Abstract
OBJECTIVE— This study assessed insulin and glucagon secretion in relation to insulin sensitivity in Caucasian women who develop impaired glucose tolerance (IGT) versus those who maintain normal glucose tolerance (NGT) over a 12-year period.
RESEARCH DESIGN AND METHODS— At baseline and after 3, 8, and 12 years, glucose tolerance (75-g oral glucose tolerance test), insulin sensitivity (euglycemic-hyperinsulinemic clamp), and insulin and glucagon secretion (2- to 5-min responses to 5 g arginine i.v. at fasting, 14 and >25 mmol/l glucose) were determined in 53 healthy Caucasian women (aged 58 years at baseline) who all had NGT at baseline.
RESULTS— During the 12-year period, 26 subjects developed IGT, whereas the remaining 27 subjects maintained NGT throughout the 12-year period. Subjects developing IGT had lower insulin sensitivity than those maintaining NGT in the tests preceding diagnosis of IGT (P ≤ 0.05). When judged in relation to insulin sensitivity, β-cell glucose sensitivity and maximal insulin secretion were lower in those who later developed IGT than in those maintaining NGT at all tests (P ≤ 0.05). Furthermore, subjects who developed IGT had defective suppression of glucagon secretion by glucose in the test preceding diagnosis of IGT when they still had NGT (P ≤ 0.05).
CONCLUSIONS— β- and α-cell dysfunction are evident several years before diagnosis of IGT, and islet dysfunction is manifested as impaired glucose sensitivity of the β- and α-cells and reduced maximal insulin secretion.
Insulin resistance is compensated by increased insulin secretion, and impaired glucose tolerance (IGT) and type 2 diabetes develop when insulin secretion is not able to fully compensate for insulin resistance (1–4). Hence, defective islet adaptation to insulin resistance is a main event underlying development of type 2 diabetes. Longitudinal studies when insulin secretion and insulin sensitivity have been sequentially determined over time have shown that β-cell dysfunction is an early manifestation during development of type 2 diabetes. Thus, insulin secretion, in relation to insulin sensitivity, has been shown to drop in association with the development of IGT in Pima Indians (5), in Mexican Americans (6), in Caucasians (7), and in subjects of white, black, and Hispanic ethnicity in the U.S. (8). This is also supported by the U.K. Prospective Diabetes Study, which demonstrated that islet β-cell dysfunction, as judged by indirect approaches, is reduced already at the time of diagnosis of type 2 diabetes (9,10).
Insulin secretion is regulated by several factors, such as nutrients, metabolites, gut hormones, and autonomic nerves (11). Furthermore, insulin secretion can be subdivided in a sensing and triggering action by glucose and amplifying or inhibitory modulatory effects by other factors that affect insulin secretion (12). It is currently not known to what extent the various factors or β-cell mechanisms contribute to the defective islet function during the early stages of development of IGT. We have previously shown that both a defective glucose sensitivity of β-cells as well as reduced maximal insulin secretion exist in subjects with IGT (13). However, whether these two defects contribute to the development of IGT and therefore are seen also at early stages (i.e., before even IGT develops) is not known.
In addition to defective β-cell function, dysfunction of glucagon secretion is also of relevance for development of IGT and type 2 diabetes (14). Thus, it was already demonstrated in 1970 that a higher glucagon level during meal ingestion exists in subjects with type 2 diabetes compared with healthy control subjects (15). Furthermore, it has been demonstrated that subjects with type 2 diabetes have elevated levels of glucagon throughout the day (16) and, moreover, that IGT and type 2 diabetes are associated with impaired suppression of glucagon secretion (13,17,18). In addition, we have previously reported that an inappropriately high glucagon secretory response to a standardized intravenous arginine challenge in normal subjects predicts development of IGT over a 3-year period (7). Hence, augmented α-cell secretion, together with impaired β-cell function, is an early defect in the development of type 2 diabetes. However, the sequential changes in defective glucagon secretion during development of IGT has not been established, and it is not known whether the high glucagon secretion is explained by impaired glucose sensing of the α-cells (i.e., the defective ability of glucose to suppress glucagon secretion).
