Abstract
OBJECTIVE— Based on rodent studies, we examined the hypothesis that increased adipose tissue (AT) mass in obesity without an adequate support of vascularization might lead to hypoxia, macrophage infiltration, and inflammation.
RESEARCH DESIGN AND METHODS— Oxygen partial pressure (AT pO2) and AT temperature in abdominal AT (9 lean and 12 overweight/obese men and women) was measured by direct insertion of a polarographic Clark electrode. Body composition was measured by dual-energy X-ray absorptiometry, and insulin sensitivity was measured by hyperinsulinemic-euglycemic clamp. Abdominal subcutaneous tissue was used for staining, quantitative RT-PCR, and chemokine secretion assay.
RESULTS— AT pO2 was lower in overweight/obese subjects than lean subjects (47 ± 10.6 vs. 55 ± 9.1 mmHg); however, this level of pO2 did not activate the classic hypoxia targets (pyruvate dehydrogenase kinase and vascular endothelial growth factor [VEGF]). AT pO2 was negatively correlated with percent body fat (R = −0.50, P < 0.05). Compared with lean subjects, overweight/obese subjects had 44% lower capillary density and 58% lower VEGF, suggesting AT rarefaction (capillary drop out). This might be due to lower peroxisome proliferator–activated receptor γ1 and higher collagen VI mRNA expression, which correlated with AT pO2 (P < 0.05). Of clinical importance, AT pO2 negatively correlated with CD68 mRNA and macrophage inflammatory protein 1α secretion (R = −0.58, R = −0.79, P < 0.05), suggesting that lower AT pO2 could drive AT inflammation in obesity.
CONCLUSIONS— Adipose tissue rarefaction might lie upstream of both low AT pO2 and inflammation in obesity. These results suggest novel approaches to treat the dysfunctional AT found in obesity.
Both insulin resistance and β-cell failure are present in individuals with type 2 diabetes. Insulin resistance is closely linked to adiposity with a central or visceral pattern, providing a greater risk of insulin resistance and metabolic dysfunction. Adipose tissue (AT) serves as an endocrine organ secreting a variety of autocrine, paracrine, and endocrine factors that can produce or prevent insulin resistance (1). The failure of AT to adequately proliferate and/or differentiate to sequester lipids away from liver, skeletal muscle, and the pancreatic β-cell has been proposed as a precursor to type 2 diabetes, broadening the number of potential mechanisms linking obesity to insulin resistance (2).
The increase in body fat in obesity should be accompanied by an increase in vascularization, in order to provide adequate oxygen and nutrients (3). In contrast to expectations, obese mice have lower AT capillary density (rarefaction, also known as capillary drop out) and decreased vascular endothelial growth factor (VEGF), the most potent angiogenic factor (4,5). Consistent with this model, preclinical studies suggest that obese AT is hypoxic (6); however, the hypothesis that AT rarefaction might lead to hypoxia remains untested.
In humans, short-term whole-body hypoxia decreases insulin sensitivity (7) and short-term whole-body hyperoxygenation increases insulin sensitivity (8). In mice, obesity is associated with lower oxygen partial pressure in subcutaneous and visceral AT (6,9). Studies in postsurgical patients support the idea that AT oxygen partial pressure (AT pO2) is lower in obesity (10).
In vitro hypoxic adipocytes secrete inflammatory molecules such as tumor necrosis factor (TNF) α, interleukin (IL)1, IL6, macrophage inflammatory protein (MIP), and plasminogen activator inhibitor-1 (6,11). Increased AT inflammation is a feature of obesity and type 2 diabetes (12). Hypoxic cells secrete chemokines, which attract macrophages, presumably to clear out necrotic cells and tissue (13). This suggests the hypothesis that the increase in AT macrophage content seen in human obesity (12) might be due to AT hypoxia.
These preclinical and cell culture experiments suggest that hypoxia might play a role in the inflammation and insulin resistance observed in human obesity. To test this hypothesis, we measured subcutaneous abdominal AT oxygenation (AT pO2) in lean and overweight/obese human subjects and related AT pO2 to the structure and function of AT.
RESEARCH DESIGN AND METHODS
Twenty-one subjects were recruited and screened based on their BMI: lean (20–25 kg/m2) or overweight/obese (27–35 kg/m2). Recruiting was conducted via newsprint, postcards, and the Pennington Biomedical Research Center (PBRC) webpage. Subjects were excluded if they had significant renal, cardiac, liver, lung, or neurological disease. Hypertension was acceptable if blood pressure was <140/90 mmHg on medications. Subjects were excluded for prior use of thiazolidinediones or injectable antihyperglycemic medication; drugs known to affect lipid metabolism, energy metabolism, or body weight; alcohol or other drug abuse; and smoking. The protocol was approved by the institutional review board at the PBRC, and all volunteers gave written informed consent.
