Abstract
Background
Aging in men is characterized by a progressive decline in levels of anabolic hormones, such as testosterone, insulinlike growth factor 1 (IGF-1), and dehydroepiandrosterone sulfate (DHEA-S). We hypothesized that in older men a parallel age-associated decline in bioavailable testosterone, IGF-1, and DHEA-S secretion is associated with higher mortality independent of potential confounders.
Methods
Testosterone, IGF-1, DHEA-S, and demographic features were evaluated in a representative sample of 410 men 65 years and older enrolled in the Aging in the Chianti Area (InCHIANTI) study. A total of 126 men died during the 6-year follow-up. Thresholds for lowest-quartile definitions were 70 ng/dL (to convert to nanomoles per liter, multiply by 0.0347) for bioavailable testosterone, 63.9 ng/mL (to convert to nanomoles per liter, multiply by 0.131) for total IGF-1, and 50 μg/dL (to convert to micromoles per liter, multiply by 0.027) for DHEA-S. Men were divided into 4 groups: no hormone in the lowest quartile (reference) and 1, 2, and 3 hormones in the lowest quartiles. Kaplan-Meier survival and Cox proportional hazards models adjusted for confounders were used in the analysis.
Results
Compared with men with levels of all 3 hormones above the lowest quartiles, having 1, 2, and 3 dysregulated hormones was associated with hazard ratios for mortality of 1.47 (95% confidence interval [CI], 0.88-2.44), 1.85 (95% CI, 1.04-3.30), and 2.29 (95% CI, 1.12-4.68), respectively (test for trend, P <.001). In the fully adjusted analysis, only men with 3 anabolic hormone deficiencies had a significant increase in mortality (hazard ratio, 2.44; 95% CI, 1.09-5.46 (test for trend, P <.001).
Conclusions
Age-associated decline in anabolic hormone levels is a strong independent predictor of mortality in older men. Having multiple hormonal deficiencies rather than a deficiency in a single anabolic hormone is a robust biomarker of health status in older persons.
It is currently believed that anabolic-catabolic imbalance that favors catabolism is a key factor of accelerated aging in men.1 The imbalance is mostly related to abnormalities in 3 anabolic endocrine axes, gonadal, adrenal, and somatotropic, with a decline in testosterone, dehydroepiandrosterone sulfate (DHEA-S), and insulinlike growth factor 1 (IGF-1), respectively.1 Anabolic impairment may facilitate the decline in muscle mass, increase in fat mass, development of insulin resistance, and several medical conditions that, in turn, affect mortality.1,2
The contribution of single hormonal deficiency to age-related morbidity and mortality has been described in previous studies. Shores et al3 showed that testosterone, the main anabolic hormone in men, is a predictor of mortality in male veterans. The extent to which this finding is valid for the general population is still in question. According to Roth et al,4 DHEA-S has been considered one of the mediators of the relationship between caloric restriction and longevity in both animals and humans. Barrett-Connor et al5 found that low DHEA-S levels predict cardiovascular mortality in older men, and Cappola et al6 found that in older disabled women the relationship between DHEA-S and mortality is not linear. However, further studies7 performed in the older population were unable to validate these findings. Signaling of IGF-1 has been considered a determinant of longevity, probably because of beneficial effects on muscle, vasculature, and metabolism.8 However, although IGF-1 was shown to be a predictor of cardiovascular mortality in older men,9,10 the role of IGF-1 as a single determinant of longevity is still debated.11
On the basis of these contradictory data, it is unlikely that a single anabolic hormone deficiency could be considered a reliable index of the aging process. Interestingly, plurihormonal dysregulation rather than single hormonal derangement has been associated with the frailty syndrome12,13 or the metabolic syndrome in older men.14 In men with chronic heart failure, deficiency of more than 1 anabolic hormone identifies patients with higher mortality rates.15 However, data concerning the relationship between parallel deficiency of anabolic hormones and mortality in a general population of older men, regardless of specific disease, are still lacking. Using data from the Aging in the Chianti Area (InCHIANTI) study, we hypothesized that a decline in the level of multiple anabolic hormones rather than in a single hormone predicts mortality during a 6-year follow-up in older men.