The aim of this study was to establish whether impaired insulin secretion and increased glucagon secretion already occur before the onset of IGT and, if so, the characteristics of the dysfunction. To that end, a 12-year longitudinal study was undertaken in Caucasian postmenopausal women with normal glucose tolerance (NGT) who, during this time period, developed IGT or maintained NGT. Insulin and glucagon secretion were studied using the glucose-dependent arginine stimulation test (19,20). This test establishes 1) basal and maximal insulin secretion and the glucose dependency of insulin secretion and 2) basal glucagon secretion and glucose-induced suppression of glucagon secretion. The results were related to insulin sensitivity as determined with the euglycemic-hyperinsulemic clamp (21).
RESEARCH DESIGN AND METHODS
The study was composed of 53 nondiabetic women who belonged to a study group of a total of 108 women who in 1993 were randomly selected from a large cohort of 841 postmenopausal women born in 1935 and living in Malmö, Sweden, to participate in a longitudinal study on islet function and insulin sensitivity (7,13,22). All subjects were healthy at the time of the baseline examination in 1993/1994, and none was taking any medication known to affect carbohydrate metabolism. None of the subjects had a first-degree relative with type 2 diabetes. During the 12-year follow-up, euglycemic-hyperinsulinemic clamps, glucose-dependent arginine stimulation tests, and conventional 75-g World Health Organization oral glucose tolerance tests (OGTTs) were undertaken at baseline and after 3, 8, and 12 years in all individuals for determination of insulin sensitivity, insulin and glucagon secretion, and glucose tolerance. Baseline data with characteristics of insulin and glucagon secretion in this population were published in 2000 (13). Furthermore, the 3-year follow-up was published in 2000, showing the changes in insulin and glucagon secretion during the first 3 years of the study (7). In the present study, follow-up after 8 and 12 years was also undertaken but has not been published before. A total of 71 women had NGT at the baseline examination (7). The present study reports 12-year follow-up data in 53 of these subjects. Eighteen subjects were lost to follow-up by the following reasons: 4 subjects had died, 2 were excluded because of corticosteroid treatment, 2 were excluded due to malignancies, in 2 subjects it was not possible to establish the necessary intravenous lines at the follow-up examinations, and 10 subjects had moved out of town or did not want to participate in the follow-up. These 18 women did not differ from the 53 participants at baseline regarding body weight, BMI, glucose tolerance, insulin sensitivity, or insulin or glucagon secretory capacity.
OGTT.
An intravenous catheter was inserted into an antecubital vein. After a baseline sample was taken, an OGTT was performed with a standard World Health Organization 75-g glucose load. Blood samples were taken after 120 min for analysis of glucose. The subjects spent 2 h in a semirecumbent position.
Glucose-dependent arginine stimulation test.
Insulin and glucagon secretion were determined with the intravenous arginine stimulation at three glucose levels (fasting and 14 and >25 mmol/l) as previously described (19,20). After an overnight fast, intravenous catheters were inserted into antecubital veins in both arms. One arm was used for the infusion of glucose and the other arm for intermittent sampling. The sampling catheter was kept patent by slow infusion of 0.9% saline when not in use. Baseline samples were taken at −5 and −2 min. A maximally stimulating dose of arginine hydrochloride (5 g) was then injected intravenously over 45 s. Samples were taken at 2, 3, 4, and 5 min. A variable-rate 20% glucose infusion was then initiated to raise and maintain blood glucose at 14 mmol/l. Blood glucose was determined every 5 min bedside, and the glucose infusion adjusted to reach the desired blood glucose level in 20–25 min. New baseline samples were taken; then arginine (5 g) was again injected and new 2-, 3-, 4-, and 5-min samples were taken. A 2.5-h resting period was then allowed, after which new baseline samples were obtained and a high-speed (900 ml/h) 20% glucose infusion for 25–30 min was used to raise blood glucose to >25 mmol/l, as determined bedside. At this blood glucose level, new baseline samples were taken and arginine (5 g) injected, followed by final 2-, 3-, 4-, and 5-min samples.
Euglycemic-hyperinsulinemic clamp.
