Abstract
OBJECTIVE—The physiology of counterregulatory responses during hypoglycemia in intensively treated type 2 diabetic subjects is largely unknown. Therefore, the specific aims of the study tested the hypothesis that 1) 6 months of intensive therapy to lower A1C <7.0% would blunt autonomic nervous system (ANS) responses to hypoglycemia, and 2) antecedent hypoglycemia will result in counterregulatory failure during subsequent hypoglycemia in patients with suboptimal and good glycemic control.
RESEARCH DESIGN AND METHODS—Fifteen type 2 diabetic patients (8 men/7 women) underwent 6-month combination therapy of metformin, glipizide XL, and acarbose to lower A1C to 6.7% and 2-day repeated hypoglycemic clamp studies before and after intensive therapy. A control group of eight nondiabetic subjects participated in a single 2-day repeated hypoglycemic clamp study.
RESULTS—Six-month therapy reduced A1C from 10.2 ± 0.5 to 6.7 ± 0.3%. Rates of hypoglycemia increased to 3.2 episodes per patient/month by study end. Hypoglycemia (3.3 ± 0.1 mmol/l) and insulinemia (1,722 ± 198 pmol/l) were similar during all clamp studies. Intensive therapy reduced (P < 0.05) ANS and metabolic counterregulatory responses during hypoglycemia. Antecedent hypoglycemia produced widespread blunting (P < 0.05) of neuroendocrine, ANS, and metabolic counterregulatory responses during subsequent hypoglycemia before and after intensive therapy in type 2 diabetic patients and in nondiabetic control subjects.
CONCLUSIONS—Intensive oral combination therapy and antecedent hypoglycemia both blunt physiological defenses against subsequent hypoglycemia in type 2 diabetes. Prior hypoglycemia of only 3.3 ± 0.1 mmol/l can result in counterregulatory failure in type 2 diabetic patients with suboptimal control and can further impair physiological defenses against hypoglycemia in intensively treated type 2 diabetes.
Large randomized controlled multicenter clinical trials have demonstrated the benefit of improved glycemic control on microvascular complications in both type 1 and type 2 diabetes (1,2). These compelling data have produced a paradigm shift in the treatment of diabetes (particularly type 2 diabetes) striving for A1C values <7.0% (3). The major drawbacks of tight metabolic control in patients with type 1 diabetes are well documented and include increased hypoglycemia and weight gain (4–8).
Recently, three large studies have investigated the effects of rigorous metabolic control (A1C <7.0%) on the prevalence of macrovascular disease in type 2 diabetes (9–11). The overall conclusion of these studies was that A1C values <7.0% did not produce a statistically significant reduction in macrovascular events but did produce a marked increase in hypoglycemia in type 2 diabetes. The effects of intensive therapy on physiological counterregulatory responses during hypoglycemia in type 2 diabetes have not been thoroughly investigated. Burge et al. (12) demonstrated that improving glycemic control during an 8-day in-patient admission could lower symptom responses and plasma glucose levels for activation of epinephrine during hypoglycemia. Levy et al. (13), using a cross-sectional study design, also concluded that improved glycemic control in type 2 diabetes shifts the thresholds for counterregulatory hormone release to lower plasma glucose concentrations during hypoglycemia. Korzon-Burakowska et al. (14) improved A1C from 11.3 ± 1.1 to 8.1 ± 0.9% during a 4-month period. Thresholds (i.e., plasma glucose values) for counterregulatory hormone release and epinephrine and cortisol responses were lowered by improved glycemic control. Spyer et al. (15), investigating a group of seven type 2 diabetic patients with an A1C of 7.4%, also found that the glycemic thresholds for counterregulatory hormone release were reduced from elevated to normal physiological glucose levels. However, similar to some (13,16) but not all studies (17), there was no difference in values of the key counterregulatory hormones, epinephrine and glucagon, during hypoglycemia when compared with nondiabetic control subjects. Studies investigating the mechanisms regulating counterregulatory responses during hypoglycemia in type 2 diabetes are even fewer. Segel et al. (16) determined that antecedent hypoglycemia in a group of type 2 diabetic patients with an A1C of 8.1% resulted in hypoglycemia-associated autonomic failure similar to patients with type 1 diabetes. Despite the above data, two questions remain unanswered: 1) What are the effects of a period of rigorous glycemic control to reduce A1C <7.0% on counterregulatory responses in type 2 diabetes, and 2) what are the effects of antecedent hypoglycemia on autonomic nervous system (ANS), neuroendocrine, and metabolic counterregulatory mechanisms before and after a period of rigorous metabolic control in type 2 diabetes? In the present study, we tested the hypothesis that 6-month intensive therapy to lower A1C <7.0% would impair counterregulatory response to hypoglycemia and that antecedent hypoglycemia would further impair key homeostatic counterregulatory mechanisms during subsequent hypoglycemia in type 2 diabetes.
RESEARCH DESIGN AND METHODS
Fifteen patients (8 men/7 women) with type 2 diabetes (age 47 ± 2 years), BMI of 33 ± 2 kg/m2, glycosylated hemoglobin of 10.2 ± 0.5% (normal range 4–6.5%), anti-GAD and anti–islet cell antibody negative, and disease duration of 6 ± 2 years were studied. Patients were receiving diet and exercise (n = 2) or oral agent monotherapy [metformin (mean 750 mg/day, n = 10) or sulfonylurea (glyburide or glipizide mean 5 mg/day, n = 3)]. Eight nondiabetic control subjects (4 men/4 women), aged 48 ± 3 years, BMI 30 ± 2 kg/m2, and A1C 5.1 ± 0.2% were also studied. Each subject had a normal blood count, plasma electrolytes, and liver and renal function. All gave written informed consent. Studies were approved by the Vanderbilt University Human Subjects Institutional Review Board.
Type 2 diabetic subjects.
