Abstract
Pre-clinical studies suggest that both omega-6 and omega-3 fatty acids have beneficial effects on peripheral nerve function. Rats feed a diet rich in polyunsaturated fatty acids (PUFAs) showed modification of phospholipid fatty acid composition in nerve membranes and improvement of sciatic nerve conduction velocity (NCV). We tested the hypothesis that baseline plasma omega-6 and omega-3 fatty acids levels predict accelerated decline of peripheral nerve function. Changes between baseline and the 3-year follow-up in peripheral nerve function was assessed by standard surface ENG of the right peroneal nerve in 384 male and 443 female participants of the InCHIANTI study (age range: 24–97 years). Plasma concentrations of selected fatty acids assessed at baseline by gas chromatography. Independent of confounders, plasma omega-6 fatty acids and linoleic acid were significantly correlated with peroneal NCV at enrollment. Lower plasma PUFA, omega-6 fatty acids, linoleic acid, ratio omega-6/omega-3, arachidonic acid and docosahexanoic acid levels were significantly predicted a steeper decline in nerve function parameters over the 3-year follow-up. Low plasma omega-6 and omega-3 fatty acids levels were associated with accelerated decline of peripheral nerve function with aging.
Keywords: aging, docosahexaenoic acid, InCHIANTI study, omega-6 fatty acids, peripheral nerves
Introduction
In vitro and animal studies have clearly demonstrated that omega-3 fatty acids have antiarrhythmic [1] as well as anticonvulsant properties [2]. Addition of polyunsaturated fatty acid (PUFA), particularly omega-3 fatty acids, in the perfusion medium of human cardiac myocytes and neurons reduces the random fluctuation of their transmembrane potential; therefore, stabilizing their electrical activity. Both in cardiac myocytes and in neurons, this effect of omega-3 is mediated by a modulation of the voltage-dependent inactivation of sodium and calcium currents [3–5]. In addition, there is an evidence that omega-3 fatty acids are neuroprotective, perhaps because of their anti-inflammatory properties [6], and both omega-3 and omega-6 fatty acids have an important role in neurite outgrowth [7]. Alpha-linolenic acid prevents neuronal death in animal models of transient global ischemia, even when administered soon after the insult [8]. In the Rotterdam Study, high dietary intake of PUFA was associated with reduced risk of developing Parkinson's disease [9].
Recently, evidence has emerged suggesting that both omega-6 and omega-3 fatty acids are also important for peripheral nerve health and function. In diabetic rats, the administration of linoleic acid, a n-6 fatty acid, improves nerve conduction velocity (NCV) of the sciatic nerve [10]. Gamma-linolenic acid (GLA), a n-6 fatty acid, has shown promising results in the treatment of diabetic complications in several human and animal studies [11]. Also docosahexaenoic acid (DHA), n-3 fatty acids, has shown properties on myelinogenesis, especially in patients with generalized peroxisomal disorders, a disorder of membrane phospholipids [12]. Based on this evidence, researchers have suggested that the administration of both omega-6 and omega-3 fatty acids may be beneficial in patients with polyneuropathy [13]. However, whether circulating levels of omega-3, omega-6 fatty acids, or total PUFA, correlate with peripheral nerve function is currently unknown. This information is an essential pre-requisite to the design of clinical trials testing the therapeutic efficacy of omega-6 and omega-3 fatty acids on peripheral nerve diseases.
Using longitudinal data from a population-based sample of men and women enrolled in the InCHIANTI study, we tested the hypothesis that, independent of potential confounders, total plasma PUFA levels and/or specific fatty acids are cross-sectionally correlated with peripheral nerve function, and low compared with normal total PUFA levels and/or specific fatty acids levels predict accelerated decline of peripheral nerve function over a 3-year follow-up.
Methods
Study population
Invecchiare in Chianti, aging in the Chianti area (InCHIANTI) is a longitudinal study of factors affecting mobility in late life, conducted in the Tuscany Region of Italy [14]. Of the 1530 subjects originally sampled, 1453 (94%) agreed to participate in the study. Of the 1343 participants who provided a blood sample, 1260 (87%) underwent standard surface electroneurography of the right peroneal nerve and had complete baseline data for the cross-sectional analyzes shown in this report. Of these 1260 participants, 73 died, 13 moved away from the area, and 98 refused to participate in the 3-year follow-up. Of the 1076 participants who were reevaluated at the 3-year follow-up, 854 underwent a new electroneurographic study and 827 (73.2%) had a new interview at the 3-year follow-up. Thus, a complete baseline and follow-up data were available for 384 men and 443 women with an average age of 68.2 (range: 24–97).
The National Institute on Research and Care of the Elderly Institutional Review Board approved the study protocol. Participants consented to participate and to have their blood samples analyzed for scientific purposes. For those who unable to fully consent, surrogate consents were obtained from close relatives.
