Abstract
Background
Aging of the peripheral nervous system is associated with several morphologic and functional changes, including a decrease of the nerve conduction velocity. There is evidence that these changes contribute to age-related-decline in muscle strength, sensory discrimination, and autonomic responses. The aim of this study was to characterize the decline in nerve conduction velocity in the peripheral nervous system over the aging process and to identify factors that, independent of age, affect nerve conduction velocity.
Methods
We measured motor nerve conduction velocity of the right superficial peroneal nerve using a standard neurophysiologic technique in a population-based sample of subjects aged between 20 and 103 years old enrolled in the InCHIANTI study.
Results
Average conduction velocities in the peripheral nerve decreased linearly with age in both sexes. We found that diabetes, cognitive impairment, uric acid, sIL-6R and α-tocopherol were significant predictors of nerve conduction velocity independently of the potential confounding effect of age, sex, sex × age interaction term, height, lymphocytes, neutrophils number, α1 and α2-globulin serum protein.
Conclusion
Our findings are consistent with the hypothesis that inflammation and inadequate antioxidant defenses are associated with accelerated decline of nerve conduction velocity over the aging process.
Keywords: Inflammation, Vitamin E, Peripheral nervous system, Aging
1. Introduction
Aging is characterized by a decline in function of multiple physiological systems and progressive exhaustion of their functional reserve. The causes of this decline are still unclear but it has been proposed that increased oxidative stress, disturbances in energy metabolism, and a primary dysregulation of the immune system might play an important role [22].
A number of observational studies have shown an age-associated decline in peripheral nervous system (PNS) function. Longitudinal assessment performed in subjects of different ages demonstrated that nerve conduction velocity (NCV) and signal amplitude decrease with age even in subjects free of diabetes and other major diseases [24]. Such decline has been attributed to structural changes, such as loss of myelinated and unmyelinated fibers and decreased production of the major myelin proteins with subsequent myelin deterioration [33].
Factors that have been associated with reduced NCV include diabetes, inflammatory disease, smoking, alcohol abuse and chronic infection [1,23]. However, no previous study tested the hypothesis that independently of age, a proinflammatory and pro-oxidative state, characterized by circulating levels of inflammatory markers and low circulating levels of antioxidants, is associated with reduced NCV.
The aim of this study was to evaluate whether the decline in NCV occurs linearly over the entire life span and to verify whether, independent of age and chronic diseases, high levels of inflammatory markers and low levels of Vitamin E, the most important lipophilic antioxidant, are associated with lower NCV.
2. Population and methods
InCHIANTI is an epidemiological study of factors contributing to the decline of mobility in late life. The InCHIANTI study population is a representative sample of the population living in Greve in Chianti and Bagno a Ripoli, two small towns located in the Chianti countryside of Tuscany, Italy. The participants were all European of Caucasian race. The study design and data collection have been previously described elsewhere [6]. Briefly, 1270 persons aged 65 years or more were randomly selected from the population registry of the two sites. Another 29 subjects were selected randomly from among those who were aged 90 years or older. Finally, men and women sampled randomly from the age strata 20–29, 30–39, 40–49, 50–59 and 60–64 years were sequentially invited to participate in the study until at least 30 men and 30 women for each decade from 20 to 59, and 10 men and 10 women aged 60 to 64 had been enrolled (Table 1).
Table 1.
Participation rate in the different sections of the InCHIANTI baseline evaluation, according to sex and age group
| Greve | Bagno | Total | Interview |
EPIC |
Blood sample |
Medical examination |
Functional evaluation |
pQTC |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | % | n | %a | n | %a | n | %a | n | %a | n | %a | |
| Men | |||||||||||||||
| <65 years | 90 | 86 | 176 | 143 | 81 | 142 | 99 | 136 | 95 | 134 | 94 | 134 | 94 | 131 | 92 |
| 65–69 years | 76 | 83 | 159 | 142 | 89 | 142 | 100 | 136 | 96 | 136 | 96 | 136 | 96 | 129 | 91 |
| 70–74 years | 86 | 72 | 158 | 142 | 90 | 141 | 99 | 134 | 94 | 132 | 93 | 130 | 92 | 127 | 89 |
| 75–79 years | 55 | 48 | 103 | 102 | 99 | 102 | 100 | 96 | 94 | 94 | 92 | 94 | 92 | 91 | 89 |
| 80–84 years | 26 | 28 | 54 | 49 | 91 | 47 | 96 | 43 | 88 | 41 | 84 | 40 | 82 | 37 | 76 |
| 85–89 years | 21 | 23 | 44 | 39 | 89 | 39 | 100 | 32 | 82 | 33 | 85 | 32 | 82 | 24 | 62 |
| 90+ years | 15 | 20 | 35 | 23 | 66 | 21 | 91 | 18 | 78 | 18 | 78 | 18 | 78 | 13 | 57 |
| Total men | 369 | 360 | 729 | 640 | 88 | 634 | 99 | 595 | 93 | 588 | 92 | 584 | 91 | 552 | 86 |
| Women | |||||||||||||||
| <65 years | 97 | 94 | 191 | 155 | 81 | 154 | 99 | 152 | 98 | 148 | 95 | 148 | 95 | 144 | 93 |
| 65–69 years | 104 | 86 | 190 | 171 | 90 | 169 | 99 | 160 | 94 | 157 | 92 | 157 | 92 | 152 | 89 |
| 70–74 years | 83 | 85 | 168 | 155 | 92 | 155 | 100 | 145 | 94 | 144 | 93 | 144 | 93 | 134 | 86 |
| 75–79 years | 73 | 86 | 159 | 131 | 82 | 131 | 100 | 124 | 95 | 121 | 92 | 120 | 92 | 117 | 89 |
| 80–84 years | 42 | 48 | 90 | 85 | 94 | 85 | 100 | 73 | 86 | 71 | 84 | 70 | 82 | 67 | 79 |
| 85–89 years | 41 | 45 | 86 | 72 | 84 | 72 | 100 | 57 | 79 | 54 | 75 | 53 | 74 | 45 | 63 |
| 90+ years | 24 | 29 | 53 | 44 | 83 | 44 | 100 | 37 | 84 | 34 | 77 | 32 | 73 | 21 | 48 |
| Total women | 464 | 473 | 937 | 813 | 87 | 810 | 100 | 748 | 92 | 729 | 90 | 724 | 89 | 680 | 84 |
| Total | 833 | 833 | 1666 | 1453 | 87 | 1444 | 99 | 1343 | 93 | 1317 | 91 | 1308 | 90 | 1232 | 85 |
Percentage of those interviewed.
