Abstract
Heterozygous loss of function mutations at the glucosecerebrosidase locus have recently been shown to be a potent risk factor for Lewy body disease. Based on this observation, we have re-evaluated the likelihood that the different PARK loci (defined using clinical criteria for disease) may be misleading attempts to find common pathways to pathogenesis. Rather, we suggest, grouping the different loci which lead to different Lewy body disease may be more revealing. Doing this, we suggest that several of the genes involved in disparate Lewy body diseases impinge on ceramide metabolism and we suggest that this may be a common theme for pathogenesis.
Parkinson’s disease (PD) is a common neurodegenerative disease which affects over 1% of people over the age of 65 years [1]. Clinical manifestations include bradykinesia, rigidity, tremor and postural instability. From a pathological perspective, PD is characterized by dopamine neuron degeneration, which leads to depigmentation of the substantia nigra. Additionally, typical PD cases have intracellular proteinaceous inclusions called Lewy bodies and Lewy neurites in the brainstem and cortical areas.
Genetic research in the past decade has changed the view of PD from an archetypical non-genetic disease to one having a clear genetic basis in a percentage of patients [2]. Five genes have been cloned in which mutations cause parkinsonism in a mendelian fashion [3–8] (Table 1).
Table 1.
Genes that cause parkinsonism in a mendelian fashion
| Locus | Inheritance | Age onset | Chr | Pathology | Gene |
|---|---|---|---|---|---|
| PARK-1 | AD | 35–65 | 4q | Lewy Body inclusions | SNCA |
| PARK-2 | AR | 7–60 | 6p | Usually no Lewy Body inclusions | PRKN |
| PARK-6 | AR | 36–60 | 1p36 | Unknown | PINK1 |
| PARK-7 | AR | 27–40 | 1p36 | Unknown | DJ-1 |
| PARK-8 | AD | 45–57 | 12q12 | Usually Lewy Body inclusions | LRRK2 |
Classically, the approach taken to the study of genetic forms of PD has relied on a clinical definition of disease and PARK loci have been assigned on this clinical basis. It is known what clinical features are primarily associated with each locus and a great deal of attention has been focused on this association [9]. However, if one wants to identify pathways of pathogenicity for a given disorder, arguably, one should start by analyzing the genetics of disease based on pathology. In this review, we start from the position that it is more likely to find a common pathway if there is a common pathology rather than common clinical characteristics. We and others have suggested that, for the early onset recessive diseases (encoded at the parkin, PINK1 and DJ-1 loci), in which Lewy bodies are either usually absent (parkin) or where no neuropathological data is available (PINK1 and DJ-1), the evidence for a mitochondrial pathway to cell death is overwhelming [2].
The inspiration for our attempt to re-evaluate a Lewy body pathway to cell death has come from the recent observation that mutations in glucosecerebrosidase (GBA) which when homozygous, lead to Gaucher’s disease but when heterozygous, predispose to PD [10]. GBA catalyses the breakdown of glucosecerebroside to ceramide and glucose. Gaucher’s disease is caused by a lysosomal build up of glucosecerebroside, but this occurs only when GBA activity is almost completely lost. In the heterozygous state this is unlikely to be a problem. We therefore began to consider that ceramide metabolism, more generally, may be an initiating problem in PD.
The genes associated with Lewy bodies that will be dealt with within this review are presented in Table 2. These are divided in three categories: the first are genes clearly involved in ceramide metabolism and that cause diseases where Lewy bodies are known to be abundant; the second category groups genes that may be involved in ceramide metabolism and cause diseases where Lewy bodies have been described; the third category presents genes for which, while they do give rise to Lewy body disease, there is currently little or no evidence suggesting a role in ceramide metabolism.
Table 2.