Methods
Study Sample
The InCHIANTI study is an epidemiologic study of a representative sample of the population living in Tuscany, Italy. We selected from this population 410 men 65 years and older (age range, 65-92 years) who had complete data on levels of testosterone, DHEA-S, IGF-1, sex hormone–binding globulin (SHBG), and albumin. Six-year vital status for the original InCHIANTI cohort was ascertained with data from the Tuscany Region Mortality General Registry maintained by the Registry Office of the Tuscany Region, including death certificates. One hundred twenty-six men died during the 6-year follow-up period. None of the men in the study were taking DHEA, testosterone, or growth hormone, as determined from examination of all prescription and over-the-counter medication containers. The Italian National Institute of Research and Care on Aging institutional review board ratified the study protocol. Participants consented to participate and to have their blood samples analyzed for scientific purposes.16
Biological Samples
At an initial home interview, participants were given a plastic container and received detailed instructions for a 24-hour urine collection. Blood samples were obtained from participants after a 12-hour fast and after a 15-minute rest. Aliquots of serum and 24-hour urine were stored at −80°C and were not thawed until analyzed.
Hormone Measurement
Total testosterone and DHEA-S levels were assayed using commercial radioimmunologic kits (Diagnostic Systems Laboratories, Webster, Texas). For total testosterone, the minimum detection limit was 0.86 ng/dL (to convert to nanomoles per liter, multiply by 0.0347); intra-assay and interassay coefficients of variation for 3 different concentrations were 9.6%, 8.1%, and 7.8% and 8.6%, 9.1%, and 8.4%, respectively. For DHEA-S, the minimum detection limit was 1.7 μg/dL (to convert to nanomoles per liter, multiply by 27.2); intra-assay and interassay coefficients of variation for the 3 different concentrations were 4.1%, 5.3%, and 4.7% and 4.8%, 7.0%, and 4.6%, respectively. The SHBG was measured by radioimmunoassay (Diagnostic Products Corporation, Los Angeles, California), which has a sensitivity of 0.000046 μg/dL (to convert to nanomoles per liter, multiply by 34.7). In our laboratory, interassay and intra-assay coefficients of variation for 3 concentrations were less than 4%.17 Serum albumin was measured with a commercial enzymatic test (Roche Diagnostics GmbH, Mannheim, Germany). Bioavailable testosterone (serum-free and albumin-bound testosterone but not SHBG-bound testosterone) and free testosterone were calculated with the Vermeulen formula.18 Serum concentrations of total IGF-1 were measured in duplicate from frozen specimens by immunoradiometric assay, using commercial reagents (Diagnostic Systems Laboratories). Interassay and intra-assay coefficients of variation for 3 concentrations (low, medium, and high) were all less than 10%. Serum interleukin 6 (IL-6) was measured in duplicate by high-sensitivity enzyme-linked immunosorbent assays (BioSource, Camarillo, California). The lowest detectable concentration was 0.1 pg/mL, and the interassay coefficient of variation was 4.5%.
