Abstract
OBJECTIVE— Some obese youth with a clinical diagnosis of type 2 diabetes have evidence of islet cell autoimmunity with positive autoantibodies. In this study, we investigated the differences in insulin sensitivity and secretion between autoantibody-negative (Ab−) and -positive (Ab+) youth with clinically diagnosed type 2 diabetes in comparison with control subjects.
RESEARCH DESIGN AND METHODS— Sixteen Ab− and 26 Ab+ clinically diagnosed type 2 diabetic patients and 39 obese control youth underwent evaluation of insulin sensitivity (3-h hyperinsulinemic-euglycemic clamp), substrate oxidation (indirect calorimetry), first- and second-phase insulin secretion (2-h hyperglycemic clamp), body composition and abdominal adiposity (dual energy X-ray absorptiometry and computed tomography scan, respectively), and glucose disposition index (first-phase insulin secretion × insulin sensitivity).
RESULTS— Insulin-stimulated total, oxidative, and nonoxidative glucose disposal, and suppression of fat oxidation during hyperinsulinemia were significantly lower in Ab− compared with Ab+ clinically diagnosed type 2 diabetic and control subjects with no difference between the latter two. First- and second-phase insulin secretion and C-peptide were lower in Ab+ compared with Ab− type 2 diabetes. Glucose disposition index was not different between the Ab− and Ab+ clinically diagnosed type 2 diabetic patients, but both were significantly lower than that in control subjects. Systolic blood pressure and alanine aminotransferase were higher in Ab− versus Ab+ clinically diagnosed type 2 diabetic patients, whereas the frequency of ketonuria at diagnosis was higher in Ab+ versus Ab− patients.
CONCLUSIONS— Islet-cell Ab− clinically diagnosed type 2 diabetic youth are characterized by severe insulin resistance and relative insulin deficiency, whereas Ab+ youth have severe insulin deficiency and β-cell failure. The former group has additional features of insulin resistance. These important metabolic differences could influence the natural history of hyperglycemia, insulin dependence, and clinical outcomes in these youth.
The clinical presentation of type 2 diabetes in youth is diverse, from minimal symptomatology to severe clinical manifestations with evidence of hyperglycemia with or without ketosis (1). Diabetes in humans is classified into two main types: type 1 diabetes, where the pathophysiology is autoimmune destruction of the pancreatic β-cells; and type 2 diabetes, where insulin resistance is central to the disease process together with a nonimmune-mediated β-cell failure relative to insulin resistance (2). Limited data in the pediatric literature suggest that the pathophysiology of youth type 2 diabetes is a combination of severe insulin resistance and relative insulin deficiency (3–6).
The diagnosis of youth type 2 diabetes is typically made using clinical criteria where obesity is the major diagnostic entity (7). However, with the increasing rates of obesity in childhood, particularly in children with type 1 diabetes, this clinical distinction has become ever more difficult and imperfect (8,9). A number of youth with a clinical diagnosis of type 2 diabetes have evidence of islet-cell autoimmunity, with autoantibodies present in 10–75% of patients (10–15). Several theories and terminologies have been proposed, such as hybrid diabetes, double diabetes, diabetes type 1.5, and latent autoimmune diabetes of youth, to refer to and to try to explain the underlying pathophysiology in this subset of young patients with a clinical phenotype consistent with type 2 diabetes and evidence of autoimmunity consistent with type 1 diabetes (8,13,15–18). Efforts to identify distinguishing features of antibody-positive (Ab+) and -negative (Ab−) clinically diagnosed type 2 diabetes in youth, which are typically focused on clinical features such as obesity, acanthosis nigricans, symptoms at diagnosis, ketonuria, A1C, and insulin requirements, have not revealed any unique distinctive features that would differentiate one from the other (10,12,15). To our knowledge, no data exist in the pediatric literature regarding the metabolic characteristics of youth with a clinical diagnosis of type 2 diabetes with versus without islet cell antibodies. Therefore, the aim of the present investigation was to test the hypothesis that youth with clinically diagnosed type 2 diabetes and positive islet cell antibodies have greater impairment of β-cell function and are less insulin resistant than their peers with clinically diagnosed type 2 diabetes who are autoantibody negative. Our objectives were 1) to compare in vivo insulin sensitivity and secretion in Ab− versus Ab+ youth with clinically diagnosed type 2 diabetes and in obese control subjects and 2) to assess whether differences exist between the two groups of clinically diagnosed type 2 diabetic patients in clinical and/or laboratory features at the time of diagnosis or during the research evaluation.
RESEARCH DESIGN AND METHODS
Study population.