Genes associated with Lewy body inclusions and their role potential role in ceramide metabolism
| Gene | Chr | Function | Disease | |
|---|---|---|---|---|
| Ceramide metabolism and Lewy body inclusions | GBA | 1q21 | Lysosomal Hydrolase | Gaucher disease/Parkinson’s disease in heterozygotes |
| PANK2 | 20p13-p12.3 | Pantothenate kinase | Neurodegeneration with brain iron accumulation type 1 (NBIA-1) | |
| PLA2G6 | 22q13.1 | A2 phospholipase | Neurodegeneration with brain iron accumulation 2 (NBIA2) | |
| Probably Ceramide metabolism ; Possibly Lewy body inclusions | NPC1 | 18q11-q12 | Regulation of intracellular cholesterol trafficking | Niemann-Pick disease type C1 |
| SPTLC1 | 9q22.1-22.3 | Transferase activity | Hereditary sensory neuropathy type I (HSN1) | |
| ATP13A2 | 1p36 | ATPase | Kufor-Rakeb Syndrome | |
| Possibly Ceramide metabolism; Definite Lewy body inclusions |
SNCA | 4q21 | Dopamine transmissionand synaptic vesicle dynamics | Parkinson’s disease |
| Unknown Ceramide; Usually Lewy body inclusions | LRRK2 | 12q12 | Protein Kinase | Parkinson’s disease |
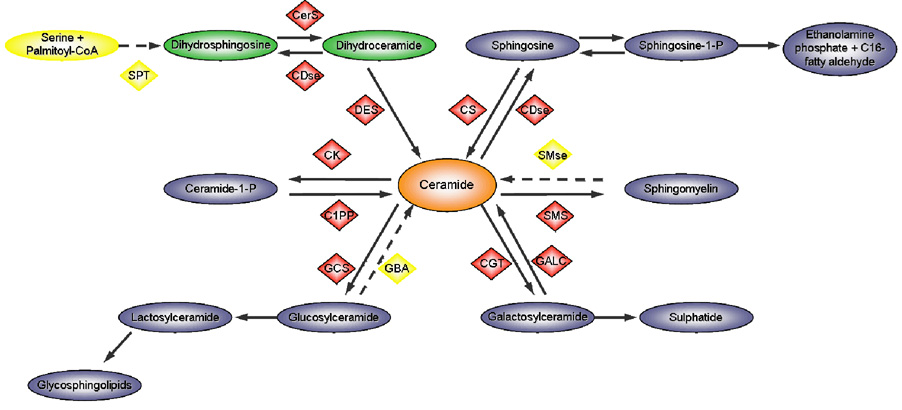
Levels of cellular ceramide are regulated by the de novo pathway and the recycling pathway. The former relates to the synthesis of ceramide through the condensation of palmitate and serine in a series of reactions that are ultimately dependent on Co-Enzyme A. The latter pathway is slightly more intricate, since several outcomes are possible depending on the enzymes involved. The simplified metabolism is shown in Figure 1.
Figure 1.
Simplified representation of ceramide metabolism. SPT, serine palmitoyl transferase; CerS, ceramide synthase; CDse, Ceramidase; DES, Desaturase; CS, Ceramide synthase; CK, Ceramide Kinase; SMse, sphingomyelinase; C1PP, Phosphatase; GCS, glucosylceramide synthase; GBA, glucosylceramidase; CGT, UDP glycosyltransferase; GALC, Galactosylceramidase; SMS, sphingomyelin synthase. Yellow represents enzymes directly involved in ceramide metabolism, in which mutations are associated with Lewy body inclusions. Adapted from [56].
The gene GBA encodes a lysosomal enzyme, glucocerebrosidase, that catalyses the breakdown of the glycolipid glucosylceramide to ceramide and glucose [11]. Over 200 mutations have been described in GBA, most of which are known to cause Gaucher disease, in the homozygous or compound heterozygous condition (for a review see [12]). Gaucher patients typically present enlarged macrophages resulting from the intracellular accumulation of glucosylceramide. The fact that these patients show increased levels of the enzyme’s substrate indicates that pathogenic variants act as loss-of-function mutations. GBA mutations, in addition to causing Gaucher disease when homozygous, have recently been established to act as a risk factor for PD [13, 14] and for Lewy body disorders [15].