Nerve conduction studies
Standard surface electroneurographic (ENG) studies of the right peroneal nerve were conducted within 3 weeks of the home interview by a trained geriatrician [14]. All studies were performed on an ENG-neuro MYTO device (E.B. Neuro S.p.A, Florence, Italy) using standard ENG-neuro disposable electrodes. A detailed description of the methods is reported elsewhere [15]. Briefly, a flexible measuring tape was used for measuring distances between stimulating and recording points. The recording electrode was placed on the skin above the extensor digitorum brevis muscle. Stimulation was performed using standard supramaximal technique, proximally at the fibular head and distally over the anterior ankle. The measurements were obtained while dorsal foot skin temperature was between 30 and 34°C [15]. Parameters of nerve conduction studies considered in the analysis were: (i) the amplitude of the distal compound muscle action potential (CMAP) and (ii) the NCV [16].
Biological samples
Blood samples were collected from participants after a 12-h fast, and after a 15-min rest. Aliquots of serum and plasma were stored at −80°C and not thawed until analyzed. Serum total cholesterol and triglycerides were assessed by commercial enzymatic tests and a Roche-Hitachi 917 Autoanalyzer (Roche Diagnostics, GmbH, Mannheim, Germany). For total cholesterol the analytical sensitivity (lower detection limit) was 3.0 mg/dl. The intra-assay coefficients of variation (CV) was 0.8%, the inter-assay CV was 3.3%. For triglycerides the analytical sensitivity (lower detection limit) was 4.0 mg/dl. The intra-assay CV was 1.8%, the inter-assay CV was 3.1%. High-density lipid-cholesterol (HDL-C) level was determined by the liquid homogeneous HDL-C assay (Alifax S.p.A, Padova, Italy). The intra-assay CV was 0.8%, the inter-assay CV was 1.3%.
Polyunsaturated fatty acids
Serum and plasma were analyzed after 2 years from their collection. It has been demonstrated that fatty acids are stable at this temperature for at least 4 years [17]. A detailed description of the methods is reported elsewhere [6]. Briefly, the amount of plasma fatty acids (ranging from C14:0 to C24:1) was quantified based on the amount of fatty acid methyl esters (FAME), analyzed by using a HP-6890 gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) [7,18,19], that was recovered and expressed as percentages of total fatty acids based on mg/l values. The correlation coefficients for the calibration curves of 20 fatty acids were in all cases higher than 0.998. The intra- and inter-assay CV were 1.6% and 3.3%, respectively. Plasma saturated fatty acids, monounsaturated fatty acids (MUFA), PUFA were used in this study. Furthermore, the following specific fatty acids were selected among the PUFA: (i) omega-6 fatty acids (n-6), linoleic acid (18:2n-6), and arachidonic acid (20:4n-6); (ii) omega-3 fatty acids (n-3), linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3) and DHA (22:6n-3) and the ratio n-6/n-3 was used in this study. The correlations among these selected fatty acids were from r = 0.29 to r = 0.77, P < 0.0001.
Other measures
Weight and height were measured in standardized position and body mass index (BMI) was calculated as weight (kg)/height (m2). Alcohol intake was assessed using the Food Frequency Questionnaire originally developed for the European Prospective Investigation into Cancer and Nutrition [20]. Diseases included in the current analysis were diabetes, defined as having hyperglycemia >126 mg/dl and peripheral artery disease (PAD), defined as an ankle-arm index of 0.9 or less [21,22].
Statistical analysis
Because of skewed distribution, log-transformed values of linolenic acid were used in all analyzes. Continuous values were reported as means ± SD. Age- and sex-adjusted partial correlation coefficients and Spearman partial rank-order correlation coefficients were used to study the correlation between baseline characteristics and baseline and follow-up NCV and CMAP. Partial correlations coefficient with follow-up NCV and CMAP were also adjusted for, respectively, baseline NCV and baseline CMAP. NCV and CMAP groups were coded according to thresholds that are generally considered clinically significant (for NCV: <40 m/s, 40–42 m/s, and >42 m/s; and for CMAP: <3 mV, 3–4 mV, and >4 mV) [15]. In previous research, we demonstrated a strong association between these thresholds and neurologic signs obtained during a medical visit [15]. Mean values of the main classes (saturated, monounsaturated, polyunsaturated, n-3, and n-6) and specific (linoleic, linolenic, arachidonic, eicosapentanoic and docosahexaenoic) fatty acids were compared across groups of NCV and CMAP at enrollment. Multivariate regression analyzes were performed, where NCV (or CMAP) was the dependent variable and fatty acids concentration were independent variables.
