Abstract
OBJECTIVE—Obesity is associated with insulin resistance and type 2 diabetes, although the mechanisms linking these pathologies remain undetermined. Recent studies in rodent models revealed endoplasmic reticulum (ER) stress in adipose and liver tissues and demonstrated that ER stress could cause insulin resistance. Therefore, we tested whether these stress pathways were also present in obese human subjects and/or regulated by weight loss.
RESEARCH DESIGN AND METHODS—Eleven obese men and women (BMI 51.3 ± 3.0 kg/m2) were studied before and 1 year after gastric bypass (GBP) surgery. We examined systemic insulin sensitivity using hyperinsulinemic-euglycemic clamp studies before and after surgery and collected subcutaneous adipose and liver tissues to examine ER stress markers.
RESULTS—Subjects lost 39 ± 9% body wt at 1 year after GBP surgery (P < 0.001), which was associated with a marked improvement in hepatic, skeletal muscle, and adipose tissue insulin sensitivity. Markers of ER stress in adipose tissue significantly decreased with weight loss. Specifically, glucose-regulated protein 78 (Grp78) and spliced X-box binding protein-1 (sXBP-1) mRNA levels were reduced, as were phosphorylated elongation initiation factor 2α (eIF2α) and stress kinase c-Jun NH2-terminal kinase 1 (JNK1) (all P values <0.05). Liver sections from a subset of subjects showed intense staining for Grp78 and phosphorylated eIF2α before surgery, which was reduced in post-GBP sections.
CONCLUSIONS—This study presents important evidence that ER stress pathways are present in selected tissues of obese humans and that these signals are regulated by marked weight loss and metabolic improvement. Hence, this suggests the possibility of a relationship between obesity-related ER stress and metabolic dysfunction in obese humans.
Increased adiposity is associated with a group of chronic metabolic disorders, including insulin resistance, type 2 diabetes, and nonalcoholic fatty liver disease (1). The prevalence of this cluster of abnormalities has increased significantly in the past few decades following the marked rise of obesity worldwide (2,3). The mechanisms responsible for the emergence of these disorders have been an intense area of investigation. In the past decade, it was recognized and established that chronic inflammatory and stress responses are a central feature of obesity, insulin resistance, and type 2 diabetes and contribute to the metabolic imbalance (4). However, the pathways and mechanisms giving rise to the chronic inflammatory responses remain to be elucidated.
Recently, we have identified endoplasmic reticulum (ER) dysfunction as a significant contributor to the development of experimental insulin resistance in obese tissues (5). The ER is a critical intracellular organelle that coordinates the synthesis, folding, and trafficking of proteins. All transmembrane and secreted proteins pass through the ER en route to cellular destinations; proteins that fail to fold into proper structures are removed from the ER through degradation. Under stress conditions, unfolded proteins accumulate in the ER and initiate an adaptive response known as the unfolded protein response (UPR). The UPR is initiated by three ER transmembrane sensors [namely PKR-like ER-regulated kinase (PERK), inositol-requiring enzyme-1 (IRE-1), and activating transcription factor-6 (ATF-6)], which activate an adaptive response that results in cessation of protein translation and transcriptional increase in protein-folding chaperones and ER-associated degradation genes (6). If the stress is too severe, the UPR may also induce cellular apoptosis through several different mechanisms involving the various branches of the UPR. In mouse models of obesity, ER stress is present in liver and adipose tissues, as evidenced by increased activity of both the IRE-1 and PERK branches (7). The UPR is also able to induce activation of the c-Jun NH2-terminal kinase (JNK) pathway and thereby inhibit insulin signaling through the subsequent phosphorylation and/or degradation of IRS1 (7–9). Data obtained from mouse models demonstrate that genetically compromised ER folding capacity induces ER stress, activates JNK, and leads to whole-body insulin resistance in the mouse (7). In contrast, enhancing ER capacity in obese mice through the use of chemical or molecular chaperones relieves ER stress in adipose tissue and liver, reduces intrahepatic fat accumulation, and restores glucose homeostasis (10).