Body composition was measured by dual-energy X-ray absorptiometry (DEXA) on a Hologic Dual Energy X-ray Absorptiometer in the fan beam mode (QDR 4500; Hologic, Waltham, MA). The coefficient of variation for the measurement of percentage of body fat is 1.7%. Two days prior and during an in-patient stay, participants were fed a standardized diet (50% carbohydrate, 15% protein, and 35% fat). The number of calories to be provided (and consumed) was calculated as 1.3 × basal energy expenditure using Harris-Benedict equation (665.10 + [9.56 × weight in kg] + [1.85 × height in cm] − [4.68 × age in years for female and 66.47 + {13.75 × weight in kg} + {5.0 × height in cm}] − [6.76 × age in years] for male subjects).
Measurement of subcutaneous abdominal AT pO2 and AT temperature.
AT pO2 was measured using a polarographic micro Clark-type electrode, and AT temperature was measured using a thermocouple concomitantly during two distinct procedures. The measurements were done with a single oxygen probe (cat. no. C1), a single temperature probe (cat. no. C8), or a combined oxygen and temperature probe (cat. no. CC1.P1; Integra Life Sciences, Plainsboro, NJ). The probes were connected to an electronic unit (LICOX CMP; brain oxygen-monitoring unit). This unit displays the AT temperature in °C and the AT pO2 in mmHg (after controlling for the contribution of temperature on AT pO2). After insertion, the system was allowed to equilibrate for 30 min. Recording was stopped when the difference between measurements done at 5-min interval was <1 mmHg (steady state); values from the last 10 min were averaged (supplementary Fig. 3 [available in an online appendix at http://dx.doi.org/10.2337/db08-1098). All measurements were made supine, on the left side of the abdomen, at one-third the distance between the umbilicus and the superior iliac crest (supplemental Fig. 1) and with the skin uncovered at an ambient room temperature of ∼25°C.
First, we measured AT pO2 (mmHg) with the probes inserted into a gas-permeable silastic tubing implanted in the subdermal space as described by Hopf (14) and optimized in our laboratory (indirect method, supplemental Fig. 2). Second, AT pO2 was measured using a direct insertion method developed by our laboratory (supplemental Fig. 2B). After cleaning the skin with povidone-iodine solution and removal of the dried iodine with sterile saline on gauze, a skin wheal was raised with 1% lidocaine. A combined oxygen and temperature probe was inserted through a 3.2-cm-long 14-Ga IV catheter (Medex) to a depth of 1 cm. The values obtained with the two methods were highly correlated (R = 0.64, P < 0.01; n = 13). However, the second method was less invasive and provided less discomfort to the volunteers. Therefore, after 13 subjects we only used the second method to measure AT pO2 and AT temperature. All values in this manuscript are from the “direct” measurement technique.
Euglycemic-hyperinsulinemic clamp.
The clamp was performed as previously described (15). Briefly, intravenous catheters were inserted in an antecubital vein for infusions and in a vein on the dorsum of the contra-lateral hand for sampling of arterialized blood. After baseline sampling, a primed-continuous insulin infusion (80 mU/m2 per min) was continued for 3–4 h. Insulin was infused for at least 1 h after reaching a concentration of glucose ∼90 mg/dl. Plasma glucose was measured every 5 min and maintained by a variable 20% glucose infusion. The mean rate of exogenous glucose infusion during steady-state (last 30 min) was corrected for changes in glycemia and divided by fat free mass to assess insulin sensitivity.
Laboratory measures.
The following assays were done on blood drawn after an overnight fast. Glucose was analyzed using a Beckman Coulter DXC 600 Pro (Brea, CA) and insulin via immunoassay on the Siemens Immulite 2000 (Siemens, Los Angeles, CA).
AT biopsy.
The skin was anesthetized with a mixture of lidocaine (2%) and bupivocaine (0.025%). AT was obtained using a blunt-ended needle designed for liposuction (3- to 4-mm diameter “mercedes” liposuction needle; M.D. Resource, Hayward, CA) and processed at the bedside by washing in 37°C PBS and snap frozen or preserved in 10% formalin for paraffin blocking. The biopsies obtained from the first completed 14 subjects (8 female and 6 male subjects) were used for the following procedures.
Short-term AT release of cytokines.
As previously described (16), fresh AT was collected into pregassed 37°C medium 199 and minced into 2- to 5-mg fragments and washed over a nylon mesh. Aliquots were incubated for 3 h in medium 199 containing 1% BSA under a 95% O2 to 5% CO2 atmosphere in a shaking water bath (60 cycles/min, 37°C).
MIP1α (chemokine [C-C motif] ligand 3), VEGF, TNFα, leptin, IL1α, and monocyte chemotactic protein-1 were measured in the condition media using the Luminex system (cat. no. HCYT060K03; MIllipore). The conditioned media was assayed for lactate dehydrogenase and confirmed the absence of cell lysis. Of 14 subjects, 1 subject had no biopsy tissue for the assay (because of technical limitations) and 1 subject had the assay compromised during processing and therefore could not be used. Subsequently, cytokine release was measured in 12 subjects (5 lean and 7 overweight/obese, from which 5 were male and 7 were female). Conditioned media concentrations of VEGF, TNFα, and leptin were below the detection level of the assay (data not shown).
Capillary density.
