Abstract
Background
N-3 fatty acids (FA) have an important role in brain development and function. However, there is conflicting evidence concerning the relationship between n-3 FA and dementia in older persons.
Methods
In the Invecchiare in Chianti (InCHIANTI) study, we measured plasma FA by gas chromatography in 935 community-dwelling older persons randomly extracted from the population of two towns near Florence, Italy. Cognitive impairment was measured using the Mini-Mental Status Examination. Participants who scored ≤26 underwent a detailed clinical and neuropsychological evaluation. The diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders, Third Revision (DSM-III-R) criteria. The population was divided in three groups: persons with normal cognitive function, persons with cognitive impairment not demented, and persons with dementia.
Results
After adjustment for age, gender, education, body mass index, weight loss, smoking status, cholesterol and triglycerides levels, daily intake of alcohol, FA and total energy, cardiovascular disease, depression and other FA levels, participants with dementia had significantly lower n-3 FA levels (2.9% vs 3.2%; p < .05), particularly alpha-linolenic acid levels (0.34% vs 0.39%; p < .05), than did participants with normal cognitive function.
Conclusions
Dementia is associated with low plasma n-3 FA relative concentrations. The possibility that higher n-3 FA intake is associated with a lower risk of cognitive impairment should be further investigated in prospective studies.
There is growing evidence linking polyunsaturated fatty acids (FA) of the omega-3 family (n-3 FA) to cognitive function in humans (1). Prospective epidemiological studies found that a low dietary intake of fish, the major source of long-chain n-3 FA (2,3), as well as a low intake of n-3 FA in general (4), was associated with higher risk of developing cognitive impairment and dementia. However this prospective association was not confirmed by other studies (5). Plasma FA levels are good markers of dietary intake (6). Although plasma FA have been usually considered markers of short-term intake, recent studies show that they also reflect long-term intake (7), because their levels are consistent with changes in FA composition of adipose tissue. Moreover, circulating levels might be a better measure of exposure than dietary intake because they are less liable to lack precision and potential biases typical of food frequency questionnaires. Studies that explored the relationship between plasma FA and cognitive function found that individuals with dementia tend to have low plasma levels of n-3 FA (8,9). A lower proportion of n-3 FA in the erythrocyte membranes was predictive of cognitive decline (10). In contrast, a recent Canadian study failed to detect any association between plasma n-3 FA and both prevalence and incidence of cognitive impairment and dementia (11).
Due to these conflicting results, it is unclear whether low plasma levels of n-3 FA are associated with cognitive impairment (12). The aim of our study was to evaluate the relationship between plasma FA and dementia in a large sample of older participants enrolled in the InCHIANTI study.
Methods
Study Sample
InCHIANTI is an epidemiological study performed in two Italian towns located in the Chianti countryside (Greve in Chianti and Bagno a Ripoli), the main aim of which is to identify the factors contributing to the decline of mobility in late life. A detailed description of the study has been previously published (13). Only participants aged ≥ 65 years were selected for this analysis. All participants underwent a clinical examination and a detailed interview. A proxy was interviewed when the participant was unable to provide the required information. Functional status was assessed by means of the basic activities of daily living (ADL) and instrumental activities of daily living (IADL) scales. Education was considered as a continuous variable (years of education). Participants were classified as “non-smokers” or “smokers” (including former and current smokers). Body mass index (BMI) was calculated as kilograms per meters squared. Weight loss was assessed by asking the participant whether he or she had lost more than 10 pounds (5 kg) over the last 12 months. Diseases were ascertained according to standard, pre-established criteria that combined information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations, and blood tests. Diseases included in the analysis were coronary heart disease (CHD, including angina and myocardial infarction), congestive heart failure (CHF), cerebrovascular disease (CVD, including transient ischemic attack [TIA] and stroke), diabetes, hypertension, and depression. The food frequency questionnaire was the instrument developed and used by the Italian sites of the European Prospective Study into Cancer and Nutrition (EPIC) (14). It was administered to the participant or to a proxy informant, if the participant obtained a score lower than 5 in the items of the Mini-Mental State Examination (MMSE) (15) exploring orientation in place and time. The variables used were total energy (kcal), alcohol (grams), and saturated, monounsaturated, polyunsaturated, alpha linolenic, and linoleic FA (grams), all expressed as daily intake. Depressive symptoms were measured by the 20-item Center for Epidemiological Studies Depression Scale (CES-D) (16). Participants with a CES-D score ≥16 were considered to be depressed. The study protocol was approved by the INRCA (Istituto Nazionale per la Ricerca e Cura dell'Anziano – National Institute for Research and Care of the Elderly) ethical committee. All participants received a description of the study and gave their written informed consent.