Comorbidity and Other Variables
Diseases were ascertained by an experienced physician according to preestablished criteria that combine information from self-reported physician diagnoses, current pharmacologic treatment, medical records, clinical examinations, and blood tests. Diseases included in the current analysis were coronary heart disease (including angina and myocardial infarction), congestive heart failure, stroke, diabetes mellitus, hypertension, Parkinson disease, peripheral artery disease, asthma, cancer, and chronic obstructive pulmonary disease (COPD). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Smoking history was determined from self-report and dichotomized in the analysis as current smoking vs ever smoked or never smoked. Educational level was recorded as years of school. Cognitive function was evaluated using the Mini-Mental State Examination, and the total score was adjusted for educational level and age.19
Depressive symptoms were measured with the 20-item Center for Epidemiologic Studies–Depression scale. Participants with a Center for Epidemiologic Studies–Depression scale score of 16 or more were considered to be depressed.20 Physical activity in the year before the interview was coded as (1) sedentary: completely inactive or light-intensity activity less than 1 hour per week; (2) light physical activity: light-intensity activity 2 to 4 hours per week; and (3) moderate to high physical activity: light activity at least 5 hours per week or more or moderate activity at least 1 to 2 hours per week. Daily total energy intake (in kilocalories) and alcohol intake (in grams) were estimated by the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire.21
Statistical Analysis
Variables are reported as mean±SD, median and interquartile range, or number and percentage. Because of skewed distributions, log-transformed values for DHEA-S, total IGF-1, bioavailable testosterone, and IL-6 were used in regression analyses. Participants' main characteristics according to the number of dysregulated hormones were determined by age-adjusted linear regression models (analysis of covariance) and the Mantel-Haenszel χ2 test. Survival analysis (Kaplan-Meier) and Cox proportional hazards models adjusted for age, BMI, log(IL-6), educational level, cognitive function, depression, physical activity, caloric and alcohol intake, smoking, coronary heart disease (including angina and myocardial infarction), congestive heart failure, stroke, diabetes, hypertension, Parkinson disease, peripheral artery disease, asthma, cancer, and COPD were used to assess the effects of single or multiple hormones in the lowest quartile on mortality. Covariates for adjustment were included in the model if in univariate analysis they were statistically associated with mortality. All analyses were performed with the SAS statistical package, version 9.1 (SAS Institute Inc, Cary, North Carolina).
Results
Serum levels used to define the lowest quartiles of the hormones considered in this study were as follows: 70 ng/dL for bioavailable testosterone, 50 μg/dL for DHEA-S, and 63.86 ng/mL for total IGF-1 (to convert to nanomoles per liter, multiply by 0.131). Four groups were created according to baseline serum levels of these hormones: no hormone in the lowest quartiles (reference group) (n=207) and 1 hormone (n=126), 2 hormones (n=57), and 3 hormones in the lowest quartiles (n=20) (the group with plurihormonal deficiency). Compared with living participants, men who died during the 6 years of follow-up were older and more likely to have a history of sedentary lifestyle, stroke, peripheral vascular disease, cognitive impairment, and higher serum log(IL-6) levels (data not shown).
Compared with men with levels of all hormones above the lowest quartile, men with 1, 2, or 3 hormones in the lowest quartiles were older and more likely to have a history of sedentary lifestyle (Table 1).
Table 1. Characteristics of the General Population at Baseline According to Anabolic Status Calculated as the Number of Anabolic Hormones in the Lowest Quartilesa.
| Characteristic | No. of Hormones in the Lowest Quartiles | P Valueb | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| No. of patients | 207 | 126 | 57 | 20 | ||
| Age, y | 72.27 (5.20) | 75.13 (6.81) | 79.52 (7.56) | 82.97 (7.69) | <.001 | |
| BMI | 27.44 (3.21) | 27.13 (3.01) | 26.56 (2.99) | 26.69 (3.40) | .90 | |
