Abstract
OBJECTIVE—Skeletal muscle insulin resistance is associated with lipid accumulation, but whether insulin resistance is due to reduced or enhanced flux of long-chain fatty acids into the mitochondria is both controversial and unclear. We hypothesized that skeletal muscle–specific overexpression of the muscle isoform of carnitine palmitoyltransferase 1 (CPT1), the enzyme that controls the entry of long-chain fatty acyl CoA into mitochondria, would enhance rates of fatty acid oxidation and improve insulin action in muscle in high-fat diet insulin-resistant rats.
RESEARCH DESIGN AND METHODS—Rats were fed a standard (chow) or high-fat diet for 4 weeks. After 3 weeks, in vivo electrotransfer was used to overexpress the muscle isoform of CPT1 in the distal hindlimb muscles (tibialis anterior and extensor digitorum longus [EDL]). Skeletal muscle insulin action was examined in vivo during a hyperinsulinemic-euglycemic clamp.
RESULTS—In vivo electrotransfer produced a physiologically relevant increase of ∼20% in enzyme activity; and although the high-fat diet produced insulin resistance in the sham-treated muscle, insulin action was improved in the CPT1-overexpressing muscle. This improvement was associated with a reduction in triacylglycerol content, the membrane-to-cytosolic ratio of diacylglycerol, and protein kinase C θ activity. Importantly, overexpression of CPT1 did not affect markers of mitochondrial capacity or function, nor did it alter skeletal muscle acylcarnitine profiles irrespective of diet.
CONCLUSIONS—Our data provide clear evidence that a physiological increase in the capacity of long-chain fatty acyl CoA entry into mitochondria is sufficient to ameliorate lipid-induced insulin resistance in muscle.
The pathogenesis of insulin resistance is a well-investigated area of research, but the precise molecular mechanisms that lead to this disorder are not fully understood. Emerging evidence suggests that insulin resistance, at least in skeletal muscle, is caused by dysregulated signaling processes secondary to lipid accumulation (1–4). Although the increase in lipid content is manifested as an increase in triacylglycerol (TAG), it is likely that elevated TAG may only serve as a marker of dysfunctional muscle fatty acid metabolism and that accumulation of bioactive lipids such as diacylglycerol (DAG) and/or ceramide is actually responsible for the insulin resistance (2,3,5). DAG can activate several isoforms of protein kinase C (PKC), which can impair insulin signal transduction via serine phosphorylation of insulin receptor substrate (IRS)-1 (6,7). Ceramides can cause insulin resistance by preventing insulin-stimulated Akt serine phosphorylation and activation and translocation of Akt to its substrate (8,9). In addition, ceramide initiates inflammatory signaling pathways, leading to the activation of both c-jun NH2-terminal kinase (JNK) and nuclear factor κB/inducer of κ kinase (10), which have been implicated in the development of insulin resistance (11–13).
Several factors may contribute to increased lipid deposition in muscle. An increase in fatty acid uptake without any change in oxidation could lead to cytosolic lipid accumulation (14). Conversely, an impaired ability to utilize fat as a fuel source because of reduced activity of enzymes of oxidative metabolism and fatty acid utilization could also result in increased cytosolic lipids (15–17). Recently, the concept of defective fatty acid oxidation causing insulin resistance has been challenged. Muoio and colleagues (18) have suggested that the increased flux of long-chain fatty acids into the mitochondria is not accompanied by complete β-oxidation because of the inability of the tricarboxylic acid (TCA) cycle to cope with the increase in the demand on fatty acid metabolism. This leads to intramitochondrial metabolite accumulation, mitochondrial stress, and cellular insulin resistance (18). Thus, the role of fatty acid oxidation in regulating insulin sensitivity is controversial and mechanisms remain unresolved. Carnitine palmitoyltransferase 1 (CPT1) is a mitochondrial transmembrane enzyme thought to be rate limiting for long-chain fatty acid entry into the mitochondria for β-oxidation (16,19). Inhibition of CPT1 with the chemical etomoxir increases lipid deposition and exacerbates insulin resistance when animals are placed on a high-fat diet (20), whereas overexpression of CPT1 protects myotubes against lipid-induced insulin resistance (21,22), arguing that alterations in fatty acid flux into the mitochondria are critical in regulating lipid effects on insulin sensitivity. Thus, to test whether increasing the capacity for fatty acid flux into the mitochondria is, in itself, sufficient to increase fat oxidation and alter insulin action, we used an approach in which we selectively overexpressed the muscle isoform of CPT1 in skeletal muscle in vivo. The results show that a physiological increase in CPT1 activity is sufficient to improve insulin resistance caused by a high-fat diet, suggesting that entry of long-chain fatty acids into the mitochondria is more critical in the regulation of fatty acid oxidation than the downstream capacity of the β-oxidation and TCA cycle.
RESEARCH DESIGN AND METHODS
Vector construction.
The human CPT1B cDNA was a gift from Dr. Feike van der Leij (van Hall University of Applied Sciences, Leeuwarden, the Netherlands) and was constructed as previously described (23).
Animal maintenance and surgery.
Male Wistar rats (∼200 g; Animal Resources Centre, Perth, Australia) were acclimatized at 22 ± 0.5°C under a 12-h light/12-h dark cycle for 1 week on a standard chow diet (8% calories from fat, 21% calories from protein, and 71% calories from carbohydrate; Gordon's Specialty Stock Feeds, Yanderra, New South Wales, Australia) with ad libitum water. Thereafter, one-half of the animals were switched to a high-fat diet (45% of calories from fat [lard]), 20% calories from protein, and 35% calories from carbohydrates; 4.7 kcal/g; based on Rodent Diet D12451; Research Diets, New Brunswick, NJ for 4 weeks. One week before study, the right and left jugular veins of rats designated to undergo euglycemic-hyperinsulinemic clamp studies were cannulated as previously described (24). Rats were single housed and handled daily to minimize stress. Body weight was recorded daily, and only those rats that had fully recovered their presurgery weight were subsequently studied. All experimental procedures were approved by the Garvan Institute/St. Vincent's Hospital Animal Experimentation Ethics Committee and were in accordance with the National Health and Medical Research Council (NHMRC) of Australia Guidelines on Animal Experimentation.
In vivo electrotransfer.