Of the 1530 subjects originally sampled, 1453 (94%) agreed to participate in the study. Of these, 1263 underwent standard surface electroneurography of the right peroneal nerve. Due to missing data 57 subject were excluded from the subsequent analyses.
The final study population included 1206 persons, 543 men and 663 women, dispersed over a wide age-range (21–96 years). The study protocol was examined and approved by the INRCA ethical committee. All participants received a detailed description of the purpose and design of the study and all signed informed participation consent.
2.1. Assessment
The study protocol included: a home interview, a validated food frequency questionnaire [26], a clinical test session, a medical examination and a blood sample collection after at least 8 h of fasting. Both cytokines and Vitamin E were measured from frozen (−80 °C) specimens.
2.2. Electromyography
The measure of nerve motor conduction velocity was performed on the right superficial peroneal nerve using standard neurophysiologic equipment (EMG Myto, EBNeuro, ESAOTE Florence, Italy). The temperature of testing room was kept at 26–27 °C so that the testing leg is maintained at physiologic temperature. The measurements were obtained while dorsal foot skin temperature was between 30 and 34 °C [13,27]. If necessary the skin over the muscle was warmed up with an infrared lamp.
A disposable surface recording electrode was placed on the dorsum of the foot, over the belly of the extensor digitorum brevis (in the anterior lateral aspect of the proximal midtarsal area); the peroneal nerve was stimulated with a bipolar electrode, distal stimulation was applied about 8 cm proximal to the pickup, just lateral to the tibialis anterior tendon. More proximally the nerve was stimulated just below the head of the fibula as the nerve curves around the bone. A ground electrode was positioned in the between the stimulating and the recording electrodes [3,10] (Fig. 1).
Fig. 1.
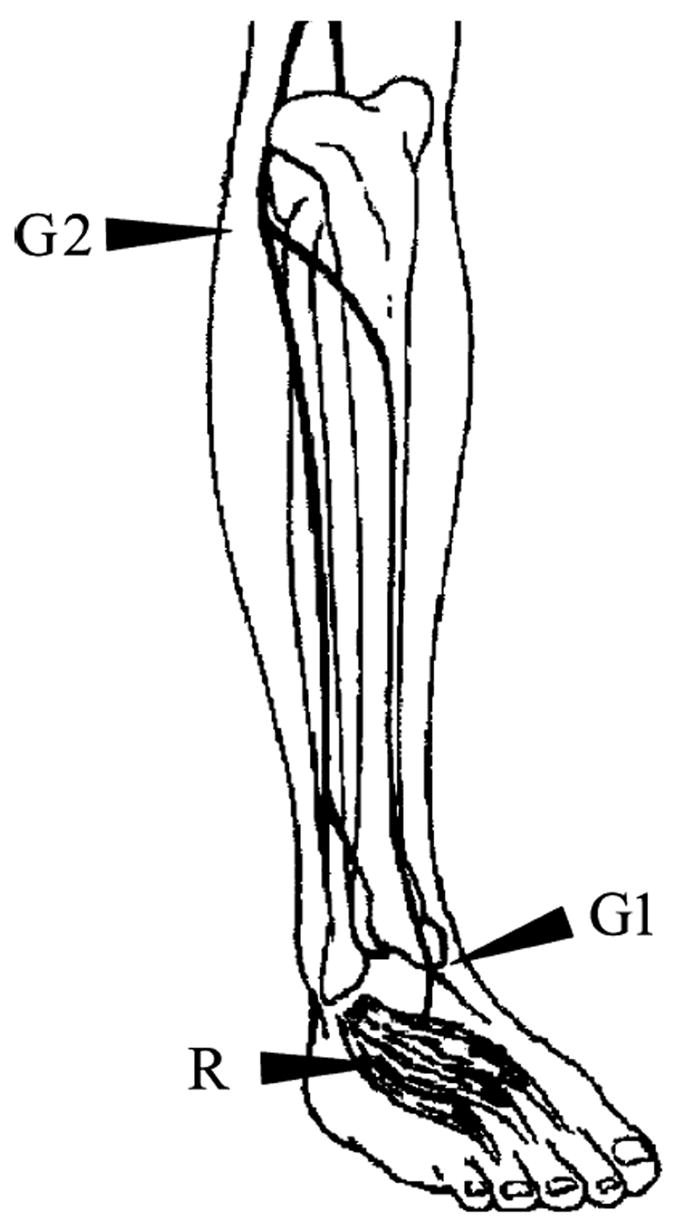
Motor nerve conduction of the peroneal nerve. For recording (R), a surface electrode is placed on the extensor digitorum brevis muscle. For stimulation, the peroneal nerve is stimulated proximally at the fibular head (G2) and distally over the anterior ankle (G1) [13].