| Bioavailable testosterone, ng/dL | 111.88 (32.43) | 89.90 (36.34) | 52.77 (30.57) | 39.02 (18.72) | <.001 | |
| Total insulinlike growth factor 1, ng/mL | 141.05 (51.45) | 122.75 (50.06) | 100.24 (54.90) | 45.83 (14.43) | <.001 | |
| Dehydroepiandrosterone sulfate, μg/dL | 109.01 (55.52) | 69.91 (62.65) | 41.26 (31.60) | 21.75 (14.90) | <.001 | |
| Interleukin 6, median (IQR), pg/mL | 0.36 (−0.16 to 0.77) | 0.48 (−0.07 to 0.90) | 0.50 (0.09 to 1.13) | 1.02 (0.17 to 2.01) | .49 | |
| Cardiovascular disease, No. (%) | 8 (3.86) | 10 (7.94) | 1 (1.75) | 1 (5.00) | .92 | |
| Stroke, No. (%) | 11 (5.31) | 11 (8.73) | 5 (8.77) | 2 (10.00) | .41 | |
| Parkinson disease, No. (%) | 3 (1.45) | 1 (0.79) | 1 (1.75) | .26 | ||
| Hypertension, No. (%) | 54 (26.09) | 41 (32.54) | 20 (35.09) | 4 (20.00) | .07 | |
| Cancer, No. (%) | 9 (4.35) | 4 (3.17) | 4 (7.02) | 1 (5.0) | .62 | |
| COPD, No. (%) | 28 (13.53) | 22 (17.46) | 11 (19.30) | 4 (20.00) | .17 | |
| Mini-Mental State Examination score | 25.88 (3.55) | 25.33 (4.34) | 23.26 (6.14) | 22.45 (6.16) | .64 | |
| CES-D total score | 9.05 (6.52) | 10.76 (8.18) | 9.62 (6.01) | 11.06 (6.78) | .19 | |
| Peripheral artery disease, No. (%) | 16 (7.73) | 12 (9.52) | 6 (10.53) | 1 (20.00) | .32 | |
| Caloric intake, g/d | 2278.83 (581.89) | 2123.32 (527.69) | 1981.49 (572.97) | 1929.25 (566.48) | .33 | |
| Alcohol intake, g/d | 24.71 (18.16) | 24.47 (21.79) | 17.14 (17.20) | 20.71 (11.88) | .32 | |
| Smoking (current vs ever or never smoked) | 47 (22.71) | 27 (21.43) | 8 (14.04) | 3 (15.00) | .16 | |
| Physical activity | ||||||
| Sedentary level | 17 (8.21) | 19 (15.08) | 18 (31.58) | 3 (15.00) |
|
|
| Light level | 170 (82.13) | 99 (78.57) | 35 (61.40) | 15 (75.00) | .002 | |
| Moderate-high level | 20 (9.66) | 8 (6.35) | 4 (7.02) | 2 (10.00) | ||
| Educational level, y | 6.16 (3.15) | 6.43 (3.81) | 5.83 (3.90) | 4.35 (1.53) | .20 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiologic Studies–Depression; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
SI conversion factors: To convert testosterone to nanomoles per liter, multiply by 0.0347; insulinlike growth factor 1 to nanomoles per liter, multiply by 0.131; and dehydroepiandrosterone sulfate to micromoles per liter, multiply by 0.027.
Values are expressed as mean±SD unless otherwise indicated.
Differences in variables among patients with different anabolic status were tested using age-adjusted analysis of variance.
After adjusting for age, we did not find a statistically significant relationship between lowest quartiles of bioavailable testosterone and IGF-1 and all-cause mortality (Table 2), whereas a low DHEA-S level was associated with significantly higher mortality. Using the hormone levels as continuous variables, none of the 3 hormones were associated with significantly higher mortality. The hazard ratios (HRs) for mortality associated with having a hormone level in the lowest quartile vs no hormone level in the lowest quartile were 1.48 (95% confidence interval [CI], 0.89-2.45) for bioavailable testosterone, 1.36 (95% CI, 0.78-2.38) for IGF-1, and 1.37 (95% CI, 0.84-2.37) for DHEA-S. However, the association of DHEA-S with mortality was no longer significant after adjusting for potential confounders, including age, BMI, educational level, smoking, alcohol and caloric intake, physical activity, log(IL-6) levels, and chronic disease including diabetes, hypertension, peripheral artery diseases, coronary heart disease, congestive heart failure, stroke, Parkinson disease, COPD, asthma, and cancer (Table 3).
Table 2. Relationship Between Hormonal Dysregulation and All-Cause Mortality in Older Men: Unadjusted Analysisa.
| Characteristic | Hazard Ratio (95% Confidence Interval) | Age-Adjusted P Value | P Value for Trendb |
|---|---|---|---|
| Bioavailable testosterone <70 ng/dLc | 1.31 (0.84-2.02) | .23 | .65 |
| Insulinlike growth factor 1 <63.86 ng/dLc | 1.30 (0.82-2.07) | .27 | .32 |
| Dehydroepiandrosterone sulfate <50 ng/dLc | 1.70 (1.13-2.55) | .01 | .28 |
| Multiple hormonal dysregulationd | <.001 | ||
| 0 (n=207) | |||
| 1 (n=106) | 1.47 (0.88-2.44) | .14 | |
| 2 (n=57) | 1.85 (1.04-3.30) | .04 | |
| 3 (n=20) | 2.29 (1.12-4.68) | .02 |
SI conversion factors: To convert testosterone to nanomoles per liter, multiply by 0.0347; insulinlike growth factor 1 to nanomoles per liter, multiply by 0.131; and dehydroepiandrosterone sulfate to micromoles per liter, multiply by 0.027.