Evaluation of Cognitive Status
Dementia was ascertained using a two-stage screening procedure, as previously described (17). Participants were first evaluated using the MMSE; those who scored ≤26 underwent a detailed clinical and neuropsychological evaluation. Participants who reported difficulty in performing ADL or IADL were asked questions aimed at understanding whether the cause of disability was, at least in part, cognitive impairment. A diagnosis of “dementia syndrome” was established using Diagnostic and Statistical Manual of Mental Disorders, Third Revision (DSM-III-R) criteria (18). On request, the authors can provide a detailed description of the neuropsychological battery used.
For this analysis we divided the population into three groups: (i) participants with normal cognitive function (i.e., MMSE score ≥ 23, no diagnosis of dementia, and no disability attributable to cognitive impairment) (n = 725); (ii) participants with cognitive impairment but not dementia (i.e., those with an MMSE score < 23 and/or any degree of disability in ADLs or IADLs that was judged to be related to impaired cognitive function) (n = 153); and (iii) participants suffering from dementia (n = 57).
Quantification of Plasma Fatty Acids
Blood samples were collected in the morning after a 12-hour fast. Plasma samples were stored at −80° C until analyzed. A detailed description of the method used has been published already (19). The amount of plasma FA (ranging from C14:0 to C24:1) is expressed as percentage of total FA based on milligram per liter values. Plasma saturated FA (SFAs), monounsaturated FA (MUFAs), total polyunsaturated FA (PUFAs), omega-3 FA (n-3), omega-6 FA (n-6), and the n-6/n-3 ratio were calculated. For the present analysis we considered a limited number of individual FA: palmitic acid (16:0) and stearic acid (18:0) among SFAs; oleic acid (18:1n-9) among MUFAs; alpha linolenic acid (18:3n-3, ALA), eicosapentaenoic acid (20:5n-3, EPA), and docosahexaenoic acid (22:6n-3, DHA) for the n-3 family; and linoleic acid (18:2n-6, LA) and arachidonic acid (20:4n-6, AA) for the n-6 family.
Statistical Analysis
Data are presented as mean ± standard deviations (SD) or percentage. All variables were normally distributed with the exception of ALA, EPA, total n-3 FA, and n-6/n-3 ratio, which were log-transformed. Differences between groups were tested with an unpaired t test, analysis of variance (ANOVA), or chi-square test, as appropriate. Correlation between variables was assessed using the Pearson correlation coefficient. Differences in the plasma relative concentrations of FA between different cognitive groups were evaluated with analysis of covariance, controlling for potential confounders. The statistical analysis was performed using SAS (version 8.1; Cary, NC).
Results
Of 1299 sampled participants aged ≥ 65 years, 1155 (88.9%) agreed to participate in the study. Plasma levels of FA were available in 935 participants (81.0%). The descriptive characteristics of the whole sample and of the three cognitive status groups are reported in Table 1. Participants with an MMSE score between 23 and 26 had a mean age of 75 years and a mean level of education of 5 years. These values were intermediate compared to those of participants with an MMSE score > 26 (mean age 72 years and mean education 7 years) and an MMSE score < 23 (mean age 82 years and mean education 3 years).
Table 1.