The tissue was cleaned in PBS, fixed in 10% formalin, and embedded in paraffin. Before staining, 3-μm sections were deparaffinized and dehydrated by incubation in Xylene (cat. no 247642; Sigma-Aldrich, MO) for 20 min followed by incubation with 100, 90, 80, 70, and 60% ethanol. Slides were rinsed with PBS (20 min). The sections were incubated for 30 min in a dark moist container with the staining solution containing lectin fluorescein isothiocyanate (FITC) conjugate (from Griffonia simplicifolia [GS]) 25 μg/ml (cat. no. L2895; Sigma-Aldrich), lectin tetramethylrhodamine isothiocyanate conjugate (from Ulex europaeus [UAE]), 10 μg/ml (cat. no. L4889; Sigma-Aldrich), and 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) 0.3 μ (cat. no. D9564; Sigma-Aldrich). The GS lectin stains the plasmalemma, UEA stains the capillaries (17), and DAPI stains nuclei (18). In a preliminary experiment, the AT sections were stained with DAPI in addition to GS lectin and UEA lectin, showing that UEA lectin does not stain nuclei (supplemental Fig. 4). The sections were rinsed with PBS (40 min) and then mounted on microscope slides with a water-soluble mounting medium (cat. no. M7644-1; Cardinal Health). Images of the stained sections were taken with a Zeiss Axioplan 2 upright microscope (Intelligent Imaging Innovations) using Zeiss Axioplan 2 with a Photometrics CoolSnap HQ CCD camera and a Sutter Lambda LS 175W Xenon arc lamp. A planar Apochromat 20×/0.75 objective lens, three filter sets (DAPI-EX 360/40, FITC-EX HQ487/25, and CY3-EX HQ535/50) and Slidebook Software version 2.0 were used for image capture. Microvessels were counted using MBF ImageJ Bundle software. Microvessel density was expressed as the number of microvessels per millimeter of section area, averaged across 6–10 images acquired from each section.
Quantitative RT-PCR.
Human total RNA from ∼100 mg AT was isolated by column purification (Qiagen). All primers and probes were designed using Primer Express version 2.1 (Applied Biosystems). Sequences of primers and probes are shown in a supplementary table. Leptin and GLUT1 were from ABI (cat. no. Hs00174877_m1, Hs00197884_m1). GLUT1 mRNA was not detectable in the AT samples. Real-time quantitative RT-PCRs (qRT-PCRs) (19) were performed as one-step reactions in ABI PRISM 7900 (Applied Biosystems) using the following parameters: one cycle of 48°C for 30 min, then 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The relative standard curve method was used to calculate the quantity of the target gene for each tissue extract with an internal control. The “housekeeping gene” cyclophilin B was previously demonstrated to be “stable” across lean and obese subjects (20–22). Therefore, each sample value was divided by the quantity of cyclophilin B as previously described (20–23).
Adipocyte size.
Mean adipocyte size was measured as previously described (24). Tissue was fixed in osmium tetroxide. Dissociation and digestion of proteins was performed with 8 mol/l urea/NaCl. Cells were filtered over a 10-μm nylon screen then recollected in a Triton X-100 solution. Approximately 2,500 cells from each sample were analyzed on a Coulter Counter using a 400-μm aperture.
Statistical methods.
Comparison between the lean and overweight/obese subjects was performed using an unpaired t test. Statistical significance was defined relative to a nominal two-sided 5% type-1 error rate. Values are presented as means ± SD. All analyses were performed in JMP (version 5.0.1; SAS, Cary, NC).
RESULTS
Subject characteristics.
Subject characteristics are listed in Table 1. The subjects were men (n = 11) and women (n = 10) with diverse ethnicity: Caucasian (n = 10), African American (n = 10), or Chinese (n = 1). The lean subjects were matched for age with the overweight/obese subjects without diabetes; however, both goups were younger than the overweight/obese subjects with diabetes (P < 0.05). Waist circumference was significantly larger in the overweight/obese versus lean group (P < 0.05). The range of BMI was 20.4–23.8 kg/m2 in the lean group and 28.9–34.7 kg/m2 in the overweight/obese group. By design, the overweight/obese group had greater BMI compared with the lean group (P < 0.05) and greater percent fat (P < 0.05). As expected, overweight/obese subjects had lower insulin sensitivity, as shown by the glucose disposal rates compared with lean subjects (P < 0.05).
TABLE 1.
