The dose-response function is modulated by a time-dependent potentiation factor, P(t), accounting for several modulators of insulin secretion (e.g., exposure to hyperglycemia, nonglucose substrates, and gastrointestinal hormones); thus, Sg(t) = P(t)f(G). The increment of potentiation during the OGTT was quantified as the ratio of the value of P(t) at the end of the test to that at baseline. The second insulin secretion component, Sd(t), represents a dynamic dependence of insulin secretion, proportional to the rate of change in glucose concentration. This proportionality constant is termed rate sensitivity and is related to early insulin release (28). From the model, the basal insulin secretion rate (pmol · min−1 · m−2) was also calculated.
Production of PET tracers.
For production of [15O] (t1/2 = 123 s), a low-energy deuteron accelerator was used. [15O] was produced in the 14N(d,n)15O reaction using nitrogen gas as target material. Radiochemical purity of [15O]O2 exceeded 97%. [15O]H2O was produced based on the membrane technique using sterile exchangeable tubing in the device as previously described (29). Sterility and pyrogenity tests were performed daily to verify the purity of the product. [18F]FDG (t1/2 = 110 min) was synthesized with a computer-controlled apparatus according to a modified method of Hamacher et al. (30). The specific radioactivity at the end of synthesis was better than 70 GBq/μmol, and the radiochemical purity exceeded 98%.
Image acquisition and hyperinsulinemic-euglycemic clamp.
Subjects were lying in the supine position during the PET study. At 0 min, a standard primed hyperinsulinemic-euglycemic clamp (31) was started for at least 220 min using 1 mU · kg−1 · min−1 intravenous insulin infusion. Normoglycemia was maintained using variable rates of 20% glucose infusion, adjusted according to plasma glucose. At 50 min, [15O]H2O (0.5–0.8 GBq) was injected in over 30 s, and femoral muscle perfusion was measured with a dynamic 6-min scan (6× 5 s, 6× 15 s, and 8× 30 s frames). To obtain the input function, radioactivity was measured with a channel detector in arterial blood, withdrawn with a pump at the rate of 6 ml/min. At 120 min, [18F]FDG (0.23–0.29 GBq) was injected over 15 s, and a dynamic scan was started at 200 min (frames 6× 300 s). One of the subjects in group 1 failed to lie still during the skeletal muscle glucose uptake measurement, and the data were not obtained. Arterial blood samples were drawn after the [18F]FDG injection, and radioactivity was assessed with an automatic gamma counter. All the images were acquired with a PET scanner that gives 35 transaxial planes with axial resolution of 4.7 mm and in-plane resolution of 5.5 mm. The PET scanner and other devices were cross-calibrated.
Measurement of skeletal muscle perfusion and glucose uptake.
Radioactivity was corrected for decay to the injection and expressed in kilobecquerels per milliliter. An autographic method was applied to calculate muscle perfusion pixel by pixel (32). Data from [18F]FDG images were reconstructed to 128 × 128 pixel matrix using a planar Hann filter and an axial ramp filter. A graphical model of [18F]FDG kinetics was used as previously described (32). The glucose uptake rate was obtained by multiplying the fractional rate of tracer transport and phosphorylation (Ki) by the plasma glucose concentration after the [18F]FDG injection and divided by lumped constant 1.2 as previously validated for skeletal muscle (33). The lumped constant accounts for the differences in the transport and phosphorylation between [18F]FDG and glucose. Glucose extraction was calculated by dividing the glucose uptake with blood flow. Regions of interest were drawn both in quadriceps femoris and to hamstring muscles, carefully avoiding large blood vessels. The localization of muscle compartments was verified by comparing [18F]FDG and [15O]H2O images with the tissue density in transmission image.
Measurement of energy expenditure.
The whole-body oxygen consumption and the carbon dioxide production was measured in the fasting state with an open-system indirect calorimeter in a quiet, dimly lit room. Resting energy expenditure was calculated from a 10-min period after 20 min of initial stabilization (34).
Measurement of the m.3243A>G mutation heteroplasmy.
A buccal epithelial cell sample was obtained from all the subjects (Table 1). The mutation heteroplasmy in skeletal muscle was available for 12 of the 15 patients with m.3243A>G (25). The m.3243A>G mutation was detected by cleavage of an amplified DNA fragment with the restriction enzyme Apa I. The digested samples were electrophoresed, and the acrylamide gel was dried and autoradiographed. The reproducibility was controlled by including, in each electrophoresis run, a sample with the m.3243A>G mutation (25).