Although ER stress is associated with obesity and metabolic dysfunction in rodent models, the importance of ER stress in the pathogenesis of obesity-related metabolic disease and the potential regulation of ER function by weight loss in human subjects is not known. Therefore, the purpose of the present study was to evaluate the effect of marked weight loss induced by gastric bypass (GBP) surgery on insulin sensitivity and ER stress in key metabolic tissues in obese human subjects. A euglycemic-hyperinsulinemic clamp procedure, in conjunction with stable isotopically labeled tracer infusions, was used to assess insulin action in liver, skeletal muscle, and adipose tissue; and UPR markers from among the three response branches were evaluated in liver and adipose tissue samples obtained before and 1 year after GBP surgery.
RESEARCH DESIGN AND METHODS
Subjects and GBP surgery.
Eleven obese subjects (mean BMI 51.3 ± 3.0 kg/m2; two men and nine women) who were scheduled to undergo GBP surgery participated in this study. All subjects completed a comprehensive medical evaluation, which included a detailed history and physical examination, routine blood tests, and a 12-lead electrocardiogram. No subjects had diabetes, had other metabolic diseases, or were taking medications that affect insulin action or glucose and fatty acid metabolism. All subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office and the Center for Applied Research Sciences Advisory Committee of Washington University School of Medicine.
All GBP procedures were performed by the same surgeon (J.C.E.) using standard surgical techniques. All patients had an open GBP procedure, which involved constructing a small (∼20 ml) proximal gastric pouch by stapling across the stomach. A 150-cm Roux-Y limb was constructed by transecting the jejunum 30 cm distal to the ligament of Treitz and creating a jejunojejunostomy 150 cm distal to the transection.
Metabolic studies and sample analyses.
Insulin sensitivity was examined by using the hyperinsulinemic-euglycemic clamp procedure in 10 of the 11 subjects before and 1 year after GBP surgery. Subjects were admitted to the Intensive Research Unit at Washington University School of Medicine on the evening before the clamp procedure. The following morning, after subjects fasted overnight, a one-stage hyperinsulinemic-euglycemic clamp procedure was performed; [6,6-2H2]glucose and [2,2-2H2]palmitate were infused for 6 h (0- to 3-h basal period followed by 3- to 6-h insulin infusion at a rate of 15 mU · m−2 body surface area [BSA] · min−1, initiated with a priming dose of 60 mU · m−2 BSA · min−1 for 5 min and then 30 mU · m−2 BSA · min−1 for 5 min. Euglycemia was maintained at a blood glucose concentration of ∼5.6 mmol/l (100 mg/dl) throughout the clamp procedure by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose. Blood samples were obtained before beginning the tracer infusion to determine background plasma glucose and palmitate tracer-to-tracee ratios and every 10 min during the final 30 min of the basal period and of the clamp procedure to determine glucose and insulin concentrations and substrate kinetics. Plasma insulin was determined by a radioimmunoassay and plasma glucose and palmitate tracer-to-tracee ratios were determined by using gas chromatography–mass spectroscopy (MSD 5973 system with capillary column; Hewlett-Packard, Palo Alto, CA), as previously described (11,12). Glucose and palmitate kinetics were determined by using Steele's equation for steady-state conditions (13,14), and hepatic insulin sensitivity was determined by the reciprocal of the hepatic insulin resistance index [10,000/(basal glucose rate of appearance (Ra) in μmol · kg−1 · min−1 × basal insulin concentration in mU/l)], as previously described (15,16).
Subcutaneous abdominal adipose tissue samples were collected during basal postabsorptive conditions in all subjects by using a 14-gauge needle; samples were immediately frozen in liquid nitrogen. Liver tissue samples were obtained by needle biopsy during GBP surgery and by percutaneous needle biopsy 1 year after GBP surgery in four subjects and placed in formalin for further analyses.
mRNA isolation and quantitative PCR.
Frozen adipose tissue samples were homogenized in TRIzol Reagent (Invitrogen) for total RNA isolation according to the manufacturer's protocol. cDNA synthesis was performed using 1 μg sample RNA reverse transcribed with the high-capacity cDNA archive system (Applied Biosystems, Foster City, CA). Real-time, quantitative PCR was performed using SybrGreen reagent in the ABI 7300 real-time PCR system (Applied Biosystems) to determine the mRNA levels of the following genes in adipose tissue samples obtained before and after GBP surgery: 18S, C/EBP homologous protein (CHOP), spliced X-box binding protein-1 (XBP-1), glucose-regulated protein 78 (Grp78), and interleukin-6 (IL-6). Primer sequences used to detect these human genes are as follows: 18S forward, GTAACCCGTTGAACCCCATT; 18S reverse, CCATCCAATCGGTAGTAGCG; Grp78 forward, CATCACGCCGTCCTATGTCG; Grp78 reverse, CGTCAAAGACCGTGTTCTCG; sXBP forward, GGTCTGCTGAGTCCGCAGCAGG; sXBP reverse, GGGCTTGGTATATATGTGG; CHOP forward, GGAGAACCAGGAAACGGAAAC; CHOP reverse, TCTCCTTCATGCGCTGCTTT; IL-6 forward, AAATTCGGTACATCCTCGACGG; and IL-6 reverse, GGAAGGTTCAGGTTGTTTTCTGC.