Characteristics of the Participants According to Their Cognitive Status
| Variable | Total Sample (N = 935) |
Group 1 (Normal Cognitive Function) (N = 725) |
Group 2 (Cognitive Impairment) (N = 153) |
Group 3 (Dementia) (N = 57) |
|---|---|---|---|---|
| Age, y | 75.6 ± 7.4 | 73.8 ± 6.2 | 80.6 ± 7.6*,† | 84.8 ± 7.2‡ |
| Gender (women)§ | 56% | 52% | 71% | 58% |
| Education, y | 5.3 ± 3.4 | 5.8 ± 0.5 | 3.7 ± 0.03‖ | 3.6 ± 0.5‖ |
| BMI | 27.3 ± 4 | 27.2 ± 0.6 | 27.7 ± 0.39 | 27.4 ± 0.6 |
| Cholesterol, mmol/L | 5.6 ± 1 | 5.6 ± 0.1 | 5.5 ± 0.1‖ | 5.1 ± 0.1‖ |
| LDL cholesterol, mmol/L | 3.5 ± 0.9 | 3.52 ± 0.13 | 3.47 ± 0.08 | 3.22 ± 0.3† |
| HDL cholesterol, mmol/L | 1.4 ± 0.4 | 1.46 ± 0.05 | 1.45 ± 0.03 | 1.21 ± 0.05‖,¶ |
| Triglylcerides, mmol/L | 1.4 ± 0.7 | 1.22 ± 0.11 | 1.33 ± 0.07 | 1.36 ± 0.11 |
| Weight loss# | 5.0% | 4.2% | 6.2% | 4.4% |
| Energy intake, kcal/day | 1918 ± 562 | 1923 ± 74 | 1921 ± 48* | 1842 ± 74‡ |
| Saturated FA intake, g/d | 21.8 ± 7.5 | 21.9 ± 1.1 | 21.6 ± 1.7‡ | 20.6 ± 1.1‖ |
| Monounsaturated FA intake, g/d | 32.2 ± 10.9 | 32.2 ± 1.5 | 35.6 ± 1.0‡ | 30.5 ± 1.5‡ |
| Polyunsaturated FA intake, g/d | 7.0 ± 2.2 | 7.0 ± 0.3 | 7.1 ± 0.2‡ | 6.8 ± 0.3‡ |
| ALA, g/d | 0.96 ± 0.29 | 0.97 ± 0.04 | 0.98 ± 0.03‡ | 0.94 ± 0.04‡ |
| LA, g/d | 5.6 ± 1.9 | 5.7 ± 0.3 | 5.7 ± 0.2‡ | 5.4 ± 0.3‡ |
| Alcohol intake, g/d | 14.8 ± 20.6 | 15.4 ± 2.7 | 13.3 ± 1.8* | 10.3 ± 2.7‡ |
| Smoking status§ | ||||
| Former/current smoker | 41% | 40.2% | 30.3% | 31.1% |
| CHD | 6.1% | 6.6% | 2.5%‖ | 1.6%‖ |
| CVD | 2.5% | 1.7% | 3.8% | 1.8% |
| CHF | 7.7% | 5.7% | 9.5% | 11.0% |
| Diabetes | 8.5% | 8.3% | 7.7% | 11.4% |
| Hypertension | 52.3% | 54.2% | 47.9% | 48.8% |
| Depression (CES-D score ≥16) | 29.6% | 28.3% | 33.3%† | 12.0%* |
Notes: Data for the whole population are expressed as mean ± standard deviation (SD) or percentage. Data for the three groups are expressed as mean ± standard error (SE) or percentage and are adjusted for age and gender.
p < .001 vs Group 1.
p < .001 vs Group 3.
p < .001 vs Group 1.
p < .0001.
p < .05 vs Group 1.
p < .05 vs Group 2.
Weight loss is intended as the loss of more than 10 pounds (5 kg) over the last 12 months.
BMI = body mass index; LDL = low-density lipoprotein; HDL = high-density lipoprotein; FA = fatty acid; ALA = alpha-linolenic acid; LA = linoleic acid; CHD = coronary heart disease; CVD = cerebrovascular disease; CHF = congestive heart failure; CES-D = Center for Epidemiologic Studies Depression scale.
Mean values and 95% confidence intervals (CI) of FA relative concentrations (as percentages based on mg/L of total FA) are presented in Table 2. After adjustment for confounders (i.e., age, gender, education, smoking status, BMI, weight loss, alcohol intake, total energy intake, low density lipoprotein [LDL] and high density lipoprotein [HDL] cholesterol levels, triglyceride levels, CHD, CVD, CHF, diabetes, hypertension, and depression), participants affected by dementia had higher relative concentrations of SFAs and lower relative concentration of PUFAs, as well as n-3, compared with participants with normal cognition. Moreover, participants affected by dementia had higher palmitic acid and lower ALA. Participants with cognitive impairment had intermediate FA values between those of demented and normal participants (Table 2). After adjusting for the confounders, however, none of the FA was statistically different between cognitively impaired and normal participants.