Insulin sensitivity was determined with the euglycemic-hyperinsulinemic clamp (21). After an overnight fast, intravenous catheters were inserted into antecubital veins in both arms. One arm was used for infusion of glucose and insulin. The contralateral arm was used for intermittent sampling, and the catheter was kept patent with slow infusion of 0.9% saline. A primed constant infusion of insulin (100 units/ml Actrapid; Novo Nordisk, Bagsvaerd, Denmark) with a constant infusion rate of 0.28 nmol/m2 body surface per min was started. After 4 min, a variable-rate 20% glucose infusion was added, and its infusion rate was adjusted manually throughout the clamp procedure to maintain the blood glucose level at 5.0 mmol/l. Blood glucose was determined every 5 min. Samples for analysis of the achieved insulin concentrations were taken at 60 and 120 min.
Analyses.
Blood glucose concentration was determined bedside by the glucose dehydrogenase technique with a Hemocue (Hemocue, Ängelholm, Sweden) during the euglycemic-hyperinsulinemic clamp and with an Accutrend (Boehringer Mannheim Scandinavia, Bromma, Sweden) during the glucose-dependent arginine stimulation test. Blood samples for insulin and glucose from the arginine study and for insulin from the clamp study were taken in EDTA tubes, were immediately centrifuged, and serum was frozen at −20°C. Serum insulin was analyzed with the double-antibody radioimmunoassay technique with the use of guinea pig anti-human insulin antibodies, mono-125I-tyr-human insulin, and human insulin standard (Linco Research, St. Charles, MO). Blood samples for determination of glucagon were taken in tubes containing aprotinin. They were immediately centrifuged, and plasma was separated and frozen at −20°C. Plasma glucagon was measured with double-antibody radioimmunoassay in duplicate using guinea pig anti-human glucagon antibodies specific for pancreatic glucagon, 125I-glucagon as tracer, and glucagon standard (Linco). Plasma glucose concentrations were analyzed using the glucose oxidase method. All samples were analyzed in duplicate.
Calculations and statistics.
Data are presented as means ± SE, unless otherwise stated. For the determination of insulin secretion, the acute insulin response to arginine was determined as the mean of the 2- to 5-min samples minus the mean prestimulus insulin concentration at fasting (AIR1), at 14 mmol/l (AIR2), and at >25 mmol/l glucose (AIR3). The slope between AIR1 and AIR2 was calculated as a measure of the β-cell sensitivity to glucose. Similarly, for the determination of glucagon secretion, the acute glucagon response to arginine was determined as the mean of the 2- to 5-min samples minus the mean prestimulus glucagon concentration at fasting (AGR1), at 14 mmol/l (AGR2), and at >25 mmol/l glucose (AGR3). The slope between AGR1 and AGR2 was calculated as a measure of the α-cell sensitivity to glucose. Insulin sensitivity was calculated as the glucose infusion rate per kilogram body weight during the second hour divided by the mean of the insulin levels at 60 and 120 min during the clamp (i.e., nmol glucose · kg body weight−1 · min−1 · pmol insulin−1 · l−1). To allow comparisons between groups with regard to β-cell secretion in relation to insulin sensitivity, disposition index was calculated (1–3). This was performed by multiplying the measure of insulin sensitivity times AIR1, slopeAIR, or AIR3, respectively. Statistical comparisons were undertaken between the two groups of women maintaining NGT or developing IGT by means of Mann-Whitney U test. Bivariate (Pearson's) correlation coefficients were calculated to estimate linear correlation between variables.
RESULTS
Glucose tolerance.
The fasting and 2-h glucose levels during the OGTT during the four tests are shown in Table 1. At the baseline examination, all 53 subjects had NGT. Throughout the 12-year follow-up, 26 subjects developed IGT, whereas 27 subjects remained normal glucose tolerant. Eight of the subjects developed IGT after 3 years, another 14 subjects developed IGT after 8 years, and 4 additional subjects developed IGT after 12 years. Eight subjects developed type 2 diabetes between the 8- and 12-year follow-up; these subjects had developed IGT after 3 years (two subjects) or after 8 years (six subjects). Fasting or 2-h glucose did not differ significantly between the two groups at baseline. Both fasting glucose (by 0.6 ± 0.1 mmol/l; P = 0.006) and 2-h glucose (by 0.8 ± 0.2 mmol/l; P = 0.012) increased throughout the 12-year study period in subjects who maintained NGT. Also, in subjects who developed IGT, fasting and 2-h glucose levels increased and were significantly higher than in the group who maintained NGT after 12 years (P ≤ 0.05).