Protein isolation, antibodies, and Western blotting.
Frozen adipose tissue samples were homogenized in lysis buffer containing 25 mmol/l Tris-HCl (pH 7.4), 10 mmol/l Na3VO4, 100 mmol/l NaF, 50 mmol/l Na4P2O7, 10 mmol/l EGTA, 10 mmol/l EDTA, and 1% Nonidet P-40 with protease inhibitors (Sigma). After homogenization, the tissue lysate was centrifuged at 4,000 rpm for 15 min at 4°C followed by 14,000 rpm for 20 min at 4°C. Eighty micrograms total tissue protein was used for direct immunoblotting. Rabbit polyclonal anti–phosphorylated eukaryotic initiation factor 2α (eIF2α) antibody (Invitrogen) was used at 1:1,000 dilution; mouse monoclonal anti–phosphorylated JNK (Cell Signaling) was used at 1:1,000 dilution; rabbit polyclonal anti-calnexin (Stressgen) was used at 1:1,000 dilution; β-tubulin (Santa Cruz) was used at 1:1,000 dilution. Horseradish peroxidase–linked anti-mouse or anti-rabbit secondary antibody (GE Healthcare) was used at 1:4,000 dilution.
Immunohistochemical examination of liver sections.
Liver biopsy specimens were evaluated for steatosis and inflammation by hematoxylin-eosin (H-E) staining and for markers of ER stress by using immunohistochemistry. Human liver sections were subjected to routine deparafinization/hydration process and blocked with goat serum albumin for 1 h at room temperature. Primary antibody incubation at 1:50 concentration was performed overnight at 4°C. After primary antibody incubation, sections were washed with PBS and incubated with secondary antibody at 1:200 dilution for 60 min. At the end of the secondary antibody incubation, sections were washed with PBS followed by application of mounting solution containing DAPI (Vector Lab). Secondary antibodies used in the current study were fluorescein isothiocyanate–or Texas-red–conjugated anti-rabbit IgG (Santa Cruz). Negative controls included omission of the primary antibody. Cells were examined with a fluorescence microscope (Axio Observer; Carl Zeiss). Quantification of immunohistochemical staining was performed using Axiovision Software (Carl Zeiss) from images of liver tissue captured at random and is represented as relative fluorescence intensity.
Selection of UPR markers.
We chose UPR markers from among the three branches that respond to ER stress. The transmembrane kinase PERK phosphorylates eIF2α, leading to inhibition of general protein translation. As a result of alternative translation, the proapoptotic transcription factor CHOP is induced. IRE-1 activates the stress kinase JNK1 and also possesses endoribonuclease activity, which splices an intron from the mRNA of XBP-1, creating an activated transcription factor. Spliced XBP-1, along with active ATF-6, translocates to the nucleus and induces expression of protein chaperone genes. One well-known target of both of these transcription factors is the chaperone Grp78.
Statistical analyses.
Insulin sensitivity and patient information datasets were normally distributed according to the Kolmogorov-Smirnov test, so that comparisons between subjects before and after weight loss were performed using parametric procedures. The statistical significance of the effect of insulin infusion on substrate metabolism before and after weight loss was analyzed by using the Student's t test for paired samples. Results are presented as means ± SE. All reported P values are two sided, and a P value of <0.05 was considered to be statistically significant.
RESULTS
Study subject characteristics and insulin sensitivity.