Longitudinal analyzes were rescricted to the participants who had complete baseline and 3-year follow-up data (n = 827). We performed a repeated measures analysis by using the ‘REPEATED’ statement in the SAS GLM procedure. In this analysis, ‘age group’ is considered between the subjects effect, while ‘time’ and the time by age interaction are within subjects effect.
The effect of baseline plasma PUFA levels on change over time in NCV was tested in stepwise regression models where follow-up NCV was the dependent variable, baseline plasma PUFA levels was the main independent variable and baseline NCV and other potential confounders were considered as covariates. A similar analytical approach was used to analyze the effect of baseline PUFA on changes in CMAP over 3 years follow-up. All analyzes were performed using the SAS statistical package, version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Anthropometric characteristics, biological variables, and prevalence of diabetes and PAD and their correlations with NCV and CMAP are reported in Table 1. At baseline, independent of age and sex, BMI and triglycerides were positively correlated with NCV, total cholesterol was positively and diabetes negatively correlated with both NCV and CMAP, while PADs was negatively correlated with NCV (Table 1). Restricting the analyzes to the 827 participants with complete baseline and follow-up data, and adjusting for age, sex and baseline NCV or baseline CMAP, the correlations between baseline characteristics and follow-up NCV and CMAP (Table 1) were very similar to those observed in the cross-sectional correlation analysis (Table 1).
Table 1.
Characteristics of the InCHIANTI study population at baseline (left) and limited to participants who were also evaluated at the three-year follow-up (right)
| Baseline population (n = 1260) | Follow-up population (n = 827) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Correlation coefficient with NCVa | Correlation coefficient with CMAPa | Men | Women | Correlation coefficient with NCVb | Correlation coefficient with CMAPb | |
| BMI (kg/m2) | 27.1 (3.4) | 27.2 (4.6) | 0.138* | 0.021 | 27.1 (3.2) | 27.2 (4.5) | 0.114* | −0.012 |
| Height (cm) | 177.7 (93.1) | 163.1 (85.6) | −0.025 | −0.056 | 169.9 (43.1) | 158.6 (58.4) | −0.038 | −0.051 |
| Energy intake (kcal/day) | 2290 (608) | 1806 (525) | 0.011 | 0.039 | 2357 (588) | 1839 (535) | 0.009 | 0.063 |
| Alcohol intake (g/day) | 24.0 (19.2) | 9.6 (7.5) | 0.017 | −0.004 | 24.8 (18.7) | 9.7 (7.6) | 0.020 | −0.032 |
| Total cholesterol (mg/dl) | 208.0 (39.3) | 222.7 (39.4) | 0.092* | 0.068** | 209.5 (37.1) | 225.5 (40.0) | 0.094** | 0.042 |
| HDL Cholestrol (mg/dl) | 51.4 (13.0) | 59.8 (15.3) | −0.008 | 0.012 | 52.3 (13.1) | 59.3 (14.6) | −0.008 | −0.002 |
| Tryglycerides (mg/dl) | 131.5 (88.8) | 120.6 (64.7) | 0.068** | 0.044 | 129.4 (72.9) | 120.1 (63.6) | 0.090* | −0.012 |
| Diabetes (n,%) | 65 (11.5) | 64 (9.2) | −0.081** | −0.095* | 37 (9.6) | 37 (8.4) | −0.029* | −0.092** |
| PAD (n, %) | 83 (14.7) | 71 (10.2) | −0.187* | −0.008 | 44 (11.5) | 38 (8.6) | −0.087** | −0.038 |
Partial correlation coefficient, adjusted for age and sex;
Partial correlation coefficient, adjusted for age- sex- and baseline NCV and baseline CMAP, respectively.
P < 0.01;
P < 0.05.
BMI, body mass index; CMAP, compound muscle action potential; HDL, high-density lipid; NCV, nerve conduction velocity; PAD, peripheral artery disease.