TABLE 1.
Anthropomorphic variables and fasting and 2-h glucose levels during an OGTT in Caucasian postmenopausal women who had NGT at baseline and after 3 years but developed IGT (n = 26 at baseline and after 3 and 8 years and n = 18 after 12 years) or maintained NGT (n = 27) at examinations after 8 and 12 years
| Baseline | 3 years | 8 years | 12 years | |
|---|---|---|---|---|
| Age (years) | ||||
| Glucose tolerance | ||||
| NGT | 58 | 61 | 66 | 70 |
| IGT | 58 | 61 | 66 | 70 |
| BMI (kg/m2) | ||||
| Glucose tolerance | ||||
| NGT | 24.8 ± 0.5 | 24.8 ± 0.6 | 25.8 ± 0.6 | 25.7 ± 0.6 |
| IGT | 26.0 ± 0.8* | 26.3 ± 1.1* | 27.3 ± 1.1* | 27.8 ± 1.4* |
| Fasting glucose (mmol/l) | ||||
| Glucose tolerance | ||||
| NGT | 4.5 ± 0.1 | 4.8 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 |
| IGT | 4.7 ± 0.1 | 5.1 ± 0.3 | 5.4 ± 0.2 | 5.5 ± 0.2* |
| 2-h glucose (mmol/l) | ||||
| Glucose tolerance | ||||
| NGT | 6.2 ± 0.1 | 6.6 ± 0.2 | 6.7 ± 0.2 | 6.8 ± 0.2 |
| IGT | 6.4 ± 0.2 | 6.8 ± 0.6 | 7.9 ± 0.6† | 8.9 ± 0.7‡ |
Data are means ± SE.
P < 0.05;
P < 0.01;
P < 0.001 as probability level of random difference between the groups.
BMI.
Both in subjects who developed IGT and in subjects who maintained NGT, a slight increase in BMI was observed over the 12-year study period (Table 1). At the baseline test, subjects who later developed IGT had a slightly higher BMI than those who maintained NGT (P = 0.022), and this difference persisted. Subjects developing IGT increased BMI by 1.8 ± 0.2 kg/m2, whereas subjects who maintained NGT increased BMI by 0.9 ± 0.1 kg/m2 (P = 0.08).
Insulin sensitivity.
Subjects who later developed IGT had a lower insulin sensitivity than those who maintained NGT already at the baseline examination (P = 0.021) (Table 2). Insulin sensitivity declined during the 12-year follow-up in subjects who maintained NGT, from 89 ± 18 to 73 ± 17 nmol · kg−1 · min−1 · pmol−1 · l−1 (P < 0.001). Subjects who had developed IGT had lower insulin sensitivity than those who maintained NGT at all tests (Table 2). Changes in BMI did not correlate to reduction in insulin sensitivity in subjects who maintained NGT, whereas a weak correlation was evident in those who developed IGT (r = −0.38; P = 0.039).
TABLE 2.