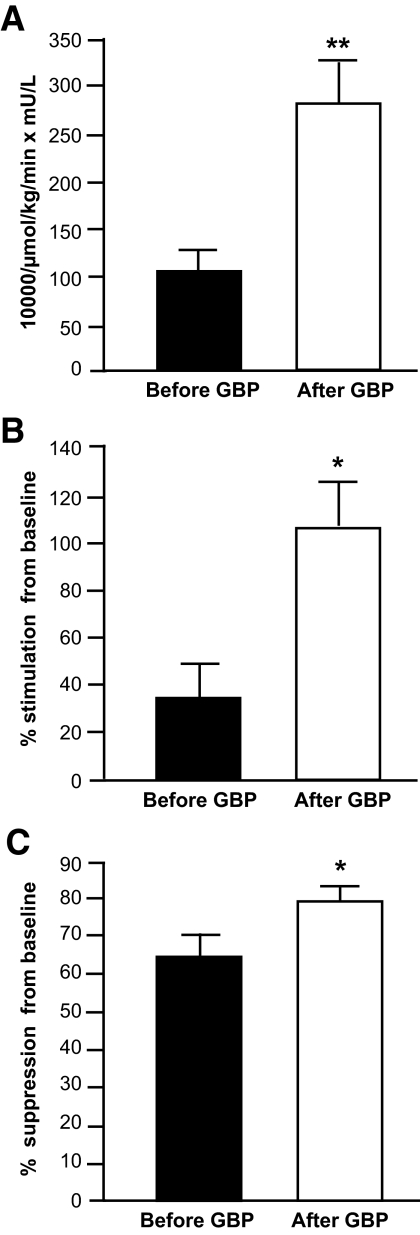
On average, subjects lost ∼40% of their initial body weight at 1 year after GBP surgery (Table 1). Weight loss caused a marked decrease in plasma glucose and insulin concentrations and in intrahepatic fat content (Table 1). The hepatic insulin sensitivity index value was 160% greater 1 year after than before GBP surgery (P < 0.01) (Fig. 1A). Insulin infusion during the clamp procedure increased plasma insulin concentration from 18.7 ± 3.1 to 43.6 ± 4.2 mU/l before and from 4.9 ± 0.8 to 27.0 ± 1.5 mU/l 1 year after GBP surgery. Plasma glucose concentrations during insulin infusion were 97.7 ± 1.7 and 99.6 ± 1.9 mg/dl during the clamp procedure performed before and after weight loss, respectively. Insulin infusion caused an increase in glucose Rd from 1,081 ± 69 to 1,418 ± 108 μmol/min before GBP surgery and from 813 ± 57 to 1,666 ± 171 μmol/min 1 year after GBP surgery. The increase in glucose Rd during insulin infusion was 200% greater after than before weight loss (P < 0.01) (Fig. 1B). Insulin infusion caused a decrease in palmitate Ra from 161 ± 13 μmol/min to 61 ± 14 μmol/min before GBP surgery and from 105 ± 9 to 21 ± 4 μmol/min 1 year after GBP surgery. Insulin-mediated suppression of palmitate Ra was 23% greater 1 year after than before weight loss (P < 0.01) (Fig. 1C). Taken together, these data demonstrate marked metabolic improvement in subjects after weight reduction by GBP surgery.
TABLE 1.
Metabolic characteristics of subjects before and 1 year after gastric bypass surgery
| Before surgery | After surgery | |
|---|---|---|
| n (men/women) | 10 (2/8) | 10 (2/8) |
| Age | 40.3 ± 2.3 | 41.3 ± 2.3 |
| Weight (kg) | 143.5 ± 9.44 | 87.6 ± 5.9* |
| BMI (kg/m2) | 51.3 ± 3.0 | 31.4 ± 2.3* |
| Glucose (mg/dl) | 98.5 ± 4.8 | 84.2 ± 2.2† |
| Insulin (mU/l) | 18.7 ± 3.1 | 4.9 ± 0.8* |
| HOMA-IR | 5.3 ± 0.9 | 1.6 ± 0.5† |
| Intrahepatic fat content (%)‡ | 19.5 ± 5.2 | 1.9 ± 2.1* |
| Systolic blood pressure (mm/Hg) | 132 ± 7 | 108 ± 5* |
| Diastolic blood pressure (mm/Hg) | 74 ± 3 | 63 ± 4† |
| Total cholesterol (mg/dl) | 160 ± 13 | 161 ± 9 |
| LDL cholesterol (mg/dl) | 85 ± 10 | 79 ± 6 |
| HDL cholesterol (mg/dl) | 48 ± 4 | 68 ± 5§ |
| Triglyceride (mg/dl) | 135 ± 19 | 72 ± 9† |
Data are means ± SE. Value significantly different from corresponding Before Surgery value;
P < 0.001;
P < 0.05;
P < 0.01.