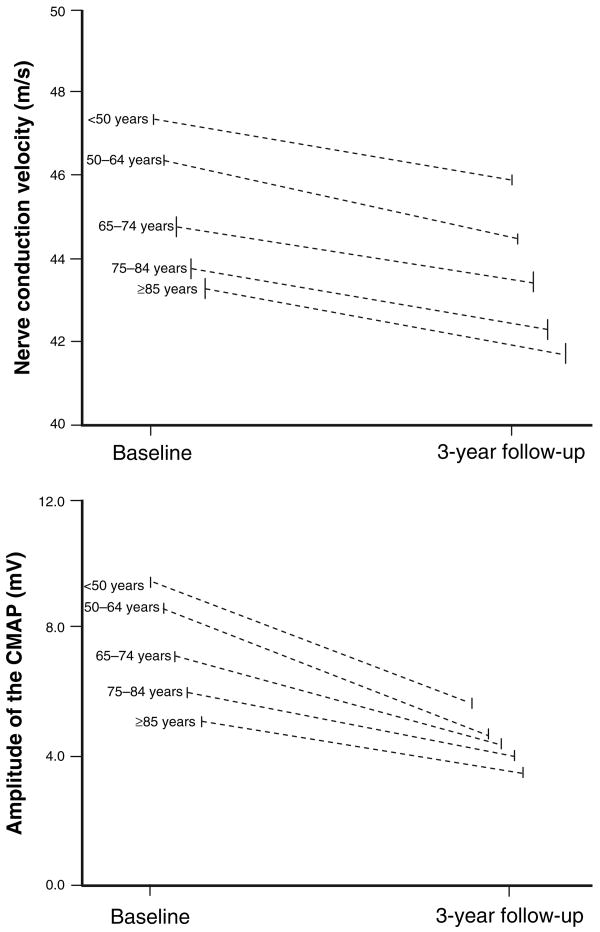
At enrollment, independent of confounders, omega-6 fatty acids and linoleic acid were positively associated with NCV (Table 2). We found that NCV was significantly different between age groups (higher in the youngest) and overall, declined significantly over the 3-year follow-up. The interaction ‘time*age-group’ was not statistically significant, indicating that the rate of decline in NCV was similar across age groups (F = 0.81; P < 0.52). For CMAP, we found that CMAP was significantly different between age groups (higher in the youngest) and overall, declined significantly over the 3-year follow-up (Table 3). However, for this variable, the interaction ‘time*age-group’ was highly statistically significant (F = 9.89; P < 0.0001), suggesting that the decline in CMAP over time is steeper in the younger participants, and becomes progressively less steep in the older participants (Fig. 1).
Table 2.
Saturated, monounsaturated and polyunsaturated fatty acids, across group of baseline NCV
| NCV (<40 m/s), (n = 129) | NCV (40–42 m/s), (n = 206) | NCV (>42 m/s), (n = 906) | Beta + SE | P* | |
|---|---|---|---|---|---|
| Saturated fatty acids (%) | 32.4 ± 3.1 | 32.8 ± 2.9 | 32.3 ± 2.8 | 0.06 ± 0.04 | 0.11 |
| Monounsaturated fatty acids (%) | 32.4 ± 3.3 | 31.2 ± 3.8 | 30.2 ± 3.8 | −0.03 ± 0.03 | 0.33 |
| Polyunsaturated fatty acids (%) | 35.2 ± 4.6 | 36.0 ± 5.0 | 37.5 ± 4.9 | 0.005 ± 0.02 | 0.83 |
| Omega-6 fatty acids(mg/l) | 963.5 ± 220.0 | 1004.1 ± 232.0 | 1079.7 ± 237.0 | 0.001 ± 0.0005 | 0.002 |
| Linoleic acid (mg/l) | 720.3 ± 166.8 | 755.5 ± 179.6 | 813.0 ± 180.9 | 0.002 ± 0.006 | 0.002 |
| Arachidonic acid (mg/l) | 239.8 ± 73.4 | 245.2 ± 75.4 | 262.8 ± 83.0 | 0.003 ± 0.001 | 0.06 |
| Omega-3 fatty acids (mg/l) | 105.1 ± 39.1 | 101.4 ± 38.5 | 110.7 ± 41.8 | 0.36 ± 0.28 | 0.21 |
| Linolenic acid (mg/l) | 14.7 ± 10.8 | 13.9 ± 9.4 | 15.0 ± 9.3 | 0.008 ± 0.01 | 0.47 |
| Eicosapentaenoic acid (mg/l) | 19.5 ± 8.3 | 18.8 ± 8.1 | 20.0 ± 8.7 | 0.018 ± 0.01 | 0.15 |
| Docosahexaenoic acid (mg/l) | 70.9 ± 27.9 | 68.7 ± 27.8 | 75.7 ± 31.2 | 0.003 ± 0.004 | 0.37 |
| Ratio n-6/n-3 | 10.0 ± 2.3 | 10.8 ± 3.1 | 10.6 ± 3.1 | 0.02 ± 0.04 | 0.54 |
Values are given as mean ± SD.
General linear model with nerve conduction velocity (NCV) considered as a continous variable and adjusted for age, sex, body mass index, total cholesterol, triglycerides, diabetes and peripheral artery disease.
Table 3.