Clinical characteristics of the study population
| Lean subjects | Overweight/ obese subjects | P | |
|---|---|---|---|
| Sex (male/female) | 9 (5/4) | 12 (6/6) | |
| Age (years) | 22.6 ± 3.3 | 38.9 ± 15.8 | <0.05 |
| Weight (kg) | 64.8 ± 7.7 | 92.0 ± 12.8 | <0.05 |
| BMI (kg/m2) | 22.1 ± 1.0 | 31.7 ± 1.9 | <0.05 |
| Waist (cm) | 73 ± 4.4 | 100 ± 10.4 | <0.05 |
| Percent fat (DEXA) | 20.9 ± 7.6 | 34.2 ± 8.2 | <0.05 |
| Fasting glucose (mg/dl) | 89 ± 3 | 110 ± 27 | <0.05 |
| Fasting insulin (μU/ml) | 4.9 ± 2.0 | 14.4 ± 9.6 | <0.05 |
| Glucose disposal rate during euglycemic-hyperglycemic clamp (mg/min × kg fat-free mass) | 11.2 ± 3.4 | 6.0 ± 2.2 | <0.05 |
| Abdominal subcutaneous AT | <0.05 | ||
| AT pO2 (mmHg) | 55.4 ± 9.1 | 46.8 ± 10.6 | <0.05 |
| AT temperature (°C) | 34.0 ± 1.0 | 32.1 ± 1.4 | <0.05 |
Data are means ± SD. Percent body fat was measured by DEXA and represents mass as the percent of total body weight. The lean group included two African Americans, six Caucasians, and one Asian. The overweight/obese group included eight African Americans and four Caucasians. *P <0.05.
Overweight/obese subjects have lower AT pO2 and AT temperature without activation of hypoxia target genes.
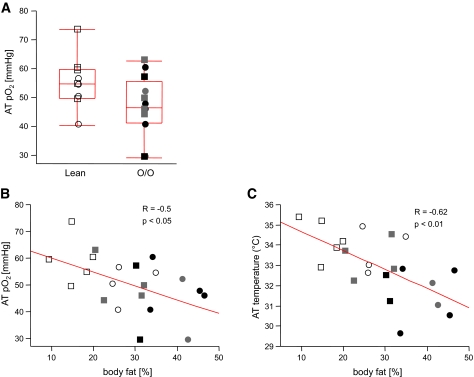
We observed lower AT pO2 in overweight/obese than lean subjects (range 29.1–62.8 vs. 40.5–73.8 mmHg; P < 0.05) (Fig. 1A; Table 1). In addition, AT pO2 was negatively correlated with percent fat (R = −0.50, P < 0.05) (Fig. 1B) and fat mass (R = −0.48, P < 0.05). AT temperature was lower in overweight/obese than lean subjects (P < 0.05) (Table 1) and was negatively correlated with percent body fat (R = −0.62, P < 0.01) (Fig. 1C) Race, sex, and age were not significant contributors to AT pO2 or AT temperature (P = NS). There was no relationship between the use of antihypertensive medication and AT pO2 (P = NS).
FIG. 1.
AT pO2 and AT temperature are inversely correlated with percent body fat. AT pO2, measured by direct insertion of a micro Clark-type electrode into abdominal subcutaneous AT, was lower in overweight/obese group (O/O) compared with lean subjects (A) and inversely correlated with percent body fat (B). AT temperature measured by a thermocouple inserted into the abdominal AT was inversely correlated with percent body fat (C). Males are represented by squares and females by circles, filled with different colors as follows: white for lean, gray for O/O without type 2 diabetes, and black for O/O with type 2 diabetes.
The differences in AT pO2 cannot be explained by differences in AT temperature. A 6°C difference in temperature leads to a 1-mmHg difference in pO2 at atmospheric pressure (suplemental Fig. 3B), a value that is trivial compared with the range of values for AT pO2 (∼35 mmHg). Next, we measured the expression of known HIF-1 targets: pyruvate dehydrogenase kinase (PDK) 1 and VEGF. We found that PDK1 and VEGF were not upregulated in overweight/obese subjects (Table 2), suggesting that the decrease in AT pO2 is not sufficient to activate the hypoxia pathway that would increase AT pO2 to normal values.
TABLE 2.
AT characteristics of lean versus overweight/obese volunteers
| Lean | Overweight/obese | P | Correlation with percent fat
|
||
|---|---|---|---|---|---|
| r | P | ||||
| n | 6 | 8 | |||
| Gene expression (AU) | |||||
| Leptin mRNA | 0.19 ± 0.11 | 0.57 ± 0.12 | <0.05 | 0.74 | <0.05 |
| PDK1 mRNA | 0.63 ± 0.24 | 0.45 ± 0.21 | <0.05 | −0.10 | |
| VEGF mRNA | 2.46 ± 1.11 | 1.04 ± 0.34 | <0.05 | −0.78 | <0.05 |
| ANG1 mRNA | 0.41 ± 0.13 | 0.69 ± 0.11 | <0.05 | 0.73 | <0.05 |
| PPARγ1 mRNA | 1.33 ± 0.36 | 0.74 ± 0.21 | <0.05 | −0.73 | <0.05 |
| COL6 mRNA | 0.32 ± 0.17 | 0.53 ± 0.16 | <0.05 | 0.55 | <0.05 |
| CD68 antigen mRNA | 0.23 ± 0.05 | 0.62 ± 0.27 | <0.05 | 0.64 | <0.05 |
| MAC2/CD163 mRNA | 0.56 ± 0.22 | 1.49 ± 0.63 | <0.05 | 0.72 | <0.05 |
| MIP1α mRNA | 0.02 ± 0.01 | 0.21 ± 0.18 | <0.05 | 0.60 | <0.05 |
| MCP1 mRNA | 0.12 ± 0.05 | 0.25 ± 0.11 | <0.05 | 0.43 | |
| Cytokine release (pg/mg tissue × h) | <0.05 | ||||
| MIP1α | 0.44 ± 0.38 | 0.96 ± 0.69 | <0.05 | 0.60 | <0.05 |
| MCP1 | 2.26 ± 0.83 | 2.36 ± 1.18 | <0.05 | 0.12 | |
| IL1α | 0.09 ± 0.08 | 0.20 ± 0.07 | <0.05 | 0.67 | <0.05 |
Data are means ± SD. MCP1, macrophage chemoattractant protein 1.