Neurodegeneration with brain iron accumulation-1 (NBIA-1), formerly known as Hallervorden-Spatz disease is a form of neurodegeneration caused by mutations in the pantothenate kinase gene, PANK2. Clinically the condition is characterized by progressive rigidity, first in the lower and later in the upper extremities. Both involuntary movements and rigidity may involve muscles supplied by cranial nerves, resulting in difficulties in articulation and swallowing. Mental deterioration and epilepsy occur in some. Onset is in the first or second decade and death usually occurs before the age of 30 years [16]. Neuropathological studies have shown that patients with NBIA-1 present extensive Lewy bodies [17–19]. Pantothenate kinase is an essential regulatory enzyme in CoA biosynthesis, catalyzing the cytosolic phosphorylation of pantothenate (vitamin B5), N-pantothenoylcysteine, and pantetheine [20]. PANK2 is also involved in ceramide metabolism as the de novo pathway for ceramide formation relies on the presence of CoA [21]. Hence, there is a direct, though not specific, connection to ceramide metabolism.
Neurodegeneration with brain iron accumulation-2 (NBIA-2) is characterized by the disruption of cellular mechanisms leading to the accumulation of iron in the basal ganglia. Mutations in the gene PLA2G6 were recently described as the cause of NBIA-2 [22]. Phenotypically similar to NBIA-1, Lewy bodies were also described in patients with NBIA-2, particularly in the brainstem nuclei and cerebral cortex [23]. PLA2G6 belongs to the family of A2 phospholipases, which catalyze the release of fatty acids from phospholipids and play a role in a wide range of physiologic functions [24]. Interestingly, it has been recently demonstrated that PLA2G6 plays a role in the ceramide pathway; activation of this enzyme promotes ceramide generation via neutral sphingomyelinase-catalyzed hydrolysis of sphingomyelins [25]. Similarly to what happens with GBA or PANK2, mutations in PLA2G6 that diminish its activity are expected to reduce the levels of ceramide formed through the breakdown of sphingomyelin.
Niemann-Pick type C (NPC) disease is an autosomal recessive lipid storage disorder characterized by progressive neurodegeneration with a highly variable clinical phenotype. Patients with the 'classic' childhood onset type C usually appear normal for 1 or 2 years with symptoms appearing between 2 and 4 years. They gradually develop neurologic abnormalities which are initially manifested by ataxia, grand mal seizures, and loss of previously learned speech. Spasticity is striking and seizures are common [26]. Approximately 95% of cases are caused by mutations in the NPC1 gene, referred to as type C1. This gene encodes a putative integral membrane protein containing motifs consistent with a role in intracellular transport of cholesterol to post-lysosomal destinations. Cells from NPC subjects show a decrease in acid sphingomyelinase activity, leading to the accumulation of sphingomyelin [27]. Since one of the pathways for ceramide recycling is the sphingomyelin pathway, it is conceivable that associated to the accumulation of sphingomyelin, a decrease of ceramide may also be present. Some cases of NPC1 were described as presenting Lewy bodies [28].
Mutations in SPTLC1 are the cause of hereditary sensory neuropathy type I (HSAN I) [29], a dominantly inherited sensorimotor axonal neuropathy with onset in the first or second decades of life. SPTLC1 is a key enzyme in sphingolipids biosynthesis, catalyzing the pyridoxal-5-prime-phosphate- dependent condensation of L-serine and palmitoyl-CoA to 3-oxosphinganine [30].
Patients usually present neuropathic arthropathy, recurrent ulceration of the lower extremities, signs of radicular sensory deficiency in both the upper and the lower extremities without any motor dysfunction [31]; restless legs and lancinating pain are other presentations of the disorder, which often results in severe distal sensory loss, and mutilating acropathy [32]. Although mutations in SPTLC1 cause neurological disease, there is, as yet, no description of the pathology of the disorder. We would hypothesize that this disease will have Lewy body pathology.
