Abstract
OBJECTIVE—Wnt signaling inhibits adipogenesis, but its regulation, physiological relevance, and molecular effectors are poorly understood. Here, we identify the Wnt modulator Dapper1/Frodo1 (Dact1) as a new preadipocyte gene involved in the regulation of murine and human adipogenesis.
RESEARCH DESIGN AND METHODS—Changes in Dact1 expression were investigated in three in vitro models of adipogenesis. In vitro gain- and loss-of-function studies were used to investigate the mechanism of Dact1 action during adipogenesis. The in vivo regulation of Dact1 and Wnt/β-catenin signaling were investigated in murine models of altered nutritional status, of pharmacological stimulation of in vivo adipogenesis, and during the development of dietary and genetic obesity.
RESULTS—Dact1 is a preadipocyte gene that decreases during adipogenesis. However, Dact1 knockdown impairs adipogenesis through activation of the Wnt/β-catenin signaling pathway, and this is reversed by treatment with the secreted Wnt antagonist, secreted Frizzled-related protein 1 (Sfrp1). In contrast, constitutive Dact1 overexpression promotes adipogenesis and confers resistance to Wnt ligand-induced antiadipogenesis through increased expression of endogenous Sfrps and reduced expression of Wnts. In vivo, in white adipose tissue, Dact1 and Wnt/β-catenin signaling also exhibit coordinated expression profiles in response to altered nutritional status, in response to pharmacological stimulation of in vivo adipogenesis, and during the development of dietary and genetic obesity.
CONCLUSIONS—Dact1 regulates adipogenesis through coordinated effects on gene expression that selectively alter intracellular and paracrine/autocrine components of the Wnt/β-catenin signaling pathway. These novel insights into the molecular mechanisms controlling adipose tissue plasticity provide a functional network with therapeutic potential against diseases, such as obesity and associated metabolic disorders.
Dysregulated adipose function, as is observed in obesity, is associated with increased risk of developing diabetes, cardiovascular diseases, and some cancers. Recently, impaired adipose tissue expandability/plasticity has been shown to be an important factor linking obesity to its metabolic complications (1–4). This has led to the hypothesis that specific regulatory mechanisms must exist to ensure that the balance between preadipocyte recruitment and differentiation tightly matches the storage demands imposed by nutritional loads. Understanding the mechanisms that control when, how, and which preadipocytes enter the program of differentiation may aid the development of rational therapeutic strategies to improve adipose tissue functionality and lipid buffering capacity and thereby prevent and/or treat obesity-associated metabolic disorders.
Adipocyte differentiation involves the sequential activation of a cascade of transcription factors that coordinate the expression of genes responsible for the adipogenic phenotype (5,6). Briefly, CCAAT/enhancer-binding protein (C/EBP) β and δ are rapidly and transiently induced in response to adipogenic stimuli. This precedes the expression of the two adipogenic transcription factors, C/EBPα and peroxisome proliferator–activated receptor (PPAR)γ. These factors act synergistically to induce expression of adipocyte-specific genes. Although in vitro adipogenesis can be synchronously induced by exposure to a defined adipogenic cocktail, in vivo adipose tissue expansion is regulated by a combination of local and endocrine factors that act to either stimulate or inhibit adipogenesis (7) in response to demands for nutritional storage. In addition, adipocyte growth is likely to be balanced with (and may precede) recruitment of new adipocytes from the progenitor pool. In healthy individuals, tight regulation of these processes is required to ensure that the appropriate number of adipocytes form and is sufficient (but not excessive) to store nutritional surplus. As a highly conserved and widely distributed intercellular signaling pathway, the Wnt signaling network and its molecular components are good candidates to contribute to this important regulated homeostatic process.
The Wnt family of secreted glycoproteins function in a paracrine and/or autocrine manner to influence cell fate and development. Binding of specific Wnt proteins to receptor/coreceptor complexes transduces intracellular signals through either β-catenin–dependent or –independent pathways. Although both pathways may be active in preadipocytes (8), it is the former that has been shown to potently inhibit adipogenesis both in vitro and in vivo (9). The Wnt/β-catenin signaling cascade comprises specific cell surface Frizzled receptors and LDL receptor–related protein 5 or 6 (LRP5/6) coreceptors, which on ligand binding transduce Wnt signals via intracellular Dishevelled (Dvl) proteins, ultimately leading to disassembly and inactivation of the β-catenin degradation complex (10,11). The resulting cytosolic accumulation and nuclear translocation of β-catenin leads to transcriptional coactivation of the T-cell factor (TCF)/lymphoid enhancer factor transcription factors. Wnt/β-catenin/TCF target genes include PPARδ, inhibitor of DNA binding 2 (Id2), CyclinD1, and c-Myc (myelocytomatosis oncogene). Some of these have been shown to exhibit antiadipogenic actions (9,12,13).
In preadipocytes, constitutive activation of Wnt/β-catenin signaling leads to maintenance of the undifferentiated state and prevents induction of C/EBPα and PPARγ (5,6). In contrast, disruption of Wnt/β-catenin signaling promotes adipogenesis (9,12,14,15). These observations have led to suggestions that Wnt proteins may act as a functional “brake” during preadipocyte recruitment into the differentiation program. Of the 19 possible Wnt ligands, Wnt10b has been most clearly implicated as the endogenous Wnt involved in regulating adipogenesis. Wnt10b is expressed in both dividing and confluent preadipocytes, but its expression decreases during adipogenesis preceding the downregulation of intracellular β-catenin. Furthermore, ectopic expression of Wnt10b activates Wnt/β-catenin signaling and potently inhibits adipogenesis (16–18). Conversely, extracellular antagonists of Wnt/β-catenin signaling such as dickkopf homolog-1 and some of the secreted Frizzled-related proteins (sFRP) have also been shown to exert proadipogenic effects (14,15).
