Abstract
OBJECTIVE—Regulatory T-cells (Tregs) have catalyzed the field of immune regulation. However, translating Treg-based therapies from animal models of autoimmunity to human clinical trials requires robust methods for the isolation and expansion of these cells—a need forming the basis for these studies.
RESEARCH DESIGN AND METHODS—Tregs from recent-onset type 1 diabetic patients and healthy control subjects were isolated by fluorescence-activated cell sorting and compared for their capacity to expand in vitro in response to anti-CD3–anti-CD28–coated microbeads and IL-2. Expanded cells were examined for suppressive function, lineage markers and FOXP3, and cytokine production.
RESULTS—Both CD4+CD127lo/− and CD4+CD127lo/−CD25+ T-cells could be expanded and used as Tregs. However, expansion of CD4+CD127lo/− cells required the addition of rapamycin to maintain lineage purity. In contrast, expansion of CD4+CD127lo/−CD25+ T-cells, especially the CD45RA+ subset, resulted in high yield, functional Tregs that maintained higher FOXP3 expression in the absence of rapamycin. Tregs from type 1 diabetic patients and control subjects expanded similarly and were equally capable of suppressing T-cell proliferation. Regulatory cytokines were produced by Tregs after culture; however, a portion of FOXP3+ cells were capable of producing interferon (IFN)-γ after reactivation. IFN-γ production was observed from both CD45RO+ and CD45RA+ Treg populations.
CONCLUSIONS—The results support the feasibility of isolating Tregs for in vitro expansion. Based on expansion capacity, FOXP3 stability, and functional properties, the CD4+CD127lo/−CD25+ T-cells represent a viable cell population for cellular therapy in this autoimmune disease.
Multiple defects within the innate and adaptive immune systems are associated with the development of type 1 diabetes (1). Collectively, these defects lead to an imbalance in immune regulation that facilitates the expansion of pathogenic autoreactive T- and B-cells, resulting in the eventual destruction of insulin-producing β-cells (2,3). In recent years, appreciation has grown for the critical role Tregs play in maintaining immune tolerance (2). Studies in animal models and humans indicate a failure of Tregs to develop or function results in the development of systemic autoimmune disease (4,5). This is most apparent in humans with mutations in the gene encoding the transcription factor FOXP3, which is necessary for the proper development and function of Tregs (6). Additionally, transfer of excess Tregs into diabetes-susceptible NOD mice has been shown to prevent and even reverse disease (7).
With this, the field of immune therapy has undergone a paradigm shift in recent years favoring treatments that elicit dominant immune regulation by Tregs over those that result in broad immunosuppression (8). One such therapy involving treatment with the anti-CD3 antibody has recently shown some efficacy in preserving β-cell function in patients with type 1 diabetes, partly by increasing immune regulation via Tregs (9). Another seemingly paradoxical approach is the use of low doses of interleukin (IL)-2, especially in conjunction with rapamycin, to enhance Treg survival and expansion (10,11). Autologous therapies using Tregs represent an attractive therapeutic approach (12); however, these therapies are limited by the relatively low frequency of Tregs in circulation (comprising roughly 5–7% of CD4+ T-cells) (13), and it remains unclear whether a sufficient number of “pure” Tregs can be generated from these patients. One means of overcoming the limiting cell numbers is by isolating and expanding autologous Tregs before adoptive cell transfer. Whereas this procedure has been widely used for effector T-cells in HIV and cancer immunotherapy, an application using Tregs for the treatment of autoimmune diseases and graft-versus-host disease has only begun to be explored (14). Inherent to this application is the requirement for robust methods to isolate sufficiently pure populations from patients that will not result in the outgrowth of potentially pathogenic T-cells.
In this study described herein, we present two protocols for isolating and expanding adult human Tregs from patients with recent-onset type 1 diabetes. Our procedures are based on a clinically relevant fluorescence-activated cell sorting (FACS)-based isolation and in vitro expansion procedure that uses our recently described CD127 marker (IL-7 receptor α-chain) in combination with CD25 (IL-2 receptor α-chain) or rapamycin (15). Moreover, we characterize the phenotypic and functional properties of these cells after in vitro expansion and suggest that these approaches lead to selective expansion of cells with immunosuppressive properties.
RESEARCH DESIGN AND METHODS
Patient population.
Tregs were isolated from nine adult individuals with recent-onset type 1 diabetes (six men/three women; mean age 26.0 ± 9.7 years, range 17–40, with a mean disease duration of 7 months, range 20 days to 11 months) at the time of blood draw (i.e., <12 months from diagnosis) and three nondiabetic healthy control subjects (one man/two women; mean age 33 ± 1.5 years, range 31–34) from the general population. All patients with type 1 diabetes were diagnosed according to American Diabetes Association criteria (16). Control subjects lacked any autoimmune disorders or related probands with type 1 diabetes. Informed consent was obtained in accordance with approved policies and procedures.
Sample processing.
Fresh peripheral blood was collected in sodium heparinized vacutainer tubes (Becton Dickinson [BD], Franklin Lakes, NJ) and processed within 24 h for isolation of peripheral blood mononuclear cells (PBMCs). Complete cell isolation and culture guidelines including cell numbers, volumes, and respective culture flasks are outlined in detail in the Supplemental Methods section (available in an online-only appendix at http://dx.doi.org/10.2337/db08-1168).
Isolation of Tregs.
Tregs were isolated on a BD FACSAria II high-speed cell sorter (BD Biosciences, San Jose, CA) with the following antibodies: CD4-PerCP (SK3), CD127-PE (hIL-7R-M21), CD25-APC (2A3), CD45RA-PE.Cy7 (L48), and CD45RO-PE.Cy5 (UCHL1). CD4+CD127lo/−CD25+ and CD4+CD127lo/− T-cells were sorted using an aseptic technique in a cGMP-level clean room facility, and the sorted populations were collected into 3 ml X-VIVO 15 media (Lonza, Walkersville, MD) containing 10% human heat-inactivated pooled AB serum (Valley Biomedical, Winchester, VA). Treg populations were analyzed for purity post-sort and determined to be 97.96 ± 1.6%, range 95–100%, for CD4+CD127lo/−CD25+ T-cells and 98.76 ± 1.17%, range 96.2–100%, for CD4+CD127lo/− T-cells.