Within the overweight/obese group, there were six overweight/obese subjects without type 2 diabetes and six overweight/obese subjects with type 2 diabetes. The groups had similar BMI and insulin sensitivity, and we found similar AT pO2 and AT temperature. Data in humans suggests that whole-body hypoxia might cause insulin resistance (7,8); however, the relationship with AT pO2 has not been explored. We found that AT pO2 did not correlate with glucose disposal rate, a gold-standard measure of skeletal muscle insulin resistance (R = 0.21, P = NS).
Evidence for rarefaction in overweight/obese AT.
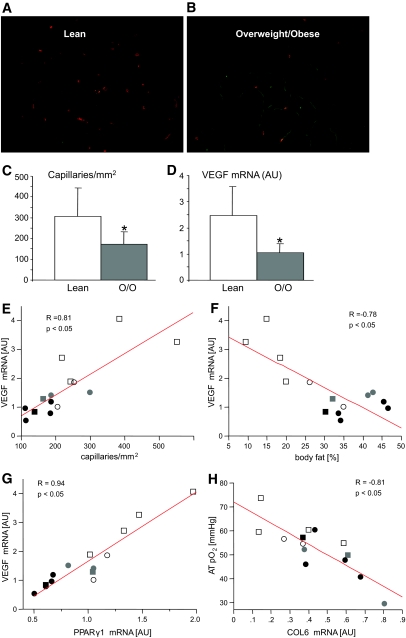
AT pO2 could be reduced via several potential mechanisms: increased demand for oxygen, decreased blood flow due to vasoconstriction, or decreased blood flow due to rarefaction. To explore the latter possibility, we measured capillary density in AT samples collected from the contra lateral abdomen. Capillary density in AT was reduced by 44% in overweight/obese subjects compared with lean subjects (172 ± 60 vs. 308 ± 135, P < 0.05) (Fig. 2C). We found that VEGF mRNA was 58% lower in overweight/obese versus lean subjects (P < 0.05) (Fig. 2D). Capillary density and VEGF expression were strongly correlated (R = 0.81, P < 0.01) (Fig. 2E). AT pO2 was also positively correlated with VEGF mRNA (R = 0.54, P < 0.05). Total body fat (%) was negatively correlated with capillary density (R = −0.69, P < 0.01) and VEGF mRNA (R = −0.78, P < 0.01) (Fig. 2F). To determine the effect of sex, we used capillary density as the dependent variable and percent fat and sex as the independent variables. The effect of sex on capillary density was not significant (data not shown).
FIG. 2.
Vascularization of AT. Representative AT sections from lean (A) and O/O (B) subjects stained with UEA lectin (orange) to label capillaries and with GS lectin (green) to label the adipocyte plasmalemma. Capillary density (C) was measured and averaged across 6–10 histological sections for each subject and VEGF mRNA expression measured by quantitative RT-PCR (D); both were lower in O/O versus lean subjects. VEGF mRNA was positively correlated with capillary density (E) and PPARγ1 mRNA (G) and inversely with percent body fat (F). H: Collagen VI (COL6) mRNA was negatively correlated with AT pO2. Males are represented by squares and females by circles, filled with different colors as follows: white for lean, gray for O/O without type 2 diabetes, and black for O/O with type 2 diabetes. (Please see http://dx.doi.org/10.2337/db08-1098 for a high-quality digital representation of this figure.)
Classically, peroxisome proliferator–activated receptor (PPAR)γ is known as a nuclear hormone receptor that increases adipogenesis and turns on lipogenesis (25). However, recent evidence strongly suggests that PPARγ also drives angiogenesis (26–28). We found that PPARγ1 was strongly correlated with VEGF (R = 0.94, P < 0.01) (Fig. 2G) and capillary density (R = 0.72, P < 0.01). Ultimately, PPARγ1 was correlated with AT pO2 (R = 0.60, P < 0.05).
Angiopoetin 1 (ANG1) is involved in vascular remodeling and is negatively correlated with the rate of body weight change in animals (4,29). We found that ANG1 negatively correlated with AT pO2 (R = −0.57, P < 0.05) and positively with percent fat (R = 0.73, P < 0.05). These suggest that overweight/obese subjects with lower AT pO2 have less vascular remodeling compared with lean subjects. Overweight/obese subjects also had a 66% greater expression of collagen VI (collagen VI α3 subunit [COL6]), a extracellular matrix collagen (P < 0.05, Table 2). Expression of COL6 was positively correlated with percent body fat (R = 0.55, P < 0.05) and inversely correlated with AT pO2 (R = −0.81, P < 0.01) Fig. 2H). In addition to increased AT mass, overweight/obese subjects had greater mean adipocyte size compared with lean subjects (0.86 ± 0.2 vs. 0.43 ± 0.13 μl, P < 0.05). Mean adipocyte size was negatively correlated with capillary density (R = −0.66, P < 0.01) and VEGF (R = −0.69, P < 0.01).