In both cases, the stimulation started with a very mild electrical impulse, to check whether the position of the electrodes was appropriate. Then, the stimulus was progressively increased, until the registered muscular response (a sinusoid) reached maximum amplitude.
The following parameters of nerve conduction studies were measured: (a) the amplitude of the compound muscle action potential (CMAP), which is related to the number of axons that conduct impulses from the stimulus point to the muscle and the number of functioning motor endplates [27]. It was measured from peak to peak of the action potential [19,27]; (b) the nerve conduction velocity (NCV), which is calculated by dividing the length of the nerve segment between the two stimulation points by the difference between the proximal and distal time latency, which reflects the conduction velocity of the fastest motor axons [13,27].
During test measurement electrical averaging was performed regularly to reduce the signal to noise ratio [13].
2.3. Electromyograph setting
According to literature electromyograph setting were: frequency 8 Hz to 8 kHz; sweep speed 5 ms/div; gain 1.000 μV [13].
2.4. Cytokines measurement
Chronic inflammation is associated with a broad spectrum of degenerative age related diseases such as Alzheimer disease, congestive heart failure, atherosclerosis, and myocardial infarction; it has been observed that aging “per se” is associated with the development of a pro-inflammatory state [2,7,8,17].
A number of cytokines were evaluated on the whole InCHIANTI sample to obtain information both the pro and anti-inflammatory response.
The serum interleukin 6 (IL-6), Tumor Necrosis Factor-α (TNF-α), interleukin 1β (IL-1β) levels were measured by enzyme linked immunosorbent assay (ELISA) using ultrasensitive commercial kits (Human Ultrasensitive, BIOSOURCE International Inc.). The detectable limits were: 0.10 pg/ml for IL-6, 0.09 pg/ml for TNF-α and 0.01 pg/ml for IL-1β.
The serum interleukin 6 receptor (sIL-6R) level was determined by ELISA kit (Cytoscreen™, Human sIL-6R BIOSOURCE International Inc.) and the minimum detectable level was 8.00 pg/ml.
The serum interleukin 1 receptor antagonist (IL-1ra) level was detected by ELISA method using commercial kits (EASIA™ ELISA Human IL-1ra, BIOSOURCE International Inc.). The minimum detectable concentration was 4.00 pg/ml. The serum interleukin 10 (IL-10) was detected by Human IL10 CytoSETS™ ELISA kits (BIOSOURCE International Inc.). The minimum detectable concentration was 1.00 pg/ml.
2.5. α-Tocopherol measurement
Vitamin E (α-tocopherol) plasma concentration was measured by reverse-phase high performance liquid chromatography (HPLC) as previously described [16]. Briefly, 100 μl of plasma were mixed with 100 μl ethanol; after vortexing, tocopherol was extracted into 500 μl hexane containing 0.002% butylated hydroxyl toluene (BHT) (Sigma St. Louis, MO). Tocol (a gift from Hoffman La Roche, Nutley, NJ), was added to the mixture as an internal standard. Samples were centrifuged at 800 rpm for 5 min at 4 °C. The supernatant was collected and dried under a stream of nitrogen gas, and reconstituted in 100 μl of methanol. Tocopherols were separated by high performance liquid chromatography (HPLC) using a 3 μm C18 reverse phase column (Perkin-Elmer, Norwalk, CT). The mobile phase, delivered at a flow rate of 1.0 ml/min, consisted of 1% water in methanol, containing 10 mmol/l lithium perclorate. Samples were injected with an autosampler, 1100 series, Hewlett Packard. Eluted peaks were detected at an applied potential of +0.6 V by a LC 4B amperometric electrochemical detector (Bioanalytical System, West Lafayette, IN). Peaks were integrated with a ChemStation software (Hewlett Packard). Tocopherol (α-tocopherol) concentration was expressed in μmol/l. Reproducibility and accuracy of the assay was tested by analyzing representative samples in triplicate from a sample provided by the American Association for Laboratory Accreditation, Washington, DC, USA containing known concentration of α-tocopherol. Intra-and inter-batch coefficients of variation were 3% and 4.2%, respectively.
2.6. Covariates
Average daily alcohol consumption was evaluated by means of the food frequency questionnaire. Smoking was categorized into: never smoker, past smoker and smoker. Based on self report, the diagnosis of several chronic medical conditions was established using pre-defined criteria that combined information from medical history, physical exam and medical records. The list of diseases includes: congestive heart failure (CHF), angina, myocardial infarction (MI), hypertension, stroke, diabetes, deep venous thrombosis, parkinson disease, peripheral artery diseases and hip fracture [25].
Cognitive function was assessed using the Mini Mental Status Examination (MMSE) corrected for age and education. Participants who scored 19 or less were considered as “cognitive impaired”.
Height was clustered according to sex specific tertiles of distribution; ranges for male were 163 and 169 cm, ranges for female were 150 and 158 cm.