The purifed CPT1 plasmid was directly injected into rat tibialis anterior muscle by a previously described protocol (23,25,26). The tibialis anterior muscle was chosen because it can be electroporated easily without the requirement of surgery, and the fiber composition is representative of the entire musculature of the rat. We have previously reported that this protocol results in a transfection rate of ∼50% of fibers (25,26). Hyperinsulinemic-euyglycemic clamps (subsequently described) were performed on animals 7 days after transfecting the tissue, a time point when we have shown peak CPT1 overexpression (23). Because we also observed overexpression in the extensor digitorum longus (EDL) muscle, which lies in close proximity to the tibialis anterior muscle, EDL was sampled from a subset of animals 7 days after electroporation, and glucose uptake and fatty acid oxidation (described below) were determined. Unless otherwise stated, the muscles were rapidly freeze-clamped using precooled tongs and stored at −80°C.
PCR and Western blot analysis.
The mRNA abundance of genes encoding the proteins involved in electron transfer/oxidative phosphorylation (ET/OxPhos), peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1 α), nuclear respiratory factor-1 (NRF-1), mitochondrial transcription factor A (tFAM), and protein abundance were analyzed using real-time RT-PCR and Western blot analysis as previously described (27,28).
CPT1 activity and palmitate metabolism.
Mitochondria were isolated using a previously described protocol (23,29,30). The forward radioisotope assay for the determination of CPT1 (EC 2.3.1.21) activity was used as described by McGarry et al. (31) with minor modifications (23). To examine palmitate metabolism in control and transfected muscles, the EDL muscle was carefully dissected into longitudinal strips from tendon to tendon by use of a 27-gauge needle. Strips were removed and assayed as previously described (23,32).
Glucose uptake and insulin signaling in isolated EDL muscle.
Glucose uptake in isolated EDL muscle strips was assayed in sealed flasks containing pregassed (95% O2/5% CO2) Krebs-Henseleit bicarbonate buffer, pH 7.4, supplemented with 2 mmol/l sodium pyruvate, 8 mmol/l mannitol, and 0.1% wt/vol BSA at 30°C. Muscles were allowed to recover for 30 min and were incubated without or with insulin (1 mU/ml) for 30 min. Glucose uptake was assayed for 15 min using [3H]-2-deoxy-d-glucose (1 mmol/l; 0.128 μCi/ml) as described previously (33). For analysis of insulin signaling, isolated EDL muscles were preincubated for 30 min and were stimulated for 15 min with 1 mU/ml insulin. Muscles were rinsed with ice-cold saline, blotted, and freeze- clamped for subsequent analysis.
Hyperinsulinemic-euglycemic clamp.
Conscious rats were studied after 5–7 h of fasting. Between 0900 and 1000 h, jugular cannulae were either connected to a sampling line or an infusion line, and after 30–40 min, hyperinsulinemic-euglycemic clamps commenced (34), incorporating administration of a bolus injection of 2-deoxy-d-[2,6-3H]glucose (GE Healthcare Life Sciences, Buckinghamshire, U.K.). Insulin was infused at a rate of 0.25 units · kg−1 · h−1. After 120 min, rats were killed by intravenous injection of sodium pentobarbital, and the tibialis anterior muscles were rapidly dissected and freeze-clamped. The area under the tracer disappearance curve of 2-deoxy-d-[2,6,-3H]glucose together with the counts of phosphorylated [3H]deoxyglucose from individual muscles were used to calculate the glucose metabolic index (Rg′). During the clamp procedure, plasma glucose was determined every 10 min.
Subcellular fractionation and PKC analysis and intramuscular lipid analysis.
Tibialis anterior muscle was homogenized in ice-cold buffer containing 20 mmol/l HEPES (pH 7.4), 2 mmol/l EGTA, 50 mmol/l ß-glycerophosphate, 1 mmol/l dithiothreitol, 1 mmol/l Na3VO4, 10% glycerol, 3 mmol/l benzamidine, 10 μmol/l leupeptin, 5 μmol/l pepstatin A, and 1 mmol/l phenylmethylsulfonylfluoride. The homogenate was centrifuged at 92,000 × g for 30 min at 4°C, and the supernatant was collected as the cytosolic fraction. The pellet was resuspended in ice-cold homogenization buffer to which 1% NP-40 was added. The resuspended pellet was incubated on ice for 30 min and was centrifuged at 50,000 × g for 60 min at 4°C. The supernatant, representing the total membrane fraction, was collected, and the protein content of both fractions determined. Western blotting was performed using antibodies specific for PKCθ, -α/β, or -δ (Cell Signaling Technology). TAG content was determined in the tibialis anterior muscles using an enzymatic colorimetric technique as previously described (23). DAG and ceramide were extracted and quantified according to the enzymatic-radiometric methods of Preiss et al. (35). DAG and ceramide content was also determined in the membrane and cytosolic fractions obtained from subcellular fractionation (described above) to examine the intracellular partitioning of these lipids.
Blood analysis.
Blood was transferred into tubes containing EDTA and centrifuged, and the plasma was stored at −80°C for later analysis. Plasma glucose was determined using an automated glucose analyzer (YSI 2300; YSI, Yellow Springs, OH). Plasma free fatty acid (FFA) was assessed by an enzymatic colorimetric method (Wako; Wako Chemicals, Richmond, VA). Plasma TAG was determined using an enzymatic colorimetric technique (Triglycerides GPO-PAP; Roche Diagnostics, Indianapolis, IN). Basal insulin was assessed using a rat/mouse insulin enzyme-linked immunosorbent assay (Linco, St. Charles, MO), and clamp insulin was assessed using human ELISA (Mercodia, Uppsala, Sweden).
Acylcarnitine analysis.
Muscle acylcarnitines were analyzed according to the method of van Vlies et al. (36). Muscle (∼60 mg) was lyophyllized and powdered before addition of stable isotope internal standards (NeoGen Cambridge Isotope Laboratory, Andover, MA) in 1 ml 80% acetonitrile. After sonication and centrifugation, the supernatant was dried under nitrogen, and the acylcarnitines were converted to their butyl esters with butanolic HCl. After evaporation of the butanol, the residue was dissolved in 100 μl 50% acetonitrile, and tandem mass spectrometric analysis was performed (Quattro LC; Waters, Milford, MA).
Statistics.
Data are reported as means ± SE. Comparisons between data from multiple treatment groups were made using factorial ANOVA followed by Tukey's post hoc analysis. To specifically examine differences in insulin sensitivity and signaling between control and transfected muscles in animals fed a high-fat diet, data were analyzed with paired t tests. Statistical significance was accepted at P < 0.05.