AT pO2 and AT inflammation.
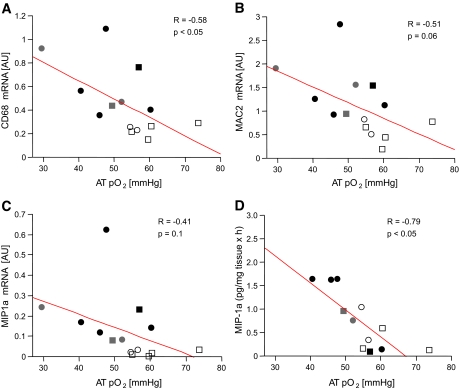
Based on in vitro studies in cultured adipocytes (6,11), we hypothesized that lower AT pO2 might lead to macrophage recruitment and the secretion of inflammatory cytokines. We have previously validated the use of macrophage-associated antigen [MAC2]/CD163 and CD68 as markers of AT macrophage infiltration (R2 = 0.77, P < 0.001) (B. Kozak, J. Gimble, S.R. Smith, unpublished data). Both MAC2/CD163 and CD68 were inversely correlated with AT pO2 (Fig. 3A and B; R = −0.66, P < 0.05 and R = −0.51, P = NS) consistent with accumulation of macrophages in AT with reduced oxygenation. Furthermore, MIP1α was higher in the overweight/obese subjects (Table 2) and AT pO2 was inversely correlated with MIP1α expression (R = −0.41, P = NS) (Fig. 3C) and MIP1α secretion in the media (R = −0.79, P < 0.05) (Fig. 3D). There was a trend toward higher monocyte chemotactic protein-1 and IL1α in obesity and negative correlations with AT pO2, but these did not reach statistical significance. However, IL1α secretion was negatively correlated with VEGF mRNA (R = −0.70, P < 0.001) and capillary density (R = −0.61, P < 0.05).
FIG. 3.
Hypoxia and AT inflammation. AT pO2 was inversely correlated with the inflammation markers CD68 mRNA (A) and MAC 2/CD163 mRNA (B), with the chemokine MIP1α mRNA expression (C) and MIP1α secretion into culture media ex vivo (D). Previous studies in our lab demonstrated a strong correlation between MAC2/CD163 and CD68 mRNA and macrophage infiltration by macrophage staining in AT sections by immunohistochemistry (R2 = 0.77, P < 0.001) (B. Kozak, J. Gimble, S.R. Smith, unpublished data). Males are represented by squares and females by circles, filled with different colors as follows: white for lean, gray for O/O without type 2 diabetes, and black for O/O with type 2 diabetes.
DISCUSSION
Trayhurn and Wood (30) first hypothesized that hypoxia of AT might play a role in insulin resistance. This hypothesis was based on experiments demonstrating that surrogate markers of hypoxia were increased in the AT of obese animals (30). We found that reduced AT pO2 in overweight/obese subjects and AT pO2 strongly correlates with percent body fat. We also found a significant reduction in AT temperature in overweight/obese subjects and a strong inverse correlation with percent total fat. This is in accordance with previous findings (31) showing a lower skin temperature in obesity. The presence of capillary rarefaction suggests that decreased AT perfusion might also play a role. Indeed, there is evidence to suggest that obese subjects have lower blood flow in abdominal adipose subcutaneous tissue (32). Further work using direct measures of both AT pO2 and direct measures of AT blood flow such as xenon washout are needed to formally address this hypothesis. There is additional evidence that obese mice have decreased oxygenation in epididymal and retroperitoneal AT (6,9) and that weight loss increases oxygenation (6). Choban et al. (33) observed an increased incidence of postsurgical wound infections in obese patients. This same group, measuring AT pO2 in the upper arm, subsequently showed that decreased oxygenation of AT contributes to the increased risk of infection (34). AT mass and adipocyte hypertrophy are closely related to the metabolic complications of obesity (35). In vivo data suggested a role for hypoxia in insulin resistance even though AT pO2 has never been measured in human subjects (7,8). We were unable to demonstrate a correlation between insulin sensitivity and AT pO2. A lack of correlation of AT pO2 and insulin sensitivity may be due to lack of power because this AT pO2 and inflammation are just two of many factors that induce insulin resistance or perhaps because there is simply no meaningful biological relationship. The correlation between macrophage content and insulin resistance is modest (36). One limitation of our study is that only one region of the body was measured and only at one depth.