Although this evidence demonstrates Wnt/β-catenin–induced modulation of the adipogenic program, less is known about the physiological regulation of Wnt/β-catenin signaling network in vivo. In this report, we identify Dapper1/Frodo1 (Dact1) as an important preadipocyte gene that controls adipocyte differentiation by regulating the Wnt/β-catenin signaling network. Previous reports identified Dact1 as an evolutionarily conserved, Dvl-interacting protein that modulates Wnt signals (19,20); however, its mechanism of action remains ambiguous (19–25). In immortalized human embryonic kidney cells (HEK293) and in some embryonic contexts, Dact1 appears to act as an antagonist of Wnt/β-catenin signaling (19,20,26). However, a positive function for Dact1 homologs in Wnt/β-catenin signaling has also been suggested by several studies in other systems, particularly knockdown and low overexpression studies in developing embryos (22,23). Hence, it has been suggested that the function of Dact family members is cell- and tissue-context–dependent (23,26–28). Because adaptor or scaffold proteins are likely to function by bringing together other signaling components to form complexes favoring one biochemical signaling cascade over another in a particular cellular, physiological, or signaling context, it may not be meaningful to categorize Dact proteins either as general activators or inhibitors. Given this consideration, it is important to note that before this study, the role of Dact1 in adipogenesis was completely unexplored.
Here, we show that Dact1 acts to alter the Wnt/β-catenin signaling tone of preadipocytes through coordinated effects on both the efficiency of intracellular signal transduction pathway and by altering the expression profiles of extracellular Wnt ligands and antagonists. The regulatory network that we define here incorporates cell-specific paracrine signals and is itself physiologically regulated, because expression levels of key components Dact1, Wnt10b, and Sfrp5 are all coordinately regulated in vivo in adipose tissue by nutritional status, by proadipogenic pharmacological stimulation, and during the development of dietary and genetic obesity.
RESEARCH DESIGN AND METHODS
Tissue culture media, insulin, isobutylmethylxanthine, dexamethasone, Oil-red-O, and puromycin were purchased from Sigma (St. Louis, MO). Tissue culture sera were from HyClone (Logan, UT). Anti-Dvl1, anti-Dvl2, anti-Dvl3, and anti–extracellular signal–regulated kinase-1/2 (-ERK1/2) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-Dact1 antibody was from Orbigen (San Diego, CA). All antibodies were used according to the manufacturer's instructions. All horseradish peroxidase–conjugated secondary antibodies were from Cell Signaling Technology (Danvers, MA). Recombinant murine Wnt3a and human SFRP1 were from R&D Systems (Minneapolis, MN).
Retroviral expression constructs and TCF4 reporter assay.
Murine Dact1 cDNA was excised from mouse-Dact1-pCMV (20) with BamH1-SnaB, Klenow filled-in, and blunt-end–ligated into the SnaB1 site of the retroviral expression vector pBabe-puro. Correct orientation was confirmed by at least three different diagnostic digests. Stable knockdown of Dact1 was achieved by retroviral expression of shRNA from the pSiren-RetroQ vector (Clontech, Mountain View, CA). Custom oligonucleotides were identified using the Dharmacon siDesign center (sequences available on request) and cloned into pSiren-RetroQ according to the manufacturer's instructions.
For TCF4 reporter assay, Dact1SH, CtrlSH, Dact1, and control 3T3-L1 cells were grown to confluence, and Topflash promoter-reporter assays were performed as previously described (15).
Animals.
Animals were housed at a density of four animals per cage in a temperature-controlled room (20–22°C) with 12-h light/dark cycles. The effects of fasting and refeeding were studied using 8-week-old male SV129 mice (whole adipose tissue) and C57/B6 mice (tissue fractionation study). For these studies, mice were deprived of chow for a 24-h period and either killed or refed for a further period of 24 h. Mice were allowed ad libitum access to water.
The effects of genetic obesity (ob/ob) were studied in tissues collected as previously reported (29). The effects of long-term high-fat diet (16 weeks) were studied in 7-month-old C57/B6 mice fed either standard laboratory chow or high-fat diet (45% calories from fat; D12451; Research Diets, New Brunswick, NJ) ad libitum from weaning. The short-term high-fat diet study was performed on 16-week-old C57/B6 mice that had been fed either chow or high-fat diet for either 3 or 28 days before they were killed. Adipose tissues were collected from all mice at the same age. The effects of thiazolidinedione (TZD) treatment were studied using male C57/B6 mice fed standard chow diet supplemented with or without 10 ppm rosiglitazone (Avandia) for 3 weeks. All animal protocols used in this study were approved by the UK Home Office and the University of Cambridge.
Preadipocyte isolation, culture differentiation, and infection.
Preadipocyte isolation from human adipose tissue biopsies was performed as previously described (15). Cambridge Research Ethics Committee approval was obtained, and all patients gave their informed consent. Murine primary preadipocytes were isolated from epididymal white adipose tissue (WAT) of 6-week-old male C57/B6 mice as described previously (29).
3T3-L1 cells were cultured, differentiated into adipocytes, and stained for Oil-red-O as described previously (12). Dact1 and control 3T3-L1 cell lines were generated with the pBabe-Puro retroviral vector system, and Dact1SH and CtrlSH 3T3-L1 cells were generated with the pSiren-RetroQ retroviral vector system as described previously (12,30).
Protein extraction and Western blotting.
Proteins were extracted from cell monolayers as previously described (12). Proteins were electroblotted nitrocellulose membrane (Amersham Biosciences). Specific proteins were detected by incubation with the appropriate primary and horseradish peroxidase–conjugated secondary antibodies. Immune complexes were detected by enhanced chemiluminescence (Amersham Biosciences).
RNA isolation and quantitative RT-PCR.
Total RNA was isolated from cultured cells using an RNeasy kit (Qiagen, Crawley, U.K.), and mRNA expression was analyzed by RT-PCR as described previously (12). The sequences of the oligonucleotides used for primers and probes are available on request.
Statistical analysis.
Data from densitometrical analysis, luciferase assays, and quantitative real-time PCR are presented as means ± SE of at least three independent experiments. Statistical significance was determined with parametric (Student) or nonparametric (Mann–Whitney) tests, as appropriate (*P < 0.05, **P < 0.01, and ***P < 0.001).
RESULTS
Dact1 is decreased during adipogenesis and is restricted to the preadipocyte-enriched fractions of adipose tissue.
