Abstract
Background
Research studies examining foods are important, because they account for biological interactions that might otherwise be lost in the analysis of individual nutrients. Single-nutrient studies are also needed to explore the mechanisms by which foods may be protective.
Objective
Our objective was to examine associations between whole grains, refined grains, and cereal fiber and chronic disease risk factors.
Design
In a cross-sectional analysis of participants in the Baltimore Longitudinal Study of Aging, associations between dietary intakes and risk factors were examined with multivariate linear regression analysis. Dietary intakes were assessed with 7-d dietary records and quantified in g/d.
Results
Compared with subjects in the lowest quintile (Q1) of whole-grain intake, subjects in the highest quintile (Q5) had lower body mass index (BMI; in kg/m2; Q1: 25.5; Q5: 24.8; P for trend <0.0001) and weight (Q1: 75.0 kg; Q5: 72.4 kg; P for trend = 0.004) and smaller waist circumference (Q1: 87.4 cm; Q5:= 85.0 cm; P for trend = 0.002). Whole grains were also inversely associated with total cholesterol (P for trend = 0.02), LDL cholesterol (P for trend = 0.04), and 2-h glucose (P for trend = 0.0006). Associations between cereal fiber and anthropometrics and plasma lipids were similar. In subgroup analyses, refined grains were positively associated with fasting insulin among women (P for trend = 0.002).
Conclusions
Similar associations of whole grains and cereal fiber with weight, BMI, waist circumference, plasma cholesterol, and 2-h glucose were observed, suggesting that cereal fiber and its constituents may in part mediate these relations. Refined grains were associated with fasting insulin among women but not men. Additional research should explore potential interaction effects with BMI, sex, age, and genes.
Keywords: Whole grains, refined grains, fiber, diet records, risk factors
Introduction
Carbohydrate nutrition, including food sources, chemical structures, and physiologic properties, is an important area of research. The role of nondigestible polysaccharides (ie, fiber) in a healthy diet has been appreciated for many decades (1), leading to statements by both the American Dietetic Association (2) and the Council on Scientific Affairs (3) that fiber consumption is related to decreased risk of several diseases, including colon cancer, heart disease, diabetes, diverticulosis, and obesity. More recently, significant associations were observed between whole-grain intakes and cardiovascular disease and stroke (4), cancer (5, 6), diabetes (7, 8), obesity (9), and the metabolic syndrome (10, 11). Refined grains are the counterpart to whole grains, but evidence is conflicting about associations with metabolic and anthropometric variables (10–13), although some evidence shows that consumption of refined grains is associated with a risk of cancer (14, 15).
Whole grains contain many bioactive components that might be responsible for their protective effect, including fiber, resistant starch, and oligosaccharides, as well as vitamins, minerals, phytate, phytoestrogens, and phytosterols (16). Research studies examining food groups such as whole grains are important, because they account for biological interactions that might otherwise be lost in an analysis of individual nutrients. However, studies examining single nutrients such as cereal fiber are also needed to increase our understanding of the mechanisms by which whole grains may be protective. Additional research is also needed to further examine whether intake of refined grains is associated with risk factors for chronic disease.
The objective of this study was to examine associations of the intakes of whole grains, refined grains, and cereal fiber measured with 7-d dietary records and quantified in gram weights with selected risk factors for chronic disease among adults participating in the Baltimore Longitudinal Study on Aging (BLSA). An additional goal was to explore whether associations with risk factors were modified by sex or body mass index (BMI; in kg/m2).
Subjects and Methods
Study population
The BLSA is an open prospective cohort study that began in 1958 with the goals of studying the physical, mental, and emotional effects of aging among healthy, active persons; the original study design and data collection have been described in detail elsewhere (17). Briefly, initial study participants were white male community-dwelling volunteers 27–88 y of age living in Baltimore, Maryland. The study protocol was expanded in 1978 to include women and minorities. Participants return approximately every 12–24 mo for repeated measurements (eg, height, weight, body composition analysis). Subjects were also invited to participate in the (optional) dietary assessment portion of the study, in which 7-d diet records were used to assess dietary intakes. The Institutional Review Boards of the Johns Hopkins Bayview Medical Research Center and the Gerontology Center approved the BLSA protocol, and all subjects gave written informed consent for their participation.
Initially, 1572 persons completed at least one 7-d diet record during the course of the study. Of those, we excluded subjects who had not completed ≥4 d of the record, as well as those with implausible energy intakes or statistical outliers for total energy intake (<2510 or >16 736 kJ/d for women; <3347 and >17 572 kJ/d for men) or who were missing information on age (n = 53). Our baseline population therefore included 1516 participants. Because of the limited number of persons with both repeated dietary data and outcome measures, this analysis only included participants from the first visit at which participants had complete dietary and outcome data. Thus, for each outcome, our sample size varied somewhat, as follows: BMI and weight (n = 1502), waist circumference (n = 1404), total cholesterol (n = 1444), HDL cholesterol (n = 1029), LDL cholesterol (n = 1025), triacylglycerols (n = 1430), diastolic and systolic blood pressures (n = 1464), fasting glucose (n = 1324), 2-h glucose (n = 882), fasting insulin (n = 460), and 2-h insulin (n = 455).
Dietary assessment
Dietary intake was assessed by 7-d dietary records, and subjects were instructed by trained dietitians how to assess portion size, weigh foods, and complete the records. Reports that detail the dietary collection methods and dietary intake in the BLSA population were published previously (18, 19). Food records were completed at home by the participant and sent back to the study center. Before 1993, subjects were given food models and a booklet of food pictures to help them assess portion size. Since 1993, subjects were given a portable scale to weigh food portions. Participants were contacted by telephone with any questions about their records.
Dietary records were originally coded and entered into a nutrient database maintained by the BLSA, whereas diet records completed since 1993 were coded and entered into the Minnesota Nutrient Database at Tufts University. Dietary data collected before 1993 were then reentered in the Minnesota Nutrient Database, and nutrient intakes were back adjusted to correct for changes in the food supply (eg, nutrient content because of fortification of cereals) with data from the US Department of Agriculture to correspond to appropriate time intervals (20, 21).
Measurements of whole grains, refined grains, and cereal fiber
For this study, we used all available dietary data from the BLSA to create a whole-grains database. In brief, all foods containing grains or mixed dishes with foods containing grains, either whole or refined, were identified and then assigned the best-matched pyramid code from the Pyramid Servings Database, a reference database of servings per 100 g from 30 food groups, including 3 grain groups (total grain, whole grain, and nonwhole grain). We then used the Continuing Survey of Food Intakes by Individuals 1994–1996 recipe database to disaggregate mixed dishes that contained grains (either whole or refined) to obtain gram weights of individual ingredients. Some foods could not be disaggregated to the ingredient level, in which case the reference gram amount of grains was determined by multiplying by the pyramid definition of a serving (eg, a grain serving of one slice of bread contains 16 g of flour and a grain serving of one serving of cereal contains 28 g of flour). Once the reference database was completed, we calculated the absolute amount of grains consumed by multiplying the gram amount eaten by the reference values/100 for each day of the dietary record, and values for all days were summed and divided by the total number of days of dietary records to obtain an average intake.
Cereal fiber was measured by summing the fiber content from cereal foods, which includes the starchy grains produced from grass plants. Thus, our quantification of cereal fiber included fiber from whole grains (eg, wheat, rice, corn, oats, etc) and all foods made from grains, including ready-to-eat and hot cereals, bread, pasta, crackers, sweet baked goods, and salty snacks. Whole grains, refined grains, and cereal fiber were adjusted for total energy intake with the nutrient residual method.