Fifteen patients participated in two 2-day hypoglycemia experiments separated by 6 months. Subjects were asked to avoid any exercise and consume their usual weight-maintaining diet for 3 days before each study. Two days before a study, sulfonylurea and metformin tablets were omitted. After 6 months when the patients were in good metabolic control, these medications were replaced with injections of regular insulin before each meal. The dose of regular insulin was carefully adjusted so that hypoglycemia (<3.9 mmol/l) and hyperglycemia (>11.1 mmol/l) were avoided in the 2 days before a study. Each subject was admitted to the Vanderbilt General Clinical Research Center (GCRC) at 5:00 p.m. on the evening before an experiment. At this time, two intravenous cannulae were inserted under 1% lidocaine local anesthesia. One cannula was placed in a retrograde fashion into a vein on the back of the hand. This hand would be placed in a heated box (55–60°C) during the study so that arterialized blood could be obtained (18). The other cannula was placed in the contralateral arm for infusions. Patients then received a standardized evening meal, and a continuous low-dose infusion of insulin was started to normalize plasma glucose. The insulin infusion was adjusted overnight to maintain blood glucose between 4.4 and 7.2 mmol/l.
Hypoglycemia experiments.
After an overnight 10-h fast at 0 min, a primed (18 μCi) continuous infusion (0.18 μCi/min) of high-performance liquid chromatography (HPLC)-purified [3-3H]glucose (11.5 mCi · mmol−1 · l−1; Perkin Elmer Life Sciences, Boston, MA) was administered via a precalibrated infusion pump (Harvard Apparatus, South Natick, MA). A period of 90 min was allowed to elapse followed by a 30-min basal control period. Plasma glucose was maintained at euglycemia during this period by continuing the overnight basal insulin. At time 120 min, a primed constant (15.0 pmol · kg−1 · min−1) infusion of insulin (Human Regular Insulin; Eli Lilly, Indianapolis, IN) was started and continued until 240 min. The rate of fall of glucose was controlled (0.07 mmol/min), and the hypoglycemic nadir (3.3 mmol/l) was achieved using a modification of the glucose clamp technique (19). During the clamp period, plasma glucose was measured every 5 min, and a 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 3.3 ± 0.1 mmol/l (20). Potassium chloride (20 mmol/l) was infused during the clamp to reduce insulin-induced hypokalemia. After completion of the 2-h test period, the insulin infusion was turned down to the basal rate, and the plasma glucose was rapidly restored to euglycemia with 20% dextrose. In the afternoon after a 2-h period of euglycemia, a second 2-h hypoglycemic clamp of 3.3 mmol/l was performed similar to the morning study. No tritiated glucose was infused during the afternoon studies. After completion of the afternoon hypoglycemic clamp, a basal insulin infusion was restarted and a standardized evening meal and snack was given. Similar to the previous night, a variable low-dose infusion was used to maintain glucose levels (4.4–7.2 mmol/l) overnight. The next morning after a 10-h overnight fast, an identical (including a tritiated glucose infusion) 2-h hypoglycemic clamp at 3.3 ± 0.1 mmol/l was performed as described for day 1.
Study medication.
After completion of the first 2-day clamp studies, patients were started on triple oral combination therapy. Metformin and acarbose were increased to 1 g twice a day and 50 mg three times a day over a 4-week period, respectively. Glipizide XL was increased to 10 mg once or twice a day over a period of 3 weeks. All patients performed intensive self-reported blood glucose monitoring before main meals, snacks, and at bedtime or any time that they felt low glucose or a second individual thought they had a low glucose. Thus, study subjects tested their glucose levels between four to seven times per day. Patients were contacted by study nurses twice a week and by a dietitian weekly to adjust diet, exercise, and medication and to discuss any treatment side effects. Patients were seen by S.N.D., study nurses, and/or a dietitian monthly. Patient adherence to protocol was assessed at monthly visits and found to be excellent. One patient moved out of state during the 6-month treatment period and was lost to follow-up. After completion of the 6-month intensive treatment period, patients were readmitted to the Vanderbilt GCRC for an identical 2-day hypoglycemic clamp protocol as described above. The nondiabetic control subjects underwent a single 2-day in-patient hypoglycemic clamp protocol similar (without overnight glucose control) to the type 2 diabetic patients.
Direct measurement of muscle sympathetic nerve activity.
Muscle sympathetic nerve activity (MSNA) was recorded from the peroneal nerve at the level of the fibular head and popliteal fossa (21,22). The approximate location of this nerve was determined by transdermal electrical stimulation (10–60 V, 0.01-ms duration). This stimulation produced painless muscle contraction of the foot. After this, a reference stainless steel microelectrode with a shaft diameter of 200 μm was placed subcutaneously. A similar tungsten electrode, with an uninsulated tip (1–5 μm) was inserted into the nerve and used for recording of MSNA.
Criteria for acceptable MSNA recordings were 1) electrical stimulation produced muscle twitches but not paresthesia, 2) nerve activity increased during phase II of the Valsalva maneuver (hypotensive phase) and was suppressed during phase IV (blood pressure overshoot), and 3) nerve activity increased in response to held expiration.
Tracer calculations.
Rates of glucose appearance (Ra), endogenous glucose production (EGP), and glucose utilization were calculated according to the methods of Wall et al. (23). EGP was calculated by determining the total Ra (this comprises both EGP and any exogenous glucose infused to maintain the desired hypoglycemia) and subtracting it from the amount of exogenous glucose infused. It is now recognized that this approach is not fully quantitative, because underestimates of total Ra and rate of glucose disposal (Rd) can be obtained. The use of a highly purified tracer and taking measurements under steady-state conditions (i.e., constant specific activity) in the presence of low glucose flux eliminates most, if not all, of the problems. In addition, to maintain a constant specific activity, isotope delivery was increased commensurate with increases in exogenous glucose infusion. During these studies, only glucose flux results from the steady-state basal and the final 30-min periods of the hypoglycemic clamps are reported.
Analytical methods.
Plasma glucose concentrations were measured in triplicate using the glucose oxidase method with a glucose analyzer (Beckman, Fullerton, CA). Glucagon was measured according to a modification of the method of Aguilar-Parada et al. (24) with an interassay coefficient of variation (CV) of 12%. Insulin was measured as previously described (25) with an interassay CV of 9%. Catecholamines were determined by HPLC (26) with an interassay CV of 12% for epinephrine and 8% for norepinephrine. Two modifications to the procedure for catecholamine determination were made: 1) a five-point rather than a one-point standard calibration curve was used, and 2) the initial and final samples of plasma with known amounts of epinephrine and norepinephrine were spiked so that accurate identification of the relevant respective catecholamine peaks could be made. Cortisol was assayed using the Clinical Assays Gamma Coat Radioimmunoassay (RIA) kit with an interassay CV of 6%. Growth hormone was determined by RIA (27) with a CV of 8.6%. Pancreatic polypeptide was measured by RIA using the method of Hagopian et al. (28) with an interassay CV of 8%. Lactate, glycerol, alanine, and β-hydroxybutyrate were measured in deproteinized whole blood using the method of Lloyd et al. (29). Nonesterified fatty acids (NEFAs) were measured using the WAKO kit adopted for use on a centrifugal analyzer (30).