Results reported in the table are from separate models.
Obtained by fitting the same model with hormone levels as continuous variables.
Top level of the lowest quartile.
Hormones in the lowest quartile.
Table 3. Relationship Between Hormonal Dysregulation and All-Cause Mortality in Older Men: Adjusted Analysisa.
| Characteristic | Hazard Ratio (95% Confidence Interval) | P Valueb | P Value for Trendc |
|---|---|---|---|
| Bioavailable testosterone <70 ng/dLd | 1.48 (0.89-2.46) | .13 | .48 |
| Insulinlike growth factor 1 <63.86 ng/mLd | 1.36 (0.78-2.38) | .28 | .91 |
| Dehydroepiandrosterone sulfate <50 μg/dLd | 1.38 (0.84-2.26) | .21 | .97 |
| Multiple hormonal dysregulatione | <.001 | ||
| 0 (n=207) | |||
| 1 (n=106) | 1.04 (0.56-1.95) | .89 | |
| 2 (n=57) | 1.34 (0.67-2.65) | .41 | |
| 3 (n=20) | 2.44 (1.09-5.46) | .03 |
Results reported in the table are from separate, fully adjusted models.
P values adjusted for age, body mass index, cancer, log(interleukin 6), education, cognitive function, depression, physical activity, caloric and alcohol intake, smoking, coronary heart disease (including angina and myocardial infarction), congestive heart failure, stroke, diabetes hypertension, Parkinson disease, peripheral artery disease, asthma, cancer, and chronic obstructive pulmonary disease.
Obtained by fitting the same model with hormone levels as continuous variables.
Top level of the lowest quartile.
Hormones in the lowest quartile.
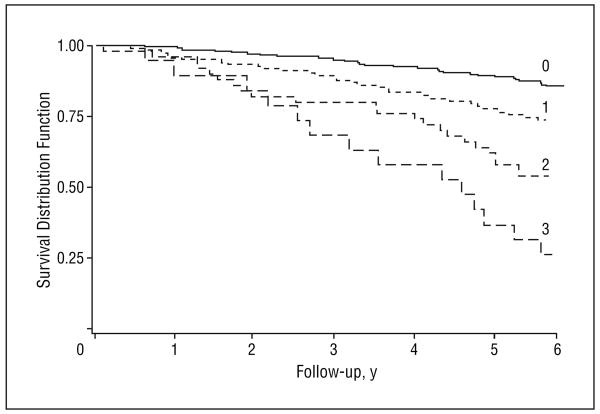
Remarkably, the HR for mortality progressively increased with the number of anabolic hormones in the lowest serum quartile level. Compared with men with levels of all hormones above the lowest quartile, men with 1, 2, or 3 hormones in the lowest quartile had an HR for mortality of 1.47 (95% CI, 0.88-2.44), 1.85 (95% CI, 1.04-3.30), and 2.29 (95% CI, 1.12-4.68) (test for trend, P=.006), respectively (Table 2). After adjusting for confounders, including age, BMI, educational level, smoking, alcohol and caloric intake, physical activity, log(IL-6) levels, and chronic disease such as diabetes, hypertension, peripheral artery diseases, coronary heart disease, congestive heart failure, stroke, Parkinson disease, COPD, asthma, and cancer, the HRs for mortality were 1.04 (95% CI, 0.56-1.95), 1.34 (95% CI, 0.67-2.65), and 2.44 (95% CI, 1.09-5.46), respectively (test for trend, P<.001) (Table 3). Survival analysis (Kaplan-Meier analysis) between the number of anabolic hormones in the lowest serum level quartile and mortality after 6 years of follow-up in older men is reported in the Figure. The mean±SD survival time expressed in years was 5.58 ± 0.07 in group 0, 5.18 ± 0.13 in group 1, 4.70±0.24 in group 2, and 3.98±0.39 in group 3 (P=.01, fully adjusted). The mortality rates (events per 100 person-years) were 2.9 in group 0, 6.2 in group 1, 16.7 in group 2, and 46.0 in group 3 (P=.007, test for trend).
