Abstract
OBJECTIVE— The pathogenesis of autoimmune pancreatitis (AIP) and fulminant type 1 diabetes remains unclear, although it is known that immune-mediated processes severely compromise the endocrine and exocrine functions in both diseases.
RESEARCH DESIGN AND METHODS— We have screened a λTriplEx2 human pancreas cDNA library with serum from a patient with AIP and obtained positive clones. Sequence analysis revealed that 7 of 10 clones were identical to human amylase α-2A. Using a recombinant COOH-terminal amylase α-2A protein, we developed an enzyme-linked immunosorbent assay system to detect autoantibodies against human amylase α-2A.
RESULTS— All 15 serum samples from patients with AIP recognized the recombinant protein, whereas sera from 25 patients with chronic alcoholic pancreatitis and sera from 25 patients with a pancreas tumor did not. Interestingly, 88% (15/17) of patients with fulminant type 1 diabetes were positive for an autoantibody against amylase α-2A. These antibodies were detected in 21% of patients with acute-onset type 1 diabetes (9 of 42) and 6% of type 2 diabetic patients (4 of 67).
CONCLUSIONS— These results suggest that an autoantibody against amylase α-2A is a novel diagnostic marker for both AIP and fulminant type 1 diabetes and that, clinically and immunologically, AIP and fulminant type 1 diabetes are closely related.
Recently, autoimmune pancreatitis (AIP), a unique form of chronic pancreatitis, has been reported as a discrete disease entity (1). It is characterized by 1) irregular narrowing of the main pancreatic duct and swelling of the pancreas, both of which are due to abundant lymphoplasmacytic inflammation to the exocrine pancreas (2); 2) the increased serum level of IgG and IgG4; 3) positive autoantibodies such as lactoferrin autoantibody or carbonic anhydrase II (CAII) autoantibody (3,4); and 4) a high prevalence of diabetes with complications (5).
We recently reported that a high proportion of pancreatic islets and exocrine pancreatic tissues were infiltrated by CD4+ or CD8+ T-cells in the inflammatory process, which might induce diabetes in AIP (6). In addition, treatment with prednisolone improved insulin secretion and glycemic control in AIP patients (5). These data support the concept that autoimmune mechanism(s) plays a pivotal role in the destruction of the endocrine and exocrine pancreas in AIP patients with diabetes.
Clinically, the most common initial symptom of AIP is jaundice, but in some patients, no symptoms or only mild symptoms, frequently without acute attacks of pancreatitis, may be present (7). It is difficult to distinguish AIP from other types of chronic pancreatitis or cancer of the pancreatic head (8). In such cases, detection of autoantibodies is useful for diagnosing AIP, but a proportion of patients with AIP are negative for autoantibodies against lactoferrin and CAII (3,4).
We encountered an AIP patient whose serum IgG and IgG4 levels were 3,498 and 2,430 mg/dl, respectively. It has been reported that median levels (5th and 95th percentiles) of IgG and IgG4 from patients with AIP were 2,389 mg/dl (1,349 and 4,310) and 742 mg/dl (265 and 1,150), respectively (3), so high concentrations of IgG in this case prompted us to search for new autoantigens associated with AIP. We also searched for the presence or absence of new autoantibodies in patients with abrupt onset and severe ketoacidosis-prone type 1 diabetes [called fulminant type 1 diabetes (9,10)], which involve the exocrine pancreas and the endocrine pancreas.
RESEARCH DESIGN AND METHODS
Serum used for screening the human pancreas cDNA library was obtained from a 67-year-old male patient (A.O.), who was admitted to our hospital complaining of slight abdominal pain and jaundice. Computed tomography revealed an enlarged pancreas, and laboratory findings showed high concentrations of IgG and IgG4. Tests for anti-lactoferrin and anti-CAII antibodies were both positive, but those for anti-nuclear antibody, anti-mitochondrial antibody, and rheumatoid factor were negative.