Outcome assessment
Our outcome variables included various risk factors for chronic disease. Anthropometric and clinical measurements were obtained by following standardized procedures (22) that have been fully described elsewhere (23). In summary, weight and height were measured for each subject at each visit, from which BMI was calculated. Waist circumference was measured with the use of an inelastic tape at the narrowest part of the torso at the end of expiration (23), roughly equivalent to the bottom of the ribcage for most persons. Blood pressure was measured from a sitting position with the use of a standard protocol (24).
For lipid measurements, an antecubital venous blood sample was drawn from study subjects after an overnight fast. As previously described (25, 26), concentrations of triacylglycerols and total cholesterol were measured by enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer; Irving, TX). HDL cholesterol was measured by the dextran sulfate–magnesium precipitation procedure (27), and LDL cholesterol was estimated by the Friedewald formula (28).
As described previously, fasting plasma glucose and 2-h glucose concentrations were obtained with the use of an oral glucose tolerance test after overnight fasting, in which the glucose dose was 40 g/m2 body surface area, corresponding to an average dose of 78 g in men and 68 g in women (29). Plasma glucose was measured by the glucose oxidase method (Beckman Instruments, Fullerton, CA). Plasma insulin was measured in duplicate by radioimmunoassay (30).
Covariate assessment
Race-ethnicity, physical activity, smoking, education, and vitamin supplement use were determined by questionnaire at the time dietary records were collected. Physical activity was measured by an adapted version of the Harvard Alumni questionnaire, which asked participants about all daily activities (eg, activities at home, work, and during recreation or sports). The amount of time spent for each activity was summed across all activities to determine the daily energy output per body weight (in kJ/kg) and was described previously (18, 32).
Statistical analysis
We first examined the relations between the intakes of whole grains, refined grains, and cereal fiber and the sample characteristics at the time of the first visit, as well as associations with nutrient intakes. We also explored associations between the 3 dietary variables with the use of Spearman correlation coefficients. Our main analyses used multivariate linear regression to estimate separately the relation between whole grains, refined grains, and cereal fiber with each of our outcome variables. As such, we built separate regression models for each dietary variable, and each was divided into quintiles of intake. For example, we fit models estimating the relation between whole grains (in quintiles) and each risk factor (BMI, weight, waist circumference, total cholesterol, HDL cholesterol, LDL cholesterol, diastolic blood pressure, systolic blood pressure, fasting glucose, 2-h glucose, fasting insulin, and 2-h insulin). Similar models were fit for refined grains and cereal fiber. We used a generalized linear model to estimate the least-squares means for each outcome, and each model was adjusted for age, sex, race, education, decade of visit, vitamin supplement use, total energy, percentage of energy from saturated fat, and alcohol. The whole-grain models were further adjusted for intakes of refined grains, and the refined-grain models were further adjusted for intakes of whole grains.
Models that predicted lipid outcomes were further adjusted for BMI, use of lipid-lowering medication, and diagnosis of hypercholesterolemia. Models that predicted blood pressure were further adjusted for BMI, use of blood pressure–lowering medication, and diagnosis of hypertension. Models that predicted glucose and insulin outcomes were further adjusted for BMI, use of oral hypoglycemic medication, and diagnosis of diabetes. We also repeated the analyses for the above outcomes excluding subjects rather than adjusting for these variables in the analysis (ie, subjects with a diagnosis of diabetes were excluded from the glucose and insulin analyses) to remove the possibility of residual confounding. We created interaction terms to test whether the effects of the intakes of whole grains, refined grains, and cereal fiber on risk factors were modified by sex in all models and whether the effects were modified by BMI in our models that estimated fasting glucose, 2-h glucose, fasting insulin, and 2-h insulin.
We performed a number of additional analyses to test the robustness of our models. First, we tested models for each relation with further adjustment for physical activity on a limited subset of subjects for whom such data were available. In addition, we tested all associations limiting our dataset to subjects entering the study in 1980 or later to reduce the potential of a cohort effect. We also added a quadratic term for age to our models to determine whether it improved model fit. All analyses were performed with the use of SAS for WINDOWS, version 9.1 (SAS Institute, Cary, NC).
Results
Age was positively related to the intakes of whole grains and cereal fiber (P for trend < 0.0001) and inversely related to the intake of refined grains (P for trend = 0.004) (Table 1). BMI was inversely related to whole-grain intake (P for trend = 0.001), and subjects in the highest quintile of whole-grain intake had the lowest prevalence of overweight (39% compared with 54% in the lowest quintile; P = 0.0003); similar associations were seen for cereal fiber intake. The highest percentages of women were found in the highest quintile of whole-grain intake (40% compared with 15% in the lowest quintile; P < 0.0001) and cereal fiber intake (38% compared with 21% in the lowest quintile; P < 0.0001) and in the lowest quintile of refined-grain intake (56% compared with 18% in the highest quintile; P < 0.0001).
Table 1.
Sample characteristics of 1516 men and women participating in the Baltimore Longitudinal Study on Aging at the time of the first visit at which participants had complete dietary data1
| Whole grains2 | Refined grains3 | Cereal fiber4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample characteristic | Q1 | Q5 | P5 | Q1 | Q5 | P5 | Q1 | Q5 | P5 |
| Age (y)6 | 52.4 ± 1.07 | 63.3 ± 0.9 | <0.0001 | 59.4 ± 1.0 | 55.4 ± 1.0 | 0.004 | 53.3 ± 1.0 | 63.8 ± 0.9 | <0.0001 |
| BMI (kg/m2)8 | 25.5 ± 0.2 | 24.5 ± 0.2 | 0.001 | 24.9 ± 0.2 | 25.2 ± 0.2 | 0.51 | 25.7 ± 0.2 | 24.3 ± 0.2 | <0.0001 |
| Weight (kg)8 | 74.9 ± 0.6 | 72.9 ± 0.7 | 0.01 | 73.5 ± 0.7 | 73.9 ± 0.7 | 0.78 | 75.5 ± 0.7 | 72.2 ± 0.7 | 0.001 |
| Waist circumference (cm)8 | 86.0 ± 0.7 | 83.5 ± 0.6 | 0.0006 | 84.1 ± 0.6 | 85.6 ± 0.7 | 0.10 | 86.1 ± 0.6 | 82.9 ± 0.6 | <0.0001 |
| Physical activity (kcal/kg)8 | 13.8 ± 0.3 | 14.3 ± 0.4 | 0.13 | 13.3 ± 0.3 | 15.0 ± 0.3 | 0.0005 | 13.4 ± 0.3 | 14.7 ± 0.3 | 0.007 |
| BMI ≥ 25 (%) | 54 | 39 | 0.0003 | 45 | 48 | 0.89 | 55 | 38 | 0.0003 |
| Hypertension (%) | 10 | 10 | 0.35 | 7 | 7 | 0.62 | 9 | 7 | 0.06 |
| Female (%) | 15 | 40 | <0.0001 | 56 | 18 | <0.0001 | 21 | 38 | <0.0001 |
| White (%) | 89 | 86 | 0.26 | 90 | 87 | 0.22 | 87 | 88 | 0.06 |
| Vitamin users (%) | 14 | 38 | <0.0001 | 32 | 31 | 0.30 | 22 | 37 | 0.001 |
| Current smokers (%) | 26 | 4 | <0.0001 | 10 | 12 | 0.23 | 22 | 4 | <0.0001 |
| College graduate (%) | 65 | 71 | 0.37 | 67 | 70 | 0.03 | 68 | 71 | 0.004 |
Sample sizes differed because of missing data, as follows: BMI and weight (n = 1500), waist circumference (n = 1106), vitamin use (n =1185), education (n = 1500), and physical activity (n = 1028). Nutrients are adjusted for total energy intake with the use of the residual approach. Q, quintile.