Results of the glucose-dependent arginine stimulation test in Caucasian postmenopausal women who had NGT at baseline but developed IGT (n = 26) or maintained NGT (n = 27) during the 12-year follow-up
| Maintaining NGT | Developing IGT | |
|---|---|---|
| n | 27 | 26 |
| Fasting insulin (pmol/l) | 48 ± 2.5 | 60 ± 8* |
| Fasting glucose (mmol/l) | 4.6 ± 0.1 | 4.7 ± 0.1 |
| Insulin at second arginine injection (pmol/l) | 221 ± 18 | 212 ± 9 |
| Glucose at second arginine injection (mmol/l) | 14.2 ± 0.1 | 14.2 ± 0.1 |
| Insulin at third arginine injection (pmol/l) | 448 ± 39 | 508 ± 36 |
| Glucose at third arginine injection (mmol/l) | 28.3 ± 0.2 | 28.8 ± 0.3 |
| AIR1 (pmol/l) | 335 ± 33 | 394 ± 106 |
| AIR2 (pmol/l) | 1,001 ± 119 | 1,018 ± 126 |
| AIR3 (pmol/l) | 994 ± 149 | 1,081 ± 162 |
| SlopeAIR (pmol/mmol) | 62 ± 8.1 | 64 ± 8.0 |
| Fasting glucagon (ng/l) | 66 ± 4.6 | 64 ± 4.2 |
| Glucagon at second arginine injection (ng/l) | 54 ± 4.6 | 53 ± 4.6 |
| Glucagon at third arginine injection (ng/l) | 42 ± 2.9 | 41 ± 2.0 |
| AGR1 (ng/l) | 84 ± 7.8 | 86 ± 11.8 |
| AGR2 (ng/l) | 36 ± 4.0 | 49 ± 6.2* |
| AGR3 (ng/l) | 24 ± 3.6 | 27 ± 4.1 |
| SlopeAGR (ng/mmol) | −5.3 ± 0.5 | −4.2 ± 0.5* |
| Glucose infusion rate during min 60–120 of the clamp (μmol/kg/min) | 60 ± 3 | 52 ± 3* |
| Insulin sensitivity (nmol · kg−1 · min−1 · pmol) | 89 ± 3 | 80 ± 4* |
Data are means ± SE.
P < 0.05 as probability level of random difference between the groups.
Insulin secretion.
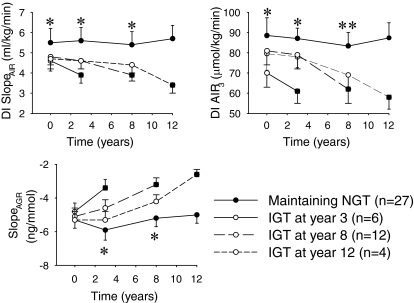
AIR1, AIR2, AIR3, and slopeAIR did not differ between the two groups at the baseline examination (Table 2). In the subjects who maintained NGT, AIR1, slopeAIR, and AIR3 progressively increased throughout the 12 years (Table 3). Insulin secretion, however, needs to be estimated in relation to insulin sensitivity (3). By calculating the disposition index (i.e., multiplying slopeAIR and AIR3 times insulin sensitivity), it was found that in the subjects who maintained NGT throughout the 12-year study period, the disposition index for the two variables remained stable. In contrast, in the subjects who developed IGT, these indexes were significantly lower than in subjects who maintained NGT in the tests preceding the IGT (P ≤ 0.05) (Fig. 1). When manifest IGT had developed, disposition index further deteriorated (Fig. 1). In contrast, disposition index for variable AIR1 did not differ between the groups throughout the study period (data not shown).
TABLE 3.
Insulin sensitivity as determined by the euglycemic-hyperinsulinemic clamp, and measures of insulin and glucagon secretion as obtained from the glucose-dependent arginine stimulation test (AIR1, slopeAIR, AIR3, and slopeAGR) in Caucasian postmenopausal women who had NGT at baseline and either maintained NGT throughout the 12-year study period or developed IGT after 3, 8, or 12 years
| 3-year test | 8-year test | 12-year test | |
|---|---|---|---|
| n | |||
| Group | |||
| NGT | 27 | 27 | 27 |
| Later IGT | 18 | 4 | 0 |
| Manifest IGT | 8 | 22 | 18 |
| Insulin sensitivity (nmol · kg−1 · min−1 · pmol) | |||
| Group | |||
| NGT | 86 ± 21 | 76 ± 18 | 73 ± 17 |
| Later IGT | 75 ± 20* | 62 ± 17† | — |
| Manifest IGT | 66 ± 22 | 69 ± 16 | 64 ± 16 |
| AIR1 (pmol/l) | |||
| Group | |||
| NGT | 309 ± 32 | 382 ± 40 | 383 ± 42 |
| Later IGT | 349 ± 38 | 360 ± 38 | — |
| Manifest IGT | 388 ± 31 | 346 ± 35 | 362 ± 68 |
| SlopeAIR (pmol/mmol) | |||
| Group | |||
| NGT | 62 ± 5.8 | 70 ± 7.8 | 78 ± 6.2 |
| Later IGT | 59 ± 4.9 | 62 ± 6.1 | — |
| Manifest IGT | 56 ± 5.1 | 58 ± 5.6 | 52 ± 5.9 |
| AIR3 (pmol/l) | |||
| Group | |||
| NGT | 1,010 ± 138 | 1,095 ± 150 | 1,195 ± 130 |
| Later IGT | 903 ± 156 | 991 ± 139 | — |
| Manifest IGT | 896 ± 86 | 922 ± 101 | 886 ± 110 |
| SlopeAGR (ng/mmol) | |||
| Group | |||
| NGT | −5.9 ± 0.6 | −5.2 ± 0.5 | −5.0 ± 0.5 |
| Later IGT | −5.1 ± 0.5* | −4.3 ± 0.4* | — |
| Manifest IGT | −3.1 ± 0.4 | −3.2 ± 0.4 | −3.1 ± 0.3 |
Data are means ± SE.