RESULTS
CPT1 overexpression increases CPT1 activity and fatty acid oxidation and reduces fatty acid incorporation into TAG.
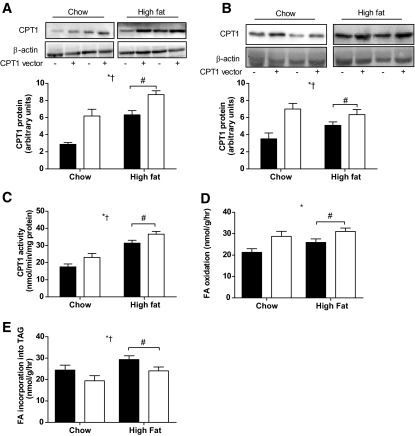
The physiological characteristics of the chow and high-fat diet–treated animals are presented in Table 1. Because the CPT1 antibody cross-reacts with both mouse and human CPT1, it recognized both the endogenous and electroporated forms of the protein. In vivo electroporation resulted in CPT1 being expressed above endogenous levels by ∼100% in the tibialis anterior and EDL (Fig. 1A and B; P < 0.05) of chow animals. CPT1 protein was increased ∼40% (Fig. 1A; P < 0.05) in the tibialis anterior and ∼30% in the EDL (Fig. 1B; P < 0.05) in high-fat diet–fed rats. CPT1 protein and activity were increased by the high-fat diet (Fig. 1A and B; P < 0.05). CPT1 activity was increased by ∼40% by CPT1 overexpression in chow animals and ∼20% in high-fat diet–fed animals (Fig. 1C; P < 0.05). Although fatty acid oxidation tended to be elevated by high-fat diet, the increase was not statistically significant (Fig. 1D; P = 0.07). Importantly, CPT1 overexpression increased fatty acid oxidation in EDL muscles obtained from chow and high-fat diet–fed animals (Fig. 1D; P < 0.05). Palmitate incorporation into DAG was unchanged in muscles that overexpressed CPT1 (chow empty vector 11.7 ± 1.0 nmol/g; chow CPT1 vector 13.4 ± 0.5 nmol/g; high-fat diet empty vector 11.6 ± 1.8 nmol/g; high-fat diet CPT1 vector 10.3 ± 1.2 nmol/g). However, palmitate incorporation into TAG was increased with high-fat diet (P < 0.05) but was reduced in muscles overexpressing CPT1 to levels comparable with chow animals (Fig. 1E).
TABLE 1.
Characteristics of rats fed a chow or high-fat diet for 4 weeks
| Chow | High fat | |
|---|---|---|
| Body mass (g) | 316 ± 13 | 340 ± 8 |
| Plasma glucose (mmol/l) | 7.1 ± 0.3 | 7.6 ± 0.3 |
| Plasma insulin (pmol/l) | 187 ± 22 | 285 ± 51* |
| Plasma FFA (μmol/l) | 0.56 ± 0.05 | 0.74 ± 0.07 |
| Plasma TAG (mmol/l) | 0.94 ± 0.03 | 1.05 ± 0.24 |
| Epididymal fat (g) | 0.95 ± 0.12 | 1.60 ± 0.15* |
| Subcutaneous fat (g) | 1.49 ± 0.14 | 2.36 ± 0.21* |
| Glucose infusion rate (mg · kg−1 · min−1) | 40.1 ± 2.6 | 25.4 ± 2.0* |
Data are means ± SE.
Significantly different from chow (P < 0.05), n = 9–12.
FIG. 1.
CPT1 overexpression increases CPT1 activity and fatty acid oxidation and reduces fatty acid incorporation into TAG. Representative immunoblots and quantification of CPT1 protein expression in tibialis anterior (A), representative immunoblots and quantification of CPT1 protein expression in EDL muscle (B), CPT1 activity in isolated mitochondria from tibialis anterior muscles (C), fatty acid oxidation rate in isolated EDL muscles ex vivo (D), and fatty acid incorporation into TAG in isolated EDL muscles ex vivo (E) from a leg electroporated with an empty vector (▪) or CPT1 vector (□) in animals placed either on a standard chow diet or high-fat diet for 4 weeks. Data are means ± SE; n = 8. *P < 0.05 main effect for CPT1 expression; †P < 0.05 main effect for diet; #P < 0.05 vs. high-fat diet control leg.
The effect of CPT1 overexpression on markers of oxidative capacity and mitochondrial function.
We observed a high-fat diet–induced increase in the activity of β-hydroxyacyl-CoA-dehydrogenase (β-HAD; Table 2; P < 0.05) and a trend for increased PGC-1α protein expression in tibialis anterior (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1078/DC1), results consistent with a previous study from our group (37). Neither the high-fat diet nor CPT1 overexpression affected the expression of the ET/OxPhos genes in tibialis anterior muscles (Table 2). We also measured the maximal activity of another important mitochondrial enzyme, citrate synthase (Table 2), and the protein abundance of complex I, II, and V proteins in the ET/OxPhos chain (supplementary Fig. 1) and observed no significant effect of either high-fat diet or CPT1 overexpression.
TABLE 2.
Markers of oxidative metabolism
| Chow
|
High fat
|
|||
|---|---|---|---|---|
| Empty vector | CPT1 vector | Empty vector | CPT1 vector | |
| Citrate synthase (μmol · min−1 · g−1 wet wt) | 3.8 ± 0.4 | 3.7 ± 0.3 | 4.0 ± 0.6 | 3.6 ± 0.4 |
| Β-HAD (μmol · min−1 · g−1 wet wt)* | 2.3 ± 0.2 | 2.2 ± 0.4 | 5.1 ± 0.4 | 4.7 ± 0.6 |
| PGC1α mRNA | 1.0 ± 0.2 | 1.3 ± 0.7 | 1.2 ± 0.3 | 1.1 ± 0.2 |
| NRF-1 mRNA | 1.0 ± 0.2 | 1.6 ± 0.9 | 1.3 ± 1.0 | 1.6 ± 0.6 |
| tFAM mRNA | 1.0 ± 0.2 | 1.2 ± 0.8 | 1.1 ± 0.4 | 0.7 ± 0.2 |
Data are means ± SE. Values for mRNA expression are expressed relative to the value obtained from the control leg of chow-fed animals using ribosomal 18S as a housekeeping gene.