Kufor-Rakeb syndrome (KRS) is a form of autosomal recessive hereditary parkinsonism with dementia. It was recently described that loss-of-function mutations in the predominantly neuronal P-type ATPase gene ATP13A2 are the cause of Kufor-Rakeb syndrome [33]. The clinical features of KRS are similar to those of idiopathic Parkinson disease and pallidopyramidal syndrome, including mask-like face, rigidity, and bradykinesia [34]. Although ATP13A2 does not play an obvious role in the ceramide pathway it is a lysosomal transport protein thought to be responsible for the maintenance of the ideal pH in the lysosome. This function, albeit potentially implying a much broader effect of mutations, might also mean that ATP13A2 may be related to the recycling pathways of ceramide metabolism. Interestingly, it has been suggested that alpha-synuclein turnover may occur via chaperone-mediated autophagy (CMA), a specialized form of lysosomal turnover [35–39]. It has also been shown that Alpha-synuclein turnover is slowed in mouse models of lysosomal storage disorders [40].
Alpha-synuclein (SNCA) is the major component of Lewy bodies and mutations in this gene are a rare cause of PD. Only three point mutations have been described to date, but duplication and triplication of the entire SNCA locus has also been discovered [3, 41–45]. PD cases with underlying SNCA mutations have extensive Lewy bodies, since these mutations are known to increase aggregation of the protein [46]. SNCA may also be involved, albeit in a more indirect manner, in the ceramide pathway. It has been shown that deletion of the gene decreases brain palmitate uptake [47] and that the presence of palmitic acid increases the de novo synthesis of ceramide significantly [48]. However, known pathogenic mutations in SNCA are likely gain-of-function mutations, suggesting that, in these cases, the mutations drive the aggregation of alpha-synuclein, while in cases where ceramide metabolism is affected, Lewy Body inclusions may be a cellular response to this altered ceramide metabolism. Also connecting the ceramide pathway to alpha-synuclein deposition is the recent description of an increase in alpha-synuclein inclusions in C. elegans when LASS2, a ceramide synthase, is knocked-down [49]. This result should obviously be taken with some caution, since it was obtained in a non-mammalian organism, but nevertheless it further connects ceramide to synuclein deposition.
Mutations in the gene encoding the leucine-rich repeat kinase 2 (LRRK2) are a common cause of PD [50–52]. The function of LRRK2 is not clear, but it has been shown to possess two enzymatic domains as well as several potential protein-protein interaction motifs [53]. The phenotype attributed to LRRK2 PD is usually not different from the idiopathic form of the disease [54]. However, discrepant results have been presented by neuropathological studies; while some cases have no Lewy bodies [55], most have typical Lewy body disease [8]. The mechanism of this variability is not clear. Similarly, it is not obvious that LRRK2 plays a role in the ceramide pathway as no studies of this question have been published to date.
With this article, we have brought together data suggesting that some of the genes involved in the genetics of Lewy body disease, have in common the fact that they impinge on ceramide metabolism. One shortfall of the present theory is the lack of neuropathological data regarding cases with PINK1 or DJ-1 mutations. However, we may see studies addressing this same issue in the near future.
A major premise of this theory is the fact that Lewy body inclusions should have a key role in our understanding of the mechanisms of the disease. We propose that pathology data will, in most cases, be more insightful than clinical data in defining the disease. This is based on what we have learned by other neurodegenerative diseases with inclusion pathology. For Alzheimer’s disease, when pathology was used as a basis to understand the disease, pathways involved became evident. This would be most unlikely to happen if, instead, clinical data was used.
These data are incomplete and there have been few relevant studies directly addressing neuronal ceramide metabolism in this context. However, the hypothesis we present has the benefit of making several predictions amongst which are:
Mutations in other genes which alter neuronal ceramide metabolism should lead to Lewy body diseases, and plausibly ATP13A2 and HSAN mutation carriers should have Lewy bodies.
α-Synuclein and LRRK2 should have roles in ceramide metabolism.
This notion also suggests that it may be profitable to consider other genes in these pathways as risk factors for Lewy body disease, and in particular, to consider whether they influence the penetrance of the GBA mutations.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; Annual Report number Z01-AG000957-05 and Portuguese FCT grant #SFRH/BD/29647/2006.