The results of blood tests were categorized according to tertiles of distribution: (a) α1 globulin (% total protein) (<2.4, 2.4–2.7, >2.7); (b) α2 globulin (% total protein) (<10.5, 10.5–11.6, >11.6); (c) albumin (% total protein) (<58.1, 58.1–60.5, >60.5); (d) total cholesterol (mg/dl) (<198, 198–230, >230); (e) tryglicerides (mg/dl) (<88, 88–131, >131); (f) uric acid (mg/dl) (<4.2, 4.2–5.4, >5.4); (g) α-tocopherol (μmol/l) (<19.5, 19.5–22.63, >22.63); (h) plasma creatinine (mg/dl) (<0.83, 0.83–0.96, >0.96); (i) glycemia (mg/dl) (<88.0, 88.0–92.0, >92.0); (j) lymphocytes (number) (<3.13, 3.13–4.02, >4.02); (k) neutrophiles (number) (<1.57, 1.57–2.07, >2.07). Cytokines were categorized according to tertiles of distribution: (a) IL-1 Ra (pg/ml) (<105.29, 105.29–159.92, >159.92); (b) IL-1β (pg/ml) (<0.09, 0.09–0.15, >0.15); c) IL-6 (pg/ml) (<0.87, 0.87–1.70, >1.70); (d) IL-6 R (ng/ml) (<74.96, 74.96–110.15, >110.15); (e) TNF-α (pg/ml) (<3.57, 3.57–6.70, >6.70); (f) since a large number of subjects had undetectable levels of IL-10, IL-10 was clustered in two groups (greater or equal to 0.1 pg/ml versus undetectable).
2.7. Statistical analysis
Differences among groups in nerves conduction velocity were evaluated by analysis of variance with generalized linear model (PROC GLM). All preliminary analyses were adjusted for age, sex and age × sex interaction, under the assumption that the effect of age on nerve conduction velocity was different in men and women. The linearity of the age-associated decline in nerve conduction velocity was verified in subsequent regression models by testing whether the combined effect of age and age-squared significantly improved the prediction of nerve conduction velocity, compared to age only.
Variables that in the univariate analyses were associated with nerve conduction velocity with a p value of 0.10 or less were entered in a linear regression model (PROC REG) predicting nerve conduction velocity.
A p value less or equal to 0.05 was used to establish statistical significance. Analysis for outliers and for leverage was also conducted.
All analyses were performed using SAS release 8.2.
3. Results
Of the 1292 subjects enrolled in the study, 573 (44.3%) were males and 719 (55.7%) females. Average NCV declined with age in both men and women, and in each age-group NCV was higher in women than in men (Fig. 2). In fact, no significant differences could be found between men and women until the 50–54 years group; after this age, the sex specific trajectories show different slopes, with men having a lower NCV, than women, in the 90+ years cluster.
Fig. 2.
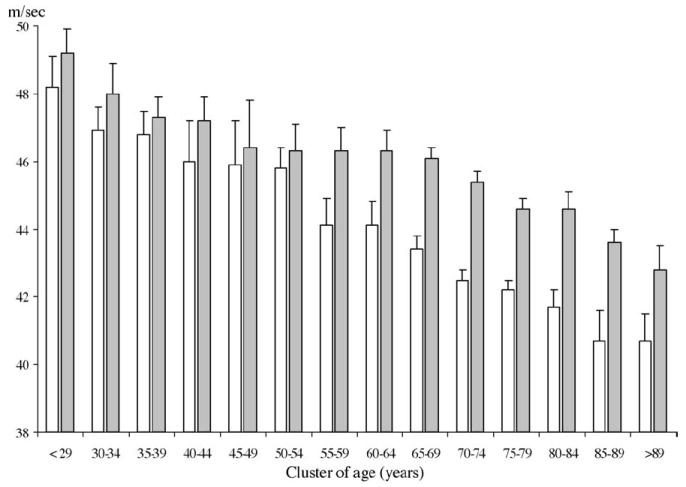
Nerve conduction velocity according to cluster of age and sex. The age-associated decline was linear. The interaction between cluster of age and sex was also significant. Nerve conduction velocity was measured in m/s. Age was clustered in groups of 5 year. White column rappresent men and grey histograms women.
There was a significant age × sex interaction in the model predicting NCV, indicating that the role of age associated decline in NCV was significant steeper in men than in women.
The age-associated decline in NCV was linear. In fact, introducing a quadratic term for age in the regression model did not significantly improve the prediction of NCV (p = 0.49 for age as quadratic term).
Of the entire population enrolled in the study, 304 (23.5%) reported to be teetotalers.
After adjusting for age and sex, participants who reported to be teetotalers and those who reported drinking alcohol had similar NCV (44.6 ± 3.8 versus 44.7 ± 4.0; F = 0.60; p = 0.44). Similarly, participants who never smoked had NCV comparable to current or former smokers (F = 3.6; p = 0.07).
Independent of age and sex, diabetes and presence of cognitive impairment were both associated with a lower NCV (Table 2). Among the cytokines considered, NCV was significantly lower in participants in the upper IL-6 and sIL-6R tertiles.
Table 2.
NCV in the InCHIANTI participants according to disease status
| Disease absent |
Disease present |
Unadjusted |
Adjusted |
|||||
|---|---|---|---|---|---|---|---|---|
| Number | Mean ± S.D. | Number | Mean ± S.D. | F | p | Fa | pa | |
| Diabetes | 1098 | 44.8 ± 3.9 | 108 | 41.9 ± 3.8 | 50.8 | <0.001 | 38.0 | <0.001 |
| Cognitive impairmentb | 1155 | 44.7 ± 4.0 | 51 | 42.2 ± 5.0 | 18.9 | <0.001 | 7.3 | 0.007 |
| Stroke | 1141 | 44.6 ± 4.0 | 65 | 42.1 ± 4.3 | 21.5 | <0.001 | 7.0 | 0.008 |
| Peripheral arterial diseases | 1149 | 44.6 ± 4.0 | 57 | 41.7 ± 3.7 | 29.8 | <0.001 | 9.3 | 0.002 |
| Congestive heart failure | 1166 | 44.6 ± 4.0 | 40 | 42.9 ± 4.1 | 7.2 | 0.007 | 1.0 | 0.31 |
| Acute myocardial infarction | 1159 | 44.6 ± 4.1 | 47 | 43.4 ± 3.6 | 2.6 | 0.11 | 0.1 | 0.75 |
| Hypertension | 703 | 44.7 ± 4.2 | 503 | 44.2 ± 3.7 | 6.0 | 0.01 | 0.3 | 0.57 |
| Angina | 1162 | 44.6 ± 4.0 | 44 | 43.7 ± 3.9 | 2.2 | 0.13 | 0.4 | 0.52 |
| Parkinson | 1195 | 44.5 ± 4.0 | 11 | 42.7 ± 8.9 | 2.2 | 0.14 | 0.4 | 0.53 |
| Hip fracture | 1180 | 44.6 ± 4.0 | 26 | 43.6 ± 3.9 | 1.9 | 0.17 | 1.4 | 0.23 |
| Deep venous thrombosis | 1174 | 44.5 ± 4.0 | 32 | 44.6 ± 3.0 | 0.0 | 0.83 | 2.0 | 0.16 |
Comparisons between groups were performed by general linear model.