Median intake: Q1, 0.68 g/d; Q5, 45.8 g/d.
Median intake: Q1, 39.0 g/d; Q5, 102.7 g/d.
Median intake: Q1, 2.2 g/d; Q5, 9.5 g/d.
Associations with continuous variables were examined with linear regression analysis (generalized linear model) and a test for trend in which subjects in each quintile were assigned the median value of intake. Associations with categorical variables were examined with a chi-square analysis.
Adjusted for sex.
x̄ ± SE (all such values).
Adjusted for age and sex.
Intake of whole grains was positively related to intakes of total energy, percentage of energy from carbohydrates, total fiber, folate, magnesium, vitamin E, and vitamin B-6 (P < 0.0001 for all) and inversely related to percentage of energy from total and saturated fats, alcohol, and cholesterol (P < 0.0001 for all); similar associations were observed with cereal fiber (P < 0.0001 for all), with the exception of vitamin B-6 (P = 0.20) (Table 2). Intake of refined grains was positively related to total energy intake and to percentage of energy from carbohydrates (P < 0.0001 for both) and inversely related to percentage of energy from protein, total fat, saturated fat, fiber, alcohol, and magnesium (P < 0.05 for all). In addition, whole grains were positively correlated with cereal fiber (r = 0.77, P < 0.0001) and negatively correlated with refined grains (r = −0.18, P < 0.0001) (data not shown).
Table 2.
Dietary intakes of 1516 men and women participating in the Baltimore Longitudinal Study on Aging at the time of the first visit at which participants had complete dietary data1
| Whole grains2 | Refined grains3 | Cereal fiber4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutrient intake | Q1 | Q5 | P5 | Q1 | Q5 | P5 | Q1 | Q5 | P5 |
| Energy (kJ)6 | 7932 ± 1117 | 9125 ± 109 | <0.0001 | 7306 ± 102 | 9868 ± 102 | <0.0001 | 8879 ± 102 | 9537 ± 103 | <0.0001 |
| Carbohydrate (% of energy)8 | 41.4 ± 0.5 | 51.7 ± 0.5 | <0.0001 | 43.1 ± 0.65 | 50.5 ± 0.5 | <0.0001 | 40.1 ± 0.5 | 52.5 ± 0.5 | <0.0001 |
| Protein (% of energy)8 | 16.1 ± 0.2 | 16.2 ± 0.2 | 0.29 | 16.7 ± 0.2 | 15.2 ± 0.2 | <0.0001 | 16.6 ± 0.2 | 16.2 ± 0.2 | 0.84 |
| Total fat (% of energy)8 | 38.9 ± 0.4 | 32.2 ± 0.4 | <0.0001 | 36.8 ± 0.5 | 34.3 ± 0.4 | <0.0001 | 39.2 ± 0.4 | 31.8 ± 0.4 | <0.0001 |
| Saturated fat (% of energy)8 | 13.7 ± 0.2 | 10.8 ± 0.2 | <0.0001 | 12.7 ± 0.2 | 11.9 ± 0.2 | 0.001 | 13.8 ± 0.2 | 10.8 ± 0.2 | <0.0001 |
| Total fiber (g)8 | 13.9 ± 0.5 | 24.7 ± 0.5 | <0.0001 | 19.8 ± 0.7 | 18.6 ± 0.5 | 0.03 | 14.5 ± 0.5 | 25.9 ± 0.5 | <0.0001 |
| Alcohol (g)8 | 15.6 ± 1.0 | 8.1 ± 1.1 | <0.0001 | 17.3 ± 1.2 | 11.2 ± 0.9 | <0.0001 | 17.5 ± 0.9 | 7.4 ± 0.9 | <0.0001 |
| Cholesterol (mg)8 | 224 ± 2 | 206 ± 2 | <0.0001 | 218 ± 3 | 218 ± 2 | 0.08 | 227 ± 2 | 203 ± 2 | <0.0001 |
| Folate (mg)8 | 315 ± 134 | 525 ± 13 | <0.0001 | 435 ± 17 | 380 ± 14 | 0.33 | 339 ± 13 | 531 ± 13 | <0.0001 |
| Magnesium (mg)8 | 243 ± 5 | 382 ± 5 | <0.0001 | 322 ± 7 | 291 ± 6 | <0.001 | 254 ± 5 | 397 ± 5 | <0.0001 |
| Vitamin E (mg)8 | 24.8 ± 6.1 | 63.1 ± 5.4 | <0.0001 | 39.7 ± 5.5 | 30.1 ± 5.4 | 0.54 | 26.9 ± 5.5 | 60.9 ± 5.5 | <0.0001 |
| Vitamin B-6 (mg)8 | 3.0 ± 0.4 | 6.2 ± 0.6 | <0.0001 | 3.7 ± 0.6 | 3.1 ± 0.6 | 0.89 | 3.0 ± 0.6 | 3.9 ± 0.6 | 0.20 |
n = 230 or 231 per quintile (Q).
Median intake: Q1, 0.68 g/d; Q5, 45.8 g/d.
Median intake: Q1, 39.7 g/d; Q5, 102.7 g/d.
Median intake: Q1, 2.2 g/d; Q5, 9.5 g/d.
Associations were examined with linear regression analysis (generalized linear model) and a test for trend in which subjects in each quintile were assigned the median value of intake.
Adjusted for age and sex.
x̄ ± SE (all such values).
Adjusted for age, sex, and total energy. Intakes from dietary supplements are not included.
Whole grains were significantly related to each of the anthropometric outcomes in multivariate adjusted models (Table 3). Compared with subjects in the lowest quintile (Q1) of intake, subjects in the highest quintile (Q5) had lower BMI (Q1: 25.5 kg/m2; Q5: 24.8 kg/m2; P for trend <0.0001) and weight (Q1: 75.0 kg; Q5: 72.6 kg; P for trend = 0.004) and smaller waist circumference (Q1: 87.4 cm, Q5:= 85.0 cm; P for trend = 0.002). Whole grains were also inversely associated with total cholesterol and LDL cholesterol (P for trend = 0.02 and P for trend = 0.04), as well as with 2-h glucose (P for trend = 0.0006). Associations with blood pressure, insulin, and fasting glucose variables were not significant. No interaction was observed between sex and whole grains in any of our models, nor was an interaction observed between BMI and whole grains in our models for fasting glucose, 2-h glucose, fasting insulin, or 2-h insulin (P > 0.05 for all interaction terms). Associations remained similar when further adjusted for physical activity.
Table 3.