Significant main effect for diet (P < 0.05), n = 5–7.
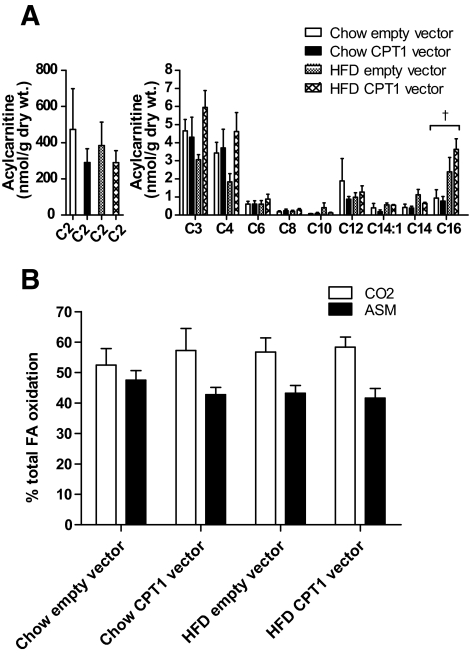
To investigate whether increased flux of fatty acid into the mitochondria would impair β-oxidation via changes in key metabolite levels, we determined the effect of a high-fat diet and increased CPT1 activity on acylcarnitine levels and the ratio of fatty acid oxidation to CO2 and acid soluble metabolites (ASMs) in EDL muscles. Using tandem mass spectrometry we analyzed several muscle acylcarnitine species ranging from C2 to C16. Free carnitine levels were not altered by diet or CPT1 overexpression (supplementary Fig. 2, available in the online appendix). We showed that most muscle acylcarnitine species were affected by neither high-fat diet nor CPT1 overexpression. However, we observed a small increase in C16 acylcarnitine in high-fat diet–fed animals (P < 0.05), a result consistent with previous observations (18). Importantly, however, CPT1 overexpression did not exacerbate C16 acylcarnitine levels in high-fat diet–fed rats (Fig. 2A). To assess whether this small increase in C16 acylcarnitine levels in high-fat diet–fed rats affected markers of mitochondrial metabolic control, we measured fatty acid oxidation to CO2 and ASM fractions, because if fatty acids are incompletely oxidized, the percentage of oxidation to CO2 should fall, leading to a concomitant increase in the percentage of fatty acid metabolites in the ASM fraction. However, we show that neither the high-fat diet nor CPT1 overexpression affected the percentage of fatty acid oxidation in either the ASM or CO2 fraction (Fig. 2B), although both high-fat diet and CPT1 overexpression increased the total oxidation of fatty acids by EDL ex vivo (Fig. 1C).
FIG. 2.
Muscle acylcarnitine levels and the ratio of fatty acid oxidation to CO2 and ASMs in overnight fasted animals. Acylcarnitine levels in tibialis anterior muscle (A) and the ratio of fatty acid oxidation to CO2 and ASMs (B) in EDL muscle from a leg electroporated with an empty vector or CPT1 vector, in animals placed either on a standard chow diet or high-fat diet (HFD) for 4 weeks. Data are means ± SE; n = 8.
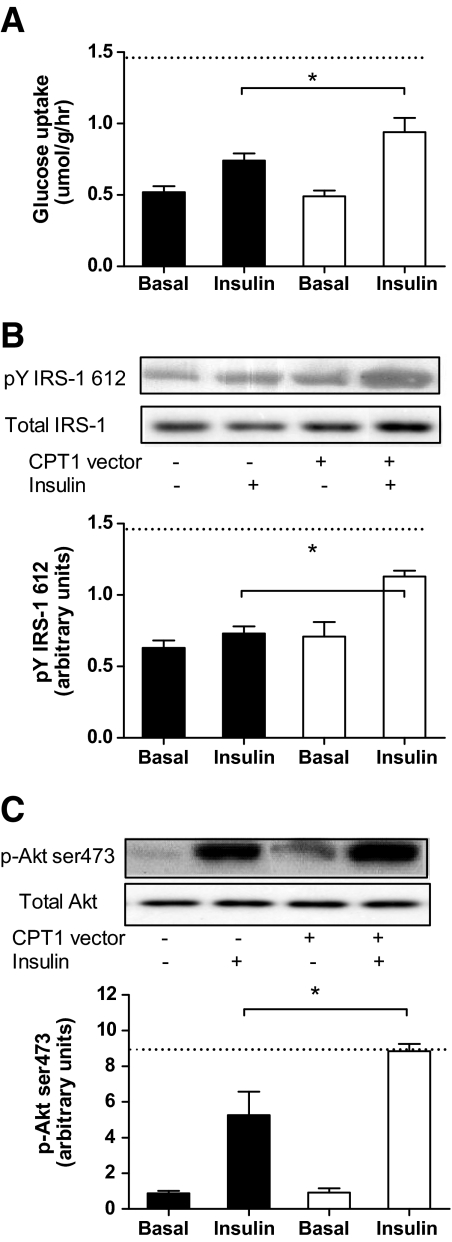
CPT1 overexpression restores insulin-stimulated glucose uptake and insulin signaling due to diet-induced obesity.
Insulin increased glucose uptake (∼twofold, P < 0.05), phosphorylation of IRS1 (Tyr612; ∼twofold, P < 0.05) and Akt (Ser473; ∼10-fold, P < 0.05) in the EDL. CPT1 overexpression had no effect on these measures in chow-fed animals (supplementary Fig. 3, available in the online appendix). However, although the high-fat diet reduced insulin-stimulated glucose uptake in the control leg by ∼30%, overexpression of CPT1 in the EDL of high-fat diet–fed animals increased insulin-stimulated glucose uptake to levels comparable with control chow-fed animals (Fig. 3A). Whereas the high-fat diet reduced the phosphorylation of IRS-1 and Akt under insulin-stimulated conditions (P < 0.05), CPT1 overexpression in the EDL of high-fat diet–fed animals restored insulin-stimulated phosphorylation of IRS-1 (Fig. 3B) and Akt (Fig. 3C) to levels observed in control chow-fed animals.
FIG. 3.