Blood for hormones and intermediary metabolites were drawn twice during the control period and every 15 min during the experimental period. Cardiovascular parameters (pulse, systolic, diastolic, and mean arterial pressure) were measured noninvasively by a Dinamap (Critikon, Tampa, FL) every 10 min throughout each study starting at 80 min.
Hypoglycemic symptoms were quantified using a previously validated semiquantitative questionnaire (31). Each individual was asked to rate his or her experience of the symptoms twice during the control period and every 15 min during experimental periods. Symptoms measured included sweaty, tremor/shaky, hot, thirsty/dry mouth, agitation/irritability, palpitations, tired/fatigued, confusion, dizzy, difficulty thinking, blurriness of vision, and sleepy. The ratings of the first six symptoms were summed to get the autonomic score, whereas the ratings from the last six symptoms provide a neuroglycopenic symptom score.
Statistical analysis.
Data are expressed as mean ± SE and were analyzed using standard, parametric, one- and two-way ANOVA, and repeated measures where appropriate (SigmaStat; SPSS Science, Chicago). Tukey's post hoc analysis was used to delineate statistical significance across time within each group and for each group compared with day 1 of the preintensive therapy group. A P value of <0.05 was accepted as statistically significant. The baseline and final 30 min of hypoglycemia were compared for most parameters, because steady-state glucose levels, insulin levels, and glucose infusion rates were achieved by this time.
RESULTS
Effects of 6-month intensive therapy on counterregulatory responses to hypoglycemia, insulin, glucose, and A1C levels.
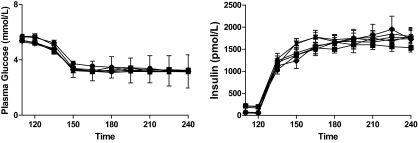
A1C levels were reduced from 10.2 ± 0.5 to 6.7 ± 0.3% during the 6 months of intensive therapy. Body weight remained stable throughout 6 months of intensive therapy (97.6 ± 7 to 96.1 ± 6 kg). Plasma glucose levels were controlled overnight and were similar at the start of the preintensive therapy and postintensive therapy (5.6 ± 0.3 and 5.3 ± 0.2 mmol/l, respectively). Basal plasma glucose levels in the control group were also equivalent at 5.3 ± 0.2 mmol/l. Plasma glucose levels (3.3 ± 0.1 mmol/l) were equivalent during preintensive therapy, postintensive therapy, and control hypoglycemic clamps (Fig. 1).
FIG. 1.
Plasma glucose and insulin levels during repeated 2-day hyperinsulinemic-hypoglycemic clamps in overnight-fasted individuals with type 2 diabetes and healthy control subjects. Type 2 diabetic patients are studied before (pre) and after (post) 6 months of intensive triple oral combination anti-diabetes therapy. •, Day 1 pre; ▪, day 2 pre; ▴, day 1 post; ▿, day 2 post; ♦, day 1 controls; ○, day 2 controls.
Basal insulin levels were 198 ± 36, 210 ± 30, and 66 ± 18 pmol/l in the preintensive therapy, postintensive therapy, and control groups, respectively. Insulin levels during the clamp studies were similar among groups (1,722 ± 198 pmol/l). C-peptide levels decreased from 0.75 ± 0.12 to 0.17 ± 0.02 ng/l during hypoglycemia in preintensive therapy. After intensive therapy, basal C-peptide levels were increased (1.2 ± 0.2 ng/ml; P < 0.05) and were higher during postintensive therapy clamps (0.3 ± 0.03 ng/l; P < 0.05) compared with preintensive therapy.
Neuroendocrine responses.
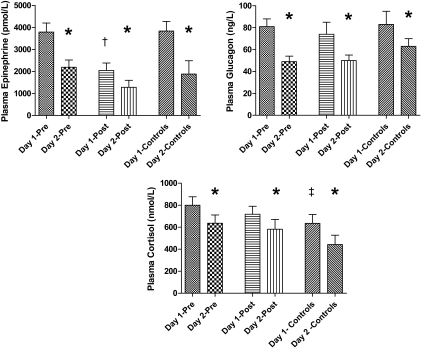
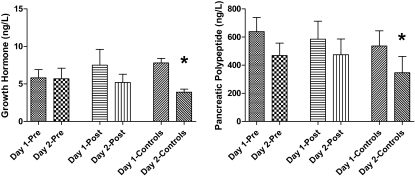
After 6-month intensive therapy, epinephrine responses were significantly blunted in postintensive therapy compared with preintensive therapy and control values (2,033 ± 343 vs. 3,788 ± 414 and 3,837 ± 441 pmol/l, respectively; P < 0.05) (Fig. 2). Intensive therapy had no effect on other neuroendocrine (glucagon, growth hormone, cortisol, norepinephrine, or pancreatic polypeptide) responses to hypoglycemia (Fig. 3). Cortisol levels were higher (P < 0.05) in preintensive therapy compared with control subjects (801 ± 77 vs. 635 ± 80 nmol/l).
FIG. 2.
Plasma epinephrine, glucagon, and cortisol levels in the final 30 min of hypoglycemia during repeated 2-day hyperinsulinemic-hypoglycemic clamps in overnight-fasted individuals with type 2 diabetes and healthy control subjects. *Plasma epinephrine, glucagon, and cortisol levels are significantly reduced (P < 0.05) after day 1 hypoglycemia in healthy control subjects and patients with type 2 diabetes both before (pre) and after (post) 6-month intensive triple oral combination anti-diabetes therapy. †Plasma epinephrine levels are significantly reduced (P < 0.05) after 6-month intensive triple oral combination anti-diabetes therapy. ‡Plasma cortisol levels are significantly reduced (P < 0.05) in healthy control subjects compared with type 2 diabetic patients before intensive therapy.
FIG. 3.
Plasma growth hormone and pancreatic polypeptide levels in the final 30 min of hypoglycemia during repeated 2-day hyperinsulinemic-hypoglycemic clamps in overnight-fasted individuals with type 2 diabetes and healthy control subjects. *Plasma growth hormone and pancreatic polypeptide levels are significantly reduced (P < 0.05) after day 1 hypoglycemia in healthy control subjects.