References
- 1.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson's disease and parkinsonism. Annals of neurology. 2006;60:389–398. doi: 10.1002/ana.21022. [DOI] [PubMed] [Google Scholar]
- 3.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science (New York, NY. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 4.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autossomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 5.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science (New York, NY. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 6.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary earlyonset Parkinson's disease caused by mutations in PINK1. Science (New York, NY. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 7.Paisán-Ruíz C, Jain S, Evans W, Gilks WP, Simón J, van der Brug M, Munain A, Aparicio S, Martínez Gil A, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Saénz Peòa A, de Silva R, Lees A, Martí-Massó JF, Pérez-Tur J, Wood NW, Singleton AB. Cloning of the Gene Containing Mutations that Cause PARK8-Linked Parkinson’s Disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Riess O, Kruger R, Schulz JB. Spectrum of phenotypes and genotypes in Parkinson's disease. Journal of neurology. 2002;249 Suppl 3 doi: 10.1007/s00415-002-1303-2. III/15-20. [DOI] [PubMed] [Google Scholar]
- 10.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Molecular genetics and metabolism. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Beutler E. Gaucher disease: new molecular approaches to diagnosis and treatment. Science (New York, NY. 1992;256:794–799. doi: 10.1126/science.1589760. [DOI] [PubMed] [Google Scholar]
- 12.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Human mutation. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 13.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. The New England journal of medicine. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 14.Bras J, Paisan-Ruiz C, Guerreiro R, Ribeiro MH, Morgadinho A, Januario C, Sidransky E, Oliveira C, Singleton A. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiology of aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mata IF, Samii A, Schneer SH, Roberts JW, Griffith A, Leis BC, Schellenberg GD, Sidransky E, Bird TD, Leverenz JB, Tsuang D, Zabetian CP. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Archives of neurology. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dooling EC, Schoene WC, Richardson EP., Jr Hallervorden-Spatz syndrome. Archives of neurology. 1974;30:70–83. doi: 10.1001/archneur.1974.00490310072012. [DOI] [PubMed] [Google Scholar]
- 17.Arawaka S, Saito Y, Murayama S, Mori H. Lewy body in neurodegeneration with brain iron accumulation type 1 is immunoreactive for alpha-synuclein. Neurology. 1998;51:887–889. doi: 10.1212/wnl.51.3.887. [DOI] [PubMed] [Google Scholar]
- 18.Neumann M, Adler S, Schluter O, Kremmer E, Benecke R, Kretzschmar HA. Alpha-synuclein accumulation in a case of neurodegeneration with brain iron accumulation type 1 (NBIA-1, formerly Hallervorden-Spatz syndrome) with widespread cortical and brainstem-type Lewy bodies. Acta Neuropathol. 2000;100:568–574. doi: 10.1007/s004010000224. [DOI] [PubMed] [Google Scholar]
- 19.Galvin JE, Giasson B, Hurtig HI, Lee VM, Trojanowski JQ. Neurodegeneration with brain iron accumulation, type 1 is characterized by alpha-, beta-, and gamma-synuclein neuropathology. The American journal of pathology. 2000;157:361–368. doi: 10.1016/s0002-9440(10)64548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hortnagel K, Prokisch H, Meitinger T. An isoform of hPANK2, deficient in pantothenate kinase-associated neurodegeneration, localizes to mitochondria. Human molecular genetics. 2003;12:321–327. doi: 10.1093/hmg/ddg026. [DOI] [PubMed] [Google Scholar]
- 21.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 22.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J, Canham N, Nardocci N, Zorzi GZ, Pasha S, Rodriguez D, Desguerre I, Mubaidin A, Bertini E, Trembath RC, Simonati A, Schanen C, Johnson CA, Levinson B, Woods CG, Wilmot B, Kramer P, Gitschier J, Maher ER, Hayflick SJ. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nature genetics. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi S, Akasaki Y, Morimura Y, Takauchi S, Sato M, Miyoshi K. An autopsy case of late infantile and juvenile neuroaxonal dystrophy with diffuse Lewy bodies and neurofibrillary tangles. Clinical neuropathology. 1992;11:1–5. [PubMed] [Google Scholar]