Biochemical analyses.
Plasma glucose was determined with a glucose oxidase method. Plasma insulin and plasma C-peptide were assessed by electrochemiluminescense immunoassay technique. Plasma creatine kinase was determined with enzymatic method. Blood glycated hemoglobin was measured with ion-exchange high-performance liquid chromatography. Serum free fatty acid concentrations were measured via an enzymatic colorimetric method.
Statistical methods.
The results are expressed as means ± SE. Before analysis, the normality of variables was assessed by the Shapiro-Wilk test. The differences among the group were identified using one-way ANOVA and Tukey-Kramer post hoc procedure. Kruskall-Wallis nonparametric test was used for group comparisons in continuous variables that were not normally distributed. Post hoc tests were carried out by using Mann-Whitney U test, and P values were corrected for multiple comparisons by the Benjamini-Hochberg method (35). A P value of <0.05 was considered statistically significant. Linear correlations were assessed with Spearman's correlation coefficients.
RESULTS
Subject characteristics.
Patients with m.3243A>G were divided into three groups based on the OGTT (Table 1). Group 1 consisted of patients with either normal or impaired glucose tolerance in OGTT (n = 5). Patients in group 2 had newly diagnosed diabetes that was detected on the basis of high 2-h glucose in OGTT (n = 3). Group 3 patients were all treated for diabetes that had been diagnosed previously (n = 7). The three patient groups and the control subjects were similar in age, physical activity, and resting energy expenditure (Table 1). Furthermore, the fasting plasma concentrations of C-peptide, uric acid, creatine kinase, and serum free fatty acids were similar in the four groups.
Insulin secretion and β-cell function.
All of the eight subjects in groups 1 and 2 had good glycemic control (GHb <6.1%). During the first 30 min of the OGTT, absolute glucose, insulin, and C-peptide concentrations were identical in the control subjects and in group 1 patients, but at 120 min, plasma glucose was significantly higher in group 1 patients and the insulin levels tended to surpass those seen in the control subjects (Fig. 1). As a consequence, the IGI and the rate sensitivity did not differ between these two groups (Table 2). These two parameters describe the rapid response of insulin secretion on the dynamic change in glucose levels. Group 2 patients had the highest basal insulin secretion rate, but the response to glucose load was blunted as the decreased IGI indicated (Table 2).
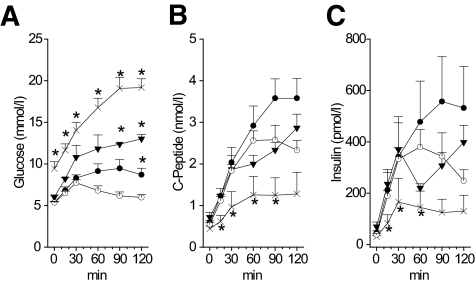
FIG. 1.
OGTT in patients with the m.3243A>G mutation. A: Glucose concentration. B: C-peptide concentration. C: Insulin concentration. ○, control subjects. Patients with m.3243A>G mutation: •, group 1; ▴, group 2; and ×, group 3. A–C: *P < 0.05 vs. healthy subjects, at respective time points.
TABLE 2.
Insulin sensitivity and β-cell function in m.3243A>G mutation
| Study group
|
||||
|---|---|---|---|---|
| Control subjects | m.3243A>G mutation
|
|||
| Group 1 | Group 2 | Group 3 | ||
| Insulin sensitivity | ||||
| M value (μmol · min−1 · kg−1) | 33 ± 10 | 24 ± 5 | 19 ± 3 | 17 ± 6* |
| Insulin secretion | ||||
| IGI (pmol · mmol−1) | 27.7 ± 8.0 | 16.8 ± 5.5 | 4.9 ± 0.8† | 3.1 ± 1.4*‡ |
| AUCI/AUCG (nmol · mol−1) | 38 ± 4.5 | 42 ± 12.8 | 22.6 ± 4.5 | 7.1 ± 3.3*‡ |
| Basal insulin secretion rate (pmol · min−1 · m−2) | 71 ± 4.8 | 78 ± 7.6 | 96 ± 15.0 | 65 ± 10.1 |
| Glucose sensitivity (pmol · min−1 · m−2 · mmol−1 · l−1) | 137 ± 15 | 106 ± 13 | 43 ± 20† | 22 ± 14*‡ |
| Rate sensitivity (pmol · m−2 · mmol−1 · l−1) | 686 ± 82 | 1,147 ± 304 | 493 ± 206 | 103 ± 50*‡ |
| Ratio of the end and start period potentiation | 1.9 ± 0.2 | 1.4 ± 0.3 | 1.3 ± 0.4 | 0.9 ± 0.1* |
| Disposition index§ | ||||
| Rate sensitivity × whole-body insulin sensitivity (pmol · min−1 · m−4 · mol−1 · l−1) | 853 ± 110 | 993 ± 340 | 323 ± 97 | 67 ± 36*¶ |
Data are means ± SE. Insulin sensitivity (M value) calculated during the steady state of hyperinsulinemic-euglycemic clamp. β-cell function is derived from an oral glucose tolerance test. Comparisons between groups were made when overall P < 0.05. A P value for significant pairwise differences is given.