Most hypoxic tissues develop strong transcriptional, metabolic, and secretory responses to reduced oxygenation in order to increase capillary density and to correct the hypoxia. Hypoxia turns on genes that act to increase oxygen availability by decreasing oxygen consumption (switching “on” anaerobic glycolysis) and stimulating angiogenesis. Hypoxia target genes expressed in AT include PDK1 and VEGF (37). Consistent with this data, VEGF and PDK1 are upregulated in adipocytes cultured in 1% oxygen (a hypoxic environment) (6,9,11). In contrast to our expectations, we found that lower AT pO2 in overweight/obese subjects did not induce an increase in hypoxia targets (PDK1, VEGF). Also, capillary density and VEGF were decreased in overweight/obese AT along with a lower AT pO2. This suggests that the transcriptional counterregulatory system was not activated. One should note, however, that the lowest value of AT pO2 in the overweight/obese group was 29 mmHg; this corresponds to 3.8% oxygen compared with the 1% oxygen used in the cell culture experiments. This suggests that overweight/obese subjects have low AT pO2 but not low enough to mount a counterregulatory response driven during a response to hypoxia. Consistent to our results, Lijnen et al. (5) found that obese mice have both lower VEGF and lower vascular density in AT. In addition, it is known that spleen, thymus, and retina have low pO2 in normal rats (38), suggesting that angiogenesis is activated at different levels of oxygenation for different types of tissue or that oxygenation could influence angiogenesis. One limitation of our study is that we did not measure VEGF protein, and this should be addressed in future experiments.
Recent data suggests that PPARγ1 might be required for angiogenesis in AT (39). PPARγ drives VEGF (and angiogenesis) (26–28). We found a strong positive correlation between PPARγ1 and VEGF and between VEGF and AT pO2. One way to interpret this data is that PPARγ1 drives angiogenesis in human AT and therefore is a key controller of AT pO2. More work is needed to test this hypothesis.
AT expansion (adipogenesis) during development is preceded by a wave of neovascularization (40). Vascular plasticity may play a role in the ability of AT to increase or decrease in size (29,41). Our data suggests that reduced capillary density might restrict the growth of AT. Our finding of reduced capillary density in subcutaneous AT in obesity suggests the hypothesis that the failure of the vasculature to expand with increasing subcutaneous obesity might limit adipogenesis in subcutaneous depot. If visceral AT were not similarly restricted, this might allow for the growth of visceral AT. Further work measuring AT pO2 and capillary density in omental and mesenteric AT is needed to test this hypothesis.
Previous studies have shown that COL6 is abundantly expressed by adipocytes (42), and obese mice have increased COL6 expression in the extracellular matrix (43). We found that overweight/obese subjects with low AT pO2 have greater expression of COL6, and COL6 expression increased with increased body fat and fat-cell size. Scherer et al. (44) suggests that proteolytic fragments of COL6 promote tumor growth through prosurvival and proliferation signaling pathways such as Akt and β-catenin. This suggests that new blood vessel formation is restricted by increased extracellular matrix or that a reduction in angiogenesis leads to increases in the formation of the extracellular matrix as exemplified by COL6. Further work is needed to separate these two possibilities.
AT inflammation has received much attention as an important factor in insulin resistance and type 2 diabetes (6,12,45). Previous in vitro and in vivo preclinical studies showed that hypoxia induces inflammation that might contribute to insulin resistance (6,11). We found that in humans, AT pO2 correlates with macrophage markers (CD68 and MAC2/CD163). In addition, AT secretion of MIP1α, a potent macrophage chemokine (46,47), increased as AT pO2 decreased. This is consistent with recent data showing upregulation of MIP1α in obesity (48). This is supportive of the hypothesis that lower oxygenation drives inflammation by upregulating adipocyte chemokine secretion but, as discussed previously, not by activating the classic hypoxia pathway and VEGF. However, it is possible that inflammation could drive hypoxia. Given that MIP1α has been implicated in angiogenesis, it is unclear why MIP1α is up when capillary density is down. Further work is needed to understand the factors regulating angiogenesis in human AT.
In summary, we provide direct evidence of lower AT pO2 in overweight/obese subjects and that the most likely causes are decreased capillary density and reduced expression of the angiogenic factors like VEGF and PPARγ1 and increased expression of COL6. This suggests that low AT pO2 in overweight/obese subjects does not result in a complete counterregulatory response to reduced AT pO2 and that neovascularization is not activated. Decreased AT pO2 was paralleled by an increase in the expression and secretion of the chemokine and markers of macrophage infiltration. These data suggests that proangiogenic therapies might reduce AT inflammation, improve insulin action, and reduce cardiovascular disease risk in obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
This work was funded by a grant from the Health and Performance Enhancement Division of PBRC and in part by an unrestricted grant from the Organization for the Study of Sex Differences. Research Support was provided by the Clinical Nutrition Research Unit Grant no. P30-DK072476.
No potential conflicts of interest relevant to this article were reported.
We acknowledge Drs. George Bray and Eric Ravussin and especially Dr. Harriet Hopf for their support, advice, and counsel.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 December 2008.