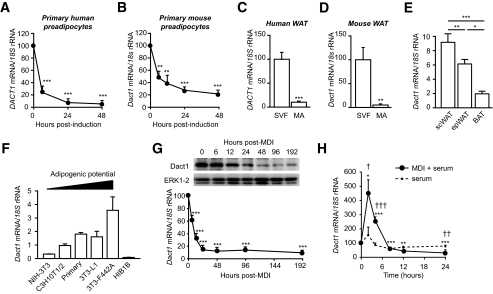
Microarray expression profiling of human preadipocyte differentiation revealed that Dact1 (DACT1) is significantly downregulated at 6 and 48 h after induction of adipogenesis. This downregulation was confirmed and also observed during differentiation of primary preadipocytes from both human and murine sources (Fig. 1A and B) and was consistent with the enrichment of Dact1 in the stroma-vascular fraction (SVF) when compared with mature adipocytes from WAT in both species (Fig. 1C and D). In adult mice, Dact1 expression was also preferentially expressed in WAT depots compared with brown adipose tissue (BAT) (Fig. 1E). Dact1 expression in different cellular models of fibroblasts and preadipocytes also correlated well with adipogenic potential and was barely detectable in the BAT cells HIB1B (Fig. 1F).
FIG. 1.
DACT1 gene expression is downregulated in early adipogenesis and is found primarily the SVF of human and mouse adipose tissue. Human DACT1 (A) and mouse Dact1 (B) mRNA levels were measured using real-time RT-PCR at the indicated time points after induction of differentiation. **P < 0.01, ***P < 0.001 vs. time 0, n = 7 experiments performed in duplicate. DACT1 and Dact1 mRNA levels were measured in stroma-vascular cells (SVF) and mature adipocytes (MA) from human subcutaneous WAT (C) and mouse epididymal WAT (D). ***P < 0.001 vs. SVF, n = 3 replicate experiments performed in duplicate. n = 3 experiments performed in duplicate. E: Normalized Dact1 mRNA levels were measured in epididydimal (epWAT), subcutaneous WAT (scWAT), and interscapular BAT of 10-week-old C57BL/6J. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3 experiments performed in duplicate. F: Dact1 mRNA levels, were measured in different cell types: fibroblast (NIH3T3), mesenchymal stem cells (C3H10T1/2), white primary preadipocytes, white preadipocyte cell lines (3T3–L1 and 3T3–F442A), and brown preadipocyte cell line (HIB1B), n = 3–4 experiments performed in duplicate. G: Dact1 mRNA levels were measured at the indicated hours after induction (with MDI) of 3T3–L1 preadipocyte differentiation. ***P < 0.001 vs. time 0, n = 4 experiments performed in duplicate. Whole-cell protein lysates were extracted at the indicated days of differentiation from 3T3 L1 cells and analyzed by immunoblotting. Representative immunoblots of Dact1 and extracellular signal–related kinase (ERK)1/2 (loading control) from three separate experiments are shown. H: Dact1 mRNA levels were measured at the indicated hours after induction of 3T3–L1 preadipocytes with either control medium (serum) or MDI. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. time 0 for MDI; †P < 0.05, ††P < 0.01, and †††P < 0.001 vs. serum, n = 3–4 experiments performed in duplicate.
As with primary preadipocyte cultures, Dact1 mRNA was also found to decrease by 90% after 24 h following adipogenic induction of the well-established 3T3-L1 preadipocyte cell line. This profile preceded the decrease in Dact1 protein in the same cells (Fig. 1G). However, profiling the early immediate response to adipogenic stimuli revealed a rapid but transient upregulation of Dact1 limited to the first 6 h after adipogenic induction (Fig. 1H).
Dact1 is required for adipocyte differentiation of 3T3-L1 preadipocytes.
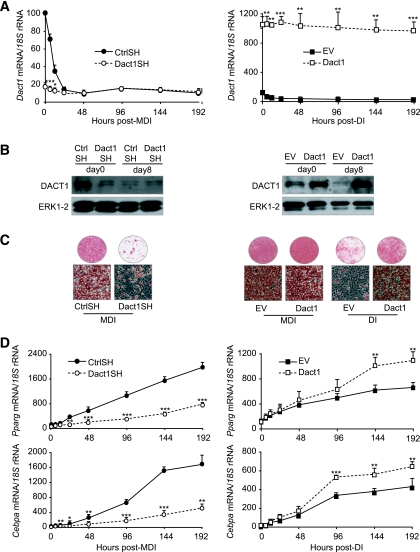
The mRNA and protein profiles of Dact1 suggest that it might be a preadipocyte marker gene whose product contributes to the maintenance of the preadipocyte phenotype. However, given that Dact1 can act in a context-dependent manner to activate or inhibit Wnt/β-catenin signaling (19,20,22,23,26), it was possible that changes in Dact1 levels reported here were functionally either pro- or antiadipogenic. Thus, we performed gain- and loss-of-function experiments using four newly established 3T3-L1 preadipocytes cell lines, engineered to constitutively express Dact1 ShRNA (Dact1SH), control ShRNA (CtrlSH), Dact1 cDNA (Dact1), or empty vector control (EV).
In Dact1SH 3T3-L1 cells, endogenous expression of Dact1 mRNA was decreased by ∼90% and remained at this level throughout the differentiation process (Fig. 2A). In contrast Dact1 3T3-L1 cells displayed a 10-fold increase of Dact1 mRNA compared with EV 3T3-L1 cells (Fig. 2A). These transcript levels were also reflected at the amount of Dact1 protein (Fig. 2B). To evaluate the impact of altered Dact1 levels on adipogenic potential, each cell line was induced to differentiate and lipid accumulation visualized with Oil Red-O staining. Knockdown of Dact1 (Dact1SH) almost completely blocked lipid accumulation (Fig. 2C). This was consistent with the reduced expression levels of the adipogenic markers C/EBPα and PPARγ in these cells (Fig. 2D). Conversely, Dact1 3T3-L1 cells and EV 3T3-L1 cells differentiated to a similar extent when induced with full induction cocktail (MDI). However, induction with submaximal stimulation (DI) revealed that Dact1 3T3-L1 cells have an increased propensity for lipid accumulation (Fig. 2C). DI-treated Dact1 3T3-L1 cells also showed increased expression of the adipogenic markers PPARγ and C/EBPα when compared with similarly treated EV 3T3-L1 cells (Fig. 2D). Taken together, these data suggest that Dact1 is proadipogenic and is required for adipogenesis.
FIG. 2.