Glucose kinetics and intermediary metabolism.
Basal EGP was similar in all three groups (preintensive therapy, 9.9 ± 1.1; postintensive therapy, 9.5 ± 1.1; control, 9.4 ± 1.1 μmol · kg−1 · min−1). EGP declined significantly in all three groups (P < 0.01). However, EGP was higher (P < 0.01) during hypoglycemia in preintensive therapy (6.6 ± 1.1 μmol · kg−1 · min−1) compared with postintensive therapy and control values where there was no measurable EGP. Rates of glucose infusion were significantly greater (P < 0.01) in postintensive therapy (10.5 ± 3.3 μmol · kg−1 · min−1) and control (16.5 ± 3.9 μmol · kg−1 · min−1) compared with preintensive therapy (2.2 μmol · kg−1 · min−1).
Intermediary metabolism.
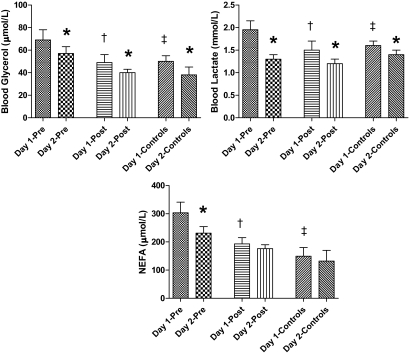
NEFAs (193 ± 22 vs. 303 ± 38 μmol/l), lactate (1.52 ± 0.17 vs. 1.96 ± 0.18 mmol/l), and glycerol (49 ± 7 vs. 69 ± 9 μmol/l) were significantly blunted (P < 0.05) during postintensive therapy compared with preintensive therapy, respectively (Fig. 4). Plasma lactate, glycerol, and blood NEFA levels were higher (P < 0.05) during preintensive therapy versus control.
FIG. 4.
Blood glycerol, lactate, and plasma NEFA levels in the final 30 min of hypoglycemia during repeated 2-day hyperinsulinemic-hypoglycemic clamps in overnight-fasted individuals with type 2 diabetes and healthy control subjects. *Blood glycerol, lactate, and plasma NEFA levels are significantly reduced (P < 0.05) in healthy control subjects and patients with type 2 diabetes both before (pre) and after (post) 6-month intensive triple oral combination anti-diabetes therapy. †Blood glycerol, lactate, and plasma NEFA levels are reduced (P < 0.05) after 6-month intensive therapy in type 2 diabetes. ‡Blood glycerol, lactate, and plasma NEFAs are significantly reduced (P < 0.05) in healthy control subjects compared with type 2 diabetes before intensive therapy.
MSNA.
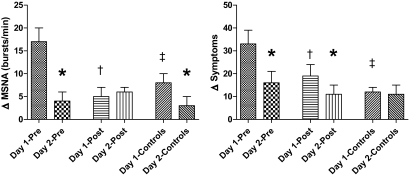
Basal rates of MSNA were similar in preintensive therapy (34 ± 3 bursts/min), postintensive therapy (31 ± 4 bursts/min), and control (38 ± 6 bursts/min). MSNA increased by 17 ± 3 bursts/min during preintensive therapy, which was increased (P < 0.05) compared with the responses during postintensive therapy (5 ± 3 bursts/min) and control clamps (8 ± 2 bursts/min) (Fig. 5).
FIG. 5.
Δ responses from baseline in MSNA and hypoglycemic symptom scores in the final 30 min of hypoglycemia during repeated 2-day hyperinsulinemic-hypoglycemic clamps in overnight-fasted individuals with type 2 diabetes and healthy control subjects. *Δ MSNA and symptom responses are significantly reduced (P < 0.05) in type 2 diabetic patients and healthy control subjects. †Δ MSNA and symptom responses are significantly reduced (P < 0.05) in type 2 diabetes after 6-month intensive triple oral combination anti-diabetic therapy. ‡Δ MSNA and symptom responses are significantly reduced (P < 0.05) in healthy control subjects compared with type 2 diabetes before intensive therapy (PRE).
Hypoglycemic symptom scores.
Total hypoglycemic scores increased by 33 ± 6 during hypoglycemia in preintensive therapy, which was increased (P < 0.01) compared with the response during postintensive therapy (19 ± 4). Symptom responses were lower (P < 0.01) in control (12 ± 2) compared with both preintensive therapy and postintensive therapy studies (Fig. 5). Intensive treatment blunted autonomic symptom responses (preintensive therapy, 18 ± 3, vs. postintensive therapy, 10 ± 4; P < 0.05) and neuroglycopenic symptom responses to hypoglycemia (15 ± 3 vs. 9 ± 3; P < 0.05).
Cardiovascular responses.
Intensive therapy significantly blunted cardiovascular responses to hypoglycemia. Systolic blood pressure increased from 120 ± 6 to 129 ± 6 mmHg (P < 0.05) during hypoglycemia in preintensive therapy but remained similar to baseline (129 ± 6 to 130 ± 7 mmHg) in postintensive therapy. Heart rate also increased during hypoglycemia in preintensive therapy (74 ± 5 to 85 ± 6 beats/min; P < 0.05) but remained similar to baseline (70 ± 4 to 73 ± 4 beats/min) in postintensive therapy. Systolic blood pressure increased by a nonsignificant amount in control subjects (119 ± 5 to 125 ± 4 mmHg), but heart rate increased significantly (62 ± 4 to 72 ± 4 beats/min; P < 0.05).
Hypoglycemic events during intensive treatment.
Self-reported blood glucose readings <3.9 mmol/l are shown in Table 1. No patient experienced any hypoglycemia in the month before the start of the study. During the study, all patients experienced hypoglycemic readings between 3.3 and 3.9 mmol/l. Thirteen patients had readings between 2.8 and 3.3 mmol/l, and frequency of hypoglycemia increased to 3.2 episodes per patient per month by the end of the 6-month study. No major episodes of hypoglycemia occurred during the study. Four patients documented hypoglycemic readings <2.8 mmol/l.
TABLE 1.