Table 2.
Mean Unadjusted Plasma Fatty Acid Relative Concentrations (% Based on mg/L of Total Fatty Acids) in Participants Who Had No Cognitive Impairment Compared With Those Who Had Cognitive Impairment and Those With Dementia
| Fatty Acid | No Cognitive Impairment (N = 725) |
Cognitive Impairment (N = 153) |
Dementia (N = 57) |
p Value for Trend |
|---|---|---|---|---|
| SFAs | 30.3 (30.1–30.5) | 30.1 (29.7–30.5)* | 31.4 (30.5–32.4)† | .04 |
| Palmitic acid (16:0) | 22.5 (22.3–22.7) | 22.4 (22.1–22.8)* | 23.7 (22.9–24.5)‡ | .02 |
| Stearic acid (18:0) | 6.5 (6.4–6.6) | 6.4 (6.3–6.6) | 6.5 (6.2–6.8) | .46 |
| MUFAs | 31.4 (31.1–31.7) | 32.5 (31.9–33) | 34 (33.2–34.8) | .49 |
| Oleic acid (18:1n-9) | 26.2 (25.9–26.5) | 26.9 (26.3–27.5) | 27.8 (27.1–28.6) | .90 |
| PUFAs | 38 (37.7–38.4) | 37.1 (36.4–37.9)* | 34.3 (33.0–35.6)‡ | .03 |
| Total n-6 | 32.6 (32.3–32.9) | 31.9 (31.2–32.6) | 29.5 (28.3–30.6)† | .10 |
| Linoleic acid (18:2) | 24.5 (24.2–24.8) | 23.7 (23.1–24.3)* | 21.7 (20.7–22.6)† | .10 |
| Arachidonic acid (20:4) | 8.0 (7.8–8.1) | 8.1 (7.8–8.4) | 7.7 (7.2–8.2) | .75 |
| Total n-3§ | 3.2 (3.1–3.3) | 3.0 (2.9–3.1)* | 2.7 (2.4–2.9)‡ | .01 |
| Linolenic acid (18:3)§ | 0.40 (0.39–0.41) | 0.35 (0.32–0.38) | 0.31 (0.27–0.34)† | .04 |
| EPA (20:5)§ | 0.59 (0.58–0.60) | 0.55 (0.52–0.57) | 0.51 (0.47–0.55) | .14 |
| DHA (22:6) | 2.28 (2.23–2.34) | 2.16 (2.06–2.26) | 2.00 (1.82–2.21) | .23 |
| n-6/n-3§ | 10.0 (9.8–10.2) | 10.5 (10.0–11.0) | 10.8 (9.9–11.9)† | .09 |
Notes: Values presented are unadjusted means (95% confidence intervals). Values of p presented here are fully adjusted for covariates, i.e., age, gender, education, smoking status, body mass index, weight loss, alcohol intake, total energy intake, low density lipoprotein and high density lipoprotein cholesterol levels, triglyceride levels, coronary heart disease, cerebrovascular disease, congestive heart failure, diabetes, hypertension, and depression.
p < .05 vs Group 3.
p < .05 vs Group 1.
p < .01 vs Group 1.
Variables were log-transformed before the analysis because they were not normally distributed.
SFAs = saturated fatty acids; MUFAs = monounsaturated FA; PUFAs = polyunsaturated FA; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid.
Figure 1 shows the mean relative concentrations of plasma FA in the three cognitive groups. Mean values, 95% CI values, and statistical comparisons between groups are adjusted for potential confounders, including other FA relative concentrations. For example, data and analysis on n-3 FA are adjusted for n-6 FA and SFAs as covariates. In this final analysis, only total n-3 FA and ALA remained significantly lower in demented participants compared to normal controls (Figure 1).
Figure 1.