Forty-two obese adolescents with a clinical diagnoses of type 2 diabetes made by the attending endocrinologist were recruited from the Diabetes Center at Children's Hospital of Pittsburgh. Screening for islet cell antibodies (details below) revealed 16 with negative antibodies and 26 with positive antibodies. Some of the Ab+ patients were initially screened for the Treatment Options of Type 2 Diabetes in Adolescents and Youth Trial (TODAY) (Children's Hospital of Pittsburgh is one of 15 participating centers in TODAY) (19) and found to be ineligible because of the presence of islet cell antibodies. Some of the Ab− patients later participated in TODAY. The control group consisted of 39 age-matched obese but otherwise healthy adolescents who were recruited from the community through a local newspaper advertisement and flyers posted on bus routes and the medical campus. Thirteen of the 42 subjects with type 2 diabetes and all of the obese control subjects were reported previously (4,20). Pubertal development was assessed by physical examination according to Tanner criteria (21). At the time of study participation, 9 subjects with clinically diagnosed type 2 diabetes were receiving lifestyle modification and no medications, 6 patients were on insulin alone, 13 were on metformin alone, and 14 were on insulin together with metformin (Table 1). This type of therapy is typical of pediatric diabetes clinical practices nationwide (22,23). Besides the type 2 diabetes treatment, none of the participants were receiving any medication that may have impacted glucose metabolism, and none of the females were on oral contraceptive pills at the time of the study. The characteristics of the study population are summarized in Table 1. All studies were approved by the institutional review board of the University of Pittsburgh. Informed consent and assent were obtained from each participant and their legal guardians. Clinical and laboratory characteristics of type 2 diabetic youth at the time of diagnosis, including presence of symptoms, ketones, glucose levels, A1C, and treatment modality and/or insulin use at diagnosis were obtained from the medical records.
TABLE 1.
Characteristics of islet cell Ab− and Ab+ obese youth clinically diagnosed with type 2 diabetes and obese control subjects
| Clinically diagnosed type 2 diabetic patients
|
Obese control subjects |
P value
|
|||
|---|---|---|---|---|---|
| Ab− | Ab+ | ANOVA | Post hoc | ||
| n | 16 | 26 | 39 | ||
| Age (years) | 14.7 ± 0.4 (10.1–17.2) | 14.9 ± 0.4 (10.2–18.3) | 14.1 ± 0.2 (12.3–17.2) | NS | NS |
| Sex (male/female) | 7/9 | 11/15 | 23/16 | NS | |
| Ethnicity (African American/Caucasian) | 6/10 | 13/13 | 20/19 | NS | |
| Tanner stages | |||||
| II-III | 2 | 3 | 10 | NS | |
| IV-V | 14 | 23 | 29 | ||
| BMI (kg/m2) | 36.0 ± 1.3 (28.3–45.4) | 33.4 ± 1.2 (24.5–48.9) | 36.0 ± 0.9 (28.0–49.6) | NS | NS |
| Body fat (%) | 40.6 ± 1.8 (24.9–48.4) | 40.8 ± 1.5 (22.6–52.9) | 42.3 ± 0.8 (32.1–51.1) | NS | NS |
| VAT (cm2) | 79.0 ± 6.9 (47.1–142.8) | 72.8 ± 8.6 (26.5–242.4) | 76.1 ± 5.9 (16.7–161.4) | NS | NS |
| Subcutaneous adipose tissue (cm2) | 520.1 ± 40.7 (291.6–776.1) | 491.5 ± 29.8 (264.9–764.4) | 556.1 ± 23.9 (321.2–890.1) | NS | NS |
| Diabetes duration (months) | 4.4 ± 1.4 (0.05–18.0) | 9.0 ± 2.1 (1.0–39.0) | NA | NS | |
| A1C (%) | 6.8 ± 0.2 (4.7–8.3) | 6.5 ± 0.2 (5.1–8.0) | 5.3 ± 0.1 (4.3–6.2) | <0.001 | NS |
| Treatment modality [n (%)] | NA | ||||
| Lifestyle modification | 4 (25) | 5 (19.2) | NS | ||
| Metformin | 6 (37.5) | 7 (26.9) | NS | ||
| Insulin | 2 (12.5) | 4 (15.4) | NS | ||
| Insulin and metformin | 4 (25) | 10 (38.5) | NS | ||
Data are means ± SEM (range) or n (%). NA, not applicable. Post hoc P value, Bonferonni correction for Ab− vs. Ab+ clinically diagnosed type 2 diabetic patients. χ2 analyses with respect to ethnicity, sex, Tanner stage, and treatment modality.
Autoantibody testing.
Glutamic acid decarboxylase 65-kDa autoantibody (GAD65-Ab) and insulinoma-associated protein-2 autoantibody (IA2 Ab) measurements were performed at the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington (Seattle, WA). This is the same reference laboratory with identical antibody determination for the National Institutes of Health (NIH)-funded multicenter TODAY study (19). Briefly, both GAD and IA2 were determined by a radioligand-binding assay, using either GAD65 or IA2 proteins produced by an in vitro transcription and translation system and labeled with [35S]methionine. Levels of both GAD65-Ab and IA2 Ab were expressed as indexes, with the cutoff for positivity set at the 99th percentile of normal. Subjects with GAD65-Ab index >0.085 and/or IA2 Ab index >0.018 were considered to be GAD65-Ab+ and/or IA2-Ab+, respectively. Patients were considered to be Ab− if both antibody indexes were below the cutoff. These are the same cutoffs used in TODAY (19).
Clamp studies.