Additional AIP sera were obtained from 14 newly diagnosed patients at the University of Yamanashi Hospital and Toranomon Hospital, Tokyo. Diagnosis of AIP was based on criteria proposed by the Japan Pancreas Society (11). Our 15 patients filled criterion 1 (narrowing of the main pancreatic duct or enlargement of pancreas by imaging studies), together with criterion 2 (high serum γ-globulin, IgG, or IgG4 or the presence of autoantibodies, such as anti-nuclear antibodies and rheumatoid factor) and/or criterion 3 (marked interlobular fibrosis and prominent infiltration of lymphocytes and plasma cells in the periductal area). Serum samples were taken from 25 patients with chronic alcoholic pancreatitis, who were diagnosed according to a history of alcohol abuse, impaired exocrine pancreatic function, and the presence of calcified precipitates in the pancreas by imaging studies [Japan Pancreas Society, criteria for chronic pancreatitis 2001 (12)]. Twenty-five serum samples were recruited from patients with pancreas tumor (cancer [n = 8] and intraductal papillary mucinous tumor [IPMT, n = 17]). Fulminant type 1 diabetes (n = 17, 13 cases at the onset and 4 cases after onset) was diagnosed by criteria (fasting C-peptide ≤0.033 nmol/l and A1C ≤8.0% or ∑C-peptide ≤0.540 nmol/l and A1C ≤8.0%) as reported previously (13,14). Fulminant type 1 diabetes associated with pregnancy (15) was excluded from the present study. Acute-onset type 1 diabetes (n = 42) (12) and type 2 diabetes (n = 67) samples were also recruited. The patients’ clinical characteristics are summarized in Table 1. Serum from patients with Hashimoto's thyroiditis (n = 47) were also studied. Diagnosis of the disease was made by elastic goiter and autoantibodies against both thyroglobulin and thyroid peroxidase. Control sera were obtained from 100 (59 male and 41 female) healthy volunteers.
TABLE 1.
Clinical characteristics of subjects
| Type of diabetes | n | Age (years) | Sex (male/female) | Duration of diabetes (months)* | Treatment by insulin* |
|---|---|---|---|---|---|
| Autoimmune pancreatitis | 15 | 66 (58–75) | 14/1 | — | 8 |
| Before PSL | 12 | ||||
| After PSL | 3 | ||||
| Chronic alcoholic pancreatitis | 25 | 63 (53–70) | 18/7 | — | 10 |
| Pancreatic tumor | 25 | 71 (63–73) | 12/13 | — | 8 |
| Cancer | 8 | ||||
| IPMT | 17 | ||||
| Fulminant type 1 diabetes | 17 | 40 (28–53) | 14/3 | 17 | |
| At onset† | 13 | 0.76 ± 0.20 | |||
| After onset | 4 | 13.5 ± 2.38 | |||
| Acute-onset type 1 diabetes | 42 | 25 (23–33) | 14/28 | 29.0 ± 45.0 | 42 |
| At onset† | 22 | 0.7 ± 0.9 | |||
| After onset | 20 | 51.0 ± 50.0 | |||
| Type 2 diabetes | 67 | 62 (58–65) | 43/24 | 130 ± 91.0 | 37 |
| Hashimoto's thyroiditis | 47 | 60 (55–62) | 6/41 | — | — |
| Control subjects | 100 | 47 (40–48) | 59/41 | — | — |
Data are medians (95% CI) or means ± SD. PSL, prednisolone; IPMT, intraductal papillary mucinous tumor.
Duration from the onset of diabetes to the time of sample collection.
At onset; within 3 months after onset.
Immunoscreening.
The λTriplEx2 human pancreas large insert cDNA library (HL5517u) and Escherichia coli XL-1 competent cells were obtained from BD Biosciences Clontech (Palo Alto, CA). The plaques on the plate were transferred to nitrocellulose filters presoaked with 10 mmol/l isopropyl-β-d-thiogalactopyranoside (IPTG), washed with Tris-buffered saline (TBS) containing 0.05% Tween 20 (TBST), and blocked with TBST containing 1% BSA. The filters were incubated overnight at 4°C with the sera from the patient with AIP (A.O.) at a dilution of 1:500. After washing four times with TBST, the filters then reacted with goat horseradish peroxidase–conjugated anti-human IgG (American Qualex, San Clemente, CA) at a dilution of 1:2,000 for 30 min at room temperature. The filters were also washed four times with TBST; positive reaction was detected with 3,3′-diaminobenzidine.
Preparation of the recombinant human AMY-2A.
A cDNA fragment of the positive clone was amplified by PCR with the sense primer, 5′-ATGGGGATCCTTGGGGTTTCGTACCTTCTGACAGA, and antisense primer, 5′-CTTCGAATTCCCAATTTAGATTCAGCATGAATTGC. The PCR product was digested with BamHI and EcoRI and then ligated into pTrc His B (Invitrogen, Carlsbad, CA). After sequencing, the plasmid was transfected into E. coli BL-21 (Novagen, Darmstadt, Germany). The production of the recombinant protein was inducted with 1 mmol/l IPTG and purified by His Bond column chromatography.