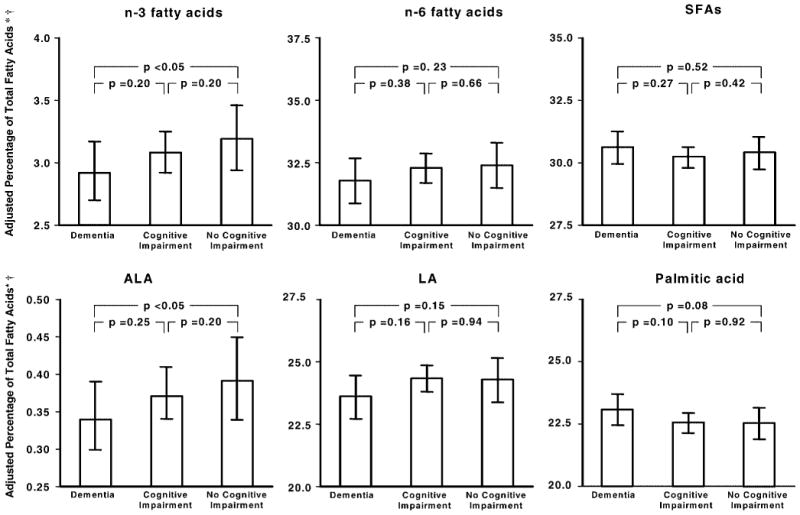
Means ± 95% confidence intervals of the relative concentration of n-3 fatty acids (FA), n-6 FA, and saturated FA (SFAs) (top); and alpha-linolenic acid (ALA), linolenic acid (LA), and palmitic acid (bottom) in participants with dementia, cognitive impairment, or normal cognitive function. *Based on mg/L total plasma FA. †Adjusted for age, gender, education, smoking status, body mass index, weight loss, alcohol intake, total energy intake, low density lipoprotein and high density lipoprotein cholesterol levels, triglyceride levels, coronary heart disease, cerebrovascular disease, congestive heart failure, diabetes, hypertension, depression, and plasma levels of the other two FA variables in the same panel.
In logistic regression models fully adjusted for confounders, a 1 SD lower n-3 FA was associated with 67% higher probability of having dementia compared to having normal cognitive function (odds ratio = 1.67; 95% CI, 1.17–2.38). Similar findings were observed for ALA (odds ratio = 1.65; 95% CI, 1.17–2.33).
Discussion
In this study, older participants with dementia had lower relative concentrations of plasma n-3 FA compared to those of participants with normal cognitive function. Our findings are consistent with studies that found an association between lower dietary intake of n-3 FA and higher prevalence and incidence of dementia (2–4), as well as between higher saturated fat intake and incident Alzheimer's disease (AD) (4). Blood levels of FA are generally considered to be good markers of dietary intake (6), although they reflect not only diet but also processes involved in their use, namely absorption and metabolism (20). Some cross-sectional studies found lower blood levels of n-3 FA, particularly DHA, in participants with dementia (8,9). Heude and colleagues (10) found an inverse association between higher n-3 FA in erythrocyte membranes and risk of cognitive decline, whereas in the Canadian Study of Health and Aging, no relationship was demonstrated between baseline serum FA levels and the prevalence and 4-year incidence of cognitive impairment or dementia (11). There is no clear explanation for these conflicting results, although caution should be used in their interpretation due to small sample sizes. As our study included a large sample of elderly participants and measured both plasma levels and dietary intake of FA, it supports the hypothesis that low levels of plasma n-3 FA are associated with dementia. Several mechanisms could explain the beneficial effect of n-3 FA on cognitive status. N-3 FA, particularly DHA, have an important role in brain development and function (21).
DHA represents a major structural component of the neuronal membrane phospholipids that improves membrane fluidity, which is important for several key functions, such as activity of membrane-bound proteins (enzymes, ion channels, and receptors), signal transduction, and neurotransmission (22). In particular, there is evidence that DHA modulates cholinergic transmission in the brain (23), and DHA inhibits neuronal apoptosis (24). A low DHA diet has been associated with the development of dendritic pathology and behavioral deficits in an AD mouse model (25), whereas DHA can decrease the amyloid toxicity in transgenic AD mice, reducing the brain plaque burden by 40% (26). This neuroprotective activity is due, at least in part, to a recently discovered DHA-derived compound, neuroprotectin D1 (27).