P < 0.01 vs. healthy subjects.
P < 0.05 vs. healthy subjects.
P < 0.05 vs. group 1.
Insulin sensitivity per body surface area multiplied with insulin secretion response to dynamic change in glucose per body surface area (rate sensitivity).
P < 0.01 vs. group 1.
The subjects in group 3 fulfilled the criteria for diabetes both in the fasting state and at 2 h in OGTT. Group 3 patients had significantly lower insulin and C-peptide already at 15 min of the OGGT than the control subjects (Fig. 1). In consequence, the IGI was lower and the rate sensitivity was strongly impaired compared with the control subjects and group 1 patients (Fig. 2).
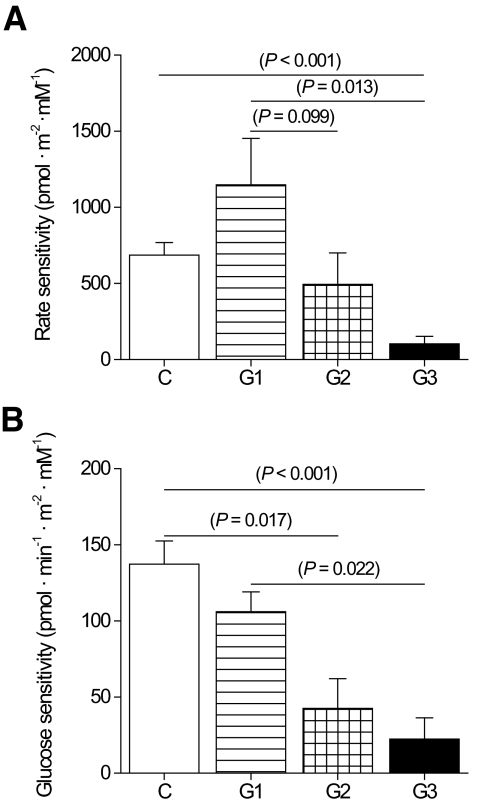
FIG. 2.
Rate sensitivity and glucose sensitivity of the β-cells during OGTT. A: Rate sensitivity is the insulin secretion response to the pace of increase in plasma glucose. Control subjects, □. Patients with m.3243A>G mutation:  , group 1;
, group 1;  , group 2; and ▪, group 3. The apparently high rate sensitivity in group 1 is partly due to the higher insulin sensitivity in the control subjects, because rate sensitivity tends to be inversely correlated with insulin sensitivity (see Table 2 for respective disposition index). B: Glucose sensitivity is the insulin dose-response function to absolute glucose level during OGTT.
, group 2; and ▪, group 3. The apparently high rate sensitivity in group 1 is partly due to the higher insulin sensitivity in the control subjects, because rate sensitivity tends to be inversely correlated with insulin sensitivity (see Table 2 for respective disposition index). B: Glucose sensitivity is the insulin dose-response function to absolute glucose level during OGTT.
In addition to the impaired rate sensitivity in group 3 patients, a mathematical modeling revealed that the β-cell glucose sensitivity was significantly lower in group 2 and 3 patients than in the control subjects (dose-response function; Fig. 2). Furthermore, a low end period–to–start period potentiation ratio was seen in group 3 patients compared with the control subjects. Disposition index was calculated by multiplying rate sensitivity with the whole-body glucose uptake. It was similar in group 1 patients and the control subjects, lower in group 2 patients than in the control subjects, and significantly lower in group 3 patients than in the control subjects or group 1 patients (Table 2). These results imply that group 2 and group 3 patients were not able to raise their insulin secretion during the OGTT in a similar manner as the control subjects did.