Dact1 is required for adipocyte differentiation of 3T3–L1 preadipocytes. Preadipocytes were infected with a retrovirus carrying Dact1 ShRNA (Dact1SH), control ShRNA (CtrlSH), Dact1 (Dact1), or vector alone (EV). A: Dact1 mRNA levels, normalized to 18S rRNA levels, were measured using real-time RT-PCR at the indicated hours of differentiation in Dact1SH- and CtrlSH-differentiated MDI and in Dact1 and EV cells differentiated with DI. *P < 0.05 and ***P < 0.001 vs. CtrlSH or EV, n = 3 experiments performed in duplicate. B: Whole-cell lysates were extracted at 0 and day 8 days of differentiation from CtrlSH, Dact1SH, EV, and Dact1 cells and analyzed by immunoblotting. Representative immunoblots of Dact1 and extracellular signal–related kinase (ERK)1/2 (loading control) from three separate experiments are shown. C: Dact1SH and CtrlSH 3T3–L1 cells were differentiated with MDI, Dact1, and EV 3T3–L1 cells were differentiated with MDI or DI. Cells were stained with Oil Red-O to visualize lipid droplets 8 days after induction, n = 5. D: Pparγ and Cebpα mRNA levels, normalized to 18S rRNA levels, were measured using real-time RT-PCR at the indicated hours of differentiation in Dact1SH and CtrlSH cells differentiated with MDI and in Dact1 and EV cells differentiated with DI. At day 0, only PPARγ mRNA was significantly altered in Dact1-SH preadipocytes. Neither PPARγ not CEBPα were significantly altered in Dact vs. EV cells at time 0 h. *P < 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CtrlSH or EV, n = 3 experiments performed in duplicate. (Please see http://dx.doi.org/10.2337/db08-1180 for a high-quality digital representation of this figure.)
Dact1 antagonizes Wnt/β-catenin signaling in 3T3-L1 cells.
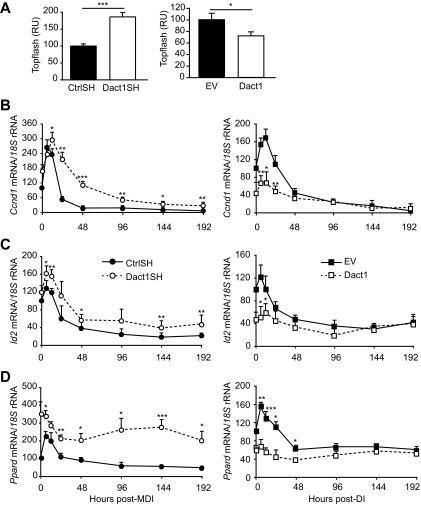
To assess the function of Dact1 in Wnt/β-catenin signaling in 3T3-L1 preadipocytes, we first investigated alterations in β-catenin/TCF promoter activity (TOPflash) in each cell line after adipogenic induction. Figure 3A shows that β-catenin/TCF promoter activity is reciprocally related to the level of Dact1 expression in each cell line, such that Dact1SH 3T3-L1 cells show significantly increased reporter activity, whereas Dact1 3T3-L1 cells show significantly reduced reporter activity. These results are further supported by parallel changes in the expression profiles of the endogenous Wnt target genes Cyclin D1, Id2, and PPARδ (Fig. 3B–D) in these cell lines. In control cells, after maximal adipogenic induction by MDI (CtrlSH cells) or submaximal induction by DI (EV cells), Wnt target gene expression was transiently upregulated between 0 and 24 h after induction (Fig. 3B–D). This response was significantly blunted in Dact1 cells, whereas Dact1SH cells maintained elevated target gene expression levels throughout the time course of adipogenesis (Fig. 3B–D). These data suggest that Dact1 inhibits Wnt/β-catenin signaling in preadipocytes and also affects the magnitude of their transcriptional response to adipogenic treatment.
FIG. 3.
Dact1 antagonizes Wnt/β-catenin signaling in 3T3–L1 cells. A: Topflash reporter activity in 3T3–L1 cells expressing control ShRNA (CtrlSH), Dact1 ShRNA (Dact1SH), empty vector (EV), or Dact1, n = 3–5 experiments performed in triplicate. Results are expressed as fold difference relative to CtrlSH. Results are the mean ± SE of at least four independent experiments. Ccnd1 (CyclinD1) (B), Id2 (C), and Pparδ (D) mRNA levels, normalized to 18S rRNA levels, were measured at the indicated hours of differentiation in Dact1SH and CtrlSH cells differentiated with MDI and in EV and Dact1 cells differentiated with DI. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CrtlSH or EV, n = 3 experiments performed in duplicate. RU, relative luciferase unit.
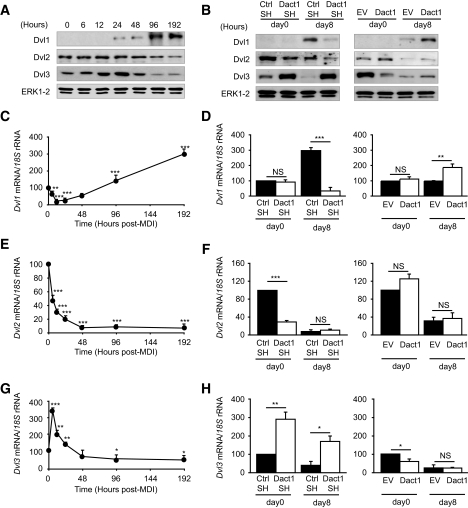
Dvl protein levels change dynamically during adipogenesis and are regulated by Dact1 in 3T3-L1 cells.
Dact1 was originally identified as a Dvl-interacting protein (19,22) and has been reported in some contexts to antagonize Wnt signaling by inducing Dvl degradation (20). The expression levels of Dvl family members have not previously been reported during adipogenesis nor has any study systematically assessed the effect of Dact1 levels on all three members of the Dvl family concurrently. Figure 4A shows the protein expression profiles of all three members of the Dvl family: Dvl1, Dvl2, and Dvl3 during adipogenesis in our in vitro models. Only Dvl2 and Dvl3 proteins are detected in confluent preadipocytes. However, during the adipogenic program each Dvl family member exhibits a distinct expression profile. The protein expression profiles closely parallel the corresponding mRNA profiles for each Dvl gene (Fig. 4B–D), suggesting that changes in Dvl protein levels are substantially regulated at the transcript level.
FIG. 4.