Chronic disease risk factors according to quintile (Q) of whole-grain intakes among men and women participating in the Baltimore Longitudinal Study of Aging
| Whole-grain intake1 | ||||||
|---|---|---|---|---|---|---|
| Risk factor | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
| BMI (kg/m2) [n = 1502] | ||||||
| Simple adjusted2 | 25.7 ± 0.23 | 25.7 ± 0.2 | 25.0 ± 0.2 | 24.7 ± 0.2 | 24.2 ± 0.2 | <0.0001 |
| Multivariate adjusted4 | 25.5 ± 0.2 | 25.7 ± 0.2 | 25.0 ± 0.2 | 24.7 ± 0.2 | 24.8 ± 0.2 | <0.0001 |
| Weight (kg) [n = 1502] | ||||||
| Simple adjusted2 | 75.4 ± 0.7 | 75.0 ± 0.6 | 73.8 ± 0.6 | 72.0 ± 0.6 | 72.2 ± 0.7 | 0.0002 |
| Multivariate adjusted4 | 75.0 ± 0.7 | 75.1 ± 0.6 | 73.7 ± 0.6 | 73.0 ± 0.6 | 72.6 ± 0.7 | 0.004 |
| Waist circumference (cm) [n = 1404] | ||||||
| Simple adjusted2 | 87.8 ± 0.6 | 87.7 ± 0.6 | 85.8 ± 0.6 | 85.6 ± 0.6 | 84.4 ± 0.6 | <0.0001 |
| Multivariate adjusted4 | 87.4 ± 0.6 | 87.6 ± 0.6 | 85.7 ± 0.6 | 85.7 ± 0.6 | 85.0 ± 0.6 | 0.002 |
| Total cholesterol (mmol/L) [n = 1444] | ||||||
| Simple adjusted2 | 5.70 ± 0.06 | 5.64 ± 0.06 | 5.52 ± 0.06 | 5.51 ± 0.06 | 5.49 ± 0.06 | 0.01 |
| Multivariate adjusted4,5 | 5.71 ± 0.06 | 5.64 ± 0.05 | 5.52 ± 0.05 | 5.50 ± 0.05 | 5.49 ± 0.06 | 0.02 |
| HDL cholesterol (mmol/L) [n = 1029] | ||||||
| Simple adjusted2 | 1.26 ± 0.02 | 1.27 ± 0.02 | 1.29 ± 0.02 | 1.25 ± 0.02 | 1.23 ± 0.02 | 0.25 |
| Multivariate adjusted4,5 | 1.27 ± 0.02 | 1.28 ± 0.02 | 1.29 ± 0.02 | 1.25 ± 0.02 | 1.22 ± 0.02 | 0.07 |
| LDL cholesterol (mmol/L) [n = 1025] | ||||||
| Simple adjusted2 | 3.15 ± 0.06 | 3.00 ± 0.06 | 2.98 ± 0.06 | 2.97 ± 0.06 | 3.00 ± 0.06 | 0.14 |
| Multivariate adjusted4,5 | 3.16 ± 0.06 | 3.02 ± 0.06 | 2.99 ± 0.06 | 2.98 ± 0.06 | 2.96 ± 0.06 | 0.04 |
| Triacylglycerols (mmol/L) [n = 1430] | ||||||
| Simple adjusted2 | 1.26 ± 0.04 | 1.30 ± 0.04 | 1.20 ± 0.04 | 1.15 ± 0.04 | 1.12 ± 0.05 | 0.005 |
| Multivariate adjusted4,5 | 1.23 ± 0.05 | 1.25 ± 0.05 | 1.21 ± 0.05 | 1.18 ± 0.05 | 1.16 ± 0.05 | 0.22 |
| Diastolic blood pressure (mm Hg) [n = 1464] | ||||||
| Simple adjusted2 | 79.8 ± 0.6 | 80.8 ± 0.5 | 78.2 ± 0.5 | 80.6 ± 0.5 | 79.1 ± 0.6 | 0.04 |
| Multivariate adjusted4,6 | 79.8 ± 0.6 | 80.8 ± 0.6 | 78.2 ± 0.6 | 80.6 ± 0.6 | 79.2 ± 0.7 | 0.42 |
| Systolic blood pressure (mm Hg) [n = 1464] | ||||||
| Simple adjusted2 | 130.9 ± 1.1 | 130.7 ± 1.1 | 125.6 ± 1.1 | 130.6 ± 1.1 | 127.8 ± 1.1 | 0.12 |
| Multivariate adjusted4,6 | 129.2 ± 1.0 | 130.0 ± 0.9 | 126.9 ± 0.9 | 131.1 ± 0.9 | 128.3 ± 1.0 | 0.79 |
| Fasting glucose (mmol/L) [n = 1324] | ||||||
| Simple adjusted2 | 5.52 ± 0.06 | 5.62 ± 0.06 | 5.51 ± 0.06 | 5.45 ± 0.06 | 5.48 ± 0.06 | 0.21 |
| Multivariate adjusted4,7 | 5.49 ± 0.06 | 5.58 ± 0.06 | 5.51 ± 0.06 | 5.51 ± 0.06 | 5.49 ± 0.06 | 0.57 |
| 2-h Glucose (mmol/L) [n = 882] | ||||||
| Simple adjusted2 | 8.34 ± 0.18 | 7.62 ± 0.18 | 7.66 ± 0.18 | 7.79 ± 0.18 | 7.32 ± 0.18 | 0.002 |
| Multivariate adjusted4,7 | 8.24 ± 0.17 | 7.59 ± 0.16 | 7.63 ± 0.16 | 7.94 ± 0.16 | 7.32 ± 0.17 | 0.006 |
| Fasting insulin (mmol/L) [n = 460] | ||||||
| Simple adjusted2 | 74.5 ± 4.0 | 70.5 ± 3.9 | 72.2 ± 3.9 | 72.1 ± 3.9 | 68.9 ± 4.0 | 0.41 |
| Multivariate adjusted4,7 | 71.6 ± 3.9 | 70.0 ± 3.7 | 73.4 ± 3.7 | 71.3 ± 3.7 | 71.8 ± 4.1 | 0.90 |
| 2-h Insulin (mmol/L) [n = 455] | ||||||
| Simple adjusted2 | 534 ± 39.2 | 399 ± 38.8 | 357 ± 38.8 | 417 ± 38.8 | 381 ± 39.2 | 0.04 |
| Multivariate adjusted4,7 | 479 ± 38.0 | 416 ± 36.0 | 360 ± 35.7 | 421 ± 35.7 | 414 ± 39.1 | 0.43 |
Quintiles were developed separately for each outcome because of differences in sample sizes. Medan values for Q1 and Q5 were as follows: BMI and weight, 0.65 and 46.0 g/d, respectively; waist circumference, 0.94 and 49.3 g/d, respectively; total cholesterol, 0.63 and 45.6 g/d, respectively; HDL cholesterol, 4.1 and 54.0 g/d, respectively; LDL cholesterol and triacylglycerols, 3.9 and 54.8 g/d, respectively; blood pressure, 0.62 and 45.4 g/d, respectively; fasting glucose, 0.56 and 45.4 g/d, respectively; 2-h glucose, 1.1 and 50.6 g/d, respectively; fasting insulin, 2.2 and 51.5 g/d, respectively; 2-h insulin, 2.4 and 51.7 g/d, respectively.
Adjusted for age, age2, sex, total energy, and decade of visit.
x̄ ± SEM (all such values) estimated with least-squares means from a linear regression analysis (generalized linear model).
Adjusted for age, age2, sex, total energy, decade of visit, race, education, vitamin supplement use, smoking, and percentage of energy from saturated fat, alcohol, and refined grains.