Muscle glucose uptake and perfusion during the hyperinsulinemic clamp.
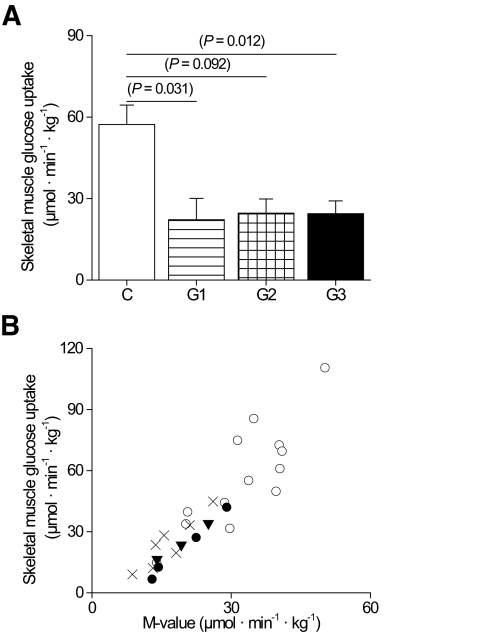
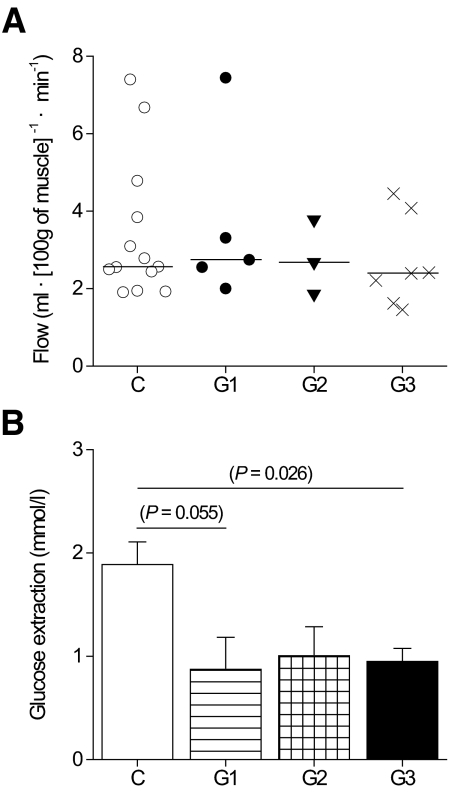
During the insulin infusion, euglycemia was maintained in all groups with no difference in the glucose or insulin concentrations. The rate of insulin-stimulated glucose uptake was twofold higher in the femoral skeletal muscle per unit tissue weight in the control subjects than in group 1, 2, and 3 patients with m.3243A>G (Fig. 3). Consequently, the whole-body glucose uptake (M value) measured as the glucose disposal rate was significantly lower in group 3 patients (Table 2) and in the patients with no previously diagnosed diabetes than in the control subjects (pooled groups 1 and 2 vs. control subjects, P = 0.030). A covariance analysis was carried out to adjust for the variation in BMI between the study groups. Analysis of the BMI-adjusted data did not change the reported differences in the means of the skeletal muscle and whole-body insulin sensitivity or in the means of β-cell rate and glucose sensitivity (data not shown). Femoral muscle perfusion per tissue weight was similar in all groups (Fig. 4), suggesting that muscle insulin resistance was caused by decreased glucose extraction rate in the patients with m.3243A>G compared with the control subjects (Fig. 4).
FIG. 3.
Skeletal muscle and whole-body glucose uptake. A: Skeletal muscle insulin-stimulated glucose uptake. B: Correlation between skeletal muscle and whole-body glucose uptake (M value) in all groups (for linear correlation r = 0.78–0.99 and P < 0.007). ○, control subjects. Patients with m.3243A>G mutation: •, group 1; ▴, group 2; and ×, group 3.
FIG. 4.
Perfusion and glucose extraction in skeletal muscle. A: Skeletal muscle blood flow per tissue weight. B: Glucose extraction; glucose uptake per blood flow in skeletal muscle.
Heteroplasmy of the m.3243A>G mutation.
