Abstract
Economic choice entails assigning values to the available options and is impaired by lesions to the orbitofrontal cortex (OFC). Recent results show that some neurons in OFC encode the value monkeys assign to different goods when they choose between them. A broad and fundamental question is how this neuronal representation of value depends on the behavioral context. Here we show that neuronal responses in OFC are typically invariant for changes of menu. In other words, the activity of a neuron in response to one particular good usually does not depend on what other goods are available at the same time. Neurons in OFC encode economic value, not relative preference. The fact that their responses are menu invariant suggests that transitivity—a fundamental trait of economic choice—may be rooted in the activity of individual neurons.
Economic choice is the behavior observed when individuals make choices solely based on subjective preferences—for example out of a restaurant menu. Behavioral evidence suggests that economic choice entails a two-stage mental process: values are initially assigned to the available options and a decision is then made between these values1-3. With respect to brain structures, lesion and imaging studies indicate that neural processes underlying economic choice partly take place in the orbitofrontal cortex (OFC)4. Indeed, lesions to the OFC impair choice behavior in various domains, leading to such deficits as eating disorders5-7, erratic choices8,9, and abnormal gambling10-12. Imaging experiments in healthy subjects show that OFC activates when individuals make choices13,14 and when they earn money15,16. Single cell recordings in non-human primates link OFC more specifically to the process of value assignment. Neurons in OFC often respond to the delivery of one particular food or juice17. However, their responses are also modulated by the juice amount18,19, by the satiation state of the animal20, and by the time until juice delivery21—all consistent with OFC neurons encoding the juice value.
In a recent study, we recorded the activity of individual neurons from OFC while monkeys engaged in economic choices22. In the experiments, monkeys chose between two beverages offered in variable amounts. Their choices provided an operational measure of the values monkeys assigned to the two juices22. We found three types of neuronal responses: offer value responses encoded the quantity or value of one of the two offered juices; chosen value responses encoded the value of the chosen juice independently of the juice type; taste responses were binary responses reflecting which one of the two juices was chosen independently of the amount22 (see Discussion). From a conceptual point of view, responses encoding the chosen value are particularly interesting because they capture two defining traits of value: value is subjective and value represents a common unit for qualitatively different goods22 (a common currency23). Neurons in OFC thus provide a neuronal representation of economic value.
A broad and fundamental question is whether and how this representation of value varies depending on the behavioral context. From a computational perspective, two seemingly opposite traits would seem desirable. On the one hand, an effective representation of value should be stable. For example, a person choosing between different brands of pasta in a grocery store might first compare brands X and Y and choose Y, and later compare brands Y and Z and choose Z; behaviorally, it is desirable for that person to be consistent and also choose Z over X. Such consistency is guaranteed if the neuronal activity representing the value of one particular good (e.g., X) does not depend on what other goods are available at the same time (e.g., Y or Z). On the other hand, values can vary by many orders of magnitude. For example, the same person can choose sometimes between different brands of pasta (worth about $3), other times between different laptops (worth about $3,000), and yet other times between different houses (worth $300,000 or more). In order to represent value efficiently in these very different situations, a neuronal representation of value should somehow adapt to the general choice context, in a way conceptually analogous to how the visual system adapts to ambient light.
Different ways in which the behavioral context can vary may thus be conceptualized as follows. On the one hand, the specific context can change rapidly from one moment to the next, as when a person compares different brands of pasta in a grocery store. We refer to these changes as changes of “menu.” On the other hand, the general context can change on a longer time scale, as when a person goes from a grocery store to a meeting with the realtor. We refer to these changes as changes of “condition”.
Previous work (T&S) shows that some neurons in OFC respond differently to the delivery of a given juice depending on the behavioral context24. In their experiments, T&S delivered to monkeys one of three types of juice (labeled A, B and C, in decreasing order of preference) in fixed amount. Trials were blocked, with only one pair of juices employed in each block. T&S found in OFC neurons that responded to juice A but not to juice B in ‘A:B’ blocks, and to juice B but not to juice C in ‘B:C’ blocks. T&S proposed that these neurons might encode the relative preference of the juices. Importantly, the experimental design used by T&S did not distinguish between OFC responses adapting to the menu or to the condition.
In the present study, we re-examined the issue of context dependence in the light of the distinction between menu and condition. In particular, we examined whether the neuronal representation of value in OFC depends on the menu. To address this question, we recorded the activity of individual OFC neurons while monkeys chose between three different juices (labeled A, B and C, in decreasing order of preference). In each trial, monkeys chose between two juices offered in variable amounts. Critically, trials with the three juice pairs (A:B, B:C and C:A) were randomly interleaved. Replicating our previous results22, we found that single-juice-pair responses encode three variables: offer value, chosen value and taste. We also found that neuronal responses in OFC are typically invariant for changes of menu. For example, if a monkey chooses between juices A, B and C offered pairwise, the activity of neurons encoding the value of juice B does not depend on whether juice B is offered against juice A or against juice C.
Results
In each trial, monkeys chose between two competing offers (Fig. 1a). For any juice pair, the quantities of the two juices varied randomly. Trials with the three juice pairs were randomly interleaved and, for any given offer type, left/right positions were counterbalanced.
Figure 1.