In vitro expansion procedure.
FACS isolated cells were plated at 2.5 × 105 Tregs per well in a 24-well plate (Costar, Cambridge, MA) and activated with anti-CD3/anti-CD28–coated microbeads (Invitrogen, Carlsbad, CA) at a 1:1 bead-to-cell ratio. CD4+CD127lo/− cells received rapamycin (100 ng/ml; Wyeth, Madison, NJ) through day 7 of culture (assuming consumption). At day 2, the culture volume was doubled and IL-2 was added (300 units/ml, Proleukin; Chiron Therapeutics, Emeryville, CA). Cells were resuspended and fresh media and IL-2 were added at days 2, 5, 7, 9, and 12 assuming consumption. On day 9, cells were restimulated with fresh anti-CD3/anti-CD28–coated beads at a 1:1 ratio.
Phenotypic analysis of expanded populations.
Freshly expanded cells were evaluated for continued expression of CD4, CD25, CD127, and FOXP3. Alexa488-conjugated anti-FOXP3 (clone 206D) was purchased from BioLegend (San Diego, CA), and intracellular staining was performed with the FOXP3 staining kit (BioLegend) according to the manufacturer's instructions and modified as follows: 5 × 105 cells were washed and fixed for 30 min at room temperature using fixation/permeabilization buffer. Cells were washed, resuspended in perm buffer containing DNase I (100 units/ml; Sigma-Aldrich, St. Louis, MO), and incubated for 30 min at room temperature, followed by two washes in perm buffer. Cells were subsequently blocked with human IgG (5 μg/test) for 5 min and stained for surface markers along with anti-human FOXP3-Alexa488 (5 μl/test) or isotype control. Flow cytometric data were collected on a FACSCalibur cytometer (BD Biosciences) and analyzed with FlowJo software (version 7.2.2; TreeStar, Ashland, OR).
In vitro suppression assays.
In repeated studies, we have observed that expanded Tregs suppress both CD4+ and CD8+ allogeneic and mitogen-stimulated T-cell responses. For the purposes of this study, in vitro suppression was assessed based on their capacity to suppress the proliferation of allogeneic CD8+ responder T-cells. To compare different Treg populations against a common responder, a normal healthy donor was apheresed and PBMCs were cryopreserved in multiple aliquots. For the carboxyfluorescein diacetate succinimidyl ester (CFSE)-based suppression assay, individual vials of standard responders (containing ∼5 × 106 cells/vial) were thawed, CFSE labeled (Invitrogen; 0.3 μmol/l), and plated in the presence of unlabeled Tregs at various ratios of Treg to responder cells. Cells were stimulated with 2 μg/ml soluble anti-CD3 (Hit3a) and anti-CD28 (28.2; BD PharMingen). Cultures were incubated for 4 days at 37°C in a 5% CO2 incubator. At day 4, cells were harvested, pooled for each condition, and stained with CD8-APC (BD Biosciences) to assess proliferation. Cells were gated by forward scatter (FSC)/side scatter (SSC) and CD8+ responders to discriminate them from Tregs. A CFSE histogram of unstimulated responder cells defined the parent population, and proliferation of activated responders was determined by calculation of precursor frequency (pf) using ModFit LT software (Verity Software House, v3.0; Topsham ME). In some instances, proliferation was also determined by the incorporation of 3H-thymidine by pulsing cultures with 1 μCi for the final 16 h of culture. Plates were harvested on a Packard FilterMate Harvester and read on a Packard TopCount Scintillation and Luminescence Counter (Perkin Elmer, Waltham, MA). For both assays, percent suppression was calculated as previously described (17).
In vitro suppression assays were conducted in RPMI-1640 (Mediatech, Manassas, VA) supplemented with 5 mmol/l HEPES, 2 mmol/l l-glutamine, penicillin/streptomycin (50 μg/ml each) (Invitrogen), 50 μmol/l 2-mercaptoethanol (Sigma), 5 mmol/l nonessential amino acids, 5 mmol/l sodium pyruvate (Mediatech), and 2.5% human serum. Cultures were maintained in 200-μl volumes in U-bottom 96-well plates (Costar, Cambridge, MA) incubated at 37°C and 5% CO2. Neutralizing antibodies to transforming growth factor (TGF)-β or IL-10 were obtained from R&D Systems (Minneapolis, MN) and control goat IgG (Jackson ImmunoResearch, West Grove, PA).
Cytokine analysis.
Tregs were assessed for cytokine production at day 14 of culture via intracellular staining and analyses of culture supernatants. For analysis of cytokines in supernatants, fresh supernatant was collected directly from expansion cultures or from a subset of cells (1 × 106 cells) reactivated in fresh media with beads (1:1) or phorbol myristate acetate (PMA) (10 ng/ml) and ionomycin (500 ng/ml) (Sigma) for an additional 5 h at 37°C.
Cytokine determinations were conducted in a multiplex format using either the Luminex platform with the cytokine multiplex kit (analytes interferon [IFN]-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, MIP-1α, RANTES, sCD40L, sIL-2Rα, and TNF-α; Millipore, Bellerica, MA) or with the Cytometric Bead Array (human Th1/Th2 cytokine kit II; BD PharMingen). TGF-β concentrations, determined using the Quantikine Human TGF-β ELISA kit (R&D Systems), showed that the human serum used for these studies contained 18.08 ng/ml TGF-β1. Intracellular cytokines were stained directly from cultures at day 14 or after 5 h of reactivation with beads or PMA/ionomycin. Intracellular cytokine staining was conducted with FOXP3 staining using the manufacturer kit reagents from BioLegend as previously described along with the anti-human cytokine antibodies IFN-γ phycoerythrin and IL-10 allophycocyanin (BD PharMingen).