P < 0.05 and
P < 0.01 as probability level of random difference between the group with NGT and the group who later developed IGT. Number of individuals in each group shown in row 2.
FIG. 1.
Insulin secretion (disposition indexes [DIs]) for slopeAIR and AIR3 and glucagon secretion (slopeAGR) as obtained from the glucose-dependent arginine-stimulation test and the euglycemic-hyperinsulinemic clamp at baseline and after 3, 8, and 12 years in healthy women with NGT at baseline (n = 53). The subjects were divided in groups who maintained NGT throughout the 12-year period (n = 27) or developed IGT after 3 years (n = 6), after 8 years (n = 12), or after 12 years (n = 4). Means ± SE are shown. Asterisks indicate the probability level of random difference between the group who maintained NGT versus the subjects with NGT who later developed IGT. *P < 0.05; **P < 0.01. ▪, indicates subjects who already have developed IGT.
Glucagon secretion.
At baseline, slopeAGR was significantly lower and AGR2 was significantly higher in subjects who later developed IGT when compared with those who maintained NGT (P < 0.05) (Table 2). In contrast, AGR1 and AGR3 did not differ between the two groups at the baseline examination. Throughout the 12-year study period, slopeAGR and AGR2 were stable in subjects who maintained NGT, whereas they were lower (slopeAGR) or higher (AGR2) in those who later developed IGT (P < 0.05) (Fig. 1).
DISCUSSION
It has earlier been shown that subjects with IGT exhibit low insulin secretion and high glucagon secretion when compared with subjects with NGT (13,17). This was also confirmed in this study. It has not previously been known, however, whether these islet dysfunctions in IGT also exist before development of IGT. The novelty of the present study, therefore, is that insulin secretion is abnormally low and glucagon secretion is abnormally high also in subjects with NGT who later develop IGT when compared with subjects who maintain NGT over a long period of time. In fact, the study shows that the combined islet dysfunction is manifest several years before the onset of IGT. Furthermore, the study also shows that the main mechanisms of the β-cell dysfunction is reduced glucose sensitivity of the β-cells (slopeAIR) and reduced maximal insulin secretory capacity (AIR3) and that the main mechanism of glucagon hypersecretion is a defective suppression by glucose of stimulated glucagon secretion rather than to baseline islet hormone secretion.
The conclusions of this study are based on a 12-year longitudinal analysis of insulin sensitivity and insulin and glucagon secretion in Caucasian postmenopausal women using the glucose-dependent arginine stimulation test for the determination of insulin and glucagon secretion (19–21). This is an elaborate test that has the advantage that it allows a detailed conclusion on glucose sensitivity as well as basal and maximal islet hormone secretion (19,20). This is of advantage when compared with the indirect techniques using algorithms from fasting levels of insulin and glucose (23,24).