Participants were admitted twice within a 1- to 4-week period (except for three control subjects within 6–8 weeks) to the Pediatric Clinical and Translational Research Center the day before the clamp studies: once for a hyperinsulinemic-euglycemic clamp and once for a hyperglycemic clamp— in random order. Each clamp evaluation was performed after a 10- to 12-h overnight fast. Metformin and long-acting insulin were discontinued 48 h before the clamp studies as reported previously (4). For each study, two intravenous catheters were inserted after the skin and subcutaneous tissues were anesthetized with eutectic mixture of local anesthetics cream (Astra Pharmaceutical Products, West Borough, MA). One catheter was placed in a vein on the forearm for administration of glucose, insulin, and stable isotopes; the second catheter was placed in a vein in the dorsum of the contralateral heated hand for sampling of arterialized venous blood (4).
In vivo glucose metabolism and insulin sensitivity.
Fasting endogenous hepatic glucose production (HGP) was measured with a primed (2.2 μmol/kg) constant rate infusion of [6,6-2H2]glucose (0.31 ± 0.01 μmol · kg−1 · min−1 in type 2 diabetic patients and 0.30 ± 0.01 μmol · kg−1 · min−1 in obese control subjects) (Isotech, Miamisburg, OH) from 7:30–9:30 a.m., as we described previously (4). Blood was sampled at the start of the stable isotope infusion (−120 min) and every 10 min from −30 to 0 min (basal period) for determination of plasma glucose, insulin, and isotopic enrichment of glucose. Calculations for fasting HGP were made over the last 30 min of the 2-h isotope infusion (−30 to 0 min).
After the 2-h baseline isotope infusion period, insulin-mediated glucose metabolism and in vivo insulin sensitivity were measured during a 3-h hyperinsulinemic-euglycemic clamp from 9:30 a.m.-12:30 p.m., in conjunction with indirect calorimetry. Intravenous crystalline insulin (Humulin Regular; Lilly, Indianapolis, IN) was infused at a constant rate of 80 mU · m−2 · min−1, and plasma glucose was clamped at 5.5 mmol/l with a variable rate infusion of 20% dextrose as before (4,20). Continuous indirect calorimetry by a ventilated hood system (Deltatrac Metabolic Monitor; Sensormedics, Anaheim, CA) was performed to measure CO2 production, O2 consumption, and respiratory quotient for 30 min at baseline and at the end of the clamp (4).
In vivo insulin secretion.
First- and second-phase insulin secretion were assessed during a 2-h hyperglycemic clamp (12.5 mmol/l) performed from 9:00–11:00 a.m. as described previously (4). Glucose, insulin, and C-peptide concentrations were measured every 2.5 min (at 2.5, 5, 7.5, 10, and 12.5 min, first phase) and then every 5 min for glucose and every 15 min for insulin and C-peptide. Before the start of the clamp, a fasting blood sample was obtained for total HDL, LDL, and VLDL cholesterol, triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase measurements.
Body composition.
Body composition was determined by dual energy X-ray absorptiometry. Subcutaneous abdominal adipose tissue and visceral adipose tissue (VAT) were examined by a single-slice computed tomography (CT) scan at L4-L5 as described previously (4,20).
Blood pressure determination.
Blood pressure was measured when the subjects were resting in the supine position in bed. Measurements were performed with an automated sphygmomanometer every 10 min for 1 h between 10:00 and 11:00 p.m. before the subject fell asleep and between 6:00 and 7:00 a.m. before awakening. The mean of seven measurements during each hour was the outcome for statistical analysis (24).
Biochemical measurements.
Plasma glucose was measured with a glucose analyzer (Yellow Springs Instrument, Yellow Springs, OH), and insulin and C-peptide were measured by radioimmunoassay as described previously (4,25). A1C was measured by high performance liquid chromatography (1998; Tosoh Medics) and lipids using the standards of the Centers for Disease Control and Prevention (4,25). Deuterium enrichment of glucose in the plasma was determined on a Hewlett-Packard 5973 mass spectrometer (Palo Alto, CA) coupled to a 6890 gas chromatograph as reported previously (20).
Calculations.
Fasting HGP was calculated during the last 30 min of the 2-h isotope infusion according to steady-state tracer dilution equations (24,25). Insulin-stimulated glucose disposal rate (Rd) was calculated during the last 30 min of the euglycemic clamp to be equal to the rate of exogenous glucose infusion. Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin level (24,25). Insulin-stimulated carbohydrate and fat oxidation rates were calculated according to the formulas of Frayn (20,24) from the indirect calorimetry data. Nonoxidative glucose disposal was estimated by subtracting the rate of glucose oxidation from the total Rd. During the hyperglycemic clamp, the first- and second-phase insulin and C-peptide concentrations were calculated as described previously (4,25). Glucose disposition index (GDI) was calculated as the product of insulin sensitivity × first-phase insulin.
Statistical analysis.
Differences in continuous variables between Ab+ and Ab− clinically diagnosed type 2 diabetic and control subjects were tested with either a one-way ANOVA or the nonparametric Kruskall-Wallis test, based on the nonviolation of statistical assumptions. Differences in categorical variables were tested using the χ2 test or the Fisher's exact test. All statistical assumptions were met. Data are presented as mean ± SE unless otherwise indicated. Statistical significance was set at P < 0.05. All statistical analyses were run using SPSS software (SPSS, Chicago).
RESULTS
Study participants.