Western blot analysis.
The 0.1% SDS–15% PAGE and transferring onto the nitrocellulose membrane was carried out as previously described (16) with slight modifications as follows: The membrane was blocked with 5% skim milk and 5% goat serum in TBS and then incubated with sera from the patients with AIP (1:500) overnight at 4°C. After washing five times with TBST, the membrane was reacted with goat horseradish peroxidase–conjugated anti-human IgG (1:2,000) for 30 min at room temperature. Positive reaction was detected by the same way as described in immunoscreening.
In vitro translation and immunoprecipitation.
A cDNA fragment of AMY-2A was amplified by PCR with the sense primer, 5′-ATGGGGATCCATGTGGGGTTTCGTACCTTCTGACAGA, and antisense primer, 5′-CTTCGAATTCCCAATTTAGATTCAGCATGAATTGC, which added an ATG codon at the NH2-terminus. The PCR product was digested with BamHI and EcoRI and then ligated into pcDNA3.1. 35S-labeled human AMY-2A was prepared with PROTEIN script II (Ambion, Austin, TX) and [35S]methionine (GE Healthcare, Piscataway, NJ). 35S–AMY-2A was incubated with patients’ sera (×100) or anti-human amylase antibody (×100; sc-12821; Santa Cruz Biotechnology, Santa Cruz, CA) in 200 μl PBS containing 1% BSA at 4°C overnight, with 10 μl GammaBind G Sepharose (GE Healthcare) added. After further incubation at room temperature for 60 min, the mixtures were centrifuged at 10,000 rpm for 5 min. The pellets were washed three times with PBS containing 0.05% Tween 20 (PBST). Final pellets were directly counted or dissolved with 10 mmol/l Tris-HCl (pH. 6.8) containing 0.1% SDS, boiled for 3 min, and loaded onto a 0.1% SDS–15% polyacrylamide gel.
Enzyme-linked immunosorbent assay for detecting autoantibody against human AMY-2A.
Autoantibody against human AMY-2A was measured by enzyme-linked immunosorbent assay (ELISA) using methods previously described (5). In brief, a microtiter plate (Coster 3590; Corning, Horseheads, NY) was coated with 50 μl 0.1 μg recombinant human AMY-2A overnight at 4°C. After washing the plate three times with PBST, the plate was incubated with 200 μl 1% BSA in PBS for 30 min. Next, the patients’ sera were tested in triplicate at dilutions of 1:200 in 1% BSA for 1 h. The bound antibody was specially reacted with goat horseradish peroxidase–conjugated anti-human IgG (1:2,000) in 1% BSA for 30 min at room temperature. After washing, the plate was incubated with 100 μl 1-Step Slow TMB-ELISA (Pierce, Rockford, IL) for 30 min. The reaction was terminated by adding 100 μl 1 mol/l H2SO4, and absorbance was determined at an optical density of 450 nm. Intra- and interassay coefficient of variation, determined with the same lot of five ELISA plates, were 4.28 and 7.72%, respectively.
Ethics.
An ethical committee approved all study protocols, and patients gave informed consent.
Statistical analysis.
Statistical analysis was carried out using Fisher's exact test (JMP, Cary, NC), in which we considered statistically significant if P values were <0.05. Receiver operating characteristic (ROC) analysis was carried out with MedCalc (MedCalc Software, Mariakerke, Belgium).
RESULTS
Cloning of cDNAs from human pancreas.
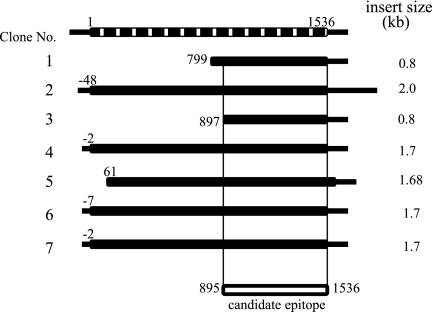
We completely screened 2 × 104 plaques with the AIP patient's serum (A.O.) and obtained 10 positive clones. Nucleotide sequencing of the insert cDNAs and a subsequent homology search revealed that 7 of 10 clones were identical to human amylase-2A (AMY-2A). When compared with the nucleotide sequence of the human AMY-2A cloned by Wise et al. (17), four of seven clones contained the full coding sequence, whereas the 5′ ends of the other three clones started from 61, 799, and 897 bp (A in ATG is designated as 1) (Fig. 1). Other nonamylase clones were those of the housekeeping genes, such as the heat shock protein and the nuclear protein.