At all the test periods, we also undertook determination of insulin sensitivity using the euglycemic-hyperinsulinemic clamp, because it is known that there is a close relation between islet function and insulin sensitivity and that insulin secretion needs to be interpreted in relation to insulin sensitivity (3). The importance of this was evident in this study in which the absolute insulin secretion was not significantly reduced in the subjects developing IGT. However, when taking into account the higher demand for insulin as caused by the insulin resistance, it is clear that subjects who developed IGT had a poor compensatory increase secretion as a sign of defective islet function as evident by the reduced disposition index. Hence, this study shows that the main defect in β-cell function in subjects developing IGT is the inability to sufficiently compensate the reduced insulin sensitivity with increased insulin secretion.
The glucose-dependent arginine stimulation test also allows conclusions on mechanisms of the β-cell dysfunction since it estimates both the glucose sensitivity of the β-cells and the maximal insulin secretion. We found that it was slopeAIR and AIR3 that were reduced in subjects who developed IGT. In contrast, basal insulin secretion (AIR1) was not reduced. This suggests that it is the glucose sensitivity of the β-cell and the maximal insulin response that are the main β-cell dysfunction mechanisms during development of IGT. The molecular mechanism of β-cell glucose sensing is a complex phenomenon that involves glucose uptake in the cell, glucose metabolism and production of energy and ATP, the opening of ATP-dependent K+ channels, and uptake of calcium (12). Furthermore, the maximal insulin secretion also involves the molecular mechanisms of storage of insulin in insulin granules and exocytosis (25). The present results therefore suggest that subjects with IGT have defects in these mechanisms already at a stage when NGT is preserved.
The present study also shows that the increase in glucagon secretion in subjects who develop IGT is evident already before IGT is manifest (i.e., this also is an early phenomenon). Hence, high glucagon secretion is indeed an important risk factor for IGT and type 2 diabetes, and impaired ability of glucose to suppress glucagon secretion seems to be a major mechanism (both slopeAGR and AGR2 are abnormal in subjects who develop IGT). The high glucagon secretion in IGT has been demonstrated before when compared with subjects with NGT (17,26,27), and a high glucagon secretion also has been documented (18) in type 2 diabetes. We have also previously reported that high glucagon predicts worsening of glucose tolerance over a 3-year study period (7). The novelty of this study is that high glucagon secretion is evident in subjects who later develop IGT when compared with subjects who maintain NGT over a long period of time. The results also suggest that the mechanism of the high glucagon secretion in the subjects who later develop IGT is impaired efficacy of glucose to inhibit glucagon secretion. This process relies on suppression of ionic channels in the α-cells, as recently reviewed (28). Another possibility, however, might be impaired suppression by paracrine factors released from the β-cells, such as insulin itself, γ-aminobutyric acid, or zinc, all of which are thought to inhibit glucagon secretion (28). Further studies are required to establish the mechanism underlying the early impairment of suppression of glucagon secretion.
The euglycemic-hyperinsulinemic clamp technique is considered the gold or reference standard for estimating insulin sensitivity in humans since it estimates the whole-body glucose disappearance in response to insulin (29). A limitation of the technique is, however, that it does not measure the action of insulin to suppress hepatic glucose production, the determination of which requires the isotope dilution technique (29). Since we did not use the isotope dilution technique in this study, it is possible that insulin sensitivity in terms of the total body effect of insulin to stimulate glucose disappearance is underestimated in subjects with insulin resistance. The degree of insulin resistance in the subjects who developed IGT would therefore be less than estimated in this study, and, consequently, the islet dysfunction when calculated as disposition index would also be less than estimated.
In conclusion, this study shows that insulin secretion (when judged in relation to insulin sensitivity) is already low before IGT is diagnosed and that this β-cell dysfunction is explained by reduced glucose sensitivity of the β-cells and the maximal β-cell secretory capacity. Furthermore, the study also shows that glucagon secretion is increased in subjects who develop IGT and that this is explained by impaired ability of glucose to suppression of glucagon secretion. Thus, the results show that islet dysfunction is critical for development of IGT and type 2 diabetes and that these pathophysiological events already start when subjects are normal glucose tolerant.
Acknowledgments
The study was supported by the Swedish Research Council (grant no. 14X-6834), the Swedish Diabetes Association, the Albert Påhlsson Foundation, Region Skåne, and the Faculty of Medicine, Lund University.