Statistics and methods of analysis.
Data analyses used GraphPad Prizm 4.00 software (GraphPad, San Diego, CA), and values at P < 0.05 were deemed significant. Cytokine concentrations were determined using SOFTmax® PRO software (Molecular Devices, Sunnyvale, CA) with four-parameter data analysis. Spearman's correlation analyses were used to compare Treg expansion to the subject's age, and Student's paired t tests were used to compare responses for CD4+CD127lo/− and CD4+CD127lo/−CD25+ T-cells.
RESULTS
Isolation and characterization of human Tregs.
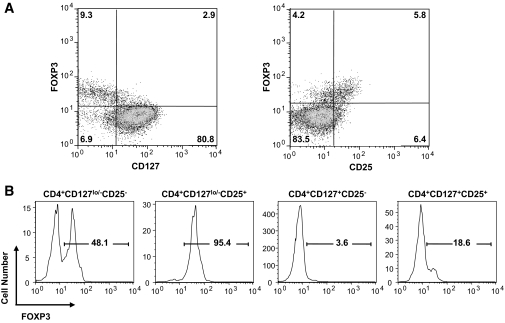
In an effort to develop an optimal protocol for expanding Tregs for therapeutic use, we examined two different strategies based on the use of a limited number of antibodies and techniques that can be adapted to cGMP-level standard operating procedures. As previously observed, the majority of FOXP3+ T-cells in peripheral blood are CD127 negative to low (Fig. 1A, left plot). Moreover, CD25 expression was positively correlated with FOXP3 expression (Fig. 1A, right plot, and Supplemental Fig. 1 and animations). CD4+ T-cells display varying levels of FOXP3 based on a combination of the markers, with the highest levels of FOXP3+ cells being within the CD4+CD127lo/−CD25+ subset (Fig. 1B, typically >95% FOXP3+). Therefore, our primary strategy consisted of isolating the highly FOXP3-expressing CD4+CD127lo/−CD25+ (15) cell population. Likewise, inclusion of the CD127 marker improves the overall yield of FOXP3+ T-cells by allowing collection of CD25lo and CD25Intermediate cells that would otherwise be lost if cells were sorted based on being solely CD4+CD25hi, as has been reported to contain cells enriched for FOXP3+ suppressors (18). As a second strategy, we examined the ability to expand all the CD4+CD127lo/− T-cells irrespective of CD25 expression, since this population represented a larger initial cell number, which we previously showed had suppressive activity when isolated directly from peripheral blood (15). The selective immunosuppressive agent, rapamycin, was used in these cultures to prevent outgrowth of contaminating nonregulatory cells (19). It is important to note that, in both procedures, the exclusion of CD127hi expressing cells made it possible to isolate Tregs devoid of memory effector T-cells (Teff) (15,20). Importantly, because the ability to broaden the cell surface phenotype to cells expressing lower levels of CD25 allowed for the isolation of the most Tregs from patients with type 1 diabetes, a patient population where CD25 has been suggested to be a susceptibility gene and where there has been a suggestion that the CD4+CD25hi population may be defective (21–23).
FIG. 1.
CD4, CD25, and CD127 identify FOXP3+ Tregs in human peripheral blood. A: CD127 expression inversely correlates with FOXP3 expression in freshly isolated human peripheral blood CD4+ T-cells (left plot), whereas CD25 expression is positively correlated with FOXP3 expression (right plot). B: Using the combination of these markers, the highest purity of FOXP3+ T-cells is found within the CD4+CD127lo/−CD25+ T-cell fraction (∼95%). One representative sample is shown. (A three-dimensional plot and animated rotations showing Treg and Teff staining are shown in Supplemental Fig. 1 and supplemental videos.)
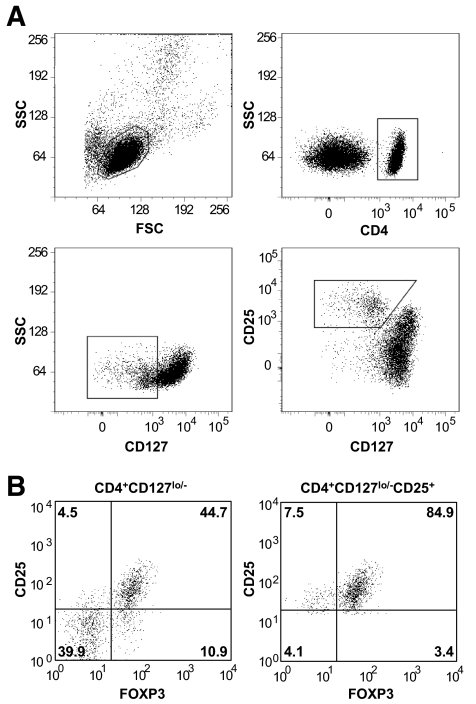
We next sought to develop a method to consistently and reproducibly isolate these cells for in vitro expansion. Procedures currently approved for clinical applications using isolated human cells rely on sorting cells using antibody-coated microbeads in an automated magnetic separation device. Whereas there are advantages to this system for isolating bulk populations using defined markers such as CD4, microbead-based sorting is limited when multiple markers are needed or when markers exhibit broad expression patterns. To overcome these limitations, we sorted Tregs in a FACS-based approach. Our sorting scheme to isolate CD4+CD127lo/−CD25+ and CD4+CD127lo/− T-cells is shown in Fig. 2A. Representative examples for each population with post-sort FOXP3 analysis are shown in Fig. 2B.
FIG. 2.
Gating strategy and FOXP3 analysis of freshly isolated CD4+CD127lo/− and CD4+CD127lo/−CD25+ T-cells. A: Tregs were isolated from peripheral blood through a FACS-based sorting procedure. The lymphocyte population was gated based on forward and side scatter (A, upper-left plot), followed by gating all CD4+ T-cells (A, upper-right plot). The two cell populations were then sorted based on CD127lo/− and CD25+ expression (A; CD4+CD127lo/− T-cells, middle-left plot, gated population, or CD4+CD127lo/−CD25+ T-cells, middle-right plot, gated population). B: FOXP3 expression from one representative sample is shown for freshly isolated CD4+CD127lo/− (left plot) and CD4+CD127lo/−CD25+ T-cells (right plot).