- 24.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. The Journal of biological chemistry. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 25.Lei X, Zhang S, Bohrer A, Bao S, Song H, Ramanadham S. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–10185. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanier MT, Millat G. Niemann-Pick disease type C. Clinical genetics. 2003;64:269–281. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 27.Tamura H, Takahashi T, Ban N, Torisu H, Ninomiya H, Takada G, Inagaki N. Niemann-Pick type C disease: novel NPC1 mutations and characterization of the concomitant acid sphingomyelinase deficiency. Molecular genetics and metabolism. 2006;87:113–121. doi: 10.1016/j.ymgme.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, Suzuki K, Hulette CM, Murayama S. Aberrant phosphorylation of alpha-synuclein in human Niemann-Pick type C1 disease. Journal of neuropathology and experimental neurology. 2004;63:323–328. doi: 10.1093/jnen/63.4.323. [DOI] [PubMed] [Google Scholar]
- 29.Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nature genetics. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- 30.Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. The Journal of biological chemistry. 2006;281:37275–37281. doi: 10.1074/jbc.M608066200. [DOI] [PubMed] [Google Scholar]
- 31.Mandell AJ, Smith CK. Hereditary sensory radicular neuropathy. Neurology. 1960;10:627–630. doi: 10.1212/wnl.10.7.627. [DOI] [PubMed] [Google Scholar]
- 32.Dyck PJ, Low PA, Stevens JC. "Burning feet" as the only manifestation of dominantly inherited sensory neuropathy. Mayo Clinic proceedings. 1983;58:426–429. [PubMed] [Google Scholar]
- 33.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nature genetics. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 34.Najim al-Din AS, Wriekat A, Mubaidin A, Dasouki M, Hiari M. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta neurological Scandinavica. 1994;89:347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 35.Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Lesuisse C, Xu Y, Troncoso JC, Price DL, Lee MK. Stabilization of alpha-synuclein protein with aging and familial Parkinson's disease-linked A53T mutation. J Neurosci. 2004;24:7400–7409. doi: 10.1523/JNEUROSCI.1370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science (New York, NY. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 39.Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type a-synuclein is degraded by chaperone mediated autophagy and macroautophagy in neuronal cells. The Journal of biological chemistry. 2008 doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A. A block of autophagy in lysosomal storage disorders. Human molecular genetics. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- 41.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber MB, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nature genetics. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 42.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science (New York, NY. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 43.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 44.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 45.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementiaq. Annals of neurology. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 46.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golovko MY, Faergeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 2005;44:8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- 48.Patil S, Melrose J, Chan C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. The European journal of neuroscience. 2007;26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS genetics. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, Pollak P, Brice A. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. The New England journal of medicine. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 51.Bras J, Guerreiro R, Ribeiro M, Januário C, Morgadinho A, Oliveira C, Cunha L, Hardy J, Singleton A. G2019S Dardarin Substitution is a Common Cause of Parkinson's Disease in a Portuguese Cohort. Mov Disord. 2005;20:1653–1655. doi: 10.1002/mds.20682. [DOI] [PubMed] [Google Scholar]
- 52.Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, Lynch J, Healy DG, Holton JL, Revesz T, Wood NW. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 53.Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. The Parkinson Disease-associated Leucine-rich Repeat Kinase 2 (LRRK2) Is a Dimer That Undergoes Intramolecular Autophosphorylation. The Journal of biological chemistry. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aasly JO, Toft M, Fernandez-Mata I, Kachergus J, Hulihan M, White LR, Farrer M. Clinical features of LRRK2-associated Parkinson's disease in central Norway. Annals of neurology. 2005;57:762–765. doi: 10.1002/ana.20456. [DOI] [PubMed] [Google Scholar]
- 55.Funayama M, Hasegawa K, Ohta E, Kawashima N, Komiyama M, Kowa H, Tsuji S, Obata F. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Annals of neurology. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 56.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature reviews. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]