Data from four subjects. HOMA-IR, homeostasis model assessment ofinsulin resistance.
FIG. 1.
Alterations in systemic insulin sensitivity in GBP subjects. Hepatic insulin sensitivity index, calculated as the reciprocal of the product of basal glucose production and insulin concentration (A); insulin-stimulated glucose disposal, an index of skeletal muscle insulin sensitivity assessed as the relative increase in glucose uptake during insulin infusion (B); and insulin-mediated suppression of palmitate Ra into plasma, an index of adipose tissue insulin sensitivity assessed as the relative decrease in palmitate Ra during insulin infusion (C), in obese subjects before (▪) and 1 year after (□) GBP surgery. *Value significantly different from the before GBP value; *P < 0.01, **P < 0.001.
Adipose tissue expression and regulation of UPR-related genes.
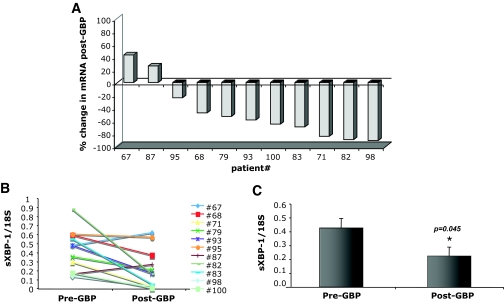
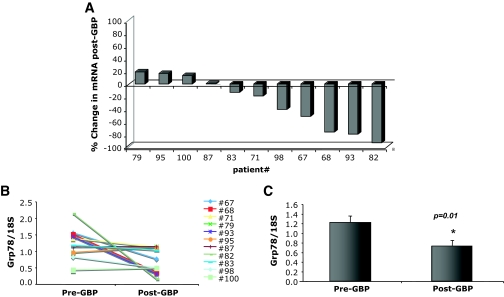
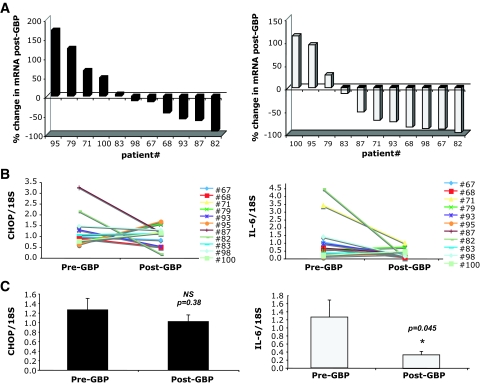
The expression of UPR induced genes in subcutaneous abdominal adipose tissue was evaluated in all 11 subjects before and 1 year after GBP surgery. We first examined the splicing of the XBP-1 mRNA, a critical transcription factor and indicator of ER stress and adaptive responses. After GBP, there was an average 47.5% reduction (P = 0.04) in spliced XBP-1 mRNA compared with levels in pre-GBP tissues (Fig. 2C). When examined individually, sXBP-1 levels were decreased in 9 of the 11 subjects (Fig. 2A and B). Next, we examined the expression of GRP78 mRNA because this chaperone can be targeted by sXBP-1 and also by ATF-6, the second transcription factor regulating the UPR. As seen in Fig. 3, the levels of Grp78 mRNA were significantly decreased in post-GBP tissues, with an average 40.1% reduction in mRNA post-GBP (P = 0.01). When examined individually, decreased Grp78 gene expression was evident in 7 of 11 subjects (Fig. 3A and B), suggesting that its regulation may be greater in variability than sXBP-1. In contrast, adipose tissue CHOP expression was not significantly different between the samples collected before or after surgery (Fig. 4). Interestingly, in rodent models of obesity, we also did not detect a significant regulation of CHOP mRNA expression between lean and obese WAT (M.F.G., G.S.H., unpublished data). Adipose tissue IL-6 expression was determined to compare the UPR changes to a well-established inflammatory alteration. Earlier studies have shown that IL-6 expression is significantly and consistently downregulated in adipose tissue after weight loss (17–21). Consistent with these observations, IL-6 mRNA levels were also significantly reduced in 8 of 11 subjects after GBP (Fig. 4).
FIG. 2.