In vitro expansion of Tregs.
Given the relatively low circulating frequency of Tregs, most applications will require in vitro expansion to impact the effective Treg to Teff cell ratio (12). We previously showed in mouse models (24) that overcoming the anergic state of Tregs for the purposes of expansion requires strong TCR and costimulatory signals in the presence of high-dose IL-2 (25). Thus, our approach took advantage of anti-CD3/anti-CD28–coated beads and IL-2 as outlined in Fig. 3A (detailed conditions included in Supplemental Methods Section).
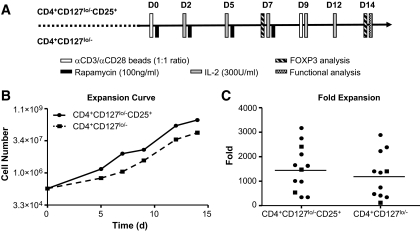
FIG. 3.
CD4+CD127lo/−CD25+ and CD4+CD127lo/− T-cells can be expanded to comparable levels. A: The diagram outlines the time points and procedures conducted for each cell population during the in vitro expansion period. Freshly sorted Tregs were activated with anti-CD3/anti-CD28–coated microbeads in the presence of exogenous IL-2 over a 14-day culture period. No immunosuppressive reagents were used in CD4+CD127lo/−CD25+ T-cell expansions, whereas CD4+CD127lo/− T-cells were grown in the presence of rapamycin (100 ng/ml) for the first 7 days of culture. B: A representative expansion curve is shown for CD4+CD127lo/−CD25+ T-cells (solid line) and CD4+CD127lo/− T-cells (dashed line). C: Tregs from a total of 12 individuals (nine recent-onset type 1 diabetes subjects [circles] and three nondiabetic healthy control subjects [squares]) were sorted and expanded. Fold expansion data are shown at the 14-day time point, with the mean indicated for CD4+CD127lo/−CD25+ T-cells (1,532-fold) and CD4+CD127lo/− T-cells (1,248-fold).
As previously discussed, the original isolation procedure greatly influences the subsequent degree of in vitro expansion required. However, this factor is offset by the observation that less-pure starting populations require more stringent growth conditions to ensure Treg expansion over the outgrowth of Teff cells (19). One means to accomplish selective growth of Tregs is through the use of rapamycin (16). However, preliminary studies suggested that the addition of this mTOR inhibitor delayed the expansion potential of T-cells (Supplemental Fig. 2). Thus, CD4+CD127lo/− T-cells were grown in the presence of rapamycin for only the first 7 days of culture. In this way, we were able to obtain a mean expansion of 1,248 ± 930-fold, median 1,347, range 117–2,886, over a 14-day period. The highly purified CD4+CD127lo/−CD25+ T-cell population, grown without rapamycin, expanded to comparable levels (mean 1,532 ± 938.7, median 1,548, range 340–3,171-fold). Representative expansion curves for both cell populations are shown in Fig. 3B, and expansion data for all subjects analyzed are graphed in Fig. 3C.
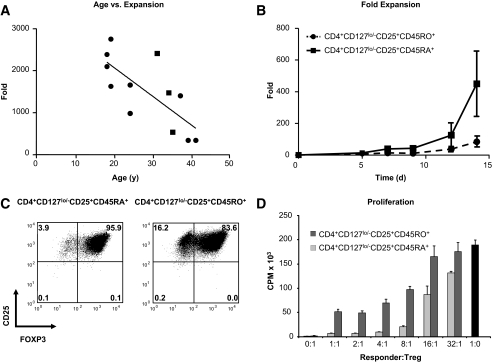
One of the surprising observations resulting from these studies was the degree of heterogeneity in expansion from one individual to the next. We hypothesized that some of the variability might be explained by the age of the donor. It has been previously reported that aging is associated with a shift in T-cell phenotype from a predominantly naïve T-cell subset (CD45RA+) to a memory (CD45RO+) T-cell phenotype (20). This shift has also been associated with increased cellular senescence, partially resulting from the shortening of telomeric DNA (26,27). Naïve T-cells have been reported to contain longer telomeres compared with antigen-experienced cells (28). In line with this notion, we observed a negative correlation between subject age and fold expansion for CD4+CD127lo/−CD25+ T-cells (Fig. 4A; r = −0.77, P = 0.0033). To determine if this observation could be influenced by the relative proportion of memory and naïve cells, CD4+CD127lo/−CD25+ T-cells were additionally sorted based on CD45RA+ versus CD45RO+ expression and expanded according to our standard protocol. We observed that CD45RA+ Tregs expanded better than their CD45RO+ counterparts (Fig. 4B, mean fold expansion 317.0 + 294.5 for CD45RA+ Tregs vs. 59.7 + 43.1-fold for CD45RO+, n = 3). This increased expansion potential was accompanied by increased purity (based on FOXP3 expression) and suppressive properties of expanded CD45RA+ Tregs compared with CD45RO+ Tregs. Representative examples of FOXP3 expression and in vitro suppression are shown in Fig. 4C and D, respectively. Thus, we conclude that expansion can be achieved with either CD4+CD127lo/−CD25+ or CD4+CD127lo/− T-cells (in the presence of rapamycin). However, the majority of the expansion results from the CD45RA+ T-cell subset, which has implications for using expanded Treg cell therapy in older individuals.
FIG. 4.