Dact1 regulates the expression of Dvl in 3T3–L1 cells. A: Whole-cell lysates were extracted at indicated times after induction of 3T3–L1 adipogenesis. Representative immunoblots of Dvl1, Dvl2, Dvl3, and ERK1/2 (loading control) from three separate experiments are shown. Dvl1 (C), Dvl2 (E), and Dvl3 (G) mRNA levels, normalized to 18S rRNA levels, were measured using real-time RT-PCR at indicated times after induction, n = 3 experiments performed in duplicate. B: Whole-cell lysates were extracted at days 0 and 8 of differentiation from Dact1SH, CtrlSH, EV, and Dact1 cells and analyzed by immunoblotting. Representative immunoblots of Dvl1, Dvl2, Dvl3, and ERK1/2 from three separate experiments are shown. Dvl1 (D), Dvl2 (F), and Dvl3 (H) mRNA levels, normalized to 18S rRNA levels, were measured at days 0 and 8 of differentiation in Dact1SH and CtrlSH cells differentiated with MDI and in differentiation in Dact1 and EV cells differentiated with DI. Complete time course data for experiments presented in D, F, and H can be found in supplementary Fig. 1, available in the online appendix. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CrtlSH or EV, n = 3 experiments performed in duplicate.
To investigate whether altered Dact1 expression affects Dvl protein levels during adipogenesis, we profiled Dvl protein and mRNA expression in both Dact1SH and Dact1 3T3-L1 cells on days 0 and 8 after induction (Fig. 4B, D, F, and H). Parallel changes were observed at both the protein and RNA levels. Before adipogenic induction (day 0), knockdown of Dact1 had no effect on levels of Dvl1 but reduced levels of Dvl2 and increased levels of Dvl3. In contrast, overexpression of Dact1 at day 0 had no significant effect on Dvl1 or Dvl2 levels but reduced levels of Dvl3. At day 8 after adipogenic treatment, the level of Dvl1 was significantly lower in Dact1SH 3T3-L1 cells but significantly higher in Dact1 3T3-L1 cells (Fig. 4B and D). In contrast, at day 8, Dvl2 levels were minimal and unaffected by Dact1 manipulation (Fig. 4B and F), whereas day 8 Dvl3 levels correlated reciprocally with that of Dact1, being significantly higher in Dact1SH 3T3-L1 cells and lower in Dact1 3T3-L1 cells (Fig. 4B and H). Hence, each of the Dvl family members shows a distinct expression profile during adipogenesis in 3T3-L1 cell lines and is likely to be primarily regulated at the level of mRNA transcription. Although levels of Dvl1 appear to correlate with extent of adipocyte conversion, alterations in Dact1 levels does impact directly on basal Dvl2 and Dvl3 expression.
Dact1 alters extracellular Wnt signals in preadipocytes and in differentiating adipocytes.
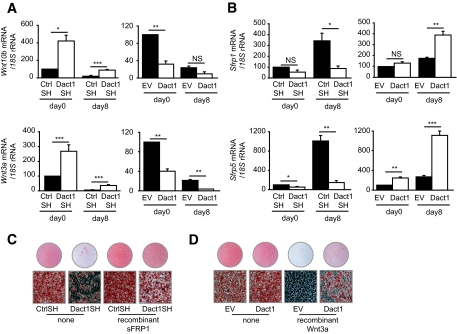
We next investigated whether extracellular components of the Wnt/β-catenin signaling cascade may be altered by changes in Dact1. In Dact1SH 3T3-L1 cells before adipogenic induction, we observed an increase in mRNA levels of Wnt10b and Wnt3a when compared with CtrlSH 3T3-L1 cells (Fig. 5A). Conversely, in Dact1-overexpressing 3T3-L1 cells, Wnt10b and Wnt3a mRNA levels are decreased when compared with EV 3T3-L1 cells (Fig. 5A). As expected, after full induction of adipogenesis by MDI (CtrlSH) or partial induction by DI (EV), Wnt10b levels fall significantly by day 8 (Fig. 5A, top). However, in Dact1SH 3T3-L1 cells, levels of Wnt10b and Wnt3a remain higher than in CtrlSH 3T3-L1 cells during adipogenic induction. Next, we examined the mRNA expression profile of two closely related secreted Wnt antagonists, Sfrp1 and Sfrp5. In contrast to the decreasing expression of Wnt10b and Wnt3a, Sfrp1 and Sfrp5 mRNA expression increased as adipocytes differentiate (Fig. 5B). The fold increase was proportional to the strength of adipogenic stimulation and conversion.
FIG. 5.
Dact1 regulates paracrine/autocrine Wnt signaling in 3T3–L1 adipocytes. Wnt10b and Wnt3a (A) and Sfrp1 and Sfrp5 (B) mRNA levels, normalized to 18S rRNA levels, were measured at the indicated hours of differentiation in Dact1SH and CtrlSH cells differentiated with MDI and in Dact1 and EV cells differentiated with DI. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. CtrlSH or EV, n = 3 experiments performed in duplicate. C: Dact1SH and CtrlSH 3T3–L1 cells were differentiated with MDI in the presence or absence of recombinant sFRP1 (75 nmol/l) from days 0–8 and stained with Oil Red-O to visualize lipid droplets 8 days after induction. D: Dact1 and EV 3T3–L1 cells were differentiated with MDI in the presence or absence of recombinant Wnt3a (0.25 nmol/l) from days 0–8 and stained with Oil Red-O at day 8, n = 3. (Please see http://dx.doi.org/10.2337/db08-1180 for a high-quality digital representation of this figure.)
To assess whether the effect of Dact1 on adipogenesis is primarily due to altered production of extracellular Wnt ligands and antagonists, we investigated the ability of recombinant SFRP1 to reverse the antiadipogenic phenotype of Dact1SH 3T3-L1 cells. As shown in Fig. 5C, Dact1SH 3T3-L1 cells treated with SFRP1 accumulate more lipids than untreated Dact1SH 3T3-L1. SFRP1 acts extracellularly by sequestering Wnt ligands, suggesting that increased Wnt and decreased SFRP production are in part responsible for the reduced adipogenic response of Dact1SH cells.
Conversely, by treating Dact1 3T3-L1 cells with recombinant Wnt ligands, we assessed whether these cells were more resistant to the antiadipogenic actions of extracellular Wnts. Treatment with recombinant Wnt3a practically eliminated the ability of EV 3T3-L1 cells to accumulate lipids, whereas Dact1 3T3-L1 cells accumulate lipid even in the presence of Wnt3a. Together with our prior assays of Wnt signaling, these data show that ectopically expressed Dact1 in 3T3-L1 cells inhibits the efficiency of Wnt/β-catenin signal transduction, thereby allowing these cells to differentiate even in the presence of exogenous Wnts. Taken together, our results suggest that changes in Dact1 protein levels alter Wnt/β-catenin signaling tone in 3T3-L1 cells through coordinated effects on both the efficiency of intracellular signal transduction pathway and by altering the expression profiles of extracellular Wnt ligands and antagonists.