Clinical trials reg. no. NCT00704197, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Smith SR, Ravussin E: Role of Adipocyte in Metabolism and Endocrine Function. DeGroot, Jameson, Endocrinology, Philadelphia, Elsevier Saunders, 2006
- 2.Danforth E Jr: Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 26: 13, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S: Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56: 1517–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Hausman GJ, Richardson RL: Adipose tissue angiogenesis. J Anim Sci 82: 925–934, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Lijnen HR, Christiaens V, Scroyen I, Voros G, Tjwa M, Carmeliet P, Collen D: Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes 55: 2698–2704, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Ye J, Gao Z, Yin J, He Q: Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL, Peters A: Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med 169: 1231–1237, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Jakobsson P, Jorfeldt L: Oxygen supplementation increases glucose tolerance during euglycaemic hyperinsulinaemic glucose clamp procedure in patients with severe COPD and chronic hypoxaemia. Clin Physiol Funct Imaging 26: 271–274, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I: Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A: Obesity decreases perioperative tissue oxygenation. Anesthesiology 100: 274–280, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Wood IS, Trayhurn P: Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455: 479–492, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr.: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdoch C, Giannoudis A, Lewis CE: Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104: 2224–2234, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hopf HW: Development of subcutaneous wound oxygen measurement in humans: contributions of Thomas K Hunt, MD. Wound Repair Regen 11: 424–430, 2003 [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Russell CD, Ricci MR, Brolin RE, Magill E, Fried SK: Regulation of the leptin content of obese human adipose tissue. Am J Physiol Endocrinol Metab 280: E399–E404, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Hausman GJ: Cytochemistry for lectins, actin, nucleotide tetrazolium reductases and several phosphatases in the porcine semitendinosus muscle: vascular development in young pigs. J Anim Sci 67: 1375–1386, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Morikawa K, Yanagida M: Light microscopic structure of DNA in solution studied by the 4′,6-diamidino-2-phenylindole staining method. J Mol Biol 152: 501–516, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Bustin SA: Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25: 169–193, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Bogacka I, Xie H, Bray GA, Smith SR: Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54: 1392–1399, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Smith SR, Bai F, Charbonneau C, Janderova L, Argyropoulos G: A promoter genotype and oxidative stress potentially link resistin to human insulin resistance. Diabetes 52: 1611–1618, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Smith SR, Gawronska-Kozak B, Janderova L, Nguyen T, Murrell A, Stephens JM, Mynatt RL: Agouti expression in human adipose tissue: functional consequences and increased expression in type 2 diabetes. Diabetes 52: 2914–2922, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR: Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115: 1934–1941, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SR, Xie H, Baghian S, Needham A, McNeil M, Bogacka I, Bray G: Pioglitazone changes the distribution of adipocyte size in type 2 diabetics. Adipocytes 2: 11–22, 2006 [Google Scholar]
- 25.Bogacka I, Xie H, Bray GA, Smith SR: The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care 27: 1660–1667, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Biscetti F, Gaetani E, Flex A, Aprahamian T, Hopkins T, Straface G, Pecorini G, Stigliano E, Smith RC, Angelini F, Castellot JJ Jr, Pola R: Selective activation of peroxisome proliferators–activated receptor (PPAR)α and PPAR γ induces neoangiogenesis through a vascular endothelial growth factor–dependent mechanism. Diabetes 57: 1394–1404, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL: Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARgamma agonist's effects on edema and weight gain. FASEB J 20: 1203–1205, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, Nishizawa Y: Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun 271: 571–574, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Dallabrida SM, Zurakowski D, Shih SC, Smith LE, Folkman J, Moulton KS, Rupnick MA: Adipose tissue growth and regression are regulated by angiopoietin-1. Biochem Biophys Res Commun 311: 563–571, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Trayhurn P, Wood IS: Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Claessens-van Ooijen AM, Westerterp KR, Wouters L, Schoffelen PF, van Steenhoven AA, van Marken Lichtenbelt WD: Heat production and body temperature during cooling and rewarming in overweight and lean men. Obesity (Silver Spring) 14: 1914–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Jansson PA, Larsson A, Smith U, Lonnroth P: Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest 89: 1610–1617, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choban PS, Heckler R, Burge JC, Flancbaum L: Increased incidence of nosocomial infections in obese surgical patients. Am Surg 61: 1001–1005, 1995 [PubMed] [Google Scholar]
- 34.Fleischmann E, Kurz A, Niedermayr M, Schebesta K, Kimberger O, Sessler DI, Kabon B, Prager G: Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg 15: 813–819, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE: Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43: 1498–1506, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA: Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54: 2305–2313, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Brahimi-Horn MC, Pouyssegur J: Oxygen, a source of life and stress. FEBS Lett 581: 3582–3591, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK: Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93: e88–97, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi TS, Suda T, Yoo OJ, Koh GY: Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res 100: e47–57, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ: Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 99: 10730–10735, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherer PE, Bickel PE, Kotler M, Lodish HF: Cloning of cell-specific secreted and surface proteins by subtractive antibody screening. Nat Biotechnol 16: 581–586, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Huber J, Loffler M, Bilban M, Reimers M, Kadl A, Todoric J, Zeyda M, Geyeregger R, Schreiner M, Weichhart T, Leitinger N, Waldhausl W, Stulnig TM: Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond ) 31: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell RG, Sasisekharan R, Trock BJ, Lippman M, Calvert VS, Petricoin EF, 3rd, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE: Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest 115: 1163–1176, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD: Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol 175: 81–92, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H: Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM: CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab 93: 3215–3221, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.