Self-reported blood glucose readings (<3.9 mmol/l) by patients during 6-month intensive treatment period
| Month
|
Total readings <3.9 mmol/l | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Level of hypoglycemia | |||||||
| <3.3–3.9 mmol/l | 12 | 26 | 27 | 33 | 26 | 34 | 158 |
| <2.9–3.3 mmol/l | 5 | 8 | 6 | 6 | 12 | 13 | 50 |
| <2.9 mmol/l | 7 | 3 | 2 | 2 | 1 | 15 | |
| Total episodes recorded | 17 | 41 | 36 | 41 | 40 | 48 | |
| Frequency per patient/month | 1.1 | 2.7 | 2.4 | 2.7 | 2.7 | 3.2 | |
All 15 patients recorded episodes of hypoglycemia (<3.9 mmol/l) during the 6-month intensive treatment period.
Effects of antecedent hypoglycemia on counterregulatory responses to hypoglycemia before and after 6-month intensive therapy
Insulin, C-peptide, and glucose levels.
Insulin levels were similar during the morning of day 1 and day 2 clamp studies in all three groups (1,534 ± 174 to 1,890 ± 222 pmol/l). Plasma glucose levels were equivalent during morning day 1 and day 2 studies (3.3 ± 0.1 mmol/l) in all groups. Antecedent hypoglycemia attenuated the fall in C-peptide during day 2 in both preintensive therapy (day 1, −0.58 ± 0.08 vs. day 2, −0.39 ± 0.11 ng/l) and postintensive therapy studies (day 1, −0.98 ± 0.15, vs. day 2, −0.61 ± 0.11 ng/l; P < 0.05).
Neuroendocrine levels.
Baseline neuroendocrine hormone levels were similar at the start of preintensive therapy, postintensive therapy, and control studies (Table 2). Despite equivalent glucose and insulin levels, antecedent hypoglycemia significantly blunted day 2 epinephrine levels in preintensive therapy (day 1, 3,788 ± 414, vs. day 2, 2,191 ± 332 pmol/l; P < 0.01), postintensive therapy (day 1, 2,033 ± 343, vs. day 2, 1,281 ± 316 pmol/l; P < 0.05), and control groups (day 1, 3,837 ± 441, vs. day 2, 1,880 ± 611 pmol/l; P < 0.05). Plasma glucagon levels were also significantly blunted by antecedent hypoglycemia in preintensive therapy (day 1, 81 ± 7, vs. day 2, 49 ± 5 ng/l; P < 0.05), postintensive therapy (day 1, 74 ± 11, vs. day 2, 50 ± 5.2 ng/l; P < 0.05), and control groups (day 1, 83 ± 12, vs. 63 ± 7 ng/l; P < 0.05). Similarly, cortisol levels were blunted by antecedent hypoglycemia in preintensive therapy (day 1, 801 ± 77 vs. 635 ± 75 nmol/l; P < 0.05), postintensive therapy (day 1, 718 ± 72, vs. day 2, 582 ± 88 nmol/l; P < 0.05), and control groups (day 1, 635 ± 80, vs. day 2, 492 ± 86 nmol/l; P < 0.05). Antecedent hypoglycemia did not significantly blunt day 2 growth hormone, pancreatic polypeptide, or norepinephrine responses in the preintensive therapy or postintensive therapy patient studies. However, growth hormone (day 1, 7.8 ± 0.6, vs. day 2, 3.9 ± 0.4 ng/l; P < 0.05) and pancreatic polypeptide (day 1, 128 ± 26, vs. day 2, 83 ± 28 ng/l; P < 0.05) levels were significantly blunted by antecedent hypoglycemia in the control group.
TABLE 2.
Baseline neuroendocrine hormone levels at the start of day 1 and day 2 hyperinsulinemic-hypoglycemic clamps in healthy individuals (control) and patients with type 2 diabetes preintensive therapy and postintensive therapy
| Type 2 diabetes
|
Healthy individuals
|
|||||
|---|---|---|---|---|---|---|
| Preintensive therapy
|
Postintensive therapy
|
Control
|
||||
| Day 1 | Day 2 | Day 1 | Day 2 | Day 1 | Day 2 | |
| Epinephrine (pg/ml) | 278 ± 82 | 180 ± 38 | 202 ± 44 | 218 ± 33 | 223 ± 48 | 184 ± 48 |
| Norepinephrine (pg/ml) | 1.07 ± 0.15 | 1.0 ± 0.14 | 0.9 ± 0.2 | 0.9 ± 0.18 | 0.7 ± 0.2 | 1.17 ± 0.26 |
| Glucagon (ng/l) | 54 ± 5 | 45 ± 5 | 48 ± 3 | 46 ± 4 | 48 ± 8 | 47 ± 6 |
| Growth hormone (μg/l) | 2 ± 1 | 2 ± 0.5 | 1 ± 0.5 | 1.5 ± 0.5 | 1 ± 0.3 | 1 ± 0.2 |
| Cortisol (pmol/l) | 345 ± 61 | 248 ± 69 | 248 ± 55 | 237 ± 44 | 254 ± 43 | 235 ± 58 |
| Pancreatic polypeptide (pmol/l) | 48 ± 19 | 31 ± 9 | 30 ± 9 | 29 ± 8 | 28 ± 8 | 25 ± 7 |
Data are means ± SE.
Glucose kinetics.
Glucose specific activity was in a steady state at the start and final 30 min of each hypoglycemic clamp (Table 3). Antecedent hypoglycemia significantly reduced EGP during preintensive therapy (day 1, 6.6 ± 0.6, vs. day 2, 0.6 ± 1.1 μmol · kg−1 · min−1; P < 0.01). EGP was not measurable during both days of postintensive therapy and control studies. Antecedent hypoglycemia resulted in greater glucose infusion rates to maintain target glucose in preintensive therapy (day 1, 2.2 ± 1.1, vs. day 2, 8.8 ± 1.6 μmol · kg−1 · min−1; P < 0.05), postintensive therapy (day 1, 10.5 ± 3.3, vs. day 2, 15.4 ± 2.7 μmol · kg−1 · min−1; P < 0.05), and control (day 1, 16.5 ± 3.3, vs. day 2, 23.1 ± 3.9 μmol · kg−1 · min−1; P < 0.05).
TABLE 3.