Saturated, monounsaturated and polyunsaturated fatty acids, across group of baseline compound muscle action potential (CMAP)
| CMAP (<3 mV), (n = 129) | CMAP (3–4 mV), (n = 206) | CMAP (>4 mV), (n = 906) | Beta+ SE | Pa | |
|---|---|---|---|---|---|
| Saturated fatty acids (%) | 32.4 ± 3.0 | 32.4 ± 3.0 | 32.4 ± 2.8 | 0.041 ± 0.036 | 0.25 |
| Monounsaturated fatty acids (%) | 31.9 ± 3.5 | 31.1 ± 3.4 | 30.2 ± 3.8 | −0.045 ± 0.28 | 0.10 |
| Polyunsaturated fatty acids (%) | 35.8 ± 4.8 | 36.5 ± 4.7 | 37.3 ± 5.0 | 0.012 ± 0.021 | 0.58 |
| Omega-6 fatty acids (mg/l) | 1020.0 ± 251.9 | 1040.4 ± 236.2 | 1065.0 ± 234.6 | 0.0007 ± 0.0004 | 0.09 |
| Linoleic acid (mg/l) | 765.2 ± 196.1 | 769.1 ± 172.8 | 803.6 ± 179.4 | 0.001 ± 0.0005 | 0.06 |
| Arachidonic acid (mg/l) | 251.2 ± 78.1 | 267.6 ± 86.4 | 269.9 ± 81.1 | 0.0009 ± 0.001 | 0.46 |
| Omega-3 fatty acids (mg/l) | 107.0 ± 42.8 | 107.3 ± 38.8 | 109.2 ± 41.2 | 0.266 ± 0.254 | 0.30 |
| Linolenic acid (mg/l) | 14.9 ± 9.7 | 14.5 ± 10.4 | 14.8 ± 9.3 | 0.006 ± 0.01 | 0.56 |
| Eicosapentaenoic acid (mg/l) | 19.7 ± 9.1 | 19.5 ± 7.4 | 19.9 ± 8.6 | 0.014 ± 0.011 | 0.21 |
| Docosahexaenoic acid (mg/l) | 72.4 ± 31.1 | 73.3 ± 28.5 | 74.5 ± 30.5 | 0.0016 ± 0.003 | 0.62 |
| Ratio n-6/n-3 | 10.5 ± 3.3 | 10.6 ± 3.6 | 10.6 ± 3.0 | 0.01 ± 0.03 | 0.64 |
Values are given as mean ± SD.
General linear model with CMAP considered as a continuous variable and adjusted for age, sex, body mass index, total cholesterol, triglycerides, diabetes and peripheral artery disease.
Figure 1.
Changes in nerve conduction parameters (NCV and CMAP) over the 3-year follow-up stratified by age.
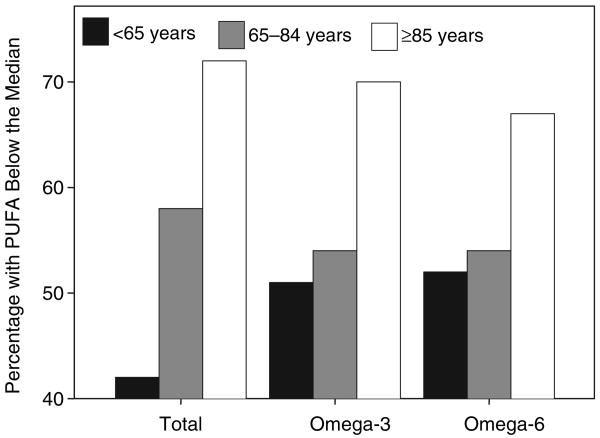
Total PUFA, omega-3 and omega-6 concentration declined with age as indicated by progressively, higher prevalence of participants with PUFA below the median level across age groups (Fig. 2). Adjusting for multiple confounders, saturated fatty acids were associated with more decline of NCV and CMAP in participants between 65 and 84 years of age (Table 4). Adjusting for multiple confounders, baseline total plasma PUFA predicted significantly less decline of NCV in participants younger than 65 years of age and docosahexanoic acid predicted significantly less decline of NCV in participants older than 85 years of age (Table 4). Higher baseline total plasma PUFA and omega-6 fatty acids predicted significantly less decline of CMAP in participants younger than 85 years of age, omega-3 fatty acids, arachidonic and DHAs predicted significantly less decline in CMAP in participants between 65 and 84 years old (Table 4). Linoleic acid predicted significantly less decline of CMAP in all participants (Table 4).
Figure 2.
Percentage of participants with PUFA below the median level, according to age groups.
Table 4.