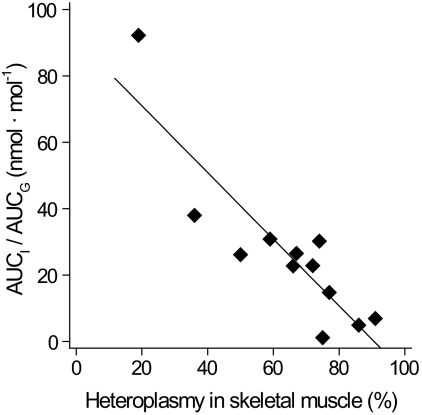
Each subject in groups 1, 2, and 3 harbored at least 19% of the m.3243A>G mutation in buccal epithelium or skeletal muscle. The mutation heteroplasmy in buccal epithelium and skeletal muscle showed a good correlation (r = 0.66, P = 0.019). AUCI/AUCG showed best negative linear correlation with mutation heteroplasmy in skeletal muscle (Fig. 5). This correlation was significant even if the patients with previously diagnosed diabetes (group 3) were excluded (r = −0.71, P = 0.02). Also, other parameters of insulin secretion—IGI, glucose sensitivity, and rate sensitivity—showed a significant negative linear correlation (r = −0.66, P = 0.02; r = −0.81, P = 0.001; and r = −0.57, P = 0.05, respectively) with mutation heteroplasmy in skeletal muscle. A negative linear correlation between rate sensitivity and heteroplasmy in buccal epithelium was also found (r = −0.54, P = 0.04). The whole-body or skeletal muscle glucose uptake as well as the basal insulin secretion did not show significant relationship with the mutation load in the m.3243A>G carriers.
FIG. 5.
β-Cell function and m.3243A>G heteroplasmy. There was an inverse relationship between dynamic insulin secretion parameters and m.3243A>G heteroplasmy. The AUCI/AUCG as a function of m.3243A>G heteroplasmy in skeletal muscle (r = −0.83; P = 0.001).
DISCUSSION
We found insulin resistance in the skeletal muscle already in the early phase of glucose intolerance in subjects harboring the m.3243A>G mutation. The dynamic β-cell response was impaired in those mutation carriers who had good glycemic control and no history of diabetes but who had diabetes in OGTT. On the other hand, the β-cell response was markedly decreased in patients with previously diagnosed diabetes. We also demonstrated a correlation between insulin secretion and mutation heteroplasmy.
This study showed that the skeletal muscle insulin sensitivity is decreased in adult subjects with the m.3243A>G mutation. The decrease was similar in subjects with and without overt diabetes, and it resembled that found in patients with newly diagnosed type 2 diabetes (29). The use of [18F]FDG and PET enabled us to measure glucose uptake directly in the skeletal muscle. Importantly, glucose uptake in muscle measured by this method is not affected by uptake in the liver or adipose tissue, whereas such measurements based on whole-body glucose disposal rate are (33). A standard hyperinsulinemic clamp technique has been used in two previous studies, both including fewer subjects with m.3243A>G than the present study. In one study (18), seven mutation carriers had lower insulin sensitivity than the control subjects. However, the authors concluded that peripheral insulin resistance did not seem to precede diabetes, even if three of the four subjects with no diabetes had insulin sensitivity lower than the mean of the control subjects. In the other study (19), slightly low M values but within normal range were reported in eight m.3243A>G patients with diabetes and one with impaired glucose tolerance. However, it is difficult to deduce the contribution of the m.3243A>G mutation to insulin sensitivity from the previous studies, because the mutation heteroplasmy of the subjects has not been reported.
The prevalence of m.3243A>G has been suggested to be 16 in 100,000 (25), making the recruitment and characterization of these patients challenging. Besides sensorineurinal hearing impairment (Table 1), myopathy and exercise intolerance are rather frequent features among patients with 3243A>G (36). In this study, a structured interview on physical activity (IPAQ) did not reveal differences in daily physical activity between the patients and the control subjects. However, we cannot exclude that the patients with m.3243A>G suffered from a subclinical myopathy even if the MRI scans of the calf (data not shown) and the PET imaging of the thigh did not show signs of it. Additionally, the m.3243A>G mutation has been shown to lead to impaired endothelial function in brain perfusion studies with [15O]H2O PET in severely ill patients (37). However, we found that the perfusion was normal in skeletal muscle at rest, suggesting no major participation of endothelial function in the insulin resistance in patients with m.3243A>G (Fig. 3).