Dact1, Wnt10b, and Sfrp5 are all regulated by nutritional status in vivo.
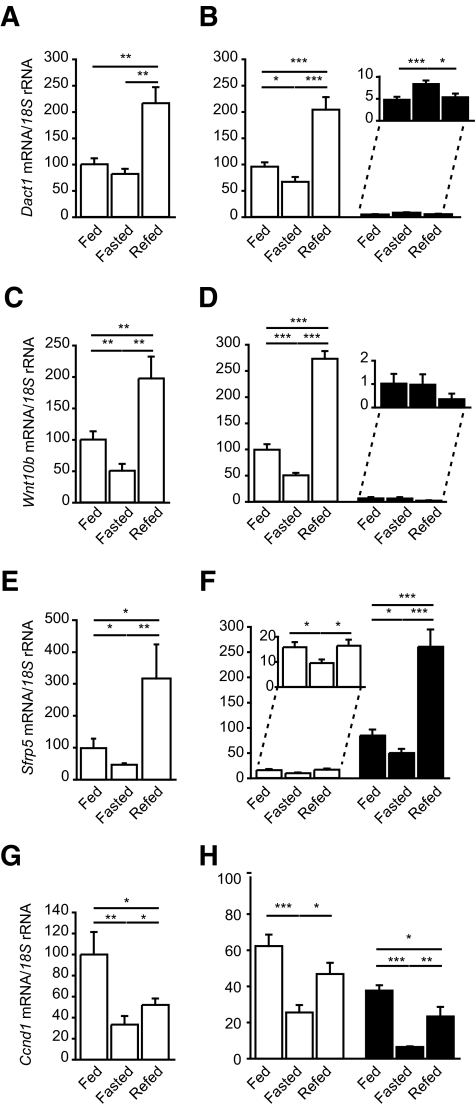
We sought to determine whether Dact1, Wnt10b, and Sfrp5 levels are regulated in vivo by nutritional status. Fasting and/or refeeding experimental conditions were selected as opposing physiological paradigms with respect to acute fuel availability. In response to fasting, the expression of Dact1, Wnt10b, and Sfrp5 were all decreased in WAT. However, in response to refeeding, the mRNA levels of all three genes were markedly increased significantly beyond the levels found in the fed state (Fig. 6). Interestingly, the expression of the Wnt/β-catenin target gene Ccnd1 was also markedly decreased after fasting but remained low following refeeding (Fig. 6G). Figure 6E–H confirmed that these changes predominantly reflected alterations in SVFs (for Dact1 and Wnt10b) and also in mature adipocytes (for Sfrp5). However, appreciable levels of Ccnd1 was found in both fractions. Taken together, these data suggest that in response to increased nutritional availability (refeeding), adipose tissue undergoes dynamic changes in the expression of key components of the Wnt/β-catenin signaling pathway, including Dact1 in preadipocytes and Sfrp5 in mature adipocytes. Furthermore, there is not only coordinated upregulation of proadipogenic signals but also a simultaneous upregulation of antiadipogenic Wnt-related signals. We speculate that the net effect of these is likely to be important for titrating the actual target gene expression and hence adipogenic potential of whole adipose tissue in vivo.
FIG. 6.
Wnt/β-catenin signals and Dact1 are regulated by nutritional status in adipose tissue in vivo. Dact1 (A and B), Wnt10b (C and D), Sfrp5 (E and F), and Ccnd1 (G and H) mRNA levels, normalized to 18S rRNA levels, were measured in whole WAT (A, C, E, and G) and fractionated adipose tissue (B, D, F, and H) from 8-week-old male C57/Bl6 mice under the following conditions: fed (n = 8–9), fasted (24 h) (n = 7–8), and refed (24 h) (n = 7–8). *P < 0.05, **P < 0.01, and ***P < 0.001.
Dact1, Wnt10b, and Sfrp5 are all regulated during dietary and genetic models of obesity and by PPARγ activators.
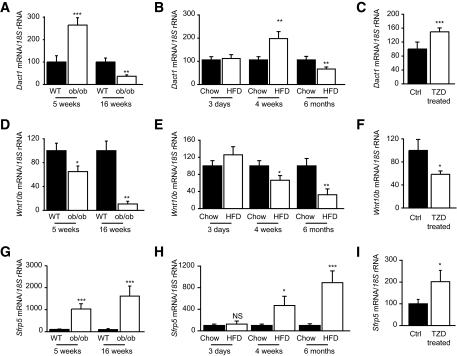
To further investigate the pathophysiological regulation of Dact1, we measured the expression profiles of Dact1, Wnt10b, and Sfrp5 in adipose tissue collected from ob/ob mice. This is a mouse model of severe positive energy balance characterized by marked hyperphagia and early-onset obesity. By 5 weeks of age, ob/ob mice are in a phase of rapid adipose accretion and are already significantly heavier than their lean wild-type littermates (2). In contrast, by 16 weeks of age the growth rate of ob/ob mice has begun to plateau, a phase that coincides with these animals being overtly obese and diabetic (29). Adipose tissue taken from 5-week-old ob/ob mice show elevated levels of proadipogenic Dact1 (Fig. 7A) and Sfrp5 (Fig. 7C) and decreased levels of antiadipogenic Wnt10b (Fig. 7B), resulting in a steady state that could be considered highly proadipogenic. In contrast, Dact1 levels in the metabolically compromised 16-week-old ob/ob animals are significantly lower than their age-matched wild-type littermates. Interestingly, these older ob/ob mice also express reduced Wnt10b, but their levels of Sfrp5 continued to rise (Fig. 7A, D, and G).
FIG. 7.
Dact1, Wnt10b, and Sfrp5 are regulated in adipose tissue by genetic and diet-induced models of obesity and by TZDs. Dact1, Wnt10b, and Sfrp5 mRNA levels were measured in whole WAT from indicated murine models as described in experimental procedures. A, D, and G: Data are normalized to 18s mRNA and represented relative to wild-type (WT) levels ± SE. Expression data from 5-week-old ob/ob mice and from 16-week-old diabetic ob/ob mice (n = 6–8 per group) *P < 0.05, **P < 0.01, and ***P < 0.001 vs. wild-type mice. B, E, and H: Expression data from C57/Bl6 mice fed either chow diet (chow) or high-fat diet (HFD) for 3 days (acute), 4 weeks (short term), or 6 months (long term) (n = 6–7 per group) after weaning. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. chow-fed mice. C, F, and I: Expression data from 4-month-old C57/B6 mice fed rodent chow supplemented with or without rosiglitazone (TZD) for 3 weeks (n = 9–10 per group). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. untreated mice.