Stepwise regression modelsa relating baseline levels of fatty acids and change in peripheral nerve functional parameter over three year follow-up
| NV velocity (m/s) | Amplitude of CMAP (mV) | |||||
|---|---|---|---|---|---|---|
| <65 years, (n = 230) | 65–84 years, (n = 569) | >85 years, (n = 26) | <65 years, (n = 230) | 65–84 years, (n = 569) | >85 years, (n = 26) | |
| Saturated fatty acids (%) | 0.14 (0.15) | −0.13 (0.07)* | −0.12 (0.16) | −0.04 (0.13) | −0.11 (0.06)* | −0.16 (0.12) |
| Monounsaturated fatty acids (%) | 0.18 (0.13) | −0.10 (0.06) | 0.09 (0.12) | 0.30 (0.12)* | 0.004 (0.05) | 0.04 (0.09) |
| Polyunsaturated fatty acids (%) | 0.21 (0.10)* | −0.01 (0.06) | −0.26 (0.10) | 0.20 (0.09)* | 0.08 (0.04)* | 0.04 (0.08) |
| Omega-6 fatty acids (mg/l) | 0.14 (0.11) | 0.03 (0.06) | 0.04 (0.73) | 0.17 (0.09)* | 0.11 (0.05)* | 0.02 (0.78) |
| Linoleic acid (mg/l) | 0.07 (0.09) | 0.07 (0.07) | 0.10 (0.13) | 0.17 (0.08)* | 0.10 (0.05)* | 0.09 (0.03)* |
| Arachidonic acid (mg/l) | −0.15 (0.19) | −0.06 (0.11) | 0.10 (0.59) | 0.10 (0.17) | 0.18 (0.09)* | 0.14 (0.15) |
| Omega-3 fatty acids (mg/l) | 1.01 (0.32) | 0.73 (0.54) | 0.15 (0.98) | 1.26 (0.91) | 0.76 (0.44)* | 0.19 (0.75) |
| Linolenic acid (mg/l) | 1.92 (1.29) | 0.27 (0.75) | 1.56 (1.40) | 0.76 (1.17) | 0.49 (0.64) | 1.33 (0.20) |
| Eicosapentaenoic acid (mg/l) | 0.81 (1.92) | 0.41 (0.87) | 0.14 (1.59) | 1.02 (1.74) | 0.12 (0.70) | 0.53 (1.21) |
| Docosahexaenoic acid (mg/l) | 0.29 (0.41) | 0.62 (0.26)* | 0.46 (0.16)* | 0.47 (0.37) | 0.40 (0.21)* | 0.35 (0.34) |
| Ratio n-6/n-3 | 0.11 (0.11) | 0.12 (0.06)* | 0.04 (0.13) | −0.06 (0.10) | −0.04 (0.05) | −0.06 (0.10) |
Values are given as B(SD).
P < 0.05.
Each coefficient is from a specific model adjusted for the baseline nerve functional parameters (NVC and CMAP) and age, sex, age-sex interaction, body mass index, total cholesterol, triglycerides, diabetes, and peripheral artery diseases.
Noteworthy, the ratio n-6/n-3 was associated with significantly less reduction of NCV after the 3-year follow-up (Table 4). When the analysis was restricted to the 753 participants free of diabetes, the results were substantially unchanged. We obtained similar results, when fatty acids concentration were expressed as percentage (%wt/wt), instead of absolute concentrations.
Discussion
Using longitudinal data from a population-based sample of older persons, we tested the hypothesis that low plasma total PUFA, or omega-3 or omega-6 levels were associated with impaired peripheral nerve function. We found that omega-6 fatty acids and marginally DHA were significantly associated with nerve peripheral parameters, independently of potential confounders. In particular, omega-6 fatty acids and linoleic acid was strongly and independently correlated with NCV in the cross-sectional analyzes. In longitudinal analyzes low total plasma PUFA levels, omega-6 fatty acids, arachidonic acid, docosahexanoic and the ratio omega-6/omega-3 predicted accelerated decline of peripheral nerve function over the 3-year follow-up.
Our findings are consistent with studies showing that high dietary intake of fatty acids prevents the development and clinical progression of nerve conduction deficits in diabetic animals as well as in the general human population [9,10]. Also, our findings are somewhat in agreement with studies showing that omega-3 and omega-6 fatty acids have an important role in the growth of neuritis [23]. In a double-blind, placebo-controlled trial of 111 patients with mild diabetic neuropathy, patients who were treated with GLA, for 1 year, showed favorable improvement in the vibration and touch sensitivity compared with controls [24], while in diabetic rats, the administration of linoleic acid, a n-6 fatty acid, improved sciatic NCV [10]. In patients with generalized peroxisomal disorders, a congenital diseases with impaired myelinogenesis, the administration of the n-3 fatty acids, DHA, significantly improved myelin formation alleviating the symptoms in these patients [12].
About 60% of the brain's structural material is lipids, almost all of it in the form of two long-chain PUFAs, DHA and arachidonic acid [25]. Thus, PUFA are the major structural components of the neuronal membrane phospholipids; and therefore, their structural and chemical characteristics influence membrane functions, such as the activity of membrane bound proteins, signal transduction and also neurotransmission [26,27]. In particular, the electrophysiologic effect of the omega-3 fatty acids seem to be the result of specific modulation of ion currents, particularly of the voltage-dependent sodium current and of the L-type calcium currents across sarcolemmal phospholipids membranes [28]. Accordingly, supplementation with sunflower oil, which contains high quantity of linoleic acid, restored NCV in diabetic rats, and this effect was accompanied by a modification of phospholipid fatty acid composition in nerve membranes [29].