Subject age and the proportion of naïve and memory T-cells influence the expansion potential and purity of Tregs. A: Spearman rank analysis indicates a negative correlation between subject age and fold expansion of CD4+CD127lo/−CD25+ Tregs at the 14-day time point (r = −0.77, P = 0.0033). B: CD4+CD127lo/−CD25+ Tregs were sorted into CD45RA+ (solid line) and CD45RO+ (dashed line) cell fractions and expanded as previously described. Expansion time-course data indicate a trend toward increased expansion of naïve compared with memory T-cell fractions (mean 317.0- vs. 59.7-fold, respectively, n = 3). C: CD45RA+ Tregs (left plot) display increased FOXP3 purity at the 14-day time point versus CD45RO+ Tregs (right plot). D: The suppression capacity of expanded CD45RA+ and CD45RO+ Tregs were compared at varying ratios of Treg to anti-CD3–and anti-CD28–stimulated PBMC responders. Results are shown as average counts per minute (CPM) of triplicates measured by the incorporation of 3H-thymidine in co-cultures and compared with responders alone (▪). C and D indicate data from one representative sample.
Stability of a regulatory T-cell phenotype after in vitro expansion.
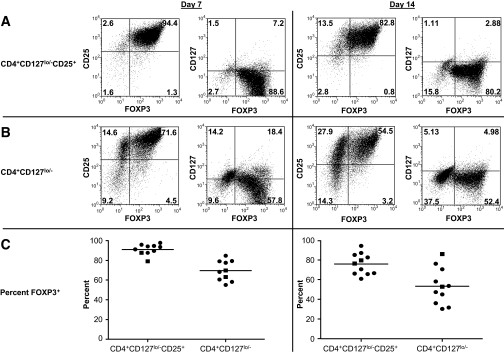
Given the comparable expansion potential of CD4+CD127lo/− and CD4+CD127lo/−CD25+ T-cells, we next sought to assess the functional properties of these cells after expansion. As the prototypical marker for Tregs, FOXP3 expression was analyzed by FACS along with CD4, CD25, and CD127 at the 7- and 14-day time points of culture (Fig. 5A, upper panels, for CD4+CD127lo/−CD25+ T-cells, and Fig. 5B, lower panels, for CD4+CD127lo/− T-cells). As predicted based on the initial higher percentage of FOXP3 within the CD4+CD127lo/−CD25+ subset, we observed a higher percentage of FOXP3+ T-cells in the expanded CD4+CD127lo/−CD25+ T-cells at day 7 (Fig. 5C, left graph, mean 91.1 ± 5.4%, median 92.4, range 79.1–97.8) compared with that obtained with CD4+CD127lo/− T-cells (69.6 ± 10.2, median 69.1, range 55.1–84.7). This trend continued at day 14 (Fig. 5C, right graph, 75.9 ± 10.7, median 76.4, range 60.8–94.3, for CD4+CD127lo/−CD25+ T-cells and 53.4 ± 18.2, median 52.8, range 30.2–86.0, for CD4+CD127lo/− T-cells).
FIG. 5.
Human Tregs retain FOXP3 expression after expansion. A: Flow cytometric plots from a representative sample showing CD25, CD127, and FOXP3 expression for CD4+CD127lo/−CD25+ T-cells (top panels) and CD4+CD127lo/− T-cells (B, middle panels). Data from days 7 and 14 of expansion are indicated (left-hand and right-hand panels, respectively). C: Individual FOXP3 percentages 7-day (left plot) and 14-day (right plot) time points from type 1 diabetic subjects (circles) and control subjects (squares) are graphed with the mean for each group indicated.
Relative FOXP3 expression within individual cells was upregulated in expanded cultures upon reactivation with either beads or PMA and ionomycin (Supplemental Fig. 3). These observations are in line with other reports suggesting that activated expanded Tregs are more potent suppressors (29), along with reports suggesting that costimulatory signals (such as those provided by CD28 and IL-2) may function to upregulate FOXP3 (30,31). Interestingly, Tregs cultured in the presence of brefeldin-A suppressed the FOXP3 increase and in fact led to downregulation of surface CD25 and FOXP3 (data not shown), suggesting that the affect of T-cell stimulation may be due to both transcriptional and translational effects. In addition to FOXP3, expanded Tregs expressed high levels of markers putatively associated with terminally differentiated Tregs including HLA-DR, CTLA-4 (BD PharMingen), and TGF-β1–LAP (R&D Systems) (Supplemental Fig. 4).
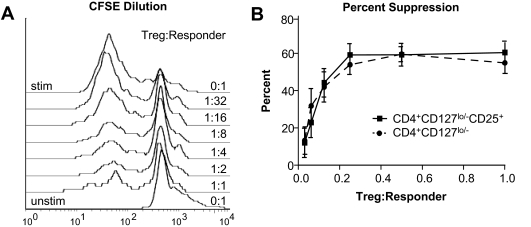
We next tested the capacity of expanded Tregs to suppress proliferation of CD8+ T-cells using a standardized allogeneic responder T-cell population stimulated with anti-CD3 and anti-CD28 (Fig. 6A). Despite more variable and often reduced numbers of FOXP3+ cells in the expanded CD4+CD127lo/− T-cell population, these cells suppressed responder T-cell proliferation to a similar degree on a per cell basis when compared with CD4+CD127lo/−CD25+ T-cells (Fig. 6B, P = NS for all ratios Treg:Tresponders). Likewise, for all ratios of Treg:Tresponders, no significant difference in suppressive capacity was observed for type 1 diabetic patient Tregs compared with normal healthy control Tregs. The mechanism of suppression of expanded Tregs was determined to be independent of IL-10 or TGF-β, since the addition of neutralizing antibodies to these cytokines during the in vitro suppression assay failed to abrogate suppression (data not shown).
FIG. 6.
Expanded Tregs retain their suppressive function after in vitro expansion. A: The plot shows a representative suppression assay using CFSE-labeled PBMCs (responders) incubated with titrating levels of CD4+CD127lo/−CD25+ Tregs. The top histogram indicates CFSE dilution of CD8+-gated responder T-cells alone that divided in response to soluble anti-CD3 and anti-CD28 over a 4-day culture period. The bottom histogram indicates the initial CFSE fluorescence peak of unstimulated responders alone with overlaid histograms showing the proliferation of responders at increasing ratios of Tregs to responders indicated (right y-axis). B: In vitro suppression from CD4+CD127lo/−CD25+ Tregs (solid lines) and CD4+CD127lo/− Tregs (dashed lines) are dose responsive. Data shown indicate the percent suppression plotted as means ± SE of for all 12 study subjects at each ratio of Treg to responder cells.