With additional adjustment for BMI, use of lipid-lowering medication, and hypercholesterolemia.
With additional adjustment for BMI, use of blood pressure-lowering medication, and hypertension.
With additional adjustment for BMI, use of oral hypoglycemic medication, and diabetes.
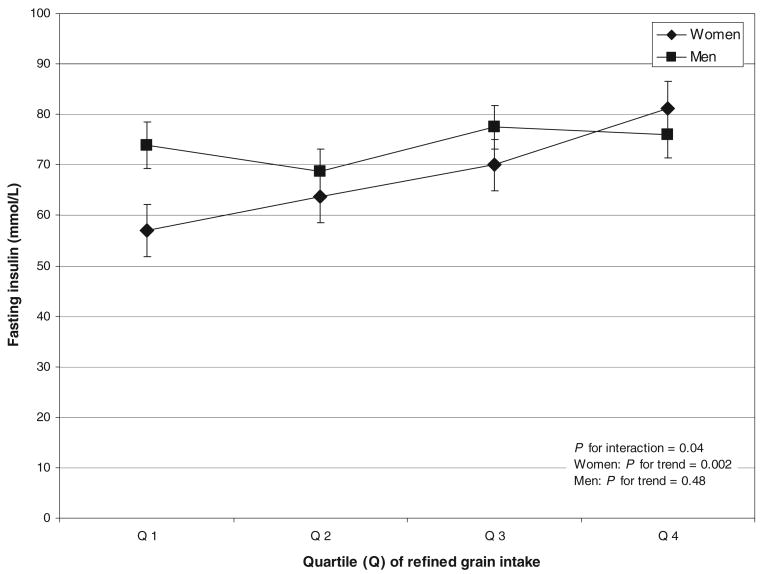
A significant interaction was observed between intake of refined grains and sex in relation to fasting insulin (P = 0.04). When a stratified analysis was performed with the use of quartiles of refined-grain intake (rather than quintiles, to preserve statistical power), a positive association was observed among women (quartile 1: 57.0; quartile 5: 81.1; P for trend = 0.002) (Figure 1), and the association remained significant and of similar magnitude when further adjusted for cereal fiber and physical activity, and when women with a diagnosis of diabetes were excluded from the analysis (data not shown). No association was seen among men (quartile 1: 73.9; quartile 4; 76.0; P for trend = 0.48). A significant interaction with sex was also detected in the analysis of refined grains and 2-h insulin (P = 0.03). However, associations among women (quartile 1: 289 mmol/L; quartile 5: 367 mmol/L; P for trend = 0.11) and men (quartile 1: 439 mmol/L; quartile 5: 528 mmol/L; P for trend = 0.29) were not significant in stratified analyses. No significant relations between refined grains and any of the other risk factors (BMI, weight, waist circumference, total cholesterol, HDL cholesterol, LDL cholesterol, triacylglycerols, systolic blood pressure, diastolic blood pressure, fasting glucose, 2-h glucose, fasting insulin, and 2-h insulin) were observed (P > 0.05 for all; data not shown).
Figure 1.
Association between quartiles of intake of refined grains (in g/d) and fasting insulin (in mmol/L) among 183 women and 277 men participating in the Baltimore Longitudinal Study of Aging. Because of differences in intakes, intake quartiles (Q1–Q4) were derived separately for women and men, as follows: women (Q1: 32.5; Q2: 46.7; Q3: 56.1; Q4: 73.7) and men (Q1: 49.4; Q2: 64.8; Q3; 80.8; Q4: 102.0). Values are x̄ ± SEM, estimated with the least-squares means from a linear regression analysis (generalized linear model). Models are adjusted for age, age2, total energy, decade of visit, race, education, vitamin supplement use, BMI, smoking, percentage of energy from saturated fat, alcohol, whole-grain intake, and diabetes diagnosis.
Similar to the findings with whole grains, cereal fiber was inversely associated with BMI (P for trend <0.0001), weight, (P for trend = 0.0004), waist circumference (P for trend <0.0001), and total cholesterol (P for trend = 0.005) (Table 4), and the associations remained significant when further adjusted for physical activity (data not shown). Cereal fiber was also inversely associated with 2-h glucose (Q1: 8.05 mmol/L; Q5: 6.48 mmol/L; P for trend = 0.02) in the multivariate-adjusted model but was no longer significant when further adjusted for physical activity (P for trend = 0.09). No interaction with sex or BMI was observed in any of the models. A quadratic term for age improved model fit (P < 0.001) and was retained in all models.
Table 4.
Chronic disease risk factors according to quintile (Q) of cereal fiber intake at the time of the first visit among men and women participating in the Baltimore Longitudinal Study of Aging
| Cereal fiber intake1 | ||||||
|---|---|---|---|---|---|---|
| Risk factor | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
| BMI (kg/m2) [n = 1502] | ||||||
| Simple adjusted2 | 25.9 ± 0.23 | 25.3 ± 0.2 | 24.4 ± 0.2 | 24.8 ± 0.2 | 24.1 ± 0.2 | <0.0001 |
| Multivariate adjusted4 | 25.7 ± 0.2 | 25.2 ± 0.2 | 24.3 ± 0.2 | 24.8 ± 0.2 | 24.3 ± 0.2 | <0.0001 |
| Weight (kg) [n = 1502] | ||||||
| Simple adjusted2 | 76.0 ± 0.6 | 74.3 ± 0.6 | 74.4 ± 0.6 | 73.4 ± 0.6 | 71.4 ± 0.7 | <0.0001 |
| Multivariate adjusted4 | 75.6 ± 0.7 | 74.1 ± 0.6 | 74.3 ± 0.6 | 73.0 ± 0.6 | 71.4 ± 0.8 | 0.0004 |
| Waist circumference (cm) [n = 1404] | ||||||
| Simple adjusted2 | 87.9 ± 0.6 | 87.2 ± 0.6 | 87.1 ± 0.6 | 85.7 ± 0.6 | 83.5 ± 0.6 | <0.0001 |
| Multivariate adjusted4 | 87.4 ± 0.6 | 86.9 ± 0.6 | 87.0 ± 0.6 | 86.0 ± 0.6 | 84.1 ± 0.6 | <0.0001 |
| Total cholesterol (mmol/L) [n = 1444] | ||||||
| Simple adjusted2 | 5.73 ± 0.06 | 5.61 ± 0.06 | 5.53 ± 0.06 | 5.57 ± 0.06 | 5.43 ± 0.07 | 0.001 |
| Multivariate adjusted4,5 | 5.73 ± 0.06 | 5.60 ± 0.06 | 5.51 ± 0.06 | 5.57 ± 0.06 | 5.44 ± 0.06 | 0.005 |
| HDL cholesterol (mmol/L) [n = 1029] | ||||||
| Simple adjusted2 | 1.23 ± 0.02 | 1.28 ± 0.02 | 1.28 ± 0.02 | 1.25 ± 0.02 | 1.26 ± 0.02 | 0.91 |
| Multivariate adjusted4,5 | 1.23 ± 0.02 | 1.30 ± 0.02 | 1.27 ± 0.02 | 1.25 ± 0.02 | 1.25 ± 0.02 | 0.59 |
| LDL cholesterol (mmol/L) [n = 1025] | ||||||
| Simple adjusted2 | 3.11 ± 0.06 | 3.07 ± 0.06 | 3.00 ± 0.06 | 2.91 ± 0.06 | 3.00 ± 0.06 | 0.09 |
| Multivariate adjusted4,5 | 3.13 ± 0.06 | 3.07 ± 0.06 | 3.01 ± 0.06 | 2.90 ± 0.06 | 2.99 ± 0.06 | 0.07 |
| Triacylglycerols (mmol/L) [n = 1430] | ||||||
| Simple adjusted2 | 1.27 ± 0.05 | 1.28 ± 0.04 | 1.18 ± 0.04 | 1.20 ± 0.04 | 1.11 ± 0.05 | 0.04 |
| Multivariate adjusted4,5 | 1.24 ± 0.05 | 1.26 ± 0.04 | 1.16 ± 0.04 | 1.23 ± 0.04 | 1.15 ± 0.05 | 0.12 |
| Diastolic blood pressure (mm Hg) [n = 1464] | ||||||
| Simple adjusted2 | 80.8 ± 0.7 | 80.7 ± 0.7 | 79.4 ± 0.7 | 79.6 ± 0.7 | 77.8 ± 0.8 | 0.009 |