CPT1 overexpression protects against high-fat feeding–induced decrements in insulin action and signaling in skeletal muscle ex vivo. Rates of basal and insulin-stimulated 2-deoxyglucose uptake in isolated EDL muscle from a leg electroporated with an empty vector (▪) or CPT1 vector (□), in animals placed on a high-fat diet for 4 weeks (A); representative immunoblots and quantification of phosphorylation of (Tyr612)/total IRS1 (B); and phosphorylation (Ser473)/total Akt (C) in isolated EDL muscle obtained from a leg electroporated with an empty vector or CPT1 vector, in animals placed on a high fat-diet for 4 weeks and then incubated in the presence (Insulin) or absence (Basal) of insulin. Dashed line represents the level of insulin-mediated glucose uptake or phosphorylation observed in the empty vector leg from chow-fed animals. Data are means ± SE; n = 7. *P < 0.05 vs. high-fat diet control leg under insulin-stimulated conditions.
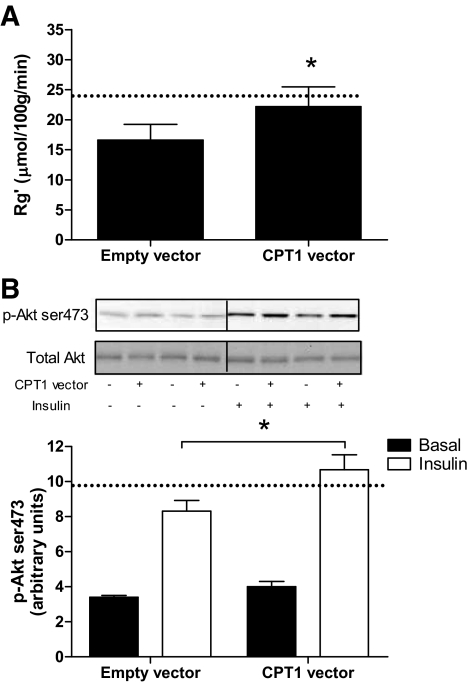
Lipid handling by muscle and its role in the etiology of insulin resistance is a process that involves tissue cross-talk that is highly influenced by circulatory factors and flux of fatty acids across the plasma membrane. Therefore, we examined the effect of CPT1 overexpression on insulin action in vivo, by conducting hyperinsulinemic-euglycemic clamps with the infusion of radiolabeled 2-deoxyglucose to measure glucose uptake (Rg′) in tibialis anterior muscles. The high-fat diet increased fat pad mass and induced whole-body insulin resistance, as shown by an increase in basal insulin levels (Table 1) and a reduction in the glucose infusion rate during the clamp. Fasting plasma glucose and FFA were not different after high-fat diet (Table 1). Rg′ was reduced in the tibialis anterior of high-fat diet–fed animals (32%, P < 0.05; Fig. 4A), but overexpression of CPT1 in the tibialis anterior of chow-fed animals had no effect on Rg′ (supplementary Fig. 4, available in the online appendix). In contrast, overexpression of CPT1 in high-fat diet–fed animals prevented the decrease in Rg′, restoring rates comparable with the control leg of chow-fed animals (24.3 ± 3.3 vs. 22.2 ± 3.3 μmol · 100 g−1 · min−1 for chow and high-fat diet, respectively; Fig. 4A). Consistent with these findings, the phosphorylation of Akt (Ser473) was enhanced in the tibialis anterior muscles of high-fat diet–fed animals overexpressing CPT1 (Fig. 4B).
FIG. 4.
CPT1 overexpression protects against high-fat feeding–induced decrements in insulin action and signaling in skeletal muscle in vivo. 2-Deoxyglucose uptake (Rg′) (A) and representative immunoblots and quantification of phosphorylation (Ser473)/total Akt (B) in tibialis anterior muscle obtained from a leg electroporated with an empty vector or CPT1 vector, in animals placed on a high-fat diet for 4 weeks and then underwent a hyperinsulinemic-euglycemic clamp for 120 min. Dashed line represents the level of insulin-mediated glucose uptake or Akt phosphorylation observed in the empty vector leg from chow-fed animals. Data are means ± SE; n = 7. †P < 0.05 main effect for diet; #P < 0.05 vs. high-fat diet control leg.
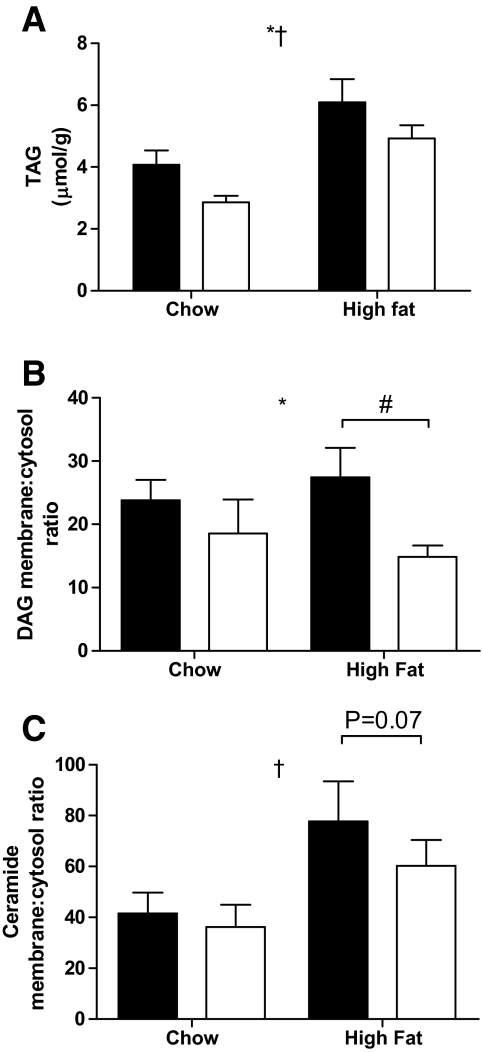
CPT1 overexpression decreases high-fat diet–induced accumulation of TAG, the membrane to cytosolic ratio of DAG, serine phosphorylation of IRS-1, and activation of PKCθ and JNK.
The high-fat diet increased TAG, whereas overexpression of CPT1 decreased TAG in both chow and high-fat diet conditions (Fig. 5A). Both DAG and ceramide content in the tibialis anterior muscle were increased with high-fat diet, but overexpression of CPT1 affected neither DAG nor ceramide content (data not shown). However, the bioactivity of deleterious lipid species is critically dependent on their cellular localization. We, therefore, measured the membrane-to-cytosolic ratio of DAG and ceramide. The membrane-to-cytosolic ratio of DAG was reduced in muscles from high-fat diet–fed animals overexpressing CPT1 (Fig. 5B). In addition, although not statistically significant, there was a trend (P = 0.07) for the same to occur in the membrane-to-cytosolic ratio of ceramide (Fig. 5C).