We also investigated Dact1 expression in mice challenged with a short- and long-term high-fat diet. Similarly to the young ob/ob mice, mice fed high-fat diet for 4 weeks show significant increases in WAT mass compared with chow-fed and age- and sex-matched littermates. Epididymal WAT weights from 4-week high-fat diet–and chow-fed mice were 1.01 ± 0.14 g compared with 0.33 ± 0.03 g, respectively, P < 0.002. At this time, adipose tissue levels of proadipogenic Dact1 (Fig. 7B) and Sfrp5 (Fig. 7H) were increased, whereas the levels of antiadipogenic Wnt10b were decreased (Fig. 7E). Furthermore, in agreement with the tendencies observed in older metabolically compromised ob/ob mice, Dact1 and Wnt10b levels were reduced (Fig. 7B–E), albeit to a lesser extent, in these wild-type mice that have become obese and metabolically compromised as a result of long-term high-fat diet (6 months).
We tested whether this Wnt-related network was modulated by pharmacological challenge with thiazolidinediones (TZDs), drugs known to promote adipogenesis and improve insulin sensitivity. TZD treatment increased the expression of adipogenic markers in adipose tissue (aP2 was increased by 1.63 ± 0.14-fold, P = 0.047). Strikingly, this was accompanied by increases in Dact1 and Sfrp5 levels (Fig. 7C and I) and decreases in Wnt10b levels (Fig. 7F). Further studies on SVFs and adipocyte fractions demonstrated that both Dact1 and Wnt10b expression was primarily altered in SVFs and preadipocytes. Furthermore, TZD feeding significantly increased Sfrp5 and Fabp4 levels primarily in adipocytes fractions (supplementary Fig. 2, available in an online appendix at http://dx.doi.org/10.2337/db08-1180). TZD-induced changes in Sfrp1 were the same as those of Sfrp5 (data not shown). Taken together, these findings suggest that Dact1 expression and key extracellular components of the Wnt/β-catenin signaling network are coordinately regulated to facilitate adipose tissue adaptation during anabolic states characterized by active adipose tissue expansion. However, in predominantly catabolic states, such as insulin resistance and diabetes, some aspects of this network may become uncoupled.
DISCUSSION
Dact1 was first identified as an evolutionarily conserved, Dvl-interacting protein (19,20); however, its mechanism of action and function remained ambiguous (19–23,25) and was reported to be cell/tissue and context dependent (23,26–28). Before this study, the presence and role of Dact1 in adipogenesis/adipose tissue was unexplored. Here, we demonstrate that Dact1 is primarily expressed in both human and murine preadipocytes and in SVFs of adipose tissue. Furthermore, Dact1 is downregulated during adipogenic conversion of primary cultures and cell lines. With this profile, Dact1 could be considered a surrogate preadipocyte marker, however, we demonstrate that its biological action contrasts with other previously described “vanishing preadipocyte” genes (e.g., Gata2/3, Pref1, and Wnt10b). Through both gain- and loss-of-function approaches, we have shown that expression of Dact1 confers proadipogenic potential and that its presence in the preadipocyte is required for efficient adipocyte differentiation.
Our observations of the effects of Dact1 on adipogenesis and on signaling in preadipocytes are most consistent with its role as an antagonist of Wnt/β-catenin signaling in this tissue. This has only previously been demonstrated in Xenopus embryos and mammalian embryonic fibroblasts (19,20,26). Resolution of the discrepancies in the apparent signaling function of Dact proteins may lie in our observations showing that manipulation of Dact1 in preadipocytes elicits a differential and specific effect on the levels of other proteins configuring a Wnt/β-catenin signaling network. This suggests that changes in Dact1 protein concentrations have downstream effects on a whole network of signaling proteins, which may contribute exquisite modulation to the strength of Wnt/β-catenin signaling tone. It is noteworthy that some of the effects of Dact1 (i.e., altering Dvl levels) are strictly cell-autonomous, whereas others (i.e., altering Wnt and sFRP levels) can also be expected to affect signaling in neighboring cells. The latter is likely to be highly relevant in vivo in an otherwise cellularly heterogeneous adipose tissue, where gene expression and differentiation state is likely to vary significantly between neighboring cells. Dact1 may play a significant role in determining not only the sensitivity of the intracellular Wnt/β-catenin pathway but also the strength of the extracellular autocrine/paracrine Wnt signals to neighboring cells. We speculate that this type of modulation, when regulated by nutrient availability, may be the basis of a local self-regulating mechanism to titrate the differentiation of an appropriate number of preadipocytes to specific storage demands.
These studies also present several new components of the Wnt/β-catenin network not previously described in adipose tissue and/or studied during adipogenesis. These include four proteins expressed primarily in preadipocytes (Dact1 Dvl2, Dvl3, and Wnt3a) and three expressed primarily by mature adipocytes (Dvl1, sFRP1, and sFRP5). Importantly, we also demonstrate that these molecules are coordinately regulated in vivo in response to nutritional and dietary challenges, in genetic forms of obesity and to pharmacological agents typically producing adipose tissue expansion and improving insulin sensitivity.
Given their intrinsic peculiarities, it is not unexpected that our studies have revealed some differences between the expression profiles obtained from in vivo and in vitro models of adipogenic regulation. One important factor that is worth considering is that the in vitro systems used here represent a homogenous population of preadipocytes that are simultaneously exposed to and synchronously respond to defined adipogenic stimuli and/or genetic manipulation, thus allowing construction of detailed temporal profiles. In contrast, whole adipose tissues as used for in vivo studies represent a heterogeneous population of cell types each with distinct gene expression profiles and sensitivities to nutritional and pathological cues and context-dependent feedback loops. These coupled with local paracrine signals are likely to affect the dynamic balance between mature adipocytes and preadipocytes. Furthermore, it is evident that adipose tissue expansion in vivo does not involve the simultaneous recruitment of all preadipocytes into the adipogenic program. Nonetheless, it is clear from our findings that key determinants of Wnt/β-catenin signaling tone are regulated in response to refeeding and that the sustained induction of Dact1 in preadipocytes could be one mechanism facilitating, on one hand, the preselection of preadipocytes for subsequent differentiation and being the source of paracrine signals that prevents the differentiation of more distal preadipocytes, resulting in an appropriate titrated adipose tissue expansion in response to nutritional status.