Glucose specific activity (dpm/mmol) during the basal period and the final 30 min of day 1 and day 2 hyperinsulinemic-hypoglycemic clamps in healthy individuals (control) and patients with type 2 diabetes preintensive therapy and postintensive therapy
| 100 min | 110 min | 120 min | 210 min | 225 min | 240 min | |
|---|---|---|---|---|---|---|
| Preintensive therapy type 2 diabetes | ||||||
| Day 1 | 286 ± 33 | 283 ± 31 | 279 ± 27 | 310 ± 27 | 314 ± 26 | 306 ± 25 |
| Day 2 | 299 ± 40 | 300 ± 35 | 305 ± 36 | 303 ± 21 | 295 ± 21 | 304 ± 22 |
| Postintensive therapy type 2 diabetes | ||||||
| Day 1 | 257 ± 51 | 266 ± 51 | 265 ± 50 | 290 ± 41 | 288 ± 43 | 290 ± 42 |
| Day 2 | 330 ± 27 | 329 ± 26 | 325 ± 26 | 332 ± 20 | 336 ± 20 | 326 ± 20 |
| Control | ||||||
| Day 1 | 373 ± 30 | 374 ± 30 | 374 ± 29 | 306 ± 26 | 320 ± 29 | 312 ± 33 |
| Day 2 | 370 ± 30 | 375 ± 36 | 363 ± 33 | 308 ± 34 | 295 ± 39 | 294 ± 38 |
Data are means ± SE.
Intermediary metabolism.
Plasma glycerol responses were blunted (P < 0.05) by antecedent hypoglycemia in preintensive therapy (day 1, 13 ± 3, vs. day 2, 3 ± 1 μmol/l), postintensive therapy (day 1, −2 ± 1, vs. day 2, −18 ± 3 μmol/l), and control studies (day 1, −22 ± 3, vs. day 2, −41 ± 4 μmol/l). Similarly, plasma lactate levels were also blunted (P < 0.05) by day 1 hypoglycemia in preintensive therapy (day 1, 1.95 ± 0.2, vs. day 2, 1.31 ± 0.2 mmol/l), postintensive therapy (day 1, 1.5 ± 0.22, vs. day 2, 1.2 ± 0.1 mmol/l), and control (day 1, 1.6 ± 0.1, vs. day 2, 1.4 ± 0.1 mmol/l).
MSNA.
Day 1 hypoglycemia reduced MSNA responses during day 2 hypoglycemia in preintensive therapy (17 ± 3 vs. 4 ± 2 bursts/min; P < 0.05) and in control (8 ± 2 vs. 3 ± 2 bursts/min; P < 0.05, respectively). MSNA responses during day 1 hypoglycemia in postintensive therapy were significantly blunted by intensive treatment and did not decline further during day 2 hypoglycemia (5 ± 2 vs. 6 ± 1 bursts/min).
Hypoglycemia symptoms.
Day 1 hypoglycemia reduced symptom responses (P < 0.05) during day 2 hypoglycemia in preintensive therapy 33 ± 6 vs. 16 ± 5) and postintensive therapy (19 ± 4 vs. 10 ± 3). Symptoms were unchanged during day 1 and day 2 hypoglycemia in control (12 ± 2 vs. 11 ± 4). Day 1 hypoglycemia blunted (P < 0.05) both autonomic (18 ± 3 vs. 9 ± 4) and neuroglycopenic (15 ± 4 vs. 7 ± 2) scores during day 2 hypoglycemia in preintensive therapy. In postintensive therapy, day 1 hypoglycemia predominantly blunted day 2 neuroglycopenic symptoms (7 ± 2 vs. 3 ± 2).
Cardiovascular responses.
Blood pressure and heart rate responses were similar during day 1 and day 2 hypoglycemia in all groups.
DISCUSSION
This study has prospectively determined the effects of 6-month intensive triple combination oral therapy with improvement of A1C to 6.7% on integrated counterregulatory responses during repeated hypoglycemia in a group of patients with type 2 diabetes. Our results demonstrate that intensive treatment with oral combination therapy substantially reduces ANS and metabolic (EGP, lipolysis) symptoms and cardiovascular responses to hypoglycemia. Additionally, antecedent hypoglycemia can produce further widespread (i.e., neuroendocrine) reductions in the above physiological counterregulatory mechanisms in type 2 diabetic patients both before and after intensive therapy.
Three recent large multicentered trials have examined the effects of intensive glucose control with an average A1C <7.0% on macro- and microvascular complications in type 2 diabetes (9–11). All three studies identified an increased prevalence of hypoglycemia with lowering of A1C in type 2 diabetes (9–11). To date, there are no published prospective studies examining the effects of intensive glucose control (i.e., A1C <7.0%) on physiological responses to hypoglycemia in type 2 diabetes. Our study demonstrates that achieving an A1C of 6.7% with triple oral therapy (glipizide XL, metformin, and acarbose) for 6 months in the absence of exogenous insulin therapy can significantly reduce neuroendocrine, metabolic, and ANS responses to hypoglycemia. Furthermore, repeated episodes of relatively mild hypoglycemia (3.3 mmol/l) can further significantly reduce counterregulatory responses in type 2 diabetic patients, thus creating a syndrome of hypoglycemia-associated autonomic failure that occurs irrespective of moderate or tight glycemic control.