Expression of cytokines by expanded regulatory T-cells.
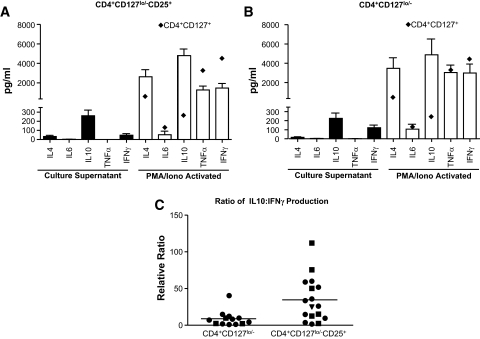
Fresh Tregs are generally noted for their lack of cytokine production when directly examined after isolation from peripheral blood. In fact, one of the clear disconnects is the relative lack of evidence that cytokines are involved in in vitro suppression by Treg, whereas multiple in vivo studies in rodent models suggest a clear role for IL-10 and TGF-β in Treg function (32,33). Therefore, we analyzed the ability of the expanded Treg populations to produce cytokines that might either enhance or abrogate Treg function in vivo. In addition, the analyses of cytokine production might also identify contaminating effector T-cells that might be a potential concern with adoptive cell therapy in autoimmune patients. Analyses of culture supernatants of individual expanded T-cell populations suggested that, in general, there was very low production of IFN-γ with relatively high expression of IL-10, when compared with effector T-cells (CD4+CD127+) expanded under the same conditions (Fig. 7). A portion of expanded Tregs were capable of producing cytokines after anti-CD3/anti-CD28–coated bead or PMA/ionomycin reactivation (Fig. 7A for CD4+CD127lo/−CD25+ T-cells and Fig. 7B for CD4+CD127lo/− T-cells; see also data from a panel of 14 cytokines and soluble receptors in Supplemental Table 1). It is generally accepted that IFN-γ represents a TH1-type effector cytokine (34), whereas IL-10 has been ascribed to exhibit multiple immunoregulatory functions (35). Therefore, we assessed the ratio of IL-10 to IFN-γ production in supernatants from stimulated CD4+CD127lo/− and CD4+CD127lo/−CD25+ T-cell populations (Fig. 7C). This analysis suggests that stimulated CD4+CD127lo/−CD25+ Tregs produce proportionately more IL-10 to IFN-γ compared with CD4+CD127lo/− T-cells (P = 0.0014).
FIG. 7.
Cytokine production profiles of in vitro expanded Tregs. A: Culture supernatants were collected from 14-day expansion cultures (▪) of CD4+CD127lo/−CD25+ T-cells and analyzed for the production of cytokines by cytometric bead array. In addition, expanded cells (1 × 106) were restimulated with PMA/ionomycin in vitro for 5 h and supernatants were collected for analysis (□). ♦, levels of cytokine detected from similarly expanded CD4+CD127+ T effector cells. Cytokine data are graphed as the means + SE for all 12 study subjects. C: Data indicate the relative ratio of IL-10/IFN-γ production from expanded CD4+CD127lo/− (left) and CD4+CD127lo/−CD25+ (right) Treg populations after 5 h reactivation with PMA/ionomycin (n = 12, 8.8 vs. 34.6, P = 0.0014).
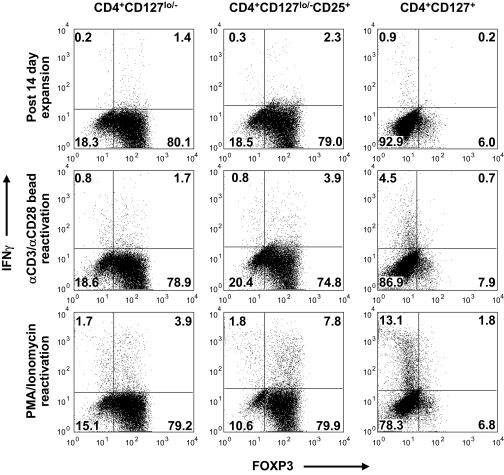
Next, we analyzed the production of the key cytokines on a per cell basis using a combination of intracellular cytokine and FOXP3 staining on expanded Treg cultures. As was previously observed in supernatants, the production of IFN-γ followed the general trend of cytokine production from the different cell populations as follows (Fig. 8; CD4+CD127+ Teff > Tregs) and likewise for stimulation signal strength (Fig. 8; PMA/ionomycin > anti-CD3/anti-CD28 beads > fresh from culture). Specifically, we observed a significant portion of the FOXP3+ T-cells produced IFN-γ after reactivation with PMA/ionomycin (median 11.6%, range 3.25–15.88, n = 6). IFN-γ production within the FOXP3+ cells was comparable in all conditions between freshly isolated and expanded cells, suggesting this does not result solely from in vitro expansion (Supplemental Table 2). It remained possible that the IFN-γ–producing T-cells were contaminating Teff cells, which can express low levels of FOXP3. Therefore, cells were split into memory (CD45RO+) and naïve (CD45RA+) T-cell fractions before expansion and cytokine analyses. Importantly, IFN-γ–producing cells were observed in the CD45RA Treg subset (albeit lower than observed from the CD45RO subset), suggesting this bona fide Treg population contains cells capable of producing cytokines. In fact, we observed that Tregs isolated and expanded from umbilical cord blood (presumably a functionally naïve repertoire) also produce IFN-γ (see Supplemental Fig. 5), suggesting human FOXP3+ T-cells are capable of producing cytokines after various activation conditions.
FIG. 8.