DHA demonstrated beneficial effects on learning ability in aged animals (22) and in a rat model of AD (28). Small clinical trials found positive effects of treatment with DHA both in patients suffering from AD (29) and in those suffering from vascular dementia (30). A recent randomized controlled trial did not find a beneficial effect on cognitive decline in mild-to-moderate AD patients treated with a mixture of DHA and EPA for 6–12 months, although positive results were observed in very mild AD (31).
Reduction in triglyceride levels, blood pressure, platelet aggregation, and expression of adhesion molecules and inflammatory cytokines, together with increased nitric oxide production and an antiarrhythmic effect are among the biological mechanisms that might account for the benefits of n-3 FA (32,33). As cardiovascular diseases are risk factors for both vascular and degenerative dementia, cardiovascular risk reduction might represent an important mechanism relating n-3 FA to reduced risk of cognitive decline. N-3 FA can inhibit the production of pro-inflammatory cytokines, i.e., interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (34), which are involved in the development of dementia. Moreover, they decrease the synthesis of pro-inflammatory eicosanoids, which derive from n-6 FA (34). According to some studies, n-3 FA could also reduce the risk of depression (35), a condition associated with dementia in late life. However, in our analysis adjustment for depression did not substantially change the outcome.
Although in our study EPA and DHA levels were reduced in older participants with dementia, the difference with normal participants became nonsignificant after multivariate adjustment. One explanation of this unexpected finding might be that the consumption of fish is low in the Chianti area and therefore there is a low variability in long-chain n-3 FA concentrations. However, levels of ALA, a source of DHA and EPA, were significantly lower in participants with dementia. ALA provides health benefits similar to those provided by longer chain n-3 FA, e.g., with respect to neuronal function in animals (36) and inflammation and cardiovascular disease in humans (37).
The causes of the reduced n-3 FA levels in participants suffering from dementia are unknown. Previous longitudinal studies demonstrated that individuals who later developed dementia had a lower intake of fish, the principal source of long-chain n-3 FA, as well of n-3 FA in general (2–4). Reduced levels of PUFA in brain membrane phospholipids (38,39) have been found in Alzheimer patients and have been attributed, at least in part, to reduced activity of the enzyme Δ 6 desaturase that is involved in the metabolism of ALA to long-chain n-3 FA. Normally, ALA is for the most part completely oxidized via β-oxidation or partially oxidized for carbon recycling into lipogenesis, whereas only a small percentage is converted to long-chain n-3 FA (37). Finally, increased oxidative stress, a process involved in dementia, might reduce PUFA levels in the membranes (40) and increase the requirement for ALA and DHA intake to compensate for this loss. Participants with dementia also had higher palmitic acid levels compared to older participants with normal cognitive function. Palmitic acid is a precursor of ceramides and sphingomyelin. Recent studies have shown that levels of long-chain ceramides are increased in brain tissue samples from AD patients compared to age-matched controls (41,42). Moreover, pharmacological inhibition of ceramide production protected hippocampal neurons from being killed by amyloid β-peptide and oxidative stress, suggesting a role for increased ceramide levels in the demise of neurons in AD (42). Thus, alterations in the levels of palmitic acid might contribute to the pathogenesis of AD.
The principal limitation of our study is its cross-sectional design, which does not allow for the establishment of a cause–effect relationship between FA and dementia. Indeed, it is equally possible that low plasma FA levels anticipated dementia as well as they decreased due to a change in dietary habits after the onset of dementia. Moreover, as we did not perform a detailed neuropsychological evaluation of participants with an MMSE score > 26, it is possible that the group labeled as cognitively normal includes participants with mild cognitive decline, and this might have prevented us from detecting a difference in FA plasma relative concentrations between normal participants and those with cognitive impairment. Another limitation of this study is that, because of the large number of fatty acids that were analyzed, a large number of tests was performed inflating the chance of a type I error. However, because our findings were consistent across different FA and are consistent with our original hypothesis, we did not apply the Bonferroni correction. Finally, the absolute difference in n-3 FA between demented and normal participants was small, but remained significant after adjustment for multiple confounders.