Regulation of spliced XBP-1 mRNA in GBP subjects. Quantitative RT-PCR was performed on RNA isolated from adipose tissue of patients before and after GBP surgery, n = 11. A: Percent change in sXBP-1 mRNA from pre-GBP levels in each individual patient. B: Pre- and post-GBP surgery levels of sXBP-1 mRNA normalized to 18S ribosomal RNA. C: Average mRNA levels for sXBP-1 in pre and post-GBP surgery groups. Data indicate means ± SE. *P < 0.05.
FIG. 3.
Regulation of Grp78 mRNA in GBP subjects. Quantitative RT-PCR was performed on RNA isolated from adipose tissue of patients before and after GBP, n = 11. A: Percent change in Grp78 mRNA from pre-GBP levels in each individual patient. B: Pre- and post-GBP surgery levels of Grp78 mRNA normalized to 18S ribosomal RNA. C: Average mRNA levels for Grp78 in pre- and post-GBP groups. Data indicate mean ± SE. *P < 0.02.
FIG. 4.
CHOP (left panel) and IL-6 (right panel) mRNA levels in GBP subjects. Quantitative RT-PCR was performed on RNA isolated from adipose tissue of patients before and after GBP, n = 11. A: Percent change in CHOP and IL-6 mRNA from pre-GBP levels in each individual patient. B: Pre- and post-GBP levels of CHOP and IL-6 mRNA normalized to 18S ribosomal RNA. C: Average mRNA levels for CHOP and IL-6 in pre- and post-GBP groups. Data indicate means ± SE. *P < 0.05.
Regulation of biochemical markers of ER stress in adipose and liver.
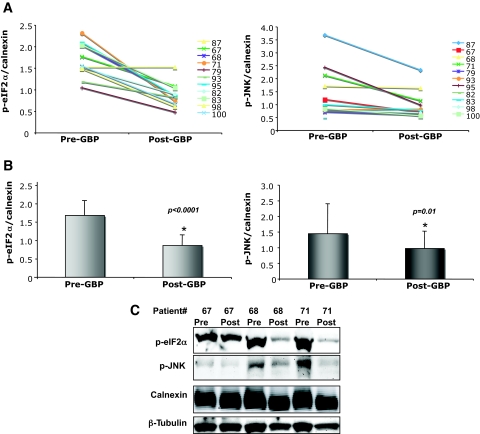
The presence of tissue ER stress in obesity in rodents is best evaluated by the phosphorylation of PERK, IRE-1, or eIF2α and stimulation of JNK activity, because these are more proximal measures of UPR activity (7). Because there are no antibodies that can detect the phosphorylated form of PERK or IRE-1 in humans yet, we first measured the phosphorylated form of eIF2α in adipose tissue before and after GBP. Figure 5 reveals the presence of robust eIF2α phosphorylation in adipose tissue in all obese subjects. Remarkably, there was a significant reduction in the phosphorylation of adipose tissue eIF2α after GBP surgery, which was observed in all of the subjects studied (P < 0.0001). We also examined the activity of JNK, which can be activated through the IRE-1 branch of the UPR during ER stress. JNK activation, as determined by phosphorylation of the JNK1 isoform, was detectable in the obese subjects before GBP surgery. As shown in Fig. 5C, JNK phosphorylation also significantly decreased in adipose tissue after GBP surgery. Taken together, these observations indicate alleviation of ER stress in adipose tissue after substantial weight loss.
FIG. 5.
Adipose tissue p-eIF2α and p-JNK levels after GBP surgery. Protein expression levels of p-eIF2α and p-JNK in adipose tissue from patients before and after GBP were detected by Western blot assay, followed by densitometric analysis. A: Pre- and post-GBP levels of p-eIF2α and p-JNK normalized to calnexin. B: Average levels of p-eIF2α and p-JNK in pre- and post-GBP groups. C: Immunoblot analyses of p-eIF2α and p-JNK expression levels in patients 67, 68, and 71 before and after GBP. Data indicate means ± SE. *P < 0.05. (Please see http://dx.doi.org/10.2337/db08-1220 for a high-quality digital representation of this figure.)