| Multivariate adjusted4,6 | 79.5 ± 0.8 | 79.8 ± 0.6 | 79.1 ± 0.7 | 80.8 ± 0.7 | 79.2 ± 0.8 | 0.90 |
| Systolic blood pressure (mm Hg) [n = 1464] | ||||||
| Simple adjusted2 | 130.8 ± 1.1 | 130.1 ± 1.1 | 128.1 ± 1.0 | 128.9 ± 1.1 | 127.6 ± 1.1 | 0.06 |
| Multivariate adjusted4,6 | 128.7 ± 1.0 | 129.0 ± 0.9 | 127.7 ± 0.9 | 130.6 ± 0.9 | 129.7 ± 1.0 | 0.27 |
| Fasting glucose (mmol/L) [n = 1324] | ||||||
| Simple adjusted2 | 5.64 ± 0.06 | 5.49 ± 0.06 | 5.54 ± 0.06 | 5.44 ± 0.06 | 5.47 ± 0.06 | 0.12 |
| Multivariate adjusted4,7 | 5.55 ± 0.05 | 5.48 ± 0.05 | 5.53 ± 0.05 | 5.49 ± 0.05 | 5.52 ± 0.05 | 0.95 |
| 2-h Glucose (mmol/L) [n = 882] | ||||||
| Simple adjusted2 | 8.23 ± 0.18 | 8.03 ± 0.18 | 7.63 ± 0.18 | 7.44 ± 0.18 | 7.40 ± 0.18 | 0.0008 |
| Multivariate adjusted4,7 | 8.05 ± 0.21 | 7.94 ± 0.20 | 7.72 ± 0.19 | 7.55 ± 0.20 | 6.48 ± 0.21 | 0.02 |
| Fasting insulin (mmol/L) [n = 460] | ||||||
| Simple adjusted2 | 70.1 ± 4.0 | 73.6 ± 3.9 | 72.4 ± 3.9 | 71.5 ± 4.0 | 70.6 ± 4.0 | 0.60 |
| Multivariate adjusted4,7 | 68.9 ± 4. | 72.2 ± 3.8 | 73.0 ± 3.7 | 71.3 ± 3.8 | 73.0 ± 4.0 | 0.68 |
| 2-h Insulin (mmol/L) [n = 455] | ||||||
| Simple adjusted2 | 492 ± 39.5 | 469 ± 38.9 | 387 ± 38.7 | 366 ± 39.3 | 374 ± 39.1 | 0.02 |
| Multivariate adjusted4,7 | 438 ± 38.8 | 477 ± 36.2 | 404 ± 35.8 | 356 ± 36.3 | 413 ± 38.2 | 0.33 |
Quintiles were developed separately for each outcome because of differences in sample sizes. Values for Q1 and Q5 were as follows: BMI and weight, 2.19 and 9.51 g/d, respectively; waist circumference, 2.31 and 9.74 g/d, respectively; total cholesterol, 2.20 and 9.56 g/d, respectively; HDL and LDL cholesterol, 2.90 and 11.49 g/d, respectively; triacylglycerols, 2.20 and 9.58 g/d, respectively; blood pressure, 2.19 and 9.43 g/d, respectively; fasting glucose, 2.27 and 9.67 g/d, respectively; 2-h glucose, 2.39 and 10.42 g/d, respectively; fasting insulin and 2-h insulin, 2.54 and 11.63 g/d, respectively.
Adjusted for age, age2, sex, total energy, and decade of visit.
x̄ ± SEM (all such values) estimated with least-squares means from a linear regression analysis (generalized linear model).
Adjusted for age, age2, sex, total energy, decade of visit, race, education, vitamin supplement use, smoking, and percentage of energy from saturated fat and alcohol.
With additional adjustment for BMI, use of lipid-lowering medication, and hypercholesterolemia.
With additional adjustment for BMI, use of blood pressure-lowering medication, and hypertension.
With additional adjustment for BMI, use of oral hypoglycemic medication, and diabetes.
Discussion
One of the main objectives of this study was to examine independent associations between whole grains and cereal fiber with risk factors for chronic disease. We found that whole grains were significantly inversely associated with anthropometric variables (weight, BMI, and waist circumference), plasma lipid measures (total cholesterol and LDL cholesterol), and 2-h glucose. Cereal fiber was also related to these variables, and the magnitude of effects was similar. The aleurone layer and cell walls of whole grains, largely composed of nonstarch polysaccharides (32), also include many additional bioactive components such as phytochemicals (eg, phytic and phenolic acids), phytoestrogens (eg, lignans and isoflavones), antioxidants, vitamins, and minerals (eg, potassium, magnesium, and selenium) (33). Indeed, many of these bioactive constituents are insoluble and bound to cell wall materials (34), and they act independently and synergistically to confer many protective health effects (16, 35–38). Our findings suggest that cereal fiber, its bioactive components, or both may mediate the associations with whole grains examined in this study.
Several other studies have shown similar inverse associations with anthropometric variables for whole grains (11, 12, 39) and cereal fiber (40, 41). Fiber contributes to satiation, satiety, and the secretion of gut hormones (9), thereby affecting body weight and body composition through its effect on energy intake. McKeown et al (10) also observed comparable associations for whole grains (odds ratio: 0.67; 95% CI: 0.48, 0.91) and cereal fiber (odds ratio: 0.62; 95% CI: 0.45, 0.86) with risk of metabolic syndrome. Unlike other studies (11, 39, 41), our study showed significant inverse associations between whole grains and cereal fibers with total cholesterol, and whole grains were also inversely associated with LDL cholesterol. Jensen et al (42) also reported a significant inverse association between whole grains and total cholesterol (P = 0.02). The relation between dietary fiber and cholesterol is thought to be mainly due to soluble fibers rather than insoluble fibers (43, 44), but phytosterols (16) and phytoestrogens (35) may also affect cholesterol metabolism. No significant associations were observed between whole grains and blood pressure in our study, as seen in 2 other cross-sectional studies (11, 39), but a small intervention study observed a decline in blood pressure after a whole-grain diet (45). Cereal fiber intake was also unrelated to blood pressure in our study. Although 2 meta-analyses reported a small but significant inverse association between total fiber and blood pressure (46, 47), neither specifically addressed the role of cereal fiber, so whether cereal fiber specifically is associated with blood pressure requires more research.