Expanded FOXP3+ T-cells are capable of producing IFN-γ after reactivation. CD4+CD127lo/− (left plots), CD4+CD127lo/−CD25+ (middle plots), and CD4+CD127+ T-cells (right plots) were stained for intracellular FOXP3 and IFN-γ. For each cell population, a dose-responsive increase in IFN-γ production was observed in cells after in vitro expansion. Plots indicate flow cytometric data from one representative subject stained immediately after a 14-day culture period (upper panels), or after an additional 5 h reactivation period with anti-CD3 and anti-CD28 microbeads (middle panels), or PMA/ionomycin (lower panels).
DISCUSSION
One potential path for an effective therapy to reverse type 1 diabetes would involve the restoration of homeostasis within the immune system normally controlled by Tregs. Compelling evidence exists from the NOD mouse that transfer of in vitro expanded Tregs can overcome intrinsic defects and restore tolerance in type 1 diabetes (12). The therapeutic benefits of restoring tolerance early in type 1 diabetes would likely result in the preservation of endogenous β-cell mass and subsequent reduction in complications resulting from hyperglycemia. Treg-based cell therapies pose several advantages over cell-depleting regimens and bone marrow transplantation protocols, in that they are unlikely to compromise host immune responses to foreign pathogens.
In this study, we demonstrated that functional Tregs can be isolated from patients with type 1 diabetes and expanded in vitro to therapeutically relevant levels. Most importantly, we demonstrated that we could attain on average 1,500-fold expansion of the highly purified CD4+CD127lo/−CD25+ T-cell population in a 14-day culture period using methods that can be adapted to cGMP procedures. Moreover, cells from both type 1 diabetic patients and healthy volunteers grown under these conditions retain the hallmark characteristics of freshly isolated Tregs (i.e., they are anergic after culture, suppressive, and continue to express high levels of the transcription factor FOXP3).
Several previous studies have reported methods for isolating and expanding natural FOXP3-expressing Tregs for therapeutic applications from healthy individuals (36,37). However, these alternative approaches can fall short of meeting clinical needs in terms of the initial cell yield, the feasibility of adapting protocols to cGMP levels, or the purity and stability of the expanded cell populations post-transfer, especially in patients with autoimmune diseases as highlighted below. We sought to address these concerns and meet these important therapeutic criteria.
The use of cellular therapies in individuals with type 1 diabetes raises concerns regarding the subsequent function of cells in patients presumably carrying a genetic predisposition toward autoimmunity. There is growing evidence from genetic studies in the NOD mouse and humans implicating pathways likely to influence Treg frequency and/or function. These genes include, but are not limited to, the IL-2 signaling axis (Il2 in the NOD mouse and CD25 in humans), the T-cell signaling molecule LYP (protein tyrosine phosphatase [PTPN22]), and the negative T-cell regulator cytotoxic T-lymphocyte antigen-4 (CTLA-4) (38–40). Susceptibility alleles in these pathways may confer deficient immune activation and subsequent control by Tregs (10,19). One means to overcome this inherent defect may be the use of matched allogeneic cells, such as that recently reported for bone marrow transplantation in a model of autoimmune arthritis (41). Although this approach may be advantageous from an immunoregulatory standpoint, the safety and efficacy profile of such an approach in patients with type 1 diabetes remains unknown. These questions require initial trials with autologous cells with the expectation that in vitro activation and IL-2 exposure will augment the functional profile of Tregs (29).
A major concern when proposing to expand Tregs from type 1 diabetic patients or other autoimmune subjects is the potential for outgrowth of activated Teff cells. Central to addressing this concern is our inclusion of the CD127 marker, which enables us to discriminate between activated Teff cells and bona fide Tregs (15). As an additional constraint, we chose to isolate Tregs through a stringent FACS-based sorting approach. Using these parameters, we were able to show expansion of functional CD4+CD127lo/− T-cells grown in the presence of rapamycin as well as highly purified CD4+CD127lo/−CD25+ T-cells. Surprisingly, both of these populations suppressed equally well during the in vitro suppression assay despite decreased numbers of FOXP3+ cells present in CD4+CD127lo/− T-cells. This may result from an unknown suppressive property of CD127lo/− T-cells, or alternatively, from the differentiation of FOXP3− T-cells into IL-10–or TGF-β–producing adaptive Tregs. This process has previously been reported to occur through exposure of conventional T-cells to Tregs in vitro or in vivo in a process commonly referred to as education (42–44).
Despite the similar suppressive properties of CD4+CD127lo/− T-cells, we believe CD4+CD127lo/−CD25+ T-cells represent the optimal polyclonal Treg population for our proposed phase I clinical trial. This conclusion is based on the highly enriched nature of the starting population and the ability to expand these cells to high purity without rapamycin. While rapamycin does selectively inhibit Teff outgrowth in vitro, there is some concern that these activated cells could expand after in vivo transfer in the absence of the drug. Therefore, when considering the optimal cell population for cellular therapies, based on the parameters of stability, FOXP3 expression, and in vitro expansion potential, we have concluded that CD4+ CD127lo/−CD25+ T-cells represent the optimal population.
Despite these promising results, several key questions still remain regarding Treg cell therapy. Little is known about how long expanded Tregs will persist upon transfer, where they track, or whether they retain their suppressive phenotype in vivo. To begin to address the issue of cell stability, we assessed the cytokine production profiles of expanded Tregs. Surprisingly, analyses of intracellular cytokines from expanded human Tregs indicate FOXP3 expression, and production of IFN-γ is not mutually exclusive. In fact, we noted that several other cytokines typically associated with effector functions could also be co-expressed with FOXP3 including IL-17 and TNF-α, whereas IL-2 and IL-4 appeared more restricted to the FOXP3− T-cell fraction (data not shown). The in vivo physiological significance of these observations in response to super-physiological stimuli is not clear, nor is the notion that these responses are detrimental. Nevertheless, these questions regarding the stability of Tregs are of great importance, and we are continuing to investigate these issues through studies with humanized mice (45), as well as FoxP3 reporter mice that can discriminate cells that are currently expressing FoxP3, or have expressed it at any point in development (46).