F and p value adjusted for age (years), sex and their interaction.
MMSE less or equal 19 corrected for school level and age.
After adjustment for age and gender, difference of NCV across IL-6 tertiles were no longer statistically significant, while differences across sIL-6R tertiles were only slightly reduced and still statistically significant (Table 3).
Table 3.
Peripheral nervous conduction in the InCHIANTI participants according to inflammatory markers tertiles
| Cytokines serum level tertilesa |
Unadjusted |
Adjusted |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower |
Intermediate |
Higher |
F | p | Fb | pb | ||||
| Number | Mean ± S.D. | Number | Mean ± S.D. | Number | Mean ± S.D. | |||||
| IL-1β (pg/ml) | 396 | 44.4 ± 4.0 | 381 | 44.5 ± 3.9 | 429 | 44.7 ± 4.3 | 1.96 | 0.16 | 2.34 | 0.13 |
| sIL-6R (ng/ml) | 400 | 44.9 ± 4.1 | 397 | 44.7 ± 4.0 | 409 | 44.0 ± 4.0 | 8.6 | 0.003 | 4.10 | 0.04 |
| IL-6 (pg/ml) | 412 | 45.4 ± 4.0 | 400 | 44.5 ± 3.9 | 394 | 43.7 ± 4.0 | 39.8 | 0.001 | 1.10 | 0.30 |
| IL-1Ra (pg/ml) | 413 | 44.6 ± 4.1 | 393 | 44.7 ± 3.9 | 400 | 44.2 ± 4.1 | 1.15 | 0.28 | 0.15 | 0.70 |
| TNF-α (pg/ml) | 414 | 44.6 ± 3.9 | 377 | 44.3 ± 4.0 | 416 | 44.6 ± 4.3 | 0.01 | 0.91 | 0.02 | 0.90 |
Comparisons between tertiles were performed by general linear model.
Lower, intermediate and higher tertiles were calculated on the entire sample (1292 subjects enrolled in the study) and were referred to the distribution of the single cytokine. Mean ± S.D. of nerve conduction velocity were reported according to tertiles of distribution of every Cytokine.
F and p value adjusted for age (years), sex and their interaction.
Independent of age and sex, participants in the highest tertiles of neutrophils number, α1 and α2-globulin serum protein, had significant lower NCV than those in the lowest tertiles. Conversely, higher total cholesterol, higher plasma α-tocopherol, higher uric acid and higher lymphocyte number were independently associated with higher NCV (Table 4).
Table 4.
Peripheral nervous conduction in the InCHIANTI participants according to tertiles of laboratory parameters
| Tertilesa |
Unadjusted |
Adjusted |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower |
Intermediate |
Higher |
F | p | Fb | pb | ||||
| Number | Mean ± S.D. | Number | Mean ± S.D. | Number | Mean ± S.D. | |||||
| α1 globulin (% total protein) | 487 | 45.2 ± 3.9 | 366 | 44.4 ± 3.9 | 352 | 43.7 ± 4.3 | 28.9 | <0.001 | 15.8 | <0.001 |
| α2 globulin (% total protein) | 398 | 44.7 ± 4.0 | 407 | 44.8 ± 4.0 | 401 | 44.1 ± 4.1 | 4.7 | 0.03 | 4.5 | 0.03 |
| Albumin (% total protein) | 407 | 44.2 ± 4.0 | 366 | 44.3 ± 4.2 | 432 | 45.0 ± 3.9 | 7.7 | 0.006 | 0.01 | 0.95 |
| Total cholesterol (mg/dl) | 397 | 44.1 ± 4.4 | 389 | 44.4 ± 4.0 | 420 | 44.9 ± 3.7 | 8.3 | 0.004 | 10.6 | 0.001 |
| Tryglicerides (mg/dL) | 399 | 44.6 ± 4.2 | 399 | 44.3 ± 4.0 | 408 | 44.6 ± 3.9 | 1.1 | 0.30 | 2.0 | 0.16 |
| α-Tocopherol (μmol/l) | 382 | 44.0 ± 4.4 | 400 | 44.7 ± 3.9 | 424 | 44.8 ± 3.8 | 8.6 | 0.003 | 9.3 | 0.002 |
| Lymphocytes (number) | 386 | 44.1 ± 4.0 | 408 | 44.3 ± 3.9 | 412 | 45.1 ± 4.2 | 14.2 | <0.001 | 6.0 | 0.01 |
| Neutrophils (number) | 410 | 44.9 ± 3.8 | 400 | 44.7 ± 3.8 | 396 | 43.9 ± 4.4 | 13.1 | <0.001 | 4.3 | 0.04 |
| Uric acid (mg/dl) | 343 | 45.0 ± 3.9 | 466 | 44.5 ± 4.1 | 397 | 44.2 ± 4.1 | 6.7 | 0.009 | 6.3 | 0.01 |
| Creatinine (mg/dl) | 405 | 45.5 ± 3.9 | 384 | 44.4 ± 3.9 | 417 | 43.6 ± 4.1 | 47.2 | <0.001 | 3.1 | 0.07 |
| Glucose (mg/dl) | 376 | 45.1 ± 4.2 | 402 | 44.9 ± 3.9 | 428 | 43.6 ± 4.0 | 25.0 | <0.001 | 1.5 | 0.22 |
Comparisons between tertiles were performed by general linear model.