Conclusion
Our results provide further evidence that older persons with dementia have lower concentration of n-3 FA. From a clinical point of view, they further support the current recommendation that the dietary intake of n-3 fatty acids should be increased.
Longitudinal analyses performed in this and other cohorts are needed to support the role of n-3 fatty acids in reducing the risk of developing cognitive impairment. Moreover, given the safety of fatty acid supplementation, the efficacy of n-3 FA in the prevention and treatment of cognitive decline and dementia should be evaluated.
Acknowledgments
This research was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and by the U.S. National Institute on Aging (contracts 263 MD 9164 13 and 263 MD 821336). It was also partially supported by an unrestricted grant by BRACCO imaging SpA, Italy. Cristina Andres-Lacueva was supported by the Ramon y Cajal program of the Ministry of Science and Technology from Spain and the ESF (European Social Fund).
Footnotes
This research was presented at the 57th Annual Scientific Meeting of The Gerontological Society of America, Washington, D.C., November 21, 2004.
References
- 1.Solfrizzi V, D'Introno A, Colacicco AM, et al. Dietary fatty acids intake: possible role in cognitive decline and dementia. Exp Gerontol. 2005;40:257–270. doi: 10.1016/j.exger.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 3.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–280. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 4.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 5.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Diet and risk of dementia: does fat matter? The Rotterdam Study. Neurology. 2002;59:1915–1921. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr. 1995;62:564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 7.Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 8.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 9.Tully AM, Roche HM, Doyle R, et al. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 10.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes–The EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 11.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis. 2003;5:315–322. doi: 10.3233/jad-2003-5407. [DOI] [PubMed] [Google Scholar]
- 12.Maclean CH, Issa AM, Newberry SJ, et al. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evid Rep Technol Assess Summ. 2005;114:1–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 14.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 17.Cherubini A, Martin A, Andres-Lacueva C, et al. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26:987–994. doi: 10.1016/j.neurobiolaging.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 19.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 20.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 21.Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging. 2004;8:163–174. [PubMed] [Google Scholar]
- 22.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Aid S, Vancassel S, Linard A, Lavialle M, Guesnet P. Dietary docosahexaenoic acid [22:6(n-3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr. 2005;135:1008–1013. doi: 10.1093/jn/135.5.1008. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 25.Calon F, Lim GP, Yang F, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim GP, Calon F, Morihara T. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr. 2005;135:549–555. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda S, Rabinovtz S, Carasso RL, Mostofsky DI. Essential fatty acids preparation (SR-3) improves Alzheimer's patients quality of life. Int J Neurosci. 1996;87:141–149. doi: 10.3109/00207459609070833. [DOI] [PubMed] [Google Scholar]
- 30.Terano T, Fujishiro S, Ban T, et al. Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids. 1999;34(Suppl):S345–S346. doi: 10.1007/BF02562338. [DOI] [PubMed] [Google Scholar]
- 31.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 32.Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- 33.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 34.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75:197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- 36.Umezawa M, Kogishi K, Tojo H, et al. High-linoleate and high-alpha-linolenate diets affect learning ability and natural behavior in SAMR1 mice. J Nutr. 1999;129:431–437. doi: 10.1093/jn/129.2.431. [DOI] [PubMed] [Google Scholar]
- 37.Sinclair AJ, Attar-Bashi NM, Li D. What is the role of alpha-linolenic acid for mammals? Lipids. 2002;37:1113–1123. doi: 10.1007/s11745-002-1008-x. [DOI] [PubMed] [Google Scholar]
- 38.Nakada T, Kwee IL, Ellis WG. Membrane fatty acid composition shows delta-6-desaturase abnormalities in Alzheimer's disease. Neuroreport. 1990;1:153–155. doi: 10.1097/00001756-199010000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 40.Urano S, Sato Y, Otonari T, et al. Aging and oxidative stress in neurodegeneration. Biofactors. 1998;7:103–112. doi: 10.1002/biof.5520070114. [DOI] [PubMed] [Google Scholar]
- 41.Han X, Holtzman DM, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 42.Cutler RG, Kelly J, Storie K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]