The clinical and physical characteristics of the study participants are depicted in Table 1. The three groups were comparable with respect to age, sex, ethnicity, pubertal development, BMI, percent body fat, and abdominal VAT and subcutaneous adipose tissue. As expected, A1C was significantly higher in clinically diagnosed type 2 diabetic patients compared with control subjects but not different between Ab− and Ab+ groups. Similarly, duration of diabetes was not different between Ab− and Ab+ groups.
Fasting metabolic profile.
Fasting plasma glucose levels were not significantly different between Ab− and Ab+ groups but were significantly higher than in obese control subjects. Fasting insulin level was lowest and HGP was highest in the Ab+ group (Table 2). Fasting lipids were not significantly different between Ab− and Ab+ groups. Rates of glucose and fat oxidation were not different between the three groups. Baseline free fatty acids were higher in the diabetic subjects compared with control subjects but not different between Ab+ and Ab− groups (Table 2).
TABLE 2.
Fasting metabolic profile of islet cell Ab− and Ab+ obese youth clinically diagnosed with type 2 diabetes and obese control subjects
| Clinically diagnosed type 2 diabetic patients
|
Obese control subjects |
P value
|
|||
|---|---|---|---|---|---|
| Ab− | Ab+ | ANOVA | Post hoc | ||
| n | 16 | 26 | 39 | ||
| Glucose (mmol/l) | 6.6 ± 0.4 (4.6–9.5) | 7.4 ± 0.4 (5.0–13.5) | 5.4 ± 0.1 (4.8–6.2) | <0.001 | NS |
| Insulin (pmol/l) | 274 ± 37 (77–690) | 183 ± 20 (49–472) | 249 ± 18 (95–573) | 0.03 | 0.05 |
| HGP (μmol · kg−1 · min−1) | 13.3 ± 0.6 (10.1–18.0) | 16.1 ± 1.1 (9.2–34.1) | 13.1 ± 0.5 (9.2–25.5) | 0.02 | NS |
| Glucose oxidation (μmol · kg−1 · min−1) | 6.5 ± 0.7 (3.8–11.4) | 7.4 ± 0.7 (2.5–17.8) | 8.6 ± 0.6 (3.1–16.1) | 0.09 | NS |
| Fat oxidation (μmol · kg−1 · min−1) | 4.7 ± 0.4 (2.2–7.8) | 4.3 ± 0.3 (1.4–7.5) | 4.1 ± 0.2 (2.1–8.8) | NS | NS |
| Free fatty acid baseline (μmol/l) | 439 ± 23 (252–598) | 427 ± 24 (216–678) | 341 ± 18 (161–607) | 0.003 | NS |
| Cholesterol (mmol/l) | 3.9 ± 0.2 (2.5–5.0) | 3.9 ± 0.1 (2.9–5.6) | 4.4 ± 0.9 (2.3–6.6) | 0.03 | NS |
| HDL (mmol/l) | 0.96 ± 0.04 (0.65–1.33) | 0.99 ± 0.04 (0.67–1.69) | 1.06 ± 0.03 (0.70–1.53) | NS | NS |
| LDL (mmol/l) | 2.3 ± 0.2 (1.3–3.1) | 2.4 ± 0.1 (1.4–4.3) | 2.7 ± 0.1 (1.1–4.5) | 0.04 | NS |
| Triglycerides (mmol/l) | 1.5 ± 0.2 (0.6–3.9) | 1.3 ± 0.1 (0.6–3.4) | 1.3 ± 0.1 (0.6–4.3) | NS | NS |
| VLDL (mmol/l) | 0.31 ± 0.04 (0.11–0.79) | 0.26 ± 0.03 (0.11–0.67) | 0.28 ± 0.03 (0.11–0.86) | NS | NS |
Data are means ± SEM (range). Post hoc P value, Bonferonni correction for Ab− vs. Ab+ clinically diagnosed type 2 diabetic patients.
In vivo insulin sensitivity.
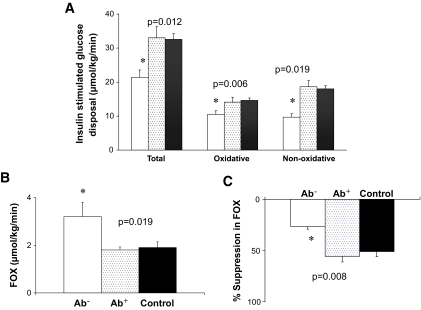
Steady-state plasma glucose and insulin concentrations during the hyperinsulinemic-euglycemic clamp were not different among the Ab−, Ab+, and obese control groups (5.6 ± 0.03, 5.6 ± 0.03, and 5.6 ± 0.02 mmol/l, respectively, and 1,677 ± 113, 1,536 ± 135, and 1,758 ± 73 pmol/l, respectively). Insulin-stimulated total, oxidative, and nonoxidative glucose disposal was significantly lower in the Ab− patients compared with their Ab+ peers and obese control subjects, with no difference between the Ab+ clinically diagnosed type 2 diabetic patients and the obese control subjects (Fig. 1A). Insulin sensitivity was lowest in the Ab− patients versus Ab+ versus control subjects (1.4 ± 0.2, 2.5 ± 0.3, and 2.0 ± 1.1 μmol · kg−1 · min−1 per pmol/l, respectively, P = 0.048). Fat oxidation during the euglycemic clamp was significantly higher in Ab− patients compared with Ab+ and control subjects, with no difference between the latter two groups (Fig. 1B). Percent suppression in fat oxidation during the euglycemic clamp was significantly lower in Ab− patients compared with Ab+ patients and control subjects (26.4 ± 0.1 in Ab− vs. 55.5 ± 6.6 in Ab+ and 50.8 ± 4.3% in control subjects, P = 0.008) (Fig. 1C). Insulin-stimulated glucose disposal (total, oxidative, and nonoxidative) and insulin sensitivity remained significantly lower and fat oxidation remained significantly higher in the Ab− patients compared with their Ab+ peers after adjusting for race, A1C, and diabetes duration at the time of the clamp studies.