Lower, intermediate and higher tertiles were calculated on the entire sample (1292 subjects enrolled in the study) and were referred to the distribution of the single laboratory parameters. Mean ± S.D. of nerve conduction velocity were reported according to tertiles of distribution of laboratory parameters.
F and p value adjusted for age (decades) and sex.
3.1. Linear regression analysis
Table 5 reports the results of the multivariable analysis. As already mentioned, in the saturated model were forced all variables that in the age and sex adjusted analysis were associated with the outcome with a p value <0.10.
Table 5.
Multiple linear regression; factors associated with peripheral nervous conduction in the InCHIANTI participants
| B | SEβ | t | P | Test for trend |
||
|---|---|---|---|---|---|---|
| t | p | |||||
| Diabetes | −2.15 | 0.35 | −6.24 | <0.001 | −6.22 | <0.001 |
| Cognitive impairmenta | 1.07 | 0.54 | 2.00 | 0.04 | 2.77 | 0.005 |
| sIl6R (tertiles) | −1.99 | 0.05 | ||||
| <74.96 (ng/ml) | Reference group | |||||
| 74.96–110.15 (ng/ml) | 0.17 | 0.24 | 0.72 | 0.47 | ||
| >110.15 (ng/ml) | −0.47 | 0.23 | −2.00 | 0.04 | ||
| α-Tocopherol (tertiles) | 2.20 | 0.03 | ||||
| <26 (μmol/l) | Reference group | |||||
| 26–32.9 (μmol/l) | 0.46 | 0.23 | 1.88 | 0.05 | ||
| >32.9 (μmol/l) | 0.49 | 0.24 | 1.98 | 0.04 | ||
| Uric acid (tertiles) | 2.57 | 0.01 | ||||
| <4.2 (mg/dl) | Reference group | |||||
| 4.2–5.4 (mg/dl) | 0.43 | 0.24 | 1.67 | 0.09 | ||
| >5.4 (mg/dl) | 0.73 | 0.28 | 2.54 | 0.01 | ||
The model shown in this table is adjusted for age, gender, their interaction, lipid levels, serum creatinine, height, lymphocytes (number), neutrophils (number), α1 and α2 proteic fractions.
Cognitive impairment was defined as a MMSE score lower or equal to 19 corrected for education and age.
The first model (Table 5, right panel, “For trend values”) showed that diabetes, cognitive impairment, sIL-6R, plasma level of α-tocopherol and uric acid were significant independent predictors of NCV, besides age, sex, their interaction, height, lymphocytes, neutrophils number, α1 and α2-globulin serum protein and serum creatinina. Participants with intermediate (26–32.9 μmol/l) and high level (>32.9 μmol/l) of α-tocopherol and high level of uric acid (5.4 mg/dl) had significantly higher in NVC compared to those in the lowest tertile. Conversely, for sIL-6R participants in the highest tertile (110 ng/ml) had a lower NCV whereas no differences was found comparing participants with intermediate and low sIL-6R levels (Table 5 left panel). The R2 of the model was 0.30; with age, sex and their interaction alone accounting for 20% of NCV variance.
4. Discussion
Using data collected in a large population-based sample of persons over a wide age range, we found that, NCV decline linearly with age both in men and in women. Diabetes, cognitive impairment, high sIL-6R, uric acid and low Vitamin E levels, were all associated with lower NCV, independent of age, sex, their interaction, height, α1 and α2-globulin serum protein, serum creatinine, lymphocytes and neutrophils.
The progressive decline of NCV with age has been already reported in the literature, but the association has been described either as linear or quadratic [4,24]. Our findings are in agreement with the notion that NCV declines linearly with age since we were unable to detect any departure from a linear decline across the entire life span.
Impaired conduction velocity with aging may be attributed to structural changes, including a marked fiber loss, involving both unmyelinated axons and myelinated fibers, and the development of abnormalities in the surviving nerve fibers. Specifically, macrophages and mast cells increase substantially in the endoneurium; Schwann cells develop bulb-like extrusion and collagen pockets. Changes in peripheral nerve metabolism habe are also been described [33].
Also progressive dysregulation of glucose metabolism that often occur in older individuals determine a loss of efficiency in the Na/K-ATPase activity, a shift to anaerobic glycolysis, accumulation of polyols, increased protein glycation also contribute to the decline in NCV in aging individuals [12]. Lastly, in older persons, a reduction in nerve blood flow can result in the paradigmatic picture of neuropathy [33].
Women had significantly faster NCV than men across all age groups considered in this study. Estrogens are known to exert a strong influence on many neurological structures [18], both during development and adult life. Although very little is known about the effects of estrogens on the peripheral nervous system, estrogen receptors have been found in motoneurons [20]. The relative estrogen deficiency that occurs in the postmenopausal period may affect the rate of NCV decline in aging women compared to men.