FIG. 5.
CPT1 overexpression decreases TAG accumulation and the membrane-to-cytosolic ratio of DAG in skeletal muscle. TAG content (A) and the membrane-to-cytosol ratio of DAG (B) and ceramide (C) in tibialis anterior muscle obtained from a leg electroporated with an empty vector (▪) or CPT1 vector (□), in animals placed either on a standard chow diet or high-fat diet for 4 weeks. Data are means ± SE; n = 5–7. *P < 0.05 main effect for CPT1 expression; †P < 0.05 main effect for diet; #P < 0.05 vs. high-fat diet control leg.
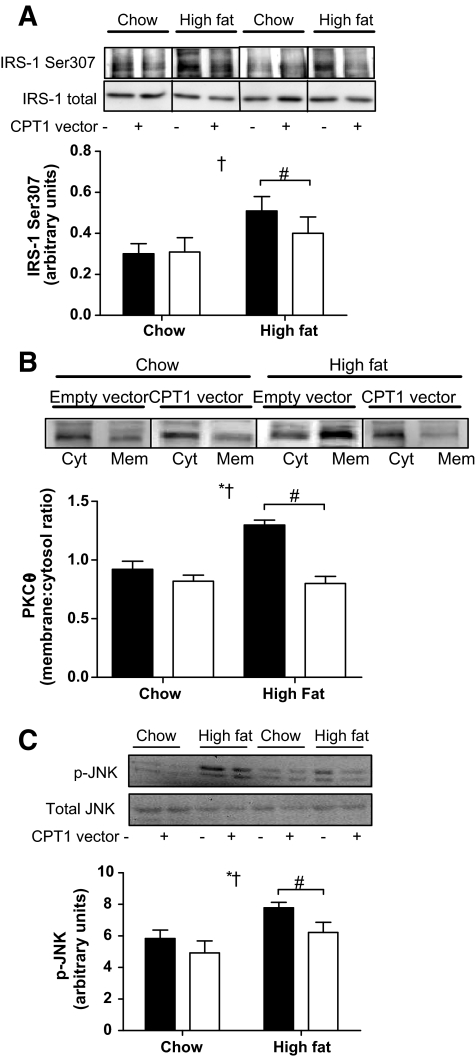
Given that ceramide and DAG act as second messengers, we next measured a raft of signal transduction pathways known to affect insulin action. The high-fat diet increased phosphorylation of IRS-1 (Ser307; Fig. 6A), the membrane-to-cytosolic ratio of PKCθ (Fig. 6B), and the phosphorylation of JNK (Thr183/Tyr185). However, these effects were ameliorated by CPT1 overexpression (Fig. 6A–C). There were no effects of either CPT1 overexpression or high-fat diet on FAT/CD36 expression, the phosphorylation status and protein expression of AMPK, ACC, IkBα, and MLK3, and membrane-associated PKCα/β or -δ (supplementary Fig. 5).
FIG. 6.
CPT1 overexpression protects against high-fat feeding–induced decrements in serine phosphorylation of IRS1 and activation of PKCθ and JNK in skeletal muscle. Representative immunoblots and quantification of phosphorylation (Ser307)/total IRS1 (A), membrane-to-cytosol ratio of PKCθ (B), and phosphorylation (Thr183/Tyr185)/total JNK (C) in tibialis anterior muscle obtained from a leg electroporated with an empty vector (▪) or CPT1 vector (□), in animals placed either on a standard chow diet or high-fat diet for 4 weeks. Data are means ± SE; n = 5–7. *P < 0.05 main effect for CPT1 expression; †P < 0.05 main effect for diet; #P < 0.05 vs. high-fat diet control leg.
DISCUSSION
Although it has been known for many years that excess lipid leads to insulin resistance, the precise molecular mechanisms linking lipid oversupply to defective insulin action are not fully elucidated. It has been suggested that enhancing fatty acid flux through the mitochondria in the absence of a concomitant “metabolic remodeling” of mitochondrial machinery may lead to deleterious effects on insulin action (38), whereas other studies report that increasing fatty acid oxidation can ameliorate insulin resistance by reducing intracellular lipid levels (22,39). Here, we report that a physiologically relevant increase in skeletal muscle CPT1 protein expression was sufficient to increase fatty acid oxidation and to prevent high-fat diet–induced fatty acid esterification into intracellular lipids, subsequently leading to enhanced muscle insulin sensitivity in high-fat–fed rats in the absence of major changes in markers of downstream capacity of the TCA cycle and ET/OxPhos pathways. The insulin-sensitizing effect of CPT1 overexpression was associated with a lower membrane-to-cytosolic ratio of DAG and reduced PKCθ activity. Furthermore, high-fat diet–induced phosphorylation of JNK and IRS-1 at Ser307 was reduced with CPT1 overexpression. Hence, using a model that selectively increases flux of fatty acids into the mitochondria by inducing a physiological overexpression of the rate-limiting enzyme for mitochondrial fatty acid flux, we clearly show amelioration of high-fat diet–induced insulin resistance.