FIG. 1.
A: Insulin-stimulated total, oxidative, and nonoxidative glucose disposal during the hyperinsulinemic-euglycemic clamp in Ab− (□) versus Ab+ (░) versus control subjects (▪). B: Fat oxidation (FOX) during the hyperinsulinemic-euglycemic clamp in the three groups. C: Percent suppression in fat oxidation during hyperinsulinemia in the three groups. P values by ANOVA. *Post hoc Bonferroni correction; P < 0.05 Ab− vs. Ab+, Ab− vs. control subjects.
In vivo β-cell function during the hyperglycemic clamp.
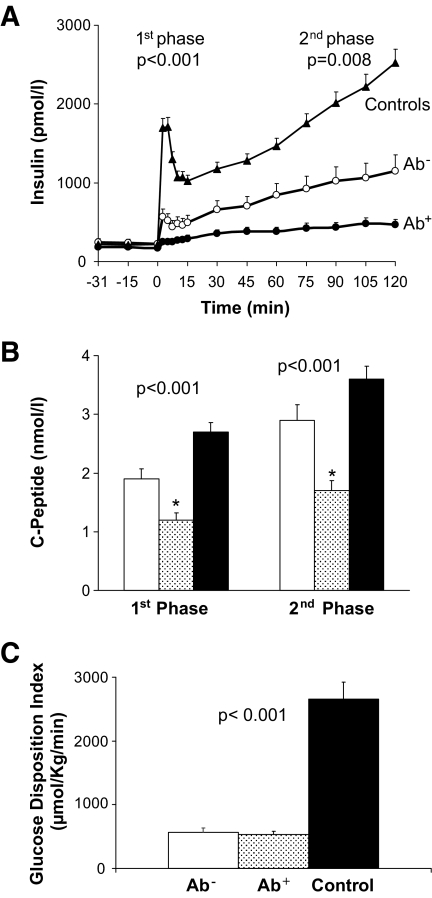
First-phase insulin and C-peptide as well as second-phase insulin and C-peptide levels were lowest in Ab+ clinically diagnosed type 2 diabetic patients compared with the other two groups (Fig. 2A and B) during hyperglycemia (first-phase glucose, 12.6 ± 0.1, 12.3 ± 0.1, and 12.2 ± 0.1 mmol/l; second-phase glucose, 12.7 ± 0.0, 12.7 ± 0.1, and 12.4 ± 0.0 mmol/l, respectively). All of these parameters of in vivo β-cell function remained significantly lower in Ab+ clinically diagnosed type 2 diabetic patients compared with their Ab− peers after adjusting for race, A1C, and diabetes duration at the time of the clamp studies. β-Cell function relative to insulin sensitivity, i.e., the glucose disposition index (GDI) did not differ between the Ab− and the Ab+ clinically diagnosed type 2 diabetic patients but were significantly lower than obese control subjects (Fig. 2C).
FIG. 2.
A: Insulin secretion during the hyperglycemic clamp in Ab− (○) versus Ab+ (•) versus control subjects (▴). B: First- and second-phase C-peptide levels during the hyperglycemic clamp in Ab− (□) versus Ab+ (░) versus control subjects (▪). C: GDI in the three groups. P values by ANOVA. *Post hoc Bonferroni correction; P < 0.05 Ab+ vs. Ab−, Ab+ vs. control subjects.
Clinical and biochemical characteristics of Ab− and Ab+ clinically diagnosed type 2 diabetic youth.
At the time of diagnosis, there were no differences between Ab− and Ab+ clinically diagnosed type 2 diabetic youth in A1C (10.1 ± 0.8 and 10.8 ± 0.6%, respectively; P = 0.4), glucose (15.4 ± 2.3 and 19.9 ± 1.2 mmol/l, P = 0.13), presence of symptoms, polyuria, polydypsia, or weight loss by patient report, and family history of diabetes. However, ketonuria was more frequent in Ab+ patients (57.7 vs. 18.8%, P = 0.01). Initiation of insulin treatment at diagnosis was comparable between the two groups (62.5% in Ab− vs. 73.1% in Ab+ patients, P = 0.51), whereas initiation of metformin therapy was more frequent among the Ab− patients (75% in Ab− vs. 34.6% in Ab+, P = 0.03). Four subjects (two Ab− and two Ab+) were started on lifestyle modification only and no medication (12.5% Ab− vs. 7.6% Ab+, P = 0.63). Ten of the 29 patients who were started on insulin treatment at diagnosis (four Ab− and six Ab+) became insulin free by the time of the clamp studies (34.5%).