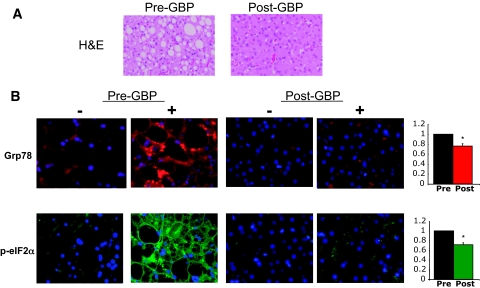
In a subgroup of four subjects, liver biopsy samples were obtained before and 1 year after GBP surgery. H-E staining of the liver sections demonstrated a 90% decrease in intrahepatic triglyceride content after GBP surgery (Table 1; Fig. 6A). In this limited number of samples, we were also able to examine the effects of weight loss on eIF2α phosphorylation and GRP78 protein levels by immunohistochemistry. Immunostaining with an anti–phospho-eIF2α antibody revealed strong immunoreactivity in liver sections obtained before GBP (Fig. 6). Similarly, immunostaining with an antibody against the ER chaperone GRP78 revealed strong staining in the obese subjects before GBP. Particularly, areas surrounding the lipid droplets revealed intense staining. In the samples obtained after weight loss, immunoreactivity against both phospho-eIF2α and GRP78 was reduced either in general intensity or number of areas. In some cases, the signal was reduced markedly (Fig. 6; supplemental Fig. 1, available in an online appendix at http://dx.doi.org/10.2337/db08-1220), and in others, it was reduced partially and with residual and scattered signal. Although variable among subjects, these results indicate that similar to adipose tissue, obesity in humans may also be associated with ER stress in liver tissue, which is ameliorated upon weight loss.
FIG. 6.
Liver histology and p-eIF2α and Grp78 immunoreactivity after GBP. Liver tissue sections from patient 67 obtained during and after GBP surgery were stained with H-E or with antibodies against p-eIF2α (green) or Grp78 (red). Nuclei are stained with DAPI (blue). The top panel illustrates severe steatosis in the pre-GBP sample and marked reduction after GBP. Staining is particularly dense around the lipid droplets (black in the stained samples). Quantification of staining is presented as relative intensity of fluorescence in pre- and post-GBP sections. *P < 0.01. (Please see http://dx.doi.org/10.2337/db08-1220 for a high-quality digital representation of this figure.)
DISCUSSION
Recent data from studies conducted in obese rodent models have demonstrated that ER stress and the activation of related stress signaling pathways may be an important mechanism underlying insulin resistance and type 2 diabetes (5). The results of the present study demonstrate that in obese humans, ER stress is present in adipose and liver tissues and is highly regulated by weight loss induced by GBP surgery.
GBP surgery is the most commonly performed bariatric surgical procedure in the U.S. and worldwide and is an effective approach for achieving weight loss in obese patients (1). Weight loss induced by GBP surgery improves or completely resolves most obesity-related medical complications and increases survival (22,23). We chose to investigate ER stress in patients who had GBP surgery so that comparisons could be made within the same individual before and after the metabolic improvements of extreme weight loss. This approach has several important strengths. First, each individual served as his/her own control, which removes confounding variables that may not necessarily be accounted for if the control group comprised a different cohort. Second, by measuring change over time within an individual, we are able to capture the state of the ER in flux. This is important given the highly dynamic nature of the ER, which adjusts its adaptive capacity to the condition of the cells and to environmental and intrinsic stress signals, such as alterations in metabolism and body weight. Given the fluctuating environment of the cell and the adaptive responses of the ER, a solitary UPR molecule may not a reliable indicator of ER functional capacity. Therefore, we measured multiple markers of ER stress to assess the different UPR pathways, ranging from proximal biochemical activities of the sensors, to their substrates, to the downstream transcriptional outcomes of target gene expression.
Our data clearly demonstrate a significant regulation of ER stress upon weight loss, as evidenced by the suppression of biochemical indicators of ER stress such as eIF2α and JNK phosphorylation, and downregulation of UPR transcriptional arms via spliced XBP-1 and Grp78. Given a relatively small patient number, we observed remarkably consistent effects on ER stress in post-GBP tissues. It is interesting that despite marked suppression of eIF2α phosphorylation in adipose tissue upon weight loss, there was no change in the levels of CHOP mRNA expression, which is generally considered to be downstream of eIF2α phosphorylation. It is possible that CHOP is regulated through other signals and does not respond to changes in body weight. In fact, in our recent work in rodent models comparing adipose tissue samples obtained from genetic and dietary models of obesity with lean controls subjects, we did not observe any change in the expression levels of CHOP mRNA (unpublished results). Nonetheless, our findings suggest that all branches of the UPR are potentially regulated by obesity and weight loss in humans.