Figure.
Relationship between the number of anabolic hormones in the lowest serum level quartile and 6-year survival in older men. The survival distribution function according to the number of dysregulated hormones is shown for the 6 years of follow-up. The solid black line indicates the survival rates for patients with 0 dysregulated hormones; short-dashed line, survival rates for patients with 1 dysregulated hormone; medium-dashed line, survival rates for patients with 2 dysregulated hormones; and long-dashed line, survival rates for patients with 3 dysregulated hormones. The thresholds for anabolic hormonal deficiency were 70 ng/dL (to convert to nanomoles per liter, multiply by 0.037) for bioavailable testosterone, 63.9 ng/mL (to convert to nanomoles per liter, multiply by 0.13) for total insulinlike growth factor 1, and 60 μg/dL (to convert to micromoles per liter, multiply by 0.027) for dehydroepiandrosterone sulfate (log rank=62.92; P<.001).
Comment
We found that independent of age and multiple potential confounders, low circulating levels of multiple anabolic hormones, including testosterone, IGF-1, and DHEA-S (in the lowest quartiles of the population), were an independent predictor of mortality during 6 years of follow-up in older men. On the contrary, serum levels of each of these hormones considered separately were not associated with significantly differential mortality.
These findings are consistent with the report of Jankowska et al15 that indicated that in patients affected by chronic heart failure, deficiency of more than 1 anabolic hormone identifies groups with a higher mortality rate. However, in contrast to the population in the study by Jankowska and colleagues, our cohort consisted of older people who were not selected according to a specific disease. In addition, we were able to show that association between multiple hormone dysregulation and mortality is not accounted for by potential confounders, such as cardiovascular risk factors and cardiovascular morbidity.
The concept that relatively low levels of anabolic hormones may be an independent cause of mortality, especially in older persons, is not surprising. Several lines of evidence suggest that the aging process is associated with a decline in anabolic hormones and increase in catabolic hormones.22 In this study, we evaluated the 3 key anabolic hormones: bioavailable testosterone, DHEA-S, and IGF-1. Each of these anabolic hormones has a direct impact on lipid and glucose metabolism.23-25 Evidence has indicated that low testosterone, IGF-1, and DHEA-S levels are good predictors of cardiovascular disease and diabetes in men.5,10,26 Furthermore, we have previously shown that a low testosterone level is inversely related with inflammatory markers and predicts the development of anemia in the older population.27,28 Both cardiovascular disease and anemia are 2 independent predictors of mortality in older men.29
Interestingly, in this study, we did not find any significant association between decline in a single hormone level and overall mortality. Two previous reports30,31 performed in older men found no association between testosterone and all-cause mortality. As already mentioned, Shores et al3 recently showed an association between testosterone and mortality in male veterans, but their study design was retrospective; therefore, the existence of a systematic bias cannot be excluded. The Veterans Affairs clinic–based population generally has greater morbidity and lower socioeconomic status than the general population, so the external validity of these findings is questionable.
Observational and intervention studies5-7,9,10 that tested the relationship between DHEA-S and IGF-1 and mortality have reported inconsistent findings. Whether these 2 hormones represent biomarkers of longevity is still an enigma.4,11 Nair et al32 recently showed that neither DHEA nor low-dose testosterone replacement in elderly people has beneficial effects on body composition, physical performance, insulin sensitivity, or quality of life.