Finally, n-3 PUFAs and the GLA, a n-6 fatty acid, have been shown to have significant anti-inflammatory properties [6, 30]. PUFAs inhibit the production of proinflammatory cytokines, i.e., Il-1β, IL6 and tumor necrosis factor-alpha by activating transcription factors, such as the peroxisome proliferator-activated receptors and nuclear factor kB [31]. As inflammation is one of the main pathophysiologic process involved in peripheral polyneuropathy [32], this activity could be extremely relevant in preventing progression of axons damage.
Polyunsaturated fatty acid are present in high concentration not only in fish oil but also in vegetable oils. For example, large quantities of omega-6 fatty acids are present in sunflowers oil, soybean corn oil and safflower oil, while large quantities of omega-3 fatty acids are present in flax oil, and hemp oil. The observation that omega-6 fatty acids appear to have a beneficial effect on peripheral nerve function than omega-3 fatty acids, require consideration. In fact, omega-6 PUFAs are generally more highly represented than omega-3 fatty acids and have major adverse effects of excess than the n-3 fatty acids [33]. Then, an optimal omega-6/omega-3 ratio is probably important for having beneficial effect of the PUFAs.
Our findings suggest that in patients affected by peripheral neuropathy, a supplementation with sunflower oil, rich in omega-6 fatty acids and a DHA, may positively influence the axonal degeneration of the nerve. Whether increasing intake of omega-6 fatty acids and DHA may be beneficial on patients with slightly peripheral neuropaty is an appealing hyphotesis that needs to be addressed in future studies.
An important limitation of this study is that n-3 and n-6 fatty acids were measured only at baseline. In addition, the effect of omega-6 fatty acids and DHA on peripheral nerve function was somewhat moderate, at least in part because of the difficulty of obtaining a precise quantitation of circulating fatty acids, and because the decline in peripheral nerve function with age is probably multifactorial. This could also explain the difference observed in the cross-sectional analyzes compared with those at follow-up. In particular, we found longitudinally that the CMAP, a markers of axonal degeneration, could be the earliest parameter affected by a reduction of the plasma PUFAs levels, with a decline in NCV only in advanced axonal peripheral neuropathy.
Furthermore, information on peripheral nerve function was limited to one motor nerve. However, this measure was objective and collected longitudinally. This is the first large population-based study of the relationship between plasma omega-3 and omega-6 fatty acids levels and peripheral nerve function using circulating level of PUFA and a gold-standard measure of peripheral nerve function.
The results of this study have important clinical and speculative implications. Based on our findings, we suggest that low omega-6 fatty acids and DHA levels may be considered a potential cause of peripheral nerve dysfunction in older persons, especially when no other plausible cause can be clearly identified [34]. The findings of this study raise the possibility that omega-6 fatty acids and DHA supplementation may be effective in treatment of peripheral neuropathy. This possibility should be carefully considered and examined in future trials of essential fatty acid supplementation.
Acknowledgments
The InCHIANTI study was supported by the Intramural Research Program, National Institute on Aging, NIH, USA and in part by the Italian Ministry of Health. This research was also partially supported by an unrestricted grant by BRACCO imaging SpA, Italy. The authors have reported no conflict of interest.