The m.3243A>G mutation affects the oxidative phosphorylation and ATP production (38), but it is unclear how mitochondrial dysfunction could induce insulin resistance in skeletal muscle. Maximal oxidative capacity (Vo2max), reflecting oxidative phosphorylation, correlates with insulin sensitivity in nondiabetic sedentary subjects (38). Vo2max is also decreased in patients with various mtDNA mutations at high heteroplasmy levels in skeletal muscle (39). However, we did not find a correlation between skeletal muscle glucose uptake and m.3243A>G mutation heteroplasmy, suggesting that other factors than the rate of oxidative phosphorylation might contribute. Low ATP production and abnormal mitochondrial morphology are present in skeletal muscle in both type 2 diabetes and in m.3243A>G patients (40,41). Furthermore, insulin-resistant subjects predisposed to type 2 diabetes have been shown to share some similarities with the m.3243A>G patients, such as low activity of the respiratory chain enzymes, especially that of Complex I, in skeletal muscle (36,40–42). Interestingly, a deficiency in Complex I activity increases free radical production (43), which has recently been shown to impair insulin signaling in mice (44). Finally, it has recently been shown that insulin resistance predisposes to the early onset of diabetes in patients with a primary defect in insulin secretion (45). Therefore, regardless of its etiology, insulin resistance might contribute to the very high penetrance of diabetes in patients with m.3243A>G (16).
Previous cross-sectional studies have suggested that insulin secretion is impaired in patients with m.3243A>G and diabetes, even if the response to arginine and glutamate is preserved (19,20,24). One OGTT study has previously included more m.3243A>G carriers than the present study (20). It demonstrated that the IGI was decreased in seven m.3243A>G carriers with normal glucose tolerance. However, in other studies, defects in insulin secretion have not been found (22,23). We found an inverse relationship between β-cell responses and the m.3243A>G mutation heteroplasmy. Lack of heteroplasmy data in previous studies may thus explain the apparently contradicting results in OGTT studies in m.3243A>G patients. Processes such as oxidative stress, impaired protein synthesis, and altered autophagy are common in other organs affected by this mutation (43,46). The onset age of diabetes in patients with m.3243A>G may be determined by a combination of the mutation load and the degenerative processes leading to a further decrease in β-cell function or mass. Interestingly, a reduced β-cell number along with absence of apoptosis has been reported in the pancreatic tissue of patients with m.3243A>G (47).
It has been proposed that the impaired ATP production due to the m.3243A>G mutation in β-cells is followed by failing K+ channel opening that leads to decreased insulin secretion in response to the glucose (38). Low redox state, low mitochondrial membrane potential, dysfunction of mitochondrial NADH shuttles, and low complex I activity could have a similar effect on glucose sensing and insulin secretion (48). The dysfunction in glucose sensing may explain the correlation of insulin secretion with tissue heteroplasmy. However, it fails to explain why none of our subjects were diabetic in their early lives. It has been claimed that impaired fatty acid β-oxidation in mitochondria may lead to inappropriate storage of triacylglycerols in the cells and increased free fatty acid and glucose levels in circulation. The following toxicity in tissues including the β-cells could then induce a decline in the β-cell function in these patients (46). Some of these hypotheses could partly be compatible with our data and the previous observations on progressive β-cell dysfunction, but they are based on data obtained from in vitro studies and need more proof of evidence in clinical studies.
Metabolic effects of mtDNA mutations affecting the respiratory chain have been poorly characterized, although several studies have implicated the role of mitochondrial dysfunction in skeletal muscle insulin resistance, in insulin secretion, and in the pathogenesis of type 2 diabetes (7,13). Our results show that both the skeletal muscle insulin sensitivity and the β-cell function are affected already before the overt diabetic state develops in patients with the m.3243A>G mutation.
Acknowledgments
The study was conducted within the “Centre of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research,” supported by the Academy of Finland, University of Turku, Turku University Hospital, and Abo Academy. Further support was obtained by grants from the Finnish Diabetes Foundation and the Sigrid Juselius Foundation.
No potential conflicts of interest relevant to this article were reported.