In accordance with our working hypothesis, the HR for mortality increased progressively with the number of anabolic hormones in the lowest quartiles. Compared with no dysregulated hormones, having 3 dysregulated anabolic hormones was a significant and independent risk factor for mortality. It has been suggested that anabolic hormonal deficiency is a surrogate of disease severity, especially in men.1,33 However, even after adjusting for extensive information on chronic morbidity, our findings were substantially unchanged.
Multisystem disorders that are widely prevalent in aging, such as the metabolic syndrome, frailty syndrome, and chronic heart failure, are more significantly associated with multiple hormonal dysregulation rather than a single hormonal derangement.12-15 Studies15 have found that the risk of death increases progressively with the number of dysregulated hormones and becomes 2.5 times higher when 3 hormones are dysregulated compared with no dysregulation.
It is conceivable that DHEA-S, testosterone, and IGF-1 have synergistic effects. For example, DHEA-S can be converted to testosterone in peripheral tissues, and some of the peripheral actions of both DHEA-S and testosterone may be mediated via tissue-generated IGF-1.25,34,35 In preliminary analyses performed in the Baltimore Longitudinal Study on Aging population, both DHEA-S and testosterone had a joint protective effect on 30-year mortality rates.36
Our study has limitations. First, testosterone, IGF-1, and DHEA-S were only measured once. Second, information on cause-specific mortality (cardiovascular and cancer) was also not available; hence, the specific mechanism underlying the relationship between anabolic hormones and mortality remains unknown. Moreover, the number of participants with 3 dysregulated hormones was small. Third, although IGF-1 is secreted from the liver under growth hormone stimulus, it is only an indirect marker of growth hormone activity. Fourth, one measure of bioavailable testosterone was estimated from total testosterone and SHBG with the Vermeulen formula, and we did not measure estradiol levels. Finally, because of the nature of the analysis, our findings cannot be directly compared with previous data from the literature.
Nevertheless, our study has several strengths. First, this is the only large, population-based prospective study, to our knowledge, to evaluate the relationship between multiple deficiencies in testosterone, DHEA-S, and IGF-1 level and mortality in older men. Second, information on hormonal levels was complemented by measures such as caloric intake, inflammatory markers, physical activity, cognition, and mood, all factors that potentially affect the relationship between anabolic hormones and mortality. Third, we used bioavailable testosterone for analysis, which is a measure of androgen bioactivity at the tissue level, is more reliable and valid than total testosterone, and was strongly correlated with free testosterone (r=0.98 for Pearson correlation coefficient; P<.001) in our sample. Finally, the existence of a relationship between plurihormonal decline and mortality, even after adjusting for multiple confounders, provides evidence of the biological plausibility of our working hypothesis. These findings strongly suggest a causal pathway between age-related deficiency in multiple anabolic hormones and high prevalence of overall mortality in older men.
The results of this study have important clinical and conceptual implications. Since previous epidemiologic studies in elderly patients focused on only a single hormonal decline rather than multiple anabolic deficiency, we suggest that assessment of these 3 anabolic hormones should always be considered in older men. Future studies should also clarify the link between impairment of these 3 different hormonal axes and cause-specific mortality in older men. The findings of this study raise the possibility that a multiple dysregulation rather than a single dysregulation of the anabolic hormones is a powerful marker of poor health status and perhaps accelerates aging in older men. Once our findings have been confirmed in other populations, the door will open to clinical trials of multiple hormonal replacement in elderly men.
Acknowledgments
Funding/Support: The InCHIANTI Study was supported as a targeted project (ICS 110.1/RS97.71) by the Italian Ministry of Health, in part by the US National Institute on Aging (contracts N01-AG-916413 and N01-AG-821336), and by the Intramural Research Program of the US National Institute on Aging (contracts 263 MD 9164 13 and 263 MD 821336).
Footnotes
Financial Disclosure: None reported.
Role of the Sponsor: None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported herein.