Whether increasing fatty acid flux into the mitochondria reduces or exacerbates insulin resistance is the subject of considerable debate. Several studies have shown that increased rates of fatty acid oxidation are associated with skeletal muscle insulin resistance (40,41). Furthermore, Muoio and colleagues (18) recently demonstrated that mice lacking the enzyme malonyl-CoA-decarboxylase (MCD−/−), an enzyme whose action theoretically promotes β-oxidation by relieving malonyl-CoA–mediated inhibition of CPT1, were protected from high-fat diet–induced insulin resistance. Together with in vitro data, these authors concluded that reducing fatty acid oxidation was beneficial with regard to insulin action. These results are in contrast with the data reported here in which increasing fatty acid oxidation alleviated insulin resistance in muscle of fat-fed rats. There are some important methodological differences that may explain this apparent anomaly. First, we used 4 weeks of high-fat diet to induce insulin resistance in rats, whereas Koves et al. (18) performed studies after 12 weeks of high-fat diet in both rats and mice. Thus, it is possible that differences between studies may be due to the duration of high-fat diet and the time of exposure of the muscle to hyperlipidemia. Furthermore, whereas our studies were focused on the tibialis anterior and EDL muscles, which are predominantly white muscles, Koves et al. (18) mostly used the gastrocnemius muscle, which is a mixture of red and white. It is also important to note that in the previous study, the model was a whole-body MCD−/− mouse, and the researchers reported insulin tolerance tests as their measure of insulin action, which cannot distinguish between insulin action in different insulin-responsive organs. They also examined the ability of MCD deletion to protect against the development of insulin resistance in fat-fed animals. In contrast, in our study, we used a technique in which we selectively overexpressed CPT1 in one hind limb of a rat and then were able to compare muscles from this hind limb with those obtained from the sham-treated, contralateral limb to see whether there were alterations to pre-existing, high-fat diet–induced insulin resistance. This technique has many advantages. First, the comparable muscles are subjected to exactly the same circulatory factors. Second, we were able to increase the activity of CPT1 in a skeletal muscle to levels seen with physiological interventions such as exercise training (29). Of note, we have consistently reported a transfection efficiency of ∼50% in the tibialis anterior muscle (25,26). We did not determine transfection efficiency of the CPT1 plasmid, but based on our previous reports (25,26), we expect that 40–50% of fibers would be overexpressing CPT1. However, given that CPT1 protein was only increased by ∼40%, it is possible that the transfection efficiency of CPT1 was lower than in our previous studies, or it may indicate that CPT1 protein has a rapid turnover rate when overexpressed. Nonetheless, the critical aspect of the study is that we achieved a physiological increase in CPT1 protein and activity that functionally translated into enhanced rates of fatty acid oxidation and, importantly, suppressed fatty acid esterification into TAG. Having performed hyperinsulinemic-euglycemic clamps with 2-deoxyglucose tracers, we were able to demonstrate that CPT1 overexpression improves insulin action in diet-induced obese models. Our results are entirely consistent with previous work in vitro, where it has been reported that a two- to threefold overexpression of CPT1 in L6 myotubes increased oxidation of the long-chain fatty acids palmitate and oleate and increased insulin stimulation of [14C]glucose incorporation into glycogen by 60% (21,22).
In the present study, high-fat diet–induced insulin resistance was associated with an increase in CPT1 activity and a trend toward elevated fatty acid oxidative capacity (Fig. 1A–C). This is consistent with previously reported data showing that rodents can adapt to a high-fat diet to coordinately increase the ability of skeletal muscle to take up (14) and utilize (37) fatty acid, this being dominant energy substrate. This is accompanied by muscle insulin resistance, which is at first glance seemingly paradoxical considering that we here demonstrate that a further enhancing of muscle CPT1 expression and fatty acid oxidation in high-fat diet–fed rats had an insulin-sensitizing effect. However, the critical difference was in the degree of fatty acid esterification (Fig. 1D). In the empty vector condition, when animals were placed on high-fat diet, fatty acid incorporation into TAG was increased compared with chow-fed rats, despite the concomitant increase in CPT1 activity and a trend toward increased fatty acid oxidation. This increase in fatty acid esterification was associated with increased lipid deposition in membranes (Fig. 6) and increases in PKCθ and JNK activation (Fig. 6), resulting in impaired insulin signal transduction. These data suggest that diet-induced obesity results in an enhanced entry of fat into the mitochondria and subsequent oxidation; however, this increase is quantitatively less than the increase in fatty acid entry into the tissue, resulting in a net increase in cellular lipid storage. In contrast, by increasing CPT1 activity in fat-fed animals, in this case due to ectopic overexpression, the exacerbated increase in fatty acid entry into the mitochondria and subsequent oxidation was sufficient to match the increased rate of fatty acid uptake into the tissue, thereby preventing any esterification into TAG and subsequent insulin resistance.
Although the total content of bioactive lipids appears to play a role in regulating insulin action in muscle, it is also emerging that the subcellular location of lipids plays a fundamental role in dictating its downstream targets and cellular responses (42). Many of the proximal steps in insulin signaling to GLUT4 translocation occur at or in close proximity to the plasma membrane (43). Thus, it is possible that excessive accumulation of lipid species, such as DAG and ceramide, in cellular membranes may affect insulin action by interfering with the activation of critical components of the insulin signaling pathway. Such an effect may be mediated by DAG and ceramide-induced displacement of cholesterol in membranes (44), which is associated with decreased Akt phosphorylation (45). In the current study, we found that the membrane-to-cytosolic ratio of ceramide was increased in muscle of high-fat–fed animals, and this was associated with impaired phosphorylation of Akt. We also observed that there was a tendency for the ceramide membrane-to-cytosolic ratio to be reduced in muscles overexpressing CPT1 from fat-fed rats, an effect that may have contributed to enhanced insulin-stimulated Akt phosphorylation. In addition, we also found that the DAG membrane-to-cytosolic ratio was decreased in muscle from fat-fed animals overexpressing CPT1.
The molecular mechanisms by which lipids induce insulin resistance in skeletal muscle remain uncertain. However, it has been suggested that lipid-induced insulin resistance in muscle is mediated by activation of a serine kinase cascade that leads to impaired insulin signaling (7). We were able to show that the high-fat diet not only induced translocation of PKCθ to the membrane but also increased JNK phosphorylation in muscle, which was associated with an increase in basal IRS-1 Ser307 phosphorylation. However, when CPT1 was overexpressed in muscles of high-fat diet–fed rats, there was a significant reduction in the membrane-to-cytosol ratio of PKCθ and phosphorylation of JNK. These changes were associated with a decrease in the phosphorylation status of IRS-1 on Ser307. Given that phosphorylation of IRS-1 at Ser307 can impair subsequent insulin-stimulated tyrosine phosphorylation (46), it is likely that the insulin-sensitizing effect of CPT1 overexpression is mediated by inhibiting the activation of serine kinases that interfere with insulin signaling. It is possible that these changes are related to the observed reduction in membrane DAG and ceramide content in muscles overexpressing CPT1.