The patients in the present study were diagnosed with type 2 diabetes for a mean of 6 ± 2 years, which was similar to Spyer et al. (15) and Israelian et al. (17) but of a shorter duration than other recent studies investigating counterregulatory responses to hypoglycemia in type 2 diabetes (12–14,16). Thus, the present results provide data in a group of more recently diagnosed individuals whom may be predicted to benefit most from rigorous metabolic control. Additionally, it should be noted that the results in this present study are applicable to a group of patients whom underwent a period of significant glycemic control with a specific triple oral therapy and lifestyle change regimen. Thus, we cannot determine whether other therapeutic regimens (i.e., with insulin, thiazolidinediones, and/or agents that increase GLP-1 axis activity) would have produced similar results. Intensive therapy in this study had marked effects on blunting ANS responses to hypoglycemia. The key counterregulatory hormone epinephrine was reduced by ∼50%. Similarly MSNA was reduced by ∼66%, and hypoglycemic symptom scores were blunted by ∼40%. Additionally, blood pressure and heart rate responses were also significantly blunted by intensive therapy. The above results demonstrate that central sensing of hypoglycemia combined with end-organ responses were both reduced by intensive therapy. The reduced sympathetic nervous system activity also affected important metabolic counterregulatory mechanisms during hypoglycemia after intensive therapy. EGP was not measurable and lipolysis (NEFA, glycerol) together with glycogenolysis (lactate) were reduced by ∼30% after intensive therapy. However, it should be noted that with the exception of epinephrine (which was lower during postintensive therapy compared with control), intensive therapy reduced the exaggerated counterregulatory responses observed in the preintensive therapy patients to the usual physiological responses observed in the control subjects. Additionally, we would like to point out that the above metabolic findings may have been caused by a combination of reduced ANS input and improvement in glucotoxicity/insulin sensitivity (32,33). We did not identify any effects of intensive therapy to significantly blunt other neuroendocrine responses (glucagon, cortisol, growth hormone, norepinephrine, and pancreatic polypeptide). With the exception of cortisol, levels of the above neuroendocrine hormones were not different from age- and BMI-matched nondiabetic individuals. Spyer et al. (15) also reported similar findings in a group of type 2 diabetes with an A1C of 7.4%. However, Israelian et al. (17) reported that both glucagon and growth hormone responses were blunted during similar hypoglycemic conditions to this study in type 2 diabetic subjects. The fact that the blunting effects of intensive therapy on counterregulatory responses in this study were confined almost exclusively to the sympathetic nervous system is different to the situation in type 1 diabetes where intensive therapy has been reported to result in a more widespread blunting of neuroendocrine responses during hypoglycemia (34). The reasons for the targeted effects on the sympathetic nervous system are not apparent from the present study. Previous work (13,14) has determined that the threshold for counterregulatory hormone release in type 2 diabetes remain elevated, even during relatively good glycemic control (A1C 7.4%). Thus, we believe that it is unlikely that the depth of hypoglycemia in our study (3.3 mmol/l) could have mitigated against finding a difference in neuroendocrine responses after intensive therapy. In addition, the magnitude of the neuroendocrine responses was sufficiently robust to have been able to determine a difference if one had been present. However, we cannot exclude the possibility that deeper hypoglycemia may have provoked a more widespread difference in maximal neuroendocrine responses between the preintensive therapy and postintensive therapy treatment groups (13,14).
The second part of our study addressed a possible mechanism for our findings of reduced counterregulatory mechanisms during hypoglycemia after intensive therapy. We tested the hypothesis that antecedent hypoglycemia was a mechanism responsible for acquired counterregulatory failure during hypoglycemia in type 2 diabetes. Self-reported blood glucose monitoring of four to six times per day had demonstrated an aggregate frequency of hypoglycemia of 36–48 episodes per month or up to 3.2 events per patient a month. More than 95% of these episodes were in the range of 2.8–3.9 mmol/l. Although this level of hypoglycemia is often considered “mild” in clinical practice, these present results clearly demonstrate the profound blunting effects of a plasma glucose of 3.3 mmol/l on subsequent physiological responses to hypoglycemia. Epinephrine and symptom responses were blunted by antecedent hypoglycemia in both preintensive therapy and postintensive therapy studies. Of note, despite equivalent insulinemia and glycemia, antecedent hypoglycemia blunted the fall of C-peptide during day 2 hypoglycemia in both preintensive therapy and postintensive therapy studies. This obviously causes some concern because suppression of endogenous insulin during hypoglycemia is a primary physiological counterregulatory defense. MSNA was substantially reduced during day 2 hypoglycemia in preintensive therapy. However, due to the inherent blunting effects of intensive therapy, MSNA was substantially suppressed during day 1 hypoglycemia in postintensive therapy and was not further reduced during day 2 hypoglycemia.
Cortisol and glucagon responses were significantly reduced by antecedent hypoglycemia in both preintensive therapy and postintensive therapy studies. In addition to the above, growth hormone and pancreatic polypeptide (a marker of parasympathetic activity) were both blunted by antecedent hypoglycemia in nondiabetic control studies. Our present findings extend the elegant studies of Segel et al. (16) whom also described blunting of ANS and neuroendocrine responses after antecedent hypoglycemia in longer duration, moderately controlled (A1C 8.6 ± 1.1%) type 2 diabetic patients. The widespread reduction of ANS and neuroendocrine responses after day 1 hypoglycemia also led to significant blunting of metabolic counterregulatory mechanisms (glucose kinetics, lipolysis, and glycogenolysis) in both preintensive therapy and postintensive therapy studies. As discussed, type 2 diabetic patients typically have higher thresholds (i.e., increased plasma glucose values) for counterregulatory defenses against hypoglycemia (7). This is an important protective mechanism that serves to reduce hypoglycemia in type 2 diabetes. The findings of increased MSNA, cortisol, and symptom scores coupled with elevated EGP, lipolysis, and glycogenolysis in preintensive therapy compared with the nondiabetic control subjects are consistent with higher plasma glucose thresholds and/or glucotoxicity providing added protection against hypoglycemia in these individuals. Thus, the finding that antecedent hypoglycemia and intensive glucose control reduced counterregulatory defenses (e.g., symptoms, EGP, and MSNA) back down to normal control values indicates that the risk for iatrogenic hypoglycemia had been increased as important physiological protective mechanisms against hypoglycemia had been removed in these type 2 diabetic patients.
A limitation of this study was that cognitive function was not formally assessed during our repeat hypoglycemia studies before and after intensive therapy. We did not identify any gross changes in cognitive function within our type 2 diabetic patients or healthy control subjects during any of our hypoglycemia studies. Whether this indicates that cognitive function is relatively preserved during repeated hypoglycemia and intensive therapy or that more sophisticated methodology is needed to detect differences in cognitive function will require further study.
In summary, this present study has demonstrated that 6-month intensive glycemic control with triple oral combination therapy to near-normal A1C levels can result in substantial reductions of epinephrine responses during hypoglycemia in individuals with type 2 diabetes. In addition, the exaggerated neuroendocrine, ANS, and metabolic counterregulatory responses that were present in the preintensive therapy group (and thus acting as increased defenses against hypoglycemia) were reduced to levels similar to the nondiabetic control group. Near normalization of A1C resulted in an increased frequency of ∼3.0 hypoglycemic episodes per patient month by study end. Our results clearly demonstrate that antecedent hypoglycemia can also induce ANS, neuroendocrine, and metabolic counterregulatory failure in type 2 diabetic patients with either elevated or near-normal glycemic control. Furthermore, the combination of repeated hypoglycemia and intensive glycemic control produces additive effects to further reduce physiological defenses against subsequent hypoglycemia. Therefore, we would conclude that antecedent hypoglycemia appears to be the likely cause of the blunted sympathoadrenal counterregulatory responses occurring during hypoglycemia after intensive treatment in our type 2 diabetic patients. Additionally, even relatively mild hypoglycemia (3.3 ± 0.1 mmol/l) can produce significant blunting of subsequent counterregulatory mechanisms in type 2 diabetes and increase the risk for future hypoglycemia. This further reinforces the therapeutic goal of achieving good glycemic control while minimizing the occurrence of any hypoglycemia.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01-DK-069803, MO1-RR-000095, P01-HL-056693, and P60-DK-020593 and by an award from the Juvenile Foundation Research Foundation International/Veterans Affairs
No potential conflicts of interest relevant to this article were reported.