FIG. 1.
Cloning of human amylase α-2A cDNAs from λTriplEx2 human pancreas cDNA library. Seven clones of human amylase α-2A cDNAs. Their lengths and 5′-ends are shown (A in ATG is designated as 1). The top bar indicates human amylase α-2A cDNA as reported by Wise et al. (17), and the common regions shared by all seven clones, from codons 299 to 512, are shown in the bottom bar.
Western blot analysis, immunoprecipitation, and ELISA system for detecting anti-human AMY-2A.
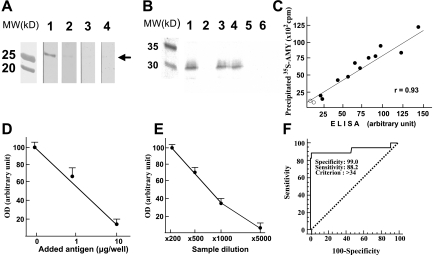
Because IgG from the AIP patient used for screening recognized four different lengths of human AMY-2A clones, we hypothesized that the regions shared by these four clones, from codons 299 to 512, might contain a common epitope for the patient's IgG (Fig. 1). Therefore, we produced histidine-tagged human AMY-2A from codons 299 to 512 (AMY-2A/299–512) in E. coli BL21 and carried out Western blot analysis (Fig. 2A). Patient's serum (A.O.) recognized the 30-kDa recombinant protein (line 1), but sera from healthy volunteers did not (lines 3 and 4). When the patient's serum was preincubated with the recombinant protein, positive staining was abolished (line 2), suggesting that the autoantibody reacted with the recombinant protein, which contains the epitope.
FIG. 2.
Western blot analysis and ELISA for detecting anti-human AMY-2A. A: Western blot analysis. Recombinant human AMY-2A (50 ng) from codons 299 to 512 (AMY-2A/299–512) was electrophoresed in 0.1% SDS–15% polyacrylamide and transferred onto a nitrocellulose filter. The filters reacted with serum (×1,000) from an AIP patient (line 1) and normal control sera (lines 3 and 4). Line 2, AIP patient's serum preincubated with 1 μg/ml AMY-2A/299–512. MW, molecular weight markers. B: Immunoprecipitation of 35S–AMY-2A with antibodies. 35S–AMY-2A was incubated with goat anti-amylase (line 1), normal goat IgG (line 2), sera from AIP patients (lines 3 and 4), and sera from healthy volunteers (lines 5 and 6) and then precipitated with protein G–sepharose. The pellets were electrophoresed in 0.1% SDS–15% polyacrylamide and analyzed with Bas 2000 image analyzer (Fujix, Tokyo). C: Correlation between the result of ELISA and that of immunoprecipitation. By coating the recombinant human AMY-2A/299–512, we developed an ELISA system for detecting anti-human AMY-2A. Sera from 11 patients with AIP (•) and two normal control subjects (○) were assayed by ELISA and immunoprecipitation for detecting the autoantibody. D: Absorption of positive ELISA signal with recombinant AMY-2A. One milliliter of a patient's serum (1:500) was preincubated with the recombinant protein at the indicated dose overnight at 4°C, and then the serum was used as the first antibody. The data are the mean of triplicate values. OD, optical density. E: Serum dilution experiment in ELISA assay. Positive serum from patient A.O. was diluted as indicated, and ELISA assay was carried out. The data are the mean of triplicate values. F: ROC analysis of the healthy volunteers and fulminant type 1 diabetic patients. We carried out ROC analysis of the healthy volunteers (n = 100) and fulminant type 1 diabetic patients (n = 17) with MedCalc.
Anti-human AMY-2A antibody produced in goat was bound to the in vitro–translated 35S–AMY-2A and was precipitated by protein G–sepharose (Fig. 2B). IgG from two patients with AIP also bound to the labeled protein and was precipitated, but the IgG from two healthy volunteers did not (Fig. 2B). This recombinant fluid phase autoantibody assay with in vitro transcription and translation of AMY-2A without additional amino acids, such as His-Tag, confirmed the specificity of the autoantibody against the protein.