References
- 1.Kang JX, Leaf A. Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation. 1996;94:1774–1780. doi: 10.1161/01.cir.94.7.1774. [DOI] [PubMed] [Google Scholar]
- 2.Leaf A, Kang JX, Xiao YF, Billman GE, Voskuyl R. The antiarrhythmic and anticonvulsant effects of dietary N-3 fatty acids. The Journal of Membrane Biology. 1999;172:1–11. doi: 10.1007/s002329900578. [DOI] [PubMed] [Google Scholar]
- 3.Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Wadman WJ. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2680–2685. doi: 10.1073/pnas.95.5.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. The Journal of Clinical Endocrinology and Metabolism. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 7.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- 8.Leaf A. The electrophysiologic basis for the antiarrhythmic and anticonvulsant effects of n-3 polyunsaturated fatty acids: heart and brain. Lipids. 2001;36(Suppl):S107–S110. doi: 10.1007/s11745-001-0691-y. [DOI] [PubMed] [Google Scholar]
- 9.de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- 10.Head RJ, McLennan PL, Raederstorff D, Muggli R, Burnard SL, McMurchie EJ. Prevention of nerve conduction deficit in diabetic rats by polyunsaturated fatty acids. The American journal of Clinical Nutrition. 2000;71:386S–392S. doi: 10.1093/ajcn/71.1.386s. [DOI] [PubMed] [Google Scholar]
- 11.Jamal GA. The use of gamma linolenic acid in the prevention and treatment of diabetic neuropathy. Diabetic Medicine. 1994;11:145–149. doi: 10.1111/j.1464-5491.1994.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 12.Martinez M, Vazquez E. MRI evidence that docosahexaenoic acid ethyl ester improves myelination in generalized peroxisomal disorders. Neurology. 1998;51:26–32. doi: 10.1212/wnl.51.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson DR, Robinson JP, Compton AM, Keen P. Essential fatty acid treatment – effects on nerve conduction, polyol pathway and axonal transport in streptozotocin diabetic rats. Diabetologia. 1989;32:655–659. doi: 10.1007/BF00274252. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of American Geriatrics Society. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 15.Lauretani F, Bandinelli S, Bartali B, et al. Axonal degeneration affects muscle density in older men and women. Neurobiology of Aging. 2006;27:1145–1154. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stalberg E, Fuglsang-Frederiksen A, Bischoff C. Quantitation and standardization in EMG and neurography. Supplements to Clinical Neurophysiology. 2000;53:101–111. doi: 10.1016/s1567-424x(09)70144-8. [DOI] [PubMed] [Google Scholar]
- 17.Hodson L, Skeaff CM, Wallace AJ, Arribas GL. Stability of plasma and erythrocyte fatty acid composition during cold storage. Clinica Chimica Acta. 2002;321:63–67. doi: 10.1016/s0009-8981(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 18.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. Journal of Lipid Research. 1986;27:114–120. [PubMed] [Google Scholar]
- 19.Rodriguez-Palmero M, Lopez-Sabater MC, Castellote-Bargallo AI, De la Torre-Boronat MC, Rivero-Urgell M. Comparison of two methods for the determination of fatty acid profiles in plasma and erythrocytes. Journal of Chromatography A. 1998;793:435–440. [PubMed] [Google Scholar]
- 20.Bartali B, Turrini A, Salvini S, et al. Dietary intake estimated using different methods in two Italian older populations. Archives of Gerontology and Geriatrics. 2004;38:51–60. doi: 10.1016/s0167-4943(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 21.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis anc classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Ferrucci L, Simonsick EM, et al. The ankle brachial index and change in lower extremity functioning over time: the women's health and aging study. Journal of American Geriatric Society. 2002;50:238–246. doi: 10.1046/j.1532-5415.2002.50054.x. [DOI] [PubMed] [Google Scholar]
- 23.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 24.Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-linolenic acid multicenter trial group. Diabetes Care. 1993;16:8–15. doi: 10.2337/diacare.16.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons A. American Association of Physical Anthropologists Meeting. Humans' head start: new views of brain evolution. Science. 2002;296:835–837. doi: 10.1126/science.296.5569.835. [DOI] [PubMed] [Google Scholar]
- 26.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. The EMBO Journal. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. International Journal of Developmental Neuroscience. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 28.Leaf A, Kang JX, Xiao YF, Billman GE, Voskuyl RA. Experimental studies on antiarrhythmic and antiseizure effects of polyunsaturated fatty acids in excitable tissues. Journal of Nutritional Biochemistry. 1999;10:440–448. doi: 10.1016/s0955-2863(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 29.Coste T, Pierlovisi M, Leonardi J, et al. Beneficial effects of gamma linolenic acid supplementation on nerve conduction velocity, Na+, K+ ATPase activity, and membrane fatty acid composition in sciatic nerve of diabetic rats. Journal of Nutritional Biochemistry. 1999;10:411–420. doi: 10.1016/s0955-2863(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor R, Huang YS. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Current Pharmaceutical Biotechnology. 2006;7:531–534. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
- 31.Calder VL, Bondeson J, Brennan FM, Foxwell BM, Feldmann M. Antigen-specific T-cell downregulation by human dendritic cells following blockade of NF-kappaB. Scandinavian Journal of Immunology. 2003;57:261–270. doi: 10.1046/j.1365-3083.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Iorio A, Cherubini A, Volpato S, et al. Markers of inflammation, vitamin E and peripheral nervous system function. The InCHIANTI study. Neurobiology of Aging. 2006;27:1280–1288. doi: 10.1016/j.neurobiolaging.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simopoulos AP, Leaf A, Salem N., Jr Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Journal of the American College of Nutrition. 1999;18:487–489. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg NR, Vermeulen M. Chronic idiopathic axonal polyneuropathy revisited. Journal of Neurology. 2004;251:1128–1132. doi: 10.1007/s00415-004-0499-8. [DOI] [PubMed] [Google Scholar]