In summary, we have shown that moderate overexpression of CPT1 is sufficient to increase fatty acid oxidation, thereby preventing fatty acid esterification, subsequently enhancing muscle insulin sensitivity in high-fat–fed rats. The insulin-sensitizing effect of CPT1 overexpression was associated with a reduction in muscle TAG content, although no difference in total DAG and ceramide levels was detected. However, the membrane-to-cytosolic ratio of DAG was reduced in muscle overexpressing CPT1, and this was associated with inhibition of PKCθ and JNK activity. Hence, we propose that increasing the capacity for fatty acid oxidation exerts an insulin-sensitizing effect in muscle by modulating the content of bioactive lipid species in membranes, preventing the activation of serine kinases and thereby relieving inhibition on insulin signaling. In addition, CPT1 overexpression did not have any major effect on markers of “metabolic remodeling,” such as changes in the protein expression of several subunits of the respiratory chain, ET/Ox Phos gene expression, maximal activity of key mitochondrial enzymes, and muscle acylcarnitine content, suggesting that under conditions of physiological increases in fatty acid flux, pathways downstream of fatty acid entry into the mitochondria were not limiting for complete oxidation of fatty acid to CO2. We conclude that CPT1 agonists are a potential therapeutic target for the treatment of obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
This work was supported by research grants from the Diabetes Australia Research Trust. C.R.B. has received a NHMRC Peter Doherty Postdoctoral fellowship. A.J.H. has received a University of New South Wales Postgraduate Award. N.T. has received a NHMRC Career Development Award. M.J.W. has received a NHMRC R Douglas Wright Fellowship. G.J.C. is a Senior Research Fellow of the NHMRC. M.A.F. is a Principal Research Fellow of the NHMRC. E.W.K. is a Senior Principal Research Fellow of the NHMRC.
No potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 December 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
REFERENCES
- 1.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH: Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglyceol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ: Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Farese RV Jr: Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Endocrinol Metab 293: E1772–E1781, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, Littman DR, Birnbaum MJ, Polakiewicz RD: Protein kinase C theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem 279: 45304–45307, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Summers SA, Garza L, Zhou H, Birnbaum MJ: Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18: 5457–5464, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz-Peiffer C, Craig DL, Biden TJ: Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Ruvolo PP: Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 47: 383–392, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS: A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE: Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA: HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A 105: 1739–1744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM: Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 51: 1477–1484, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Goodpaster B, Wing RR, Simoneau JA: Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA: Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA: Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88: 5444–5451, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Koves TR, Ussher R, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM: Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Stephens FB, Constantin-Teodosiu D, Greenhaff PL: New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581: 431–444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD: Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 50: 123–130, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Sebastián D, Herrero L, Serra D, Asins G, Hegardt FG: CPT I overexpression protects L6E9 muscle cells from fatty acid-induced insulin resistance. Am J Physiol Endocrinol Metab 292: E677–E686, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Perdomo G, Commerford SR, Richard AM, Adams SH, Corkey BE, O'Doherty RM, Brown, NF: Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem 279: 27177–27186, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Bruce CR, Brolin C, Turner N, Cleasby ME, van der Leij FR, Cooney GJ, Kraegen EW: Overexpression of carnitine palmitoyltransferase 1 (CPT1) in skeletal muscle in vivo increases fatty acid oxidation and reduces triacylglycerol esterification. Am J Physiol Endocrinol Metab 292: E1231–E1237, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Clark PW, Jenkins AB, Kraegen EW: Pentobarbital reduces basal liver glucose output and its insulin suppression in rats. Am J Physiol 258: E701–E707, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Cleasby ME, Davey JR, Reinten TA, Graham MW, James DE, Kraegen EW, Cooney GJ: Acute bidirectional manipulation of muscle glucose uptake by in vivo electrotransfer of constructs targeting glucose transporter genes. Diabetes 54: 2702–2711, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Polkinghorne E, Lau Q, Cooney GJ, Kraegen EW, Cleasby ME: Local activation of the IkappaK-NF-kappaB pathway in muscle does not cause insulin resistance. Am J Physiol Endocrinol Metab 294: E316–E325, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Watt MJ, Dzamko N, Thomas W, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR: CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 12: 541–548, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Southgate RJ, Bruce CR, Carey AL, Steinberg GR, Walder K, Monks R, Hawley JA, Watt MJ, Birnbaum MJ, Febbraio MA: PGC-1α gene expression is down-regulated by Akt mediated phosphorylation and nuclear extrusion of FoxO1 in insulin stimulated skeletal muscle. FASEB J 19: 2072–2074, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Cogswell AM, Stevens RJ, Hood DA: Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol Cell Physiol 264: C383–C389, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJF, Dyck DJ: Improved glucose tolerance in obese subjects following endurance training is associated with improved mitochondrial fatty acid oxidation and altered muscle lipid content. Am J Physiol Endocrinol Metab 291: E99–E107, 2006 [DOI] [PubMed] [Google Scholar]
- 31.McGarry JD, Mills SE, Long CS, Foster DW: Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues: demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J 214: 21–28, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruce CR, Dyck DJ: Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-α. Am J Physiol Endocrinol Metab 287: E616–E621, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hansen PA, Gulve EA, Marshall BA, Gao J, Pessin JE, Holloszy JO, Mueckler M: Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J Biol Chem 270: 1679–1684, 1995 [DOI] [PubMed] [Google Scholar]
- 34.James DE, Jenkins AB, Kraegen EW: Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol 248: E567–E574, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM: Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem 261: 8597–8600, 1986 [PubMed] [Google Scholar]
- 36.van Vlies N, Tian L, Overmars H, Bootsma AH, Kulil W, Wanders RA, Wood PA, Vaz FM: Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase-deficient mouse. Biochem J 387: 185–193, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ: Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56: 2085–2092, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Muoio DM, Koves TR: Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab 32: 874–883, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI: Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A 104: 16480–16485, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouzakri K, Austin R, Rune A, Lassman ME, Garcia-Roves PM, Berger JP, Krook A, Chibalin AV, Zhang BB, Zierath JR: Malonyl coenzyme A decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 57: 1508–1516, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP: A potential link between muscle peroxisome proliferator-activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 1: 133–144, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frec M, Cron P, Cohen P, Lucocq JM, Hemmings BA: Role of translocation in the activation and function of protein kinase B. J Biol Chem 272: 31515–31524, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Megha, London E: Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem 279: 9997–10004, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Peres C, Yart A, Perret B, Salles JP, Raynal P: Modulation of phosphoinositide 3-kinase activation by cholesterol level suggests a novel positive role for lipid rafts in lysophosphatidic acid signalling. FEBS Lett 534: 164–168, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ζick Y: Role of Ser/Thr kinases in the uncoupling of insulin signaling. Int J Obes Relat Metab Disord 27: S56–S60, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF: Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.