No potential conflicts of interest relevant to this article were reported.
The author is grateful to Dr. Hillevi Larsson, who took part in the study during the baseline and 3-year follow-up, and to Margaretha Persson and Lilian Bengtsson for expert technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 18 December 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bergman RN, Phillips LC, Cobelli C: Physiologic evaluation of factors controlling glucose tolerance in man. Measurement of insulin sensitivity and β-cell glucose sensitivity fro the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D Jr: Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B, Pacini G: Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol 150: 97–104, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Kahn SE: The relative contribution of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46: 3–19, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104: 787–794, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffner SM, Miettinen H, Gaskill SP, Stern MP: Decreased insulin action and insulin secretion predict the development of impaired glucose tolerance. Diabetologia 39: 1201–1207, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Larsson H, Ahrén B: Glucose tolerance is predicted by low insulin secretion and high glucagon secretion: outcome of a prospective study in postmenopausal Caucasian women. Diabetologia 43: 194–202, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Festa A, Williams K, Hanley AJG, Haffner SM: β-Cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes. Diabetes 57: 1638–1644, 2008 [DOI] [PubMed] [Google Scholar]
- 9.U.K. Prospective Diabetes Study 16: Overview of 6 years therapy of type II diabetes: a progressive disease. Diabetes 44: 1249–1258, 1995 [PubMed] [Google Scholar]
- 10.Holman RR: Assessing the potential for α-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract 40: (Suppl.) S21–S25 [DOI] [PubMed]
- 11.Ahrén B, Taborsky GJ Jr: Beta cell function and insulin secretion. In Ellenberg & Rifkin's Diabetes Mellitus, 6th Edition. Porte D Jr, Sherwin RS, Baron A, Eds. New York, McGraw-Hill Medical Publishing, 2002, p. 43–65
- 12.Henquin JC: Pathways in β-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes 53 (Suppl. 3): S48–S58, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Larsson H, Ahrén B: Islet dysfunction in insulin resistance involves impaired insulin secretion and increased glucagon secretion in postmenopausal women with impaired glucose tolerance. Diabetes Care 23: 650–657, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Dunning BE, Foley J, Ahrén B: Alpha-cell function in health and disease: influence of GLP-1. Diabetologia 48: 1700–1713, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Muller WA, Faloona GR, Aquilar-Parada E, Unger RH: Abnormal α-cell function in diabetes. N Engl J Med 283: 109–115, 1970 [DOI] [PubMed] [Google Scholar]
- 16.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB: Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64: 106–110, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Ahrén B, Larsson H: Impaired glucose tolerance (IGT) is associated with reduced insulin-induced suppression of glucagon concentrations. Diabetologia 44: 1998–2003, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Shah P, Vella A, Basu AS, Basu R, Schwenk WF, Rizza RA: Lack of suppression of glucagon contributes to postprandial hyperglycaemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85: 4053–4059, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr: Diminished B cell secretory capacity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 74: 1318–1328, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson H, Ahrén B: Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 41: 772–777, 1998 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 22.Larsson H, Ahrén B, Lindgärde F, Berglund G: Fasting blood glucose in determining the prevalence of diabetes in a large, homogenous population of Caucasian middle-aged women. J Intern Med 237: 537–541, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ahrén B, Pratley RE, Soubt M, Dunning BE, Foley JE: Clinical measures of islet function: usefulness to characterize defects in diabetes. Curr Diabetes Rev 4: 129–145, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Rorsman P, Renström E: Insulin granule dynamics in pancreatic beta cells. Diabetologia 46: 1029–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Larsson H, Berglund G, Ahrén B: Glucose modulation of insulin and glucagon secretion is altered in impaired glucose tolerance. J Clin Endocrinol Metab 80: 1778–1782, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Larsson H, Ahrén B: Islet dysfunction in obese women with impaired glucose tolerance. Metabolism 45: 502–509, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Quesada I, Tuduri E, Ripoll C, Nadal A: Physology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 199: 5–19, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Muniyappa R, Lee S, Chen H, Quon MJ: Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol 294: E15–E26, 2008 [DOI] [PubMed] [Google Scholar]