Antioxidants and minerals present in whole grains and cereal fiber may contribute to insulin sensitivity (9, 48), and fiber may also be related to insulin sensitivity through effects on delayed gastric emptying. We observed a significant inverse association between whole grains and cereal fiber and 2-h glucose, and the magnitude of the effects was quite similar for both variables. McKeown et al (39) observed null associations between whole grains and 2-h glucose and 2-h insulin in a cross-sectional study of persons in the Framingham Offspring Study, and whole grains were also unrelated to fasting insulin in 2 large cohort studies (42). Although Lairon et al (41) saw no significant association between whole grains and fasting glucose, Sahyoun et al (11) showed an inverse dose-response relation (P for trend = 0.01). A small crossover study among overweight persons reported improved insulin sensitivity after an intervention with cereal fiber (49), and several other studies have shown significant associations between whole grains and plasma glucose (39) and insulin sensitivity (50, 51) among subjects with higher BMI, suggesting effect modification. Inconsistent findings on associations of whole grains or cereal fiber in relation to insulin or glucose measures may be due to differences in study design and dietary assessment methods, inadequate testing of interaction effects, or chance.
We observed a significant positive association between refined grains and fasting insulin among women but not men. Interaction effects are more likely to occur by chance and often are not reproducible (52), so our findings need to be interpreted with caution. However, our result is supported by recent results from the Hypertension Genetic Epidemiology Network study, which found a quantitative trait locus influencing fasting insulin in female, but not male, subjects (53). Refined grains are major contributors to glycemic load, and several reviews support a positive association between glycemic load and insulin resistance or glycemic control (54–56). That the percentage of energy from total carbohydrate in the highest quintile of refined grains (50.5%) and whole grains (51.7%) is so similar underscores the importance of differentiating between whole and refined foods when making dietary choices. Indeed, a small randomized crossover feeding study among overweight subjects with hyperinsulinemia observed 10% lower fasting insulin when consumption with refined grains were replaced with whole grains (57), as seen in a trial that included a reduction in refined grains as part of a comprehensive dietary intervention (58, 59). Note also that we observed a significant interaction between refined grains and sex in relation to 2-h insulin. Although the observed effects were in the expected direction, we likely had inadequate power to achieve statistical significance in stratified analyses.
Refined grains were not significantly associated with any other risk factor in this study. As mentioned, findings about refined grains and risk factors for chronic disease are inconsistent. Decreasing intake of refined grains was associated with a smaller weight change in one study (12), whereas increasing intake of refined-grain breakfast cereals was inversely related to weight gain and BMI in another (13). Refined grains were positively associated with fasting glucose among older adults (11) and hypertriglyceridemic waist phenotype (60). Refined grains were also positively associated with risk of metabolic syndrome in 2 cross-sectional studies (10, 11), but neither showed associations with individual risk factors. Those results and ours suggest that the effect of refined grains on measures of insulin and glucose may be modified by age (11), BMI (4, 33), and sex, probably due to differences in glucose tolerance and insulin sensitivity.
There are several limitations to our study. First, our study is cross-sectional; thus, our results do not allow us to make inferences about the directions of our associations and may be obfuscated by reverse causation. Although a longitudinal study would be stronger, our data are limited by the small numbers of subjects who have repeated measures of both dietary and risk factor data. An additional limitation is that our study sample, which is selected from an open cohort study, includes subjects across several decades. However, a cohort effect was not observed in an earlier study in this population (19). In addition, our models were adjusted for decade of the visit, and results remained similar when we limited our study to data from 1980 or later. Our study has a small sample size for some of our outcome variables; thus, we probably had insufficient power to detect and explore potential interactions. Finally, our study did not include markers of genetic variability, which are increasingly understood to modify the effect of diet on disease risk factors such as plasma lipids (61).
In conclusion, our study shows significant and similar associations of whole grains and cereal fiber with weight, BMI, waist circumference, and total cholesterol. Refined grains were positively associated with fasting insulin among women but not men. Longitudinal studies are needed to reproduce these findings, and special attention should be dedicated to exploring potential interactions with BMI, sex, age, and genes.
Acknowledgments
We thank Nicola McKeown, PhD, for helpful comments on this manuscript.
Footnotes
Supported by the USDA contract 58-1950-7-707; the Intramural Research Program of the NIH, National Institute on Aging; and General Mills Bell Institute of Health and Nutrition. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
None of the authors had a financial interest or professional or personal affiliation that compromises the scientific integrity of this work.
The author's responsibilities were as follows—PKN: was responsible for the design and analysis for this report and drafted the manuscript; JM and PB: created the database for this study; DM and LF: contributed to data collection for the Baltimore Longitudinal Study of Aging; KLT: contributed to the database development and oversaw the HNRCA collaboration with the BLSA. All authors made critical comments during the preparation of the manuscript and fully accept responsibility for the work.
References
- 1.Bing FC. Dietary fiber–in historical perspective. J Am Diet Assoc. 1976;69:498–505. [PubMed] [Google Scholar]
- 2.Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 3.Dietary fiber and health. Council on Scientific Affairs. JAMA. 1989;262:542–6. [PubMed] [Google Scholar]
- 4.Flight I, Clifton P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. Eur J Clin Nutr. 2006;60:1145–59. doi: 10.1038/sj.ejcn.1602435. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs DR, Jr, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. 1998;30:85–96. doi: 10.1080/01635589809514647. [DOI] [PubMed] [Google Scholar]
- 6.Chatenoud L, Tavani A, La Vecchia C, et al. Whole grain food intake and cancer risk. Int J Cancer. 1998;77:24–8. doi: 10.1002/(sici)1097-0215(19980703)77:1<24::aid-ijc5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Venn BJ, Mann JI. Cereal grains, legumes and diabetes. Eur J Clin Nutr. 2004;58:1443–61. doi: 10.1038/sj.ejcn.1601995. [DOI] [PubMed] [Google Scholar]
- 8.Murtaugh MA, Jacobs DR, Jr, Jacob B, Steffen LM, Marquart L. Epidemiological support for the protection of whole grains against diabetes. Proc Nutr Soc. 2003;62:143–9. doi: 10.1079/pns2002223. [DOI] [PubMed] [Google Scholar]
- 9.Koh-Banerjee P, Rimm EB. Whole grain consumption and weight gain: a review of the epidemiological evidence, potential mechanisms and opportunities for future research. Proc Nutr Soc. 2003;62:25–9. doi: 10.1079/PNS2002232. [DOI] [PubMed] [Google Scholar]
- 10.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–46. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 11.Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr. 2006;83:124–31. doi: 10.1093/ajcn/83.1.124. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003;78:920–7. doi: 10.1093/ajcn/78.5.920. [DOI] [PubMed] [Google Scholar]