Diabetes and cognitive impairment were the only diseases independently associated with nerve conduction velocity. The impairment of nerve conduction velocity is among the earliest abnormalities detected in the early stage of diabetes and is directly correlated with the disease duration and the degree of metabolic control. Although several mechanism have been proposed to explain the genesis of diabetic neuropathy increased oxidative stress is considered to play a preeminent role [28]. In addition chronic hyperglycemia causes increased production of reactive oxygen species (ROS) leading to production of superoxide and hydroxyl radicals, consequently, the oxidation of cell structure may induce functional and structural changes [30].
There are many evidences that the oxidative stress and the consequent accumulation of free radicals could be also a causative factor in the genesis of the Alzheimer disease [32,34]. Vitamin E is the major chain-breaking antioxidant present in biological membranes and fluids and an essential factor for the development of the normal structure and function of the human nervous system and the maintenance of their integrity over the life span. Vitamin E deficiency causes symptoms of cerebellar dysfunction and peripheral neuropathy that is partially reversible by Vitamin E supplementation administered at an early stage. Interestingly, the administration of Vitamin E in an early phase of diabetes improves peripheral nerve function in diabetic rats [5] and prevents the development of nerve conduction deficits in young streptozotocin-diabetic rats [15].
There is also evidence that the administration of Vitamin E in type 2 diabetic patients slows-down the progression of peripheral neuropathy [28]. Additionally, the administration of Vitamin E reduces nerve malondialdehyde levels [29] suggesting that its protective action may be mediated, at least in part, by its antioxidant activity against free radical induced damage.
Both pre-clinical and clinical data have shown a clear connection between oxidative stress and the production of inflammatory mediators. Antioxidants, such as Vitamin E reduce the production of IL-1β, IL-8 and TNF-α by leukocytes [31].
There is also evidence that older person are often affected by a mild pro-inflammatory state that has been attributed to primary dysregulation of the immune response, and might contribute to accelerate the physiological decline associated with aging [9]. Inflammatory states are pleomorphic and involve a variety of cell types, including not only neutrophils but also macrophages and lymphocytes. These cells may act by releasing a wide range of signaling molecules, including growth factors, cytokines and chemokines. Many of these molecules, and in particular the IL-6 family cytokines through a receptor complex exert important biological effect on the nervous system cells.
The delivery of IL-6 after an insult, in the central and in the peripheral nervous system promotes neuronal survival and outgrowth, attenuates motor deficits and may accelerate nerve regeneration.
Recent evidences suggest that neuronal cells require sIL-6R to be responsive to IL-6 [11,14,21].
For example, in pre-clinical models of axonal damage, the addition of IL-6 and sIl-6R enhances the speed of axonal regeneration, while the addition of IL-6 or sIl-6R alone have virtually no effects.
It has also been demonstrated that high levels of proinflammatory cytokines and high oxidative stress are associated with neuronal apoptosis. Thus, it is conceivable that a dysregulation of the inflammatory pathway and the excessive production of ROS may negative influence neuronal function, which results clinically in lower NCV.
The main limitation of this study concerns the cross-sectional design, that does not enable us to establish a direct causal effect relationship. Additionally, because of the epidemiological nature of this study, we were able to collect only one measure of motor conduction velocity so we cannot exclude that variable associated with sensitive nerve conduction could have been different.
Our results support the hypothesis that inflammation and increased (impaired) oxidative stress accelerate the decline of function in the peripheral nervous system that is often observed during the aging process.
This hypothesis should be further confirmed in epidemiological longitudinal studies whether the chronic administration of antioxidants or anti-inflammatory agents slow-down the neuronal age-associated decline in NCV should be tested in randomized controlled trials.
Acknowledgments
The InCHIANTI Study is supported as a “target project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336).
This research was also partially supported by an unrestricted grant by BRACCO imaging SpA, Italy.
This study was conducted in the framework of the EU FP5 (EC grant QLK6-CT1999-02031) and partly supported by a grant of the Italian Ministry of Education and University (MIUR) to the Center of Excellence on Aging of the University “G. D’Annunzio”.
Angelo Di Iorio is supported by a fund cosponsored by University “G. D’Annunzio” Chieti and SANOFI-SYNTHELABO SpA.