The mechanism responsible for weight loss–induced reduction in ER stress in our subjects is not known. Potential factors may include the decrease in adipose and liver triglyceride content or the decrease in systemic plasma insulin and glucose concentrations. Although it is unclear how these factors influence ER stress, data from in vitro studies suggest that inflammation, reactive oxygen species, saturated fatty acids, or hypoxia could also be involved. These signals have all been shown to compromise ER folding capacity and/or induce ER stress in vitro and have also been described in obese, insulin-resistant tissues (5). Nonetheless, the in vivo physiological mechanisms/causes remain unknown.
Two recent studies utilizing lean and obese cohorts have demonstrated regulation of ER stress markers. Boden et al. (24) reported an increase in ER stress proteins and gene expression in subcutaneous adipose tissue in obese individuals compared with lean. A second study measured ER stress markers within a range of BMIs and found a significant positive correlation with increasing BMI (25). These data support the hypothesis that obesity leads to increased ER stress in adipose tissue and complements our findings of the effect of weight loss on tissue stress.
Interestingly, recent studies have also provided evidence that ER stress pathways are activated in pancreatic islets in type 2 diabetes and in atherosclerotic vascular lesions (26,27). Although this is a relatively young field, these observations have raised the possibility that ER dysfunction and the resulting stress responses may be important components of chronic metabolic diseases in a broader sense. The extent to which these pathways will be relevant to human metabolic diseases, particularly obesity and type 2 diabetes, remains a critical but unanswered question.
In summary, the results from the present study demonstrate that human obesity is associated with insulin resistance and ER stress in both adipose tissue and the liver. Weight loss is associated with decreased ER stress and improved insulin sensitivity, suggesting the possibility that the ER is involved in regulating insulin action in obese humans. These findings, while correlative, are consistent with data from studies conducted in rodent models, which have found that obesity is associated with ER stress and that ameliorating ER stress improves insulin action. Therefore, the ER could be an important new target for treating the metabolic complications of obesity.
Supplementary Material
Acknowledgments
M.F.G. has received a Donald and Sue Pritzker Scholar award and National Institutes of Health (NIH) Environmental Health Training Grant T32-ES-007155-24. L.Y. has received a mentor-based fellowship from the American Diabetes Association. E.F. has received a Veronica Atkins International Fellowship in Obesity Research. G.S.H. has received NIH Grant DK52539 and funding from the American Diabetes Association. S.K. has received NIH Grant DK-37948. This article was made possible by funding from Grant UL1-RR-024992 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research and NIH Grant DK-56341 (Clinical Nutrition Research Unit).
No potential conflicts of interest relevant to this article were reported.
We thank the nursing staff of the Center for Applied Research Sciences for their help in performing the studies; Freida Custodio, Jennifer Shew, and Adewole Okunade for their technical assistance; and the study subjects for their participation.
Published ahead of print at http://diabetes.diabetesjournals.org on 9 December 2008.
M.F.G. and L.Y. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 518.
REFERENCES
- 1.Klein S, Wadden T, Sugerman HJ: AGA technical review on obesity. Gastroenterology 123: 882–932, 2002 [DOI] [PubMed] [Google Scholar]
- 2. The World Health Report: Reducing Risks, Promoting Healthy Life. Geneva, World Health Organization, 2002
- 3.Hossain P, Kawar B, El Nahas M: Obesity and diabetes in the developing world: a growing challenge. N Engl J Med 356: 213–215, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS: Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Gregor MG, Hotamisligil GS: Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 48: 1905–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Ron D, Walter P: Signal integration in the ER unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS: Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Aguirre V, Uchida T, Yenush L, Davis R, White MF: The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D: Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS: Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S: Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 40: 2118–2124, 1999 [PubMed] [Google Scholar]
- 12.Patterson BW, Zhao G, Klein S: Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism 47: 706–712, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Steele R: Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 14.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S: What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 52: 1641–1648, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K: A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B: Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 85: 3338–3342, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW: Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82: 4196–4200, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G: Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745–E751, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K: Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54: 2277–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Fried SK, Bunkin DA, Greenberg AS: Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83: 847–850, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Buchwald H: Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis 1: 371–381, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Sjostrom L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study: Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S: Increase in ER stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57: 2438–2444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC: Endoplasmic reticulum stress markers are associated with obesity in non-diabetic subjects. J Clin Endocrinol Metab 93: 4532–4541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ: Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50: 752–763, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M: Increased ER stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116: 1226–1233, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.