We are thankful for the expert technical assistance of Eric Allen, Pam Venson, and Wanda Snead. We also thank the nursing staff of the Vanderbilt GCRC, Caroll Moffat, Linda Balch, and Jerri Brown for superb dietary and diabetes management of our patients.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 December 2008.
Clinical trial registry no. NCT00732862, clinicaltrials.org.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 515.
REFERENCES
- 1.Diabetes Control and Complication Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 2.U.K. Prospective Study Group: Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 3.American Diabetes Association: Standards of medical care in diabetes. Diabetes Care 31: S3–S78, 2008 [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group: Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med 90: 450–459, 1991 [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial (DCCT) Research Group: Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 46: 271–286, 1997 [PubMed] [Google Scholar]
- 6.Gabriely I, Shamoon H: Hypoglycemia in diabetes: common, often unrecognized. Cleveland Clin J Med 71: 335–342, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Briscoe VJ, Davis SN: Hypoglycemia in type 1 and type 2 diabetes: physiology, pathophysiology and management. Clinical Diabetes 24: 115–121, 2006 [Google Scholar]
- 8.Briscoe VJ, Davis SN: Hypoglycemia in Type 1 Diabetes in Adults: Principles and Practice. Jabbour S, Stephens E, Hirsch I, Goldstein B, Garg S, Riddle M, Eds. New York, Taylor 7 Francis Informa, 2008
- 9.Gerstein HC, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M, Yusuf S, HOPE Investigators: The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 48: 1749–1755, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Action to Control Cardiovascular Risk in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Burge MR, Sobhy TA, Qualls CR, Schade DS: Effect of short-term glucose control on glycemic thresholds for epinephrine and hypoglycemic symptoms. J Clin Endocrinol Metabol 86: 5471–5478, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Levy CJ, Kinsley BT, Bajaj A, Simonson DC: Effect of glycemic control on glucose counterregulation during hypoglycemia in NIDDM. Diabetes Care 21: 1330–1338, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Korzon-Burakowska A, Hopkins D, Matyka K, Lomas J, Pernet A, Macdonald I, Amiel S: Effects of glycemic control on protective responses against hypoglycemia in type 2 diabetes. Diabetes Care 21: 283–290, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Spyer G, Hattersley AT, MacDonald IA, Amiel S, MacLeod KM: Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type-2 diabetes. Lancet 365: 1970–1974, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Segel SA, Peramore DS, Cryer PE: Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes 51: 724–733, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Israelian Z, Szoke E, Woerle J, Bokhari S, Shorr M, Schwenke D, Cryer PE, Gerich J, Meyer C: Multiple defects in counterregulation of hypoglycemia in modestly advanced type 2 diabetes mellitus. Metabolism 55: 593–598, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Abumrad NN, Rabin D, Diamond MC, Lacy WW: Use of a heated superficial hand vein as an alternative site for measurement of amino acid concentration and for the study of glucose and alanine kinetics in man. Metabolism 30: 936–940, 1981 [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo R, Tobin K, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E216–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 20.Amiel SA, Tambolane WV, Simonson DC, Sherwin R: Defective glucose counterregulation after strict control of insulin-dependent diabetes mellitus. N Engl J Med 316: 1376–1383, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Wallin BG, Sundlof G, Eriksson BM, Dominiak P, Grobecker H, Lindblad LE: Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand 111: 69–73, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Sandoval DA, Ertl AC, Richardson MA, Tate DB, Davis SN: Estrogen blunts neuroendocrine and metabolic responses to hypoglycemia. Diabetes 52: 1749–1755, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Wall JS, Steele R, Debodo RD, Altszuler N: Effect of insulin on utilization and production of circulating glucose. Am J Physiol 189: 43–50, 1957 [DOI] [PubMed] [Google Scholar]
- 24.Aguilar-Parada E, Eisentraut AM, Unger RH: Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci 257: 415–419, 1969 [DOI] [PubMed] [Google Scholar]
- 25.Wide L, Porath J: Radioimmunoassay of proteins with the uses of sephadex-coupled antibodies. Biochim Biophys Acta 130: 257–260, 1966 [Google Scholar]
- 26.Causon R, Caruthers M, Rodnight R: Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem 116: 223–226, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Hunter W, Greenwood F: Preparation of [131I]-labeled human growth hormone of high specific activity. Nature 194: 495–496, 1962 [DOI] [PubMed] [Google Scholar]
- 28.Hagopian W, Lever E, Cen D, Emmonoud D, Polonsky K, Pugh W, Moosa A, Jaspan J: Predominance of renal and absence of hepatic metabolism of pancreatic polypeptide in the dog. Am J Physiol 245: 171–177, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd B, Burrin J, Smythe P, Alberti KGMM: Enzymatic fluorometric continuous flow assays for blood glucose, lactate, pyruvate, alanine, glycerol and 2-hydroxybutyrate. Clin Chem 24: 1724–1729, 1978 [PubMed] [Google Scholar]
- 30.Ho RJ: Radiochemical assay of long chain fatty acids using 63NI as tracer. Anal Biochem 26: 105–113, 1970 [DOI] [PubMed] [Google Scholar]
- 31.Deary L, Hepburn D, Macleod K, Frier BM: Partitioning the symptoms of hypoglycemia using multi-sample confirmatory factor analysis. Diabetologia 36: 771–770, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Hawkins M, Gabriely L, Wozniak R, Reddy K, Rossetti L, Shamoon H: Glycemic control determines hepatic and peripheral glucose effectiveness in type 2 diabetic subjects. Diabetes 51: 2179–2189, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Donath MY, Schumann DM, Faulenback M, Ellingsgaard H, Perren A, Ehses JA: Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31: S161–S164, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Davis MR, Mellman M, Shamoon H: Further defects in counterregulatory response induced by recurrent hypoglycemia in IDDM. Diabetes 41: 1335–1340, 1992 [DOI] [PubMed] [Google Scholar]