References
- 1.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441(1–2):1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- 2.Caruso C, Candore G, Colonna-Romano G, Lio D, Franceschi C. Inflammation and life-span. Science. 2005;307(5707):208–9. doi: 10.1126/science.307.5707.208. [DOI] [PubMed] [Google Scholar]
- 3.Checkles NS, Bailey JA, Johnson EW. Tape and caliper surface measurements in determination of peroneal nerve conduction velocity. Arch Phys Med Rehabil. 1969;50(4):214–8. [PubMed] [Google Scholar]
- 4.Choi SJ, Harii K, Lee MJ, Furuya F, Ueda K. Electrophysiological, morphological, and morphometric effects of aging on nerve regeneration in rats. Scand J Plast Reconstr Surg Hand Surg. 1995;29(2):133–40. doi: 10.3109/02844319509034329. [DOI] [PubMed] [Google Scholar]
- 5.Cotter MA, Love A, Watt MJ, Cameron NE, Dines KC. Effects of natural free radical scavengers on peripheral nerve and neurovascular function in diabetic rats. Diabetologia. 1995;38(11):1285–94. doi: 10.1007/BF00401760. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Ble A, Bandinelli S, Lauretani F, Suthers K, Guralnik JM. A flame burning within. Aging Clin Exp Res. 2004;16(3):240–3. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–9. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31(2):457–61. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez J, Easton JK, Redford JB. Conduction studies of the anterior and posterior tibial nerves. Arch Phys Med Rehabil. 1970;51(3):164–9. [PubMed] [Google Scholar]
- 11.Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta. 2002;1592(3):251–63. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa Y, Kuwabara S, Misawa S, Tamura N, Kitano Y, Ogawara K, et al. The acute effects of glycemic control on nerve conduction in human diabetics. Clin Neurophysiol. 2005;116(2):270–4. doi: 10.1016/j.clinph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Kimura J. Assessment of individual nerves. Philadelphia: F.A. Davis; 1993. [Google Scholar]
- 14.Lacroix S, Chang L, Rose-John S, Tuszynski MH. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol. 2002;454(3):213–28. doi: 10.1002/cne.10407. [DOI] [PubMed] [Google Scholar]
- 15.Love A, Cotter MA, Cameron NE. Effects of alpha-tocopherol on nerve conduction velocity and regeneration following a freeze lesion in immature diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;355(1):126–30. doi: 10.1007/pl00004910. [DOI] [PubMed] [Google Scholar]
- 16.Martin A, Zulueta J, Hassoun P, Blumberg JB, Meydani M. Effect of Vitamin E on hydrogen peroxide production by human vascular endothelial cells after hypoxia/reoxygenation. Free Radic Biol Med. 1996;20(1):99–105. doi: 10.1016/0891-5849(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–16. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- 18.Norbury R, Cutter WJ, Compton J, Robertson DM, Craig M, Whitehead M, et al. The neuroprotective effects of estrogen on the aging brain. Exp Gerontol. 2003;38(1–2):109–17. doi: 10.1016/s0531-5565(02)00166-3. [DOI] [PubMed] [Google Scholar]
- 19.Oh SJ. Clinical electromyography: nerve conduction studies. 2. Batimore: Williams &Wilkins; 1993. [Google Scholar]
- 20.Patrone C, Andersson S, Korhonen L, Lindholm D. Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci USA. 1999;96(19):10905–10. doi: 10.1073/pnas.96.19.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzi M, Sarnico I, Boroni F, Benarese M, Dreano M, Garotta G, et al. Prevention of neuron and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6 receptor/IL-6 fusion protein in organotypic hippocampal slices. Mol Cell Neurosci. 2004;25(2):301–11. doi: 10.1016/j.mcn.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Prolla TA, Mattson MP. Molecular mechanisms of brain aging and neurodegenerative disorders: lessons from dietary restriction. Trends Neurosci. 2001;24(11 Suppl):S21–31. doi: 10.1016/s0166-2236(00)01957-3. [DOI] [PubMed] [Google Scholar]
- 23.Resnick HE, Vinik AI, Heimovitz HK, Brancati FL, Guralnik JM. Age 85+ years accelerates large-fiber peripheral nerve dysfunction and diabetes contributes even in the oldest-old: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2001;56(1):M25–31. doi: 10.1093/gerona/56.1.m25. [DOI] [PubMed] [Google Scholar]
- 24.Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24(9):1134–41. doi: 10.1002/mus.1124. [DOI] [PubMed] [Google Scholar]
- 25.Simonsick EM, Maffeo CE, Rogers SK, Skinner EA, Davis D, Guralnik JM, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52(5):M264–74. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 26.Slimani N, Ferrari P, Ocke M, Welch A, Boeing H, Liere M, et al. Standardization of the 24-h diet recall calibration method used in the european prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr. 2000;54(12):900–17. doi: 10.1038/sj.ejcn.1601107. [DOI] [PubMed] [Google Scholar]
- 27.Stalberg E, Fuglsang-Frederiksen A, Bischoff C. Quantitation and standardization in EMG and neurography. Suppl Clin Neurophysiol. 2000;53:101–11. doi: 10.1016/s1567-424x(09)70144-8. [DOI] [PubMed] [Google Scholar]
- 28.Tutuncu NB, Bayraktar M, Varli K. Reversal of defective nerve conduction with Vitamin E supplementation in type 2 diabetes: a preliminary study. Diabetes Care. 1998;21(11):1915–8. doi: 10.2337/diacare.21.11.1915. [DOI] [PubMed] [Google Scholar]
- 29.van Dam PS, Bravenboer B, van Asbeck BS, Marx JJ, Gispen WH. High rat food Vitamin E content improves nerve function in streptozotocin-diabetic rats. Eur J Pharmacol. 1999;376(3):217–22. doi: 10.1016/s0014-2999(99)00376-3. [DOI] [PubMed] [Google Scholar]
- 30.van Dam PS, van Asbeck BS, Erkelens DW, Marx JJ, Gispen WH, Bravenboer B. The role of oxidative stress in neuropathy and other diabetic complications. Diabetes Metab Rev. 1995;11(3):181–92. doi: 10.1002/dmr.5610110303. [DOI] [PubMed] [Google Scholar]
- 31.van Tits LJ, Demacker PN, de Graaf J, Hak-Lemmers HL, Stalenhoef AF. α-Tocopherol supplementation decreases production of superoxide and cytokines by leukocytes ex vivo in both normolipidemic and hypertriglyceridemic individuals. Am J Clin Nutr. 2000;71(2):458–64. doi: 10.1093/ajcn/71.2.458. [DOI] [PubMed] [Google Scholar]
- 32.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130(2–3):184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 33.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 34.Yatin SM, Varadarajan S, Butterfield DA. Vitamin E prevents Alzheimer’s amyloid beta-peptide (1-42)-induced neuronal protein oxidation and reactive oxygen species production. J Alzheimers Dis. 2000;2(2):123–31. doi: 10.3233/jad-2000-2212. [DOI] [PubMed] [Google Scholar]