- 13.Bazzano LA, Song Y, Bubes V, Good CK, Manson JE, Liu S. Dietary intake of whole and refined grain breakfast cereals and weight gain in men. Obes Res. 2005;13:1952–60. doi: 10.1038/oby.2005.240. [DOI] [PubMed] [Google Scholar]
- 14.Slattery ML, Potter JD, Coates A, et al. Plant foods and colon cancer: an assessment of specific foods and their related nutrients (United States) Cancer Causes Control. 1997;8:575–90. doi: 10.1023/a:1018490212481. [DOI] [PubMed] [Google Scholar]
- 15.Levi F, Pasche C, Lucchini F, Chatenoud L, Jacobs DR, Jr, La Vecchia C. Refined and whole grain cereals and the risk of oral, oesophageal and laryngeal cancer. Eur J Clin Nutr. 2000;54:487–9. doi: 10.1038/sj.ejcn.1601043. [DOI] [PubMed] [Google Scholar]
- 16.Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62:129–34. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 17.Shock N, Greulich R, Andres R, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984. [Google Scholar]
- 18.McGandy R, Barrows C, Spanias A, Meredith A, Stone J, Norris A. Nutrient intakes and energy expenditure in men of different ages. J Gerontol. 1966;21:581–7. doi: 10.1093/geronj/21.4.581. [DOI] [PubMed] [Google Scholar]
- 19.Elahi VK, Elahi D, Andres R, Tobin JD, Butler MG, Norris AH. A longitudinal study of nutritional intake in men. J Gerontol. 1983;38:162–80. doi: 10.1093/geronj/38.2.162. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture Nutrient Database for Standard Reference, releases 10 and 11. Washington, DC: US Government Printing Office; 1993. [Google Scholar]
- 21.Watt B, Merrill A. Composition of foods: raw, processed, prepared. Washington, DC: US Department of Agriculture; 1963. [Google Scholar]
- 22.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 23.Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R. Studies in the distribution of body fat: I. Effects of age, sex, and obesity. J Gerontol. 1989;44:M66–73. doi: 10.1093/geronj/44.2.m66. [DOI] [PubMed] [Google Scholar]
- 24.Pearson JD, Morrell CH, Brant LJ, Landis PK, Fleg JL. Age-associated changes in blood pressure in a longitudinal study of healthy men and women. J Gerontol A Biol Sci Med Sci. 1997;52:M177–83. doi: 10.1093/gerona/52a.3.m177. [DOI] [PubMed] [Google Scholar]
- 25.Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Effect of aging on the relationship between multiple risk factors and waist circumference. J Am Geriatr Soc. 2000;48:788–94. doi: 10.1111/j.1532-5415.2000.tb04754.x. [DOI] [PubMed] [Google Scholar]
- 26.Iwao N, Iwao S, Muller DC, Elahi D, Shimokata H, Andres R. A test of recently proposed BMI standards with respect to old age. Aging (Milano) 2000;12:461–9. doi: 10.1007/BF03339878. [DOI] [PubMed] [Google Scholar]
- 27.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism. 2005;54:542–7. doi: 10.1016/j.metabol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Soeldner JS, Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technique. Diabetes. 1965;14:771–9. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- 31.Shimokata H, Muller D, Fleg J, Sorkin J, Ziemba A, Andres R. Age as independent determinant of glucose tolerance. Diabetes. 1991;40:44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 32.Selvendran RR. The plant cell wall as a source of dietary fiber: chemistry and structure. Am J Clin Nutr. 1984;39:320–37. doi: 10.1093/ajcn/39.2.320. [DOI] [PubMed] [Google Scholar]
- 33.Truswell AS. Cereal grains and coronary heart disease. Eur J Clin Nutr. 2002;56:1–14. doi: 10.1038/sj.ejcn.1601283. [DOI] [PubMed] [Google Scholar]
- 34.Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem. 2002;50:6182–7. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 35.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129(suppl):758S–67S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 36.Slavin JL, Jacobs D, Marquart L, Wiemer K. The role of whole grains in disease prevention. J Am Diet Assoc. 2001;101:780–5. doi: 10.1016/S0002-8223(01)00194-8. [DOI] [PubMed] [Google Scholar]
- 37.Thompson LU. Antioxidants and hormone-mediated health benefits of whole grains. Crit Rev Food Sci Nutr. 1994;34:473–97. doi: 10.1080/10408399409527676. [DOI] [PubMed] [Google Scholar]
- 38.Flight I, Clifton P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. Eur J Clin Nutr. 2006;60:1145–59. doi: 10.1038/sj.ejcn.1602435. [DOI] [PubMed] [Google Scholar]
- 39.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr. 2002;76:390–8. doi: 10.1093/ajcn/76.2.390. [DOI] [PubMed] [Google Scholar]
- 40.Koh-Banerjee P, Chu NF, Spiegelman D, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr. 2003;78:719–27. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 41.Lairon D, Arnault N, Bertrais S, et al. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr. 2005;82:1185–94. doi: 10.1093/ajcn/82.6.1185. [DOI] [PubMed] [Google Scholar]
- 42.Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Gronbaek M, Rimm EB. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr. 2006;83:275–83. doi: 10.1093/ajcn/83.2.275. [DOI] [PubMed] [Google Scholar]
- 43.Glore SR, Van Treeck D, Knehans AW, Guild M. Soluble fiber and serum lipids: a literature review. J Am Diet Assoc. 1994;94:425–36. doi: 10.1016/0002-8223(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 44.Kay RM. Dietary fiber. J Lipid Res. 1982;23:221–42. [PubMed] [Google Scholar]
- 45.Behall KM, Scholfield DJ, Hallfrisch J. Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. J Am Diet Assoc. 2006;106:1445–9. doi: 10.1016/j.jada.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–81. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 47.Streppel MT, Arends LR, van 't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165:150–6. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 48.Slavin J, Jacobs D, Marquart L. Whole-grain consumption and chronic disease: protective mechanisms. Nutr Cancer. 1997;27:14–21. doi: 10.1080/01635589709514495. [DOI] [PubMed] [Google Scholar]
- 49.Weickert MO, Mohlig M, Schofl C, et al. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006;29:775–80. doi: 10.2337/diacare.29.04.06.dc05-2374. [DOI] [PubMed] [Google Scholar]
- 50.Steffen LM, Jacobs DR, Jr, Murtaugh MA, et al. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am J Epidemiol. 2003;158:243–50. doi: 10.1093/aje/kwg146. [DOI] [PubMed] [Google Scholar]
- 51.Liese AD, Roach AK, Sparks KC, Marquart L, D'Agostino RB, Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2003;78:965–71. doi: 10.1093/ajcn/78.5.965. [DOI] [PubMed] [Google Scholar]
- 52.Willett WC. Nutritional epidemiology. 2nd. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 53.North KE, Franceschini N, Borecki IB, et al. Genotype-by-sex interaction on fasting insulin concentration: the HyperGEN study. Diabetes. 2007;56:137–42. doi: 10.2337/db06-0624. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Curr Atheroscler Rep. 2002;4:454–61. doi: 10.1007/s11883-002-0050-2. [DOI] [PubMed] [Google Scholar]
- 55.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76(suppl):274S–80S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 56.Wolever TM, Miller JB. Sugars and blood glucose control. Am J Clin Nutr. 1995;62(suppl):212S–21S. doi: 10.1093/ajcn/62.1.212S. [DOI] [PubMed] [Google Scholar]
- 57.Pereira MA, Jacobs DR, Jr, Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–55. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 58.Kaaks R, Bellati C, Venturelli E, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr. 2003;57:1079–88. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 59.Berrino F, Bellati C, Secreto G, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10:25–33. [PubMed] [Google Scholar]
- 60.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am J Clin Nutr. 2005;81:55–63. doi: 10.1093/ajcn/81.1.55. [DOI] [PubMed] [Google Scholar]
- 61.Masson LF, McNeill G, Avenell A. Genetic variation and the lipid response to dietary intervention: a systematic review. Am J Clin Nutr. 2003;77:1098–111. doi: 10.1093/ajcn/77.5.1098. [DOI] [PubMed] [Google Scholar]