We thank Anja Heikkinen for her expert technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 December 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Iozzo P, Hällsten K, Oikonen V, Virtanen KA, Kemppainen J, Solin O, Ferrannini E, Knuuti J, Nuutila P: Insulin-mediated hepatic glucose uptake is impaired in type 2 diabetes: evidence for a relationship with glycemic control. J Clin Endocrinol Metab 88: 2055–2060, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Rönnemaa T, Huupponen R, Nuutila P: Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 87: 3902–3910, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Beck-Nielsen H, Vaag A, Poulsen P, Gaster M: Metabolic and genetic influence on glucose metabolism in type 2 diabetic subjects: experiences from relatives and twin studies. Best Pract Res Clin Endocrinol Metab 17: 445–467, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG: Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322: 223–228, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K: Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI: Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56: 1376–1381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Festa A, Williams K, D'Agostino R Jr, Wagenknecht LE, Haffner SM: The natural course of β-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 55: 1114–1120, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA: Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 55: 1074–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Perry JR, Frayling TM: New gene variants alter type 2 diabetes risk predominantly through reduced beta-cell function. Curr Opin Clin Nutr Metab Care 11: 371–377, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Zeviani M, Di Donato S: Mitochondrial disorders. Brain 127: 2153–2172, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR: Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56: 720–727, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Wiederkehr A, Wollheim CB: Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 147: 2643–2649, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hirai M, Suzuki S, Onoda M, Hinokio Y, Hirai A, Ohtomo M, Chiba M, Kasuga S, Hirai S, Satoh Y, Akai H, Miyabayashi S, Toyota T: Mitochondrial deoxyribonucleic acid 3256C-T mutation in a Japanese family with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83: 992–994, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Tawata M, Hayashi JI, Isobe K, Ohkubo E, Ohtaka M, Chen J, Aida K, Onaya T: A new mitochondrial DNA mutation at 14577 T/C is probably a major pathogenic mutation for maternally inherited type 2 diabetes. Diabetes 49: 1269–1272, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Guillausseau PJ, Massin P, Dubois-LaForgue D, Timsit J, Virally M, Gin H, Bertin E, Blickle JF, Bouhanick B, Cahen J, Caillat-Zucman S, Charpentier G, Chedin P, Derrien C, Ducluzeau PH, Grimaldi A, Guerci B, Kaloustian E, Murat A, Olivier F, Paques M, Paquis-Flucklinger V, Porokhov B, Samuel-Lajeunesse J, Vialettes B: Maternally inherited diabetes and deafness: a multicenter study. Ann Intern Med 134: 721–728, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Chinnery PF, Elliott C, Green GR, Rees A, Coulthard A, Turnbull DM, Griffiths TD: The spectrum of hearing loss due to mitochondrial DNA defects. Brain 123: 82–92, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Velho G, Byrne MM, Clement K, Sturis J, Pueyo ME, Blanche H, Vionnet N, Fiet J, Passa P, Robert JJ, Polonsky KS, Froguel P: Clinical phenotypes, insulin secretion, and insulin sensitivity in kindreds with maternally inherited diabetes and deafness due to mitochondrial tRNALeu(UUR) gene mutation. Diabetes 45: 478–487, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Iizuka T, Kobayashi T, Nishikawa T, Atsumi Y, Kadowaki T, Oka Y, Kadowaki H, Taniyama M, Hosokawa K, Asahina T, Matsuoka K: Diabetes mellitus associated with the 3243 mitochondrial tRNA(Leu)(UUR) mutation: insulin secretion and sensitivity. Metabolism 46: 1019–1023, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Hinokio Y, Hirai S, Onoda M, Matsumoto M, Ohtomo M, Kawasaki H, Satoh Y, Akai H, Abe K: Pancreatic beta-cell secretory defect associated with mitochondrial point mutation of the tRNA(LEU(UUR)) gene: a study in seven families with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS). Diabetologia 37: 818–825, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Salles JE, Kasamatsu TS, Dib SA, Moises RS: Beta-cell function in individuals carrying the mitochondrial tRNA leu (UUR) mutation. Pancreas 34: 133–137, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Holmes-Walker DJ, Ward GM, Boyages SC: Insulin secretion and insulin sensitivity are normal in non-diabetic subjects from maternal inheritance diabetes and deafness families. Diabet Med 18: 381–387, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Gebhart SS, Shoffner JM, Koontz D, Kaufman A, Wallace D: Insulin resistance associated with maternally inherited diabetes and deafness. Metabolism 45: 526–531, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Brändle M, Lehmann R, Maly FE, Schmid C, Spinas GA: Diminished insulin secretory response to glucose but normal