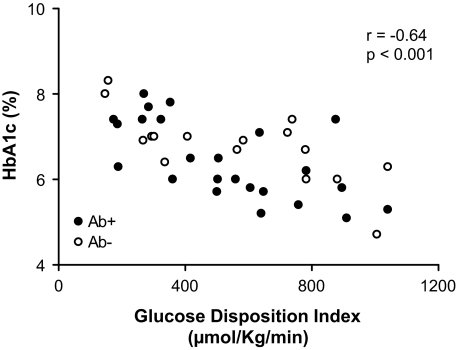
At the time of research evaluation, there were no differences between Ab− and Ab+ clinically diagnosed type 2 diabetic patients in A1C, insulin use, and/or treatment modalities (Table 1). However, nighttime systolic blood pressure (121.9 ± 2.5 vs. 113.0 ± 1.8 mmHg, respectively; P = 0.006) and fasting ALT (40.5 ± 5.0 and 28.4 ± 1.7 units/l, P = 0.03) were significantly higher in the Ab− compared with Ab+ patients. The differences in nighttime systolic blood pressure remained significant after adjusting for race, VAT, and insulin sensitivity (P = 0.011). A1C correlated with GDI (Fig. 3) but not with insulin sensitivity or insulin secretion. In a multiple regression analysis with A1C as the dependent variable and GDI, age, and VAT as the independent variables (r2 = 0.612, P < 0.001), all three contributed significantly (partial correlations, GDI −0.71, P < 0.001; age −0.52, P = 0.001; VAT 0.42, P = 0.013) and independently to explain 78% of the variance in A1C.
FIG. 3.
Relationship between GDI and A1C in Ab− (○) vs. Ab+ (•) clinically diagnosed type 2 diabetic patients.
Double Ab+ (2Ab+) versus single (1Ab+) positive.
Among the 26 Ab+ clinically diagnosed type 2 diabetic patients, 18 were positive for a single Ab (14 for GAD and 4 for IA2), and 8 were positive for double antibodies (both GAD and IA2). There was no difference in insulin-stimulated glucose disposal between 1Ab+ and 2Ab+ groups (32.8 ± 3.9 vs. 33.3 ± 4.4, NS). First- and second-phase insulin was higher in the 1Ab+ compared with the 2Ab+ patients (314.5 ± 36.1 vs. 165.0 ± 40.0 pmol/l, P = 0.02; 482.8 ± 67.1 vs. 239.0 ± 68.6 pmol/l, respectively, P = 0.03). There were no differences in age, BMI, body composition, A1C, fasting lipid profile, blood pressure, glucose level, A1C, and ketonuria at the time of diagnosis.
DISCUSSION
This study was undertaken to determine whether there are differences in insulin sensitivity and secretion between Ab+ and Ab− obese youth with a clinical diagnosis of type 2 diabetes in an effort to shed light on distinguishing pathophysiological mechanisms. Our results demonstrate that despite similar body composition and body fat topography, 1) youth with Ab− clinically diagnosed type 2 diabetes have severe impairment in insulin action, whereas those with Ab+ clinically diagnosed type 2 diabetes have severe impairment in β-cell function; 2) youth with Ab− type 2 diabetes had additional features consistent with insulin resistance/metabolic syndrome, including decreased suppression in fat oxidation during hyperinsulinemia, higher systolic blood pressure, and higher ALT; and 3) there were no clinical distinguishing features between Ab+ and Ab− youth with clinically diagnosed type 2 diabetes at the time of diagnosis except for the higher frequency of ketonuria in the former group.
Between 10 and 75% of youth diagnosed clinically with type 2 diabetes are reported to have β-cell autoantibodies (10–15). The antibodies tested and reported to be positive in these patients include ICA in 5–8% (10,12,13,15), GAD in 8–30% (10–12,15), IA-2 in 8–42% (11–13,15), and insulin antibodies in 5–35% (10–13). In one study, 11% of clinically diagnosed type 2 diabetic youth were positive for all four autoantibodies (12). These studies, however, had very limited numbers of patients from different racial groups (10–13,15). Some of these studies investigated but could not find clinically distinguishing features between patients with or without β-cell antibodies (10,15). A preliminary report of a much larger scale of 432 youth with clinically diagnosed type 2 diabetes screened in TODAY study revealed that 17.4% were positive for one or both of the antibodies GAD and IA-2 (14). Ab+ versus Ab− subjects did not differ in age, diabetes duration, BMI Z score, and lipid levels; however, there seemed to be a tendency for C-peptide to be lower and A1C to be higher in Ab+ youth (14).