Author Contributions:Study concept and design: Maggio, Lauretani, Ceda, Bandinelli, Metter, Carassale, Cazzato, Guralnik, Basaria, Valenti, and Ferrucci. Acquisition of data: Lauretani, Bandinelli, Ceresini, Guralnik, and Ferrucci. Analysis and interpretation of data: Maggio, Lauretani, Ceda, Ling, Metter, Artoni, Guralnik, Valenti, and Ferrucci. Drafting of the manuscript: Maggio, Lauretani, Bandinelli, Ling, Basaria, and Ferrucci. Critical revision of the manuscript for important intellectual content: Maggio, Ceda, Ling, Metter, Artoni, Carassale, Cazzato, Ceresini, Guralnik, Basaria, Valenti, and Ferrucci. Statistical analysis: Maggio, Lauretani, Metter, Guralnik, and Ferrucci. Obtained funding: Bandinelli and Ferrucci. Administrative, technical, and material support: Maggio, Ling, and Guralnik. Study supervision: Maggio, Ceda, Artoni, Carassale, Cazzato, Ceresini, Basaria, Valenti, and Ferrucci.
References
- 1.Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96(2):526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 2.Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30(4):997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 3.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 4.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297(5582):811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315(24):1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Xue QL, Walston JD, et al. DHEAS levels and mortality in disabled older women: the Women's Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2006;61(9):957–962. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001;86(9):4171–4177. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- 8.Ceda GP, Dall'Aglio E, Maggio M, et al. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28(1111 suppl Proceedings):96–100. [PubMed] [Google Scholar]
- 9.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–120. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 11.Anversa P. Aging and longevity: the IGF-1 enigma. Circ Res. 2005;97(5):411–414. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- 12.Maggio M, Cappola AR, Ceda GP, et al. The hormonal pathway to frailty in older men. J Endocrinol Invest. 2005;28(1111 suppl Proceedings):15–19. [PubMed] [Google Scholar]
- 13.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 14.Maggio M, Lauretani F, Ceda GP, et al. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc. 2006;54(12):1832–1838. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114(17):1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 16.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 17.Valenti G, Denti L, Maggio M, et al. Effect of DHEA-S on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(5):466–472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137(9):1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 21.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 22.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278(5337):419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 23.Ceda GP, Dall'Aglio E, Magnacavallo A, et al. The insulin-like growth factor axis and plasma lipid levels in the elderly. J Clin Endocrinol Metab. 1998;83(2):499–502. doi: 10.1210/jcem.83.2.4548. [DOI] [PubMed] [Google Scholar]
- 24.Simpson HL, Jackson NC, Shojaee-Moradie F, et al. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J Clin Endocrinol Metab. 2004;89(1):425–432. doi: 10.1210/jc.2003-031274. [DOI] [PubMed] [Google Scholar]
- 25.GISEG (Italian Study Group on Geriatric Endocrinology) Valenti G, Denti L, Saccò M, et al. Consensus document on substitution therapy with DHEA in the elderly. Aging Clin Exp Res. 2006;18(4):277–300. doi: 10.1007/BF03324662. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30(2):234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 27.Muzzarelli S, Pfisterer M, TIME Investigators Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J. 2006;152(5):991–996. doi: 10.1016/j.ahj.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 29.Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166(13):1380–1388. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley JE, Kaiser FE, Perry HM, III, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men: a prospective population-based study. Circulation. 1988;78(3):539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 32.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Veldhuis JD. A paradoxical gender dissociation within the growth hormone/insulin-like growth factor I axis during protracted critical illness. J Clin Endocrinol Metab. 2000;85(1):183–192. doi: 10.1210/jcem.85.1.6316. [DOI] [PubMed] [Google Scholar]
- 34.Valenti G, Bossoni S, Giustina A et al. Italian Study Group on Geriatric Endocrinology. Consensus Document on substitution therapy with testosterone in hypoandrogenic elderly men. Aging Clin Exp Res. 2002;6:439–464. doi: 10.1007/BF03327345. [DOI] [PubMed] [Google Scholar]
- 35.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282(3):E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 36.Maggio M, Metter EJ, Ruggiero C, Ferrucci L. Biological pathways to health and longevity: insight from the Baltimore Longitudinal Study of Aging. The Gerontologist: Program Abstracts 59th Annual Scientific Meeting; November 16-20, 2006; Dallas, TX. [Google Scholar]