insulin and glucagon secretory responses to arginine in a family with maternally inherited diabetes and deafness caused by mitochondrial tRNA(LEU(UUR)) gene mutation. Diabetes Care 24: 1253–1258, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Majamaa K, Moilanen JS, Uimonen S, Remes AM, Salmela PI, Kärppä M, Majamaa-Voltti KA, Rusanen H, Sorri M, Peuhkurinen KJ, Hassinen IE: Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63: 447–454, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tehard B, Saris WH, Astrup A, Martinez JA, Taylor MA, Barbe P, Richterova B, Guy-Grand B, Sorensen TI, Oppert JM: Comparison of two physical activity questionnaires in obese subjects: the NUGENOB study. Med Sci Sports Exerc 37: 1535–1541, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Vancauter E, Mestrez F, Sturis J, Polonsky KS: Estimation of insulin-secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41: 368–377, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Mari A, Tura A, Gastaldelli A, Ferrannini E: Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 51: S221–S226, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Hällsten K, Virtanen KA, Lönnqvist F, Sipilä H, Oksanen A, Viljanen T, Rönnemaa T, Viikari J, Knuuti J, Nuutila P: Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 51: 3479–3485, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hamacher K, Coenen HH, Stocklin G: Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27: 235–238, 1986 [PubMed] [Google Scholar]
- 31.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 32.Hällsten K, Yki-Järvinen H, Peltoniemi P, Oikonen V, Takala T, Kemppainen J, Laine H, Bergman J, Bolli GB, Knuuti J, Nuutila P: Insulin- and exercise-stimulated skeletal muscle blood flow and glucose uptake in obese men. Obes Res 11: 257–265, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Peltoniemi P, Lönnroth P, Laine H, Oikonen V, Tolvanen T, Grönroos T, Strindberg L, Knuuti J, Nuutila P: Lumped constant for [(18)F]fluorodeoxyglucose in skeletal muscles of obese and nonobese humans. Am J Physiol Endocrinol Metab 279: E1122–E1130, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Sutinen J, Yki-Järvinen H: Increased resting energy expenditure, fat oxidation, and food intake in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab 292: E687–E692, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Hochberg Y, Benjamini Y: Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57: 289–300, 1995 [Google Scholar]
- 36.Kärppä M, Herva R, Moslemi AR, Oldfors A, Kakko S, Majamaa K: Spectrum of myopathic findings in 50 patients with the 3243A>G mutation in mitochondrial DNA. Brain 128: 1861–1869, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Koga Y, Akita Y, Junko N, Yatsuga S, Povalko N, Fukiyama R, Ishii M, Matsuishi T: Endothelial dysfunction in MELAS improved by l-arginine supplementation. Neurology 66: 1766–1769, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Maassen JA, Janssen GM, 'T Hart LM: Molecular mechanisms of mitochondrial diabetes (MIDD). Ann Med 37: 213–221, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Jeppesen TD, Schwartz M, Olsen DB, Vissing J: Oxidative capacity correlates with muscle mutation load in mitochondrial myopathy. Ann Neurol 54: 86–92, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Kelley DE, He J, Menshikova EV, Ritov VB: Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Vydt TC, de Coo RF, Soliman OI, Ten Cate FJ, van Geuns RJ, Vletter WB, Schoonderwoerd K, van den Bosch BJ, Smeets HJ, Geleijnse ML: Cardiac involvement in adults with m.3243A>G MELAS gene mutation. Am J Cardiol 99: 264–269, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ: Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab 92: 1467–1473, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J: ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320: 661–664, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Hitomi H, Fukui T, Moriwaki K, Matsubara K, Sun GP, Rahman M, Nishiyama A, Kiyomoto H, Kimura S, Ohmori K, Abe Y, Kohno M: Synergistic effect of mechanical stretch and angiotensin II on superoxide production via NADPH oxidase in vascular smooth muscle cells. J Hypertens 24: 1089–1095, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Martin D, Bellanne-Chantelot C, Deschamps I, Froguel P, Robert JJ, Velho G: Long-term follow-up of oral glucose tolerance test-derived glucose tolerance and insulin secretion and insulin sensitivity indexes in subjects with glucokinase mutations (MODY2). Diabetes Care 31: 1321–1323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maassen JA, Romijn JA, Heine RJ: Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia 50: 2036–2041, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otabe S, Yasuda K, Mori Y, Shimokawa K, Kadowaki H, Jimi A, Nonaka K, Akanuma Y, Yazaki Y, Kadowaki T: Molecular and histological evaluation of pancreata from patients with a mitochondrial gene mutation associated with impaired insulin secretion. Biochem Biophys Res Commun 259: 149–156, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Maechler P, Carobbio S, Rubi B: In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol 38: 696–709, 2006 [DOI] [PubMed] [Google Scholar]