Studies investigating the pathophysiology of youth type 2 diabetes are very limited and have conflicting results (3–6,26). In one of the earliest studies, first-phase insulin response during a frequently sampled intravenous glucose tolerance test was lower in type 2 diabetic patients compared with obese control subjects with no difference in insulin sensitivity (3). This was in contrast with another study where insulin sensitivity was lower (6) in type 2 diabetes. However, despite lower insulin response to IVGTT, integrated insulin and C-peptide levels during the OGTT were not different between type 2 diabetic patients and obese as well as lean control subjects (6). In the former study, β-cell antibodies were not measured, and in the latter it is not clear which, how many, and how antibodies were measured. In our previous publication, GAD and ICA antibodies were measured commercially, and only type 2 diabetic patients adolescents with negative antibodies were reported and shown to have ∼50% lower insulin sensitivity and relative insulin deficiency (4). In another study of six adolescents with type 2 diabetes and negative GAD, IA-2, and ICA, besides marked insulin resistance, the impairment in β-cell function was highly variable (5). In another study of 10 obese youth with type 2 diabetes negative for GAD, ICA and IA2, glucose sensitivity of first- and second-phase β-cell secretion was significantly lower in type 2 diabetic patients compared with obese youth, whereas insulin sensitivity was similar between the groups (26). Potential explanations for these variable findings could be differences in study population and ethnicity, differences in diabetes duration and therapeutic modalities, differences in methodologies, and differences in measuring antibodies. In the present investigation, we elected to use the same laboratory that was deemed most appropriate for the NIH-funded multicenter TODAY study. Our current results suggest that youth who are clinically diagnosed with type 2 diabetes but negative for the two tested antibodies have severe impairment of insulin action, both in glucose and fat metabolism, which is over and beyond that due to obesity since there were no differences in total and abdominal obesity between them and the other two groups. Furthermore, the presence of higher blood pressure and higher ALT would suggest that this group has an inherent insulin resistance that is typical of the metabolic syndrome (27,28). On the other hand, patients clinically diagnosed with type 2 diabetes but positive antibodies have severe impairment in β-cell function, whereas their insulin action is significantly better than Ab− patients and near identical to obese nondiabetic control subjects. This suggests that the major abnormality is in insulin secretion and not insulin action, which is typical of autoimmune type 1 diabetes. Thus, it is our proposition that these patients are merely obese children who happen to develop autoimmune type 1 diabetes. Moreover, our limited data would suggest that there could be a dose-response phenomenon because first- and second-phase insulin secretion in patients who are positive for double antibodies is ∼50% lower than that of patients who are positive for a single antibody. Previous limited studies from a single group of investigators found that some youth clinically classified with type 2 diabetes or indeterminant diabetes (admixture of clinical features of types 1 and 2: type 1.5) show T-cell reactivity to islet proteins (12) and have type 1 diabetes–associated HLA alleles (13). Baseline C-peptide levels in the type 1.5 diabetic patients were significantly lower than those in patients classified as type 2 diabetic and higher than those in patients classified as type 1 diabetic (13). The findings in our study provide direct evidence to the more severe degree of impairment in β-cell function in Ab+ patients proposed in the aforementioned study. The more frequent ketonuria at diagnosis in Ab+ patients may be a reflection of the severity of β-cell impairment and has been previously reported (12).
It is not surprising that despite significantly more severe impairment in β-cell function in the Ab+ group, there was no difference in the frequency of insulin treatment between the two groups of clinically diagnosed type 2 diabetic patients either at diagnosis or at the time of the study (73% Ab+ vs. 62% Ab− at diagnosis, and 54% vs. 38% at the time of the study). Treatment of patients is frequently practitioner driven and not pathophysiology driven, and these rates of insulin treatment are consistent with the pediatric literature of type 2 diabetes (29,12,15). The change in BMI from diagnosis to the time of research was not different between those patients initially treated with versus without insulin (data not shown).
Despite these important differences in insulin action and secretion between Ab− and Ab+, the GDI, which is insulin secretion corrected for the degree of insulin sensitivity, is not different. Our present metabolic observations are consistent with findings in GAD+ adults with type 2 diabetes or latent autoimmune diabetes (30). GAD+ adult patients had decreased early insulin response; however, when this response was corrected for the degree of insulin sensitivity, GAD+ and GAD− patients had similar β-cell function. Thus, in the absence of careful metabolic studies using sensitive tools, it will be difficult to discern the underlying pathways: impaired insulin action versus β-cell function, which may result in similar GDI. However, such knowledge is important to help guide pathophysiology- based therapy amid the multiple terminologies used: double diabetes, hybrid diabetes, diabetes type 1.5, etc. (8,13,15–18).
In conclusion, important metabolic differences exist in insulin sensitivity and secretion between obese youth with versus without evidence of pancreatic autoantibodies. These metabolic differences highlight different underlying pathophysiological mechanisms between the two groups despite a clinical diagnosis of type 2 diabetes. Finally, it remains to be determined whether the natural history of diabetes and/or response to treatment and/or micro- and macrovascular complications differ between Ab+ and Ab− clinically diagnosed obese youth with type 2 diabetes.
Acknowledgments
F.B. has received support from the Thrasher Research Fund. N.G. has received support from the Thrasher Research Fund and the Pittsburgh Foundation. S.A. has received U.S. Public Health Service Grant RO1-HD-27503, NICHD Grant K24-HD-01357, NIDDK Grant U01-DK-61254, and the Richard L. Day Endowed Chair. This work has been supported by General Clinical Research Center Grant MO1-RR-00084 and Clinical and Translational Science Award Grant UL1-RR-024153.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in a platform session at the 67th Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 22–26 June 2007.