That being said, our in vitro observation that changes in Dact1 levels lead to reciprocal changes in Wnt10b in preadipocyte cell lines are recapitulated in adipose tissue obtained from three in vivo models that reflect actively expanding adipose tissue (short-term high-fat diet, young ob/ob, and TZD-treated wild-type mice). Conversely, our data also show that under conditions of insulin resistance when adipogenesis and fat deposition are compromised and fat accumulation plateaus, this network becomes uncoupled, but Dact1 appears to be appropriately regulated in the opposite direction. This suggests that Dact1 may act as a gate-keeper, facilitating the accurate adaptation of adipose tissue expansion to storage requirements based on nutritional and metabolic status.
In summary, we present evidence of a functional network formed by Dact1, sFRP, and Wnt ligands that facilitates cross talk in adipose tissue between preadipocytes and mature adipocytes, thereby ensuring appropriate titration of adipose tissue expansion in response to nutrient availability. We speculate that dysregulation of this network may be the pathogenic basis leading to an altered balance between the processes of adipocyte growth versus preadipocyte recruitment—ultimately leading to a spectrum of adipose tissue cellularity ranging from hypertrophy to hyperplasia. Similarly, modulation of this network by targeting Dact1 may be of therapeutic value to improve the metabolic efficiency of the adipose tissue and prevent obesity-associated metabolic complications.
Supplementary Material
Acknowledgments
C.L. has received funding from Association de Langue Française pour l'Etude du Diabète et des Maladies Métaboliques and a Marie Curie Intra-European Fellowship postdoctoral fellowship. C.C. has received a Medical Research Council (MRC) Doctoral Studentship. W.P.C. has received a MRC Doctoral Studentship. S.V. has received a MRC Doctoral Studentship. A.J.V.-P. has received funding from the Biotechnology and Biological Science Research Council (BBSRC), MRC, Diabetes UK, and the European Union Sixth Framework Programme on Hepatic and Adipose Tissue Functions in the Metabolic Syndrome (EU-FP6 HEPADIP) and has received a MRC Career Establishment Award. J.K.S. has received funding from the BBSRC, MRC, Diabetes UK, and the European Union Sixth Framework Programme on Hepatic and Adipose Tissue Functions in the Metabolic Syndrome (EU-FP6 HEPADIP).
No potential conflicts of interest relevant to this article were reported.
We thank the hospital surgeons from Addenbrooke for generously providing adipose tissue biopsies. We thank Ye-Guang Chen (Department of Biological Sciences and Biotechnology, Tsinghua University, China) for the mouse-Dact1 cDNA.
C.C. is currently affiliated with the Department of Diabetes, Endocrinology and Metabolism, Churchill Hospital, Oxford, U.K.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 December 2008.
C.L. and C.C. contributed equally to this work
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gray SL, Nora ED, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O'Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A: Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator–activated receptor γ function (P465L PPARγ) in mice. Diabetes 55: 2669–2677, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppanen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A: PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet 3: e64, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology: adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 48: 1253–1262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P: Dynamics of fat cell turnover in humans. Nature 453: 783–787, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Farmer SR: Transcriptional control of adipocyte formation. Cell Metab 4: 263–273, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen ED, MacDougald OA: Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896, 2006 [DOI] [PubMed] [Google Scholar]
- 7.MacDougald OA, Mandrup S: Adipogenesis: forces that tip the scales. Trends Endocrinol Metab 13: 5–11, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Kennell JA, MacDougald OA: Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J Biol Chem 280: 24004–24010, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Prestwich TC, Macdougald OA: Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19: 612–617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon RT, Kohn AD, De Ferrari GV, Kaykas A: WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Wodarz A, Nusse R: Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14: 59–88, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cawthorn WP, Heyd F, Hegyi K, Sethi JK: Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ 14: 1361–1373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cawthorn WP, Sethi JK: TNF-alpha and adipocyte biology. FEBS Lett 582: 117–131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA: Regulation of Wnt signaling during adipogenesis. J Biol Chem 277: 30998–31004, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O'Rahilly S, Sethi JK, Vidal-Puig A: The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci 119: 2613–2620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, De Pergola G, Stirling H, Pannacciulli N, Sethi JK, Federspil G, Vidal-Puig A, Farooqi IS, O'Rahilly S, Vettor R: WNT10B mutations in human obesity. Diabetologia 49: 678–684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR: Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J 376: 607–613, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA: Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT: Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell 2: 449–461, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Gao X, Wen J, Ning Y, Chen YG: Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem 281: 8607–8612, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Brott BK, Sokol SY: Frodo proteins: modulators of Wnt signaling in vertebrate development. Differentiation 73: 323–329, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Gloy J, Hikasa H, Sokol SY: Frodo interacts with Dishevelled to transduce Wnt signals. Nat Cell Biol 4: 351–357, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Waxman JS, Hocking AM, Stoick CL, Moon RT: Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development 131: 5909–5921, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J: Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell 12: 1251–1260, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau TO, Chan CY, Chan KL, Lee MF, Wong CM, Fan ST, Ng IO: HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene 24: 1607–1614, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hikasa H, Sokol SY: The involvement of Frodo in TCF-dependent signaling and neural tissue development. Development 131: 4725–4734, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Fisher DA, Kivimae S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN: Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn 235: 2620–2630, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Suriben R, Fisher DA, Cheyette BN: Dact1 presomitic mesoderm expression oscillates in phase with Axin2 in the somitogenesis clock of mice. Dev Dyn 235: 3177–3183, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C, Perrin C, Jimenez-Linan M, Blount M, Dixon J, Zahn D, Thresher RR, Aparicio S, Carlton M, Colledge WH, Kettunen MI, Seppanen-Laakso T, Sethi JK, O'Rahilly S, Brindle K, Cinti S, Oresic M, Burcelin R, Vidal-Puig A: The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-γ2 isoform. Diabetes 54: 1706–1716, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nawaratne R, Gray A, Jorgensen CH, Downes CP, Siddle K, Sethi JK: Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol Endocrinol 20: 1838–1852, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.