Next, by coating the protein onto the plate, we developed an ELISA system for detecting anti-amylase antibodies in the serum. When compared with the normal serum, patient sera showed strong signals, which were well correlated with immunoprecipitated 35S–AMY-2A by protein G–sepharose (Fig. 2C). This positive reaction in ELISA was displaced in a concentration-dependent fashion by AMY-2A/299–512 (Fig. 2D). When the AIP patient's serum (A.O.) was diluted, we could detect positive signals up to ×1,000 dilution (Fig. 2E). To obtain a cutoff value for positivity, we carried out ROC analysis of the healthy volunteers (n = 100) and fulminant type 1 diabetic patients (n = 17) (Fig. 2F). Table 2 shows criterion values and coordinates of the ROC curve. When the value was set as 34 (area under the ROC curve 0.92; significance level P = 0.0001), sensitivity, specificity, and positive predictive value were 88.24, 99.0, and 93.7%, respectively.
TABLE 2.
Criterion values and coordinates of the ROC curve
| Criterion | Sensitivity (%) | Specificity (%) | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| ≥3.2 | 100.00 (80.3–100.0) | 0.00 (0.0–3.7) | 14.5 | |
| >11.4 | 100.00 (80.3–100.0) | 9.00 (4.2–16.4) | 15.7 | 100 |
| >11.8 | 94.12 (71.2–99.0) | 9.00 (4.2–16.4) | 15.0 | 90.0 |
| >17.3 | 94.12 (71.2–99.0) | 53.00 (42.8–63.1) | 25.4 | 98.1 |
| >17.5 | 88.24 (63.5–98.2) | 55.00 (44.7–65.0) | 25.0 | 96.5 |
| >34.0* | 88.24 (63.5–98.2) | 99.00 (94.5–99.8) | 93.7 | 98.0 |
| >34.7 | 82.35 (56.6–96.0) | 99.00 (94.5–99.8) | 93.3 | 97.1 |
| >35.2 | 82.35 (56.6–96.0) | 100.0 (96.3–100.0) | 100.0 | 97.1 |
| >98.4 | 0.00 (0.0–19.7) | 100.0 (96.3–100.0) | 85.5 |
Data in parentheses are 95% CI.
Cutoff value for positivity.
Prevalence of autoantibody against human AMY-2A in AIP patients.
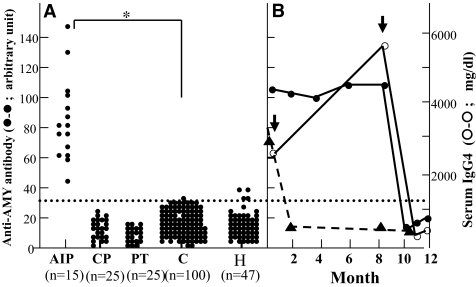
Using the ELISA system, we determined the prevalence of autoantibody against human AMY-2A in AIP patients and various pancreatic diseases (Fig. 3). All 15 IgGs from patients with AIP were positive for AMY-2A/299–512, whereas 1 of 100 IgGs from control subjects was positive for the antibody (P < 0.001, Fisher's exact test). All the IgGs from the patients with chronic alcoholic pancreatitis (n = 25) or with pancreas tumor (pancreatic cancer, n = 8; IPMT, n = 17) were negative for the antigen. Antibodies were detected in 9% (4/47) of patients with Hashimoto's thyroiditis, a representative organ-specific autoimmune disease (Fig. 3A).
FIG. 3.
Prevalence of autoantibody against human AMY-2A in patients with various pancreatic diseases. A: Prevalence of autoantibody against human AMY-2A in patients with AIP (n = 15), chronic alcoholic pancreatitis (CP, n = 25), pancreatic tumor (PT, n = 25), control subjects from healthy volunteers (C, n = 100), and Hashimoto's thyroiditis (H, n = 47) was examined by ELISA, as described in research design and methods. The data are the mean of triplicate values. The dotted line shows a cutoff value. Fisher's exact test was carried out between AIP and control groups. *P < 0.001. B: Time course of anti-AMY antibody and IgG4 of AIP patients. AIP patient (A.O.), whose IgG was used to screen λTriplEx2 human pancreas cDNA library, was treated with prednisolone (arrow). Before and after the treatment, anti-AMY antibody (•-•) and IgG4 (○-○) were measured. In the other AIP patient (T.M.), titer of the anti-AMY antibody (▴-▴) was also measured before and after prednisolone treatment (arrow).
Figure 3B shows the time course of the autoantibody titer from two AIP patients before and after prednisolone treatment. In patient A.O., IgG4 gradually increased and reached 5,540 mg/dl, but administration of prednisolone initiated a rapid decrease of IgG4 to 571 mg/dl. Before prednisolone treatment, the titer of the autoantibody against AMY remained high, and prednisolone treatment induced a rapid decrease of the titer of AMY-2A autoantibody to a normal level. The fall rate of the antibody titer seemed to be parallel to that of serum IgG4. In patient T.M., administration of prednisolone also rapidly decreased the titer of the autoantibody against AMY. The autoantibodies did not increase even at the drug maintenance dose in both cases.
Prevalence of autoantibody against human AMY-2A in patients with fulminant type 1 diabetes and acute-onset type 1 diabetes.
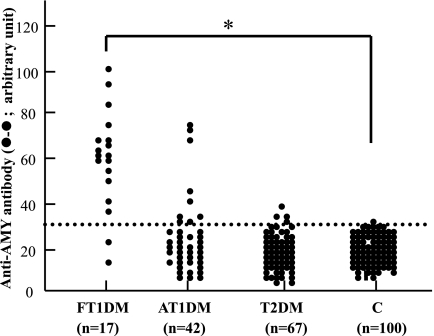
We next studied the prevalence of autoantibody against human AMY-2A in various types of diabetic patients (fulminant type 1 diabetes, n = 17; acute-onset type 1 diabetes, n = 42; and type 2 diabetes, n = 67; Fig. 4). Interestingly, 88% of patients with fulminant type 1 diabetes were positive for the autoantibody, but 1% of control was positive for the antibody (P < 0.001, Fisher's exact test). The autoantibody was detected with low frequency in patients with acute-onset type 1 diabetes (21%, 9 of 42) and patients with type 2 diabetes (6%, 4 of 67).
FIG. 4.
Prevalence of autoantibody against human AMY-2A in patients with various types of diabetes. Prevalence of autoantibody against human AMY-2A (•) in patients with fulminant type 1 diabetes (FT1DM, n = 17), acute-onset type 1 diabetes (AT1DM, n = 42), type 2 diabetes (T2DM, n = 67), and control subjects from healthy volunteers (C, n = 100) was studied by ELISA, as described in research design and methods. The data are the mean of triplicate values. The dotted line shows a cutoff value. Fisher's exact test was carried out between fulminant type 1 diabetic and control groups. *P < 0.001.
DISCUSSION
In 2002, Barera et al. (18) reported a case of an 11-year-old girl with celiac disease and hypothyroidism. Because of hyperamylasemia, she was suspected to have chronic pancreatitis, but no pancreatic damage was demonstrated. By using ELISA to detect autoantibodies to amylase, they found that she produced an autoantibody against porcine amylase and that this declined after the institution of a gluten-free diet. In the present study, we also detected an autoantibody against pancreas-specific AMY-2A in all of the AIP patients, but not in patients with chronic alcoholic pancreatitis and with pancreatic tumors.
The presence of autoantibodies against CAII, lactoferrin, and pancreatic secretory trypsin inhibitor (PSTI) has been reported (3,4,19). However, the distribution of these molecules is non–organ specific (20–22), and the prevalence of these autoantibodies against CAII, lactoferrin, and PSTI in AIP is rather low, ranging from 42∼73% (3,4,19). Using 13 serum samples from our AIP patients, we carried out ELISA assays for autoantibodies against CAII and lactoferrin. As a result, 66% (10 of 15) were positive for CAII, and 53% (8 of 15) were positive for lactoferrin. Thus, an autoantibody against AMY-2A might be a more sensitive marker for AIP than that of CAII, lactoferrin, or PSTI.
Furthermore, the adoptive transfer of amylase-specific CD4+ T-cells to rats was able to confer pancreatitis, whereas the transfer experiment with lactoferrin-specific or CAII-specific CD4+ T-cells failed to induce experimental pancreatitis (23). Our findings of a high prevalence of autoantibody against AMY-2A in human AIP and the results from the adoptive transfer experiment of amylase-specific CD4+ T-cells to rodents suggest that cellular and/or humoral autoimmunity against AMY-2A plays some role in the pathogenesis of AIP.
Approximately 80% of patients with chronic pancreatitis are alcoholic, the pathogenesis of which still remains unclear. However, it is well known that acute or chronic alcohol exposure suppresses all branches of the immune system (24), and none of our sera from patients with chronic alcoholic pancreatitis were positive for autoantibody against AMY-2A (Fig. 3). Therefore, an assay for autoantibody against AMY-2A is useful for distinguishing AIP from chronic alcoholic pancreatitis.
It is of particular interest that anti–AMY-2A autoantibody is detected in 88% of patients with fulminant type 1 diabetes. Fulminant type 1 diabetes is a recently proposed subtype of type 1B, nonimmune-mediated, or idiopathic type 1 diabetes (9,10). A nationwide survey revealed that fulminant diabetes accounted for ∼20% of Japanese type 1 diabetes with ketosis or ketoacidosis and flu-like symptoms frequently observed at onset (25). Clinical characteristics of this subtype of type 1 diabetes are 1) remarkably abrupt onset of disease; 2) very short (<1 week) duration of diabetic symptoms; 3) severe ketoacidosis at diagnosis; 4) negative status of islet-related autoantibodies, such as GADAb and anti–IA-2 antibody; 5) virtually no C-peptide secretion (10 μg/day in urine); and 6) elevated serum pancreatic enzyme levels (26). These features and the absence of insulitis in patients’ pancreases have led some to hypothesize that an autoimmune mechanism does not contribute to the development of fulminant type 1 diabetes, but rather that viral infection plays a central role in the pathogenesis of the disease (27). However, we previously demonstrated CD4+ and CD8+ T-cell infiltration to pancreatic exocrine cells and to the islet in an autopsy case deceased immediately after the onset of fulminant type 1 diabetes (28).
Imagawa and Hanafusa (27) also confirmed cellular infiltration of pancreatic islets in patients with fulminant type 1 diabetes. Shimada et al. (29) described a fulminant type 1 diabetic patient with a high serum level of CXCL10, a chemokine that induces migration of activated T-cells to local lesions and GAD-reactive CD4+ cells in the periphery. These results, and the presence of an autoantibody against AMY-2A, suggest that the disease might be autoimmune-related, involving the exocrine and the endocrine pancreas (10,28).
Exocrine dysfunction and impaired glucose tolerance are common features for both AIP and fulminant type 1 diabetes. With regard to the HLA genotype, Kawa et al. (30) demonstrated that the DRB1*0405-DQB1*0401 haplotype is closely associated with AIP in the Japanese population, and Tanaka et al. (31) revealed that the DQA1*0303-DQB1*0401 haplotype is strongly associated with fulminant type 1 diabetes in a homologous manner. When we studied the frequency of this allele in our patients with AIP, 5 of 15 patients were heterozygous for the DRB1*0405-DQB1*0401 haplotype. Although further study with larger sample sizes will be needed, these two reports and our own analysis suggest the importance of the DQB1*0401 allele in both diseases. Furthermore, we are able to detect autoantibody against AMY-2A in both with nearly the same prevalence. Although further investigation is needed, the present results suggest that clinically and immunologically, AIP and fulminant type 1 diabetes are closely related to one another.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
We acknowledge T. Hugh for his editorial work.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 520.
REFERENCES
- 1.Sarles H, Sarles JC, Muratore R, Guien C: Chronic inflammatory sclerosis of the pancreas: an autonomous pancreatic disease. Am J Dig Dis 6: 688–698, 1961 [DOI] [PubMed] [Google Scholar]
- 2.Okazaki K, Chiba T: Autoimmune related pancreatitis. Gut 51: 1–4, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K: High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344: 732–738, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Okazaki K, Uchida K, Chiba T: Recent concept of autoimmune-related pancreatitis. J Gastroenterol 36: 293–302, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Kobayashi T, Nakanishi K, Okubo M, Murase T, Hashimoto M, Takeuchi K: Corticosteroid responsive diabetes mellitus associated with autoimmune pancreatitis. Lancet 356: 910–911, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka S, Kobayashi T, Nakanishi K, Okubo M, Murase T, Hashimoto M, Watanabe G, Matsushita H, Endo Y, Yoshizaki H, Kosuge T, Sakamoto M, Takeuchi K: Evidence of primary β-cell destruction by T-cells and β-cell differentiation from pancreatic ductal cells in diabetes associated with active autoimmune chronic pancreatitis. Diabetes Care 24: 1661–1667, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Okazaki K: Clinical relevance of autoimmune-related pancreatitis. Best Pract Res Clin Gastroenterol 16: 365–378, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Finkelberg DL, Sahani D, Deshpande V, Brugge WR: Autoimmune pancreatitis. N Engl J Med 355: 2670–2676, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T: Immunology and immunogenetics of type 1 diabetes in Japan. IDF Bull 35: 34–37, 1990 [Google Scholar]
- 10.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y: A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies: Osaka IDDM Study Group. N Engl J Med 342: 301–307, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, Shimosegawa T, Koizumi M, Suda K, Shiratori K, Yamaguchi K, Yamaguchi T, Sugiyama M, Otsuki M, Research Committee of Intractable Diseases of the Pancreas: Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol 41: 626–631, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otsuki M: Chronic pancreatitis in Japan: epidemiology, prognosis, diagnostic criteria, and future problems. J Gastroenterol 38: 315–326, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hanafusa T, Imagawa A: Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab 3: 36–45, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Endo T, Aida K, Shimura H, Yokomori N, Kaneshige M, Furuya F, Amemiya S, Mochizuki M, Nakanishi K, Kobayashi T: Distinct diagnostic criterias of fulminant type 1 diabetes based on serum C-peptide response and HbA1c levels at onset. Diabetes Care 27: 1936–1941, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Shimizu I, Makino H, Imagawa A, Iwahashi H, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Maruyama T, Hanafusa T: Clinical and immunogenetic characteristics of fulminant type 1 diabetes associated with pregnancy. J Clin Endocrinol Metab 91: 471–476, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Endo T, Ohno M, Kotani S, Onaya T: Thyrotropin receptor in non-thyroid tissues. Biochem Biophys Res Commun 190: 774–779, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Wise RJ, Karn RC, Larsen SH, Hodes ME, Gardell SJ, Rutter WJ: A complementary DNA sequence that predicts a human pancreatic amylase primary structure consistent with the electrophoretic mobility of the common isozyme, Amy2 A. J Mol Biol Med 2: 307–332, 1984 [PubMed] [Google Scholar]
- 18.Barera G, Bazzigaluppi E, Viscardi M, Renzetti F, Bianchi C, Chiumello G, Bosi E. Macroamylasemia attributable to gluten-related amylase autoantibodies: a case report. Pediatrics 107: E93, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Asada M, Nishio A, Uchida K, Kido M, Ueno S, Uza N, Kiriya K, Inoue S, Kitamura H, Ohashi S, Tamaki H, Fukui T, Matsuura M, Kawasaki K, Nishi T, Watanabe N, Nakase H, Chiba T, Okazaki K: Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 33: 20–26, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M: Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med 313: 139–145, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Neville MC, Chatfield K, Hansen L: Lactoferrin secretion into mouse milk: development of secretory activity, the localization of lactoferrin in the secretory pathway, and interactions of lactoferrin with milk iron. Adv Exp Med Biol 443: 141–153, 1998 [PubMed] [Google Scholar]
- 22.Fukayama M, Hayashi Y, Koike M: Immunohistochemical localization of pancreatic secretory trypsin inhibitor in fetal and adult pancreatic and extrapancreatic tissues. J Histochem Cytochem 34: 227–235, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Davidson TS, Longnecker DS, Hickey WF: An experimental model of autoimmune pancreatitis in the rat. Am J Pathol 166: 729–736, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messingham KA, Faunce DE, Kovacs EJ: Alcohol, injury, and cellular immunity. Alcohol 28: 137–149, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Toyoda T, Maruyama T, Makino H: Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 26: 2345–2352, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y: A proposal for three distinct subtypes of diabetes mellitus based on clinical and pathological evidence. Ann Med 32: 539–543, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Imagawa A, Hanafusa T: Pathogenesis of fulminant type 1 diabetes. Rev Diabet Stud 3: 169–177, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka S, Kobayashi T, Momotsu T: A novel subtype of type 1 diabetes mellitus. N Engl J Med 342: 1835–1837, 2000 [PubMed] [Google Scholar]
- 29.Shimada A, Morimoto J, Komada K, Suzuki R, Oikawa Y, Funae O, Kasuga A, Saruta T, Narumi S: Elevated serum IP-10 levels observed in type 1 diabetes. Diabetes Care 24: 510–515, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S, Hasebe O, Kiyosawa K: HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology 122: 1264–1269, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Kobayashi T, Nakanishi K, Koyama R, Okubo M, Murase T, Odawara M, Inoko H: Association of HLA-DQ genotype in autoantibody-negative and rapid-onset type 1 diabetes. Diabetes Care 25: 2302–2307, 2002 [DOI] [PubMed] [Google Scholar]