These studies would not have been possible without the nurses and staff of the Pediatric Clinical and Translational Research Center, the devotion of the research team (Kristin Porter, RN; Sally Foster, RN, CDE; Lori Bednarz, RN, CDE; Nancy Guerra, CRNP; and Sabrina Kadri), and the laboratory expertise of Resa Stauffer; but most importantly the commitment of the study participants and their parents. We thank So Jung Lee, PhD, for the interpretation of the abdominal CT data in some of the participants.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 December 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gungor N, Hannon T, Libman I, Bacha F, Arslanian S: Type 2 diabetes mellitus in youth: the complete picture to date. Pediatr Clin North Am 52: 1579–1609, 2005 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association: Position statement: diagnosis and classification of diabetes mellitus. Diabetes Care 31 (Suppl. 1): S55–S60, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Amemiya S, Higashida K, Ishihara T, Sawanobori E, Kobayashi K, Mochizuki M, Kikuchi N, Tokuyama K, Nakazawa S: Pathogenic factors of glucose intolerance in obese Japanese adolescents with type 2 diabetes. Metabolism 49: 186–191, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S: Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care 28: 638–644, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C: Characterization of insulin secretion and resistance in type 2 diabetes of adolescents J Clin Endocrinol Metab 91: 401–404, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA: Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab 91: 185–191, 2006 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association: Type 2 diabetes in children and adolescents. Diabetes Care 23: 381–389, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ: Evidence for heterogeneous pathogenesis of insulin-treated diabetes in black and white children. Diabetes Care 26: 2876–2882, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Rosenbloom AL: Obesity, insulin resistance, β-cell autoimmunity, and the changing clinical epidemiology of childhood diabetes. Diabetes Care 26: 2954–2956, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hathout EH, Thomas W, El-Shahawy M, Nahab F, Mace JW: Diabetic autoimmune markers in children and adolescents with type 2 diabetes. Pediatrics 107: E102, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Umpaichitra V, Banerji MA, Castells S: Autoantibodies in children with type 2 diabetes mellitus. J Pediatr Endocrinol Metab 15 (Suppl. 1): 525–530, 2002 [PubMed] [Google Scholar]
- 12.Brooks-Worrell BM, Greenbaum CJ, Palmer JP, Pihoker C: Autoimmunity to islet proteins in children diagnosed with new-onset diabetes. J Clin Endocrinol Metab 89: 2222–2227, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gilliam L, Brooks-Worrell B, Palmer J, Greenbaum C, Pihoker C: Autoimmunity and clinical course in children with type1, type2, and type 1.5 diabetes. J Autoimmunity 25: 244–250, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Klinkgensmith GJ, Coombs LP, Arslanian S, Copeland K, Cuttler L, Haffner S, Kaufman F, Laffel L, Linder B, Marcovina SM, Tollefsen SE, Weinstock RS, for the TODAY study group: Autoantibody positivity in subjects screened for participation in a treatment trial for T2D in youth. Diabetes 55 (Suppl. 1): A67, 2006 [Google Scholar]
- 15.Reinehr T, Schober E, Wiegand S, Thon A, Holl R, DPV-Wiss Study Group: Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child 91: 473–477, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pozzilli P, Guglielmi C, Pronina E, Petraikina E: Double or hybrid diabetes associated with an increase in type 1 and type 2 diabetes in children and youths. Pediatr Diabetes 8 (Suppl. 9): 88–95, 2007 [DOI] [PubMed] [Google Scholar]
- 17.SEARCH Study Group: SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25: 458–471, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Wilkin TJ: The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 44: 914–922, 2001 [DOI] [PubMed] [Google Scholar]
- 19.The TODAY Study Group, Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D: Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 8: 74–87, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacha F, Saad R, Gungor N, Arslanian SA: Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care 29: 1599–1604, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Tanner JM: Growth and maturation during adolescence. Nutr Rev 39: 43–55, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Silverstein JH, Rosenbloom AL: Treatment of type 2 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab 13 (Suppl. 6): 1403–1409, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Pinhas-Hamiel O, Zeitler P: Clinical presentation and treatment of type 2 diabetes in children. Pediatr Diabetes 8 (Suppl. 9): 16–27, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Arslanian SA, Lewy VD, Danadian K: Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 86: 66–71, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Bacha F, Saad R, Gungor N, Arslanian S: Adiponectin in youth: relationship to visceral adiposity, insulin-sensitivity, and β-cell function. Diabetes Care 27: 547–552, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R: β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes 54: 1735–1743, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Gungor N, Bacha F, Arslanian S: Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30: 2091–2097, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Bacha F, Gungor N, Arslanian S: Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr 152: 177–184, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Zuhri-Yafi MI, Brosnan PG, Hardin DS: Treatment of type 2 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab 15 (Suppl. 1): 541–546, 2002 [PubMed] [Google Scholar]
- 30.Zinman B, Kahn SE, Haffner SM, O'Neill MC, Heise MA, Freed MI, ADOPT Study Group: Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 53: 3193–3200, 2004 [DOI] [PubMed] [Google Scholar]