Animal model data support the use of Tregs to halt type 1 diabetes progression and suggest transfer of Tregs may lead to reversal of ongoing disease (7,24,47). With that said, this may be challenging in subjects after the processes of epitope spreading and memory T-cell expansion. Here we propose the use of polyclonal Tregs as a necessary advancement in the use of Tregs for the treatment of type 1 diabetes. However, we acknowledge that overcoming the autoimmune attack in type 1 diabetes in the future may require even more challenging approaches involving combination therapies or the use of antigen-specific Tregs. To begin to address these issues, we are currently developing protocols for the generation of antigen-specific Tregs capable of recognizing diabetes autoantigens through the use of viral TCR-α and TCR-β gene transfer into CD4+CD127lo/−CD25+ T-cells.
In conclusion, our data indicate functional Tregs can be isolated and expanded from recent-onset type 1 diabetic patients. These Tregs can be expanded in vitro to >1,500-fold in a 2-week period using cGMP-level materials and procedures. These cells retain potent suppressive function and FOXP3 expression without the need for rapamycin. This study outlines important tools and principles for translating Treg therapies into clinical treatments for patients with type 1 diabetes and other autoimmune disorders.
Supplementary Material
Acknowledgments
This work was supported by a Juvenile Diabetes Research Foundation (JDRF) Collaborative Center for Cell Therapy Grant. Additional funding and reagent support was provided by BD Biosciences. T.M.B. was supported by a fellowship from the JDRF, as well as funding from the Dee and William Brehm Foundation.
No potential conflicts of interest relevant to this article were reported.
We wish to thank the members of the Bluestone Laboratory for their helpful comments. The authors thank Michael Haller, John Alexander, and Miriam Cintron for patient recruitment at the University of Florida. We thank Stephanie McClymont for assistance with data analysis and Carlos Garcia of Beckman Coulter for assistance with 3D flow cytometric data analysis.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 December 2008.
A.L.P. and T.M.B. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358: 221–229, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Chatenoud L, Salomon B, Bluestone JA: Suppressor T cells: they're back and critical for regulation of autoimmunity! Immunol Rev 182: 149–163, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD: B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ig mu null mice. J Exp Med 184: 2049–2053, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rudensky AY: Molecular aspects of regulatory T cell development. Semin Immunol 16: 73–80, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA: B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12: 431–440, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD: The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bluestone JA, Tang Q: Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci U S A 101 (Suppl. 2): 14622–14626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Clair EW, Turka LA, Saxon A, Matthews JB, Sayegh MH, Eisenbarth GS, Bluestone J: New reagents on the horizon for immune tolerance. Annu Rev Med 58: 329–346, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54: 1763–1769, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA: Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28: 687–697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R: Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 51: 638–645, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Brusko T, Bluestone J: Clinical application of regulatory T cells for treatment of type 1 diabetes and transplantation. Eur J Immunol 38: 931–934, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA: CD4+CD25high regulatory cells in human peripheral blood. J Immunol 167: 1245–1253, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Brusko TM, Putnam AL, Bluestone JA: Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev 223: 371–390, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA: CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203: 1701–1711, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS: Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 111: 453–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusko TM, Hulme MA, Myhr CB, Haller MJ, Atkinson MA: Assessing the in vitro suppressive capacity of regulatory T cells. Immunol Invest 36: 607–628, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA: CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp 252: 67–88, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG: Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177: 8338–8347, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M: No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes 56: 604–612, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M, Clayton DG, Wicker LS, Todd JA: Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 39: 1074–1082, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA: Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes 54: 1407–1414, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI: Defective suppressor function in CD4+ CD25+ T-cells from patients with type 1 diabetes. Diabetes 54: 92–99, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA: In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199: 1455–1465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hombach AA, Kofler D, Hombach A, Rappl G, Abken H: Effective proliferation of human regulatory T cells requires a strong costimulatory CD28 signal that cannot be substituted by IL-2. J Immunol 179: 7924–7931, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Roth A, Yssel H, Pene J, Chavez EA, Schertzer M, Lansdorp PM, Spits H, Luiten RM: Telomerase levels control the lifespan of human T lymphocytes. Blood 102: 849–857, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM: Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood 98: 597–603, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, Chiumello G, Ziegler AG, Bonifacio E: Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol 179: 5785–5792, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Chai JG, Coe D, Chen D, Simpson E, Dyson J, Scott D: In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol 180: 858–869, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Scotta C, Soligo M, Camperio C, Piccolella E: FOXP3 induced by CD28/B7 interaction regulates CD25 and anergic phenotype in human CD4+CD25- T lymphocytes. J Immunol 181: 1025–1033, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Bayer AL, Yu A, Adeegbe D, Malek TR: Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med 201: 769–777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr, Muller W, Rudensky AY: Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M: Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol 173: 6526–6531, 2004 [DOI] [PubMed] [Google Scholar]
- 34.De Maeyer E, De Maeyer-Guignard J: Interferon-gamma. Curr Opin Immunol 4: 321–326, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M: Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 104: 895–903, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann P, Boeld TJ, Eder R, Albrecht J, Doser K, Piseshka B, Dada A, Niemand C, Assenmacher M, Orso E, Andreesen R, Holler E, Edinger M: Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol Blood Marrow Transplant 12: 267–274, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P: Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet 39: 329–337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P: The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176: 6752–6761, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC: Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423: 506–511, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Roord ST, de Jager W, Boon L, Wulffraat N, Martens A, Prakken B, van Wijk F: Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood 111: 5233–5241, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH: Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med 196: 255–260, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G: Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J Exp Med 196: 247–253, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kearley J, Barker JE, Robinson DS, Lloyd CM: Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 202: 1539–1547, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shultz LD, Ishikawa F, Greiner DL: Humanized mice in translational biomedical research. Nat Rev Immunol 7: 118–130, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA: Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med 205: 1983–1991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masteller EL, Tang Q, Bluestone JA: Antigen-specific regulatory T cells: ex vivo expansion and therapeutic potential. Semin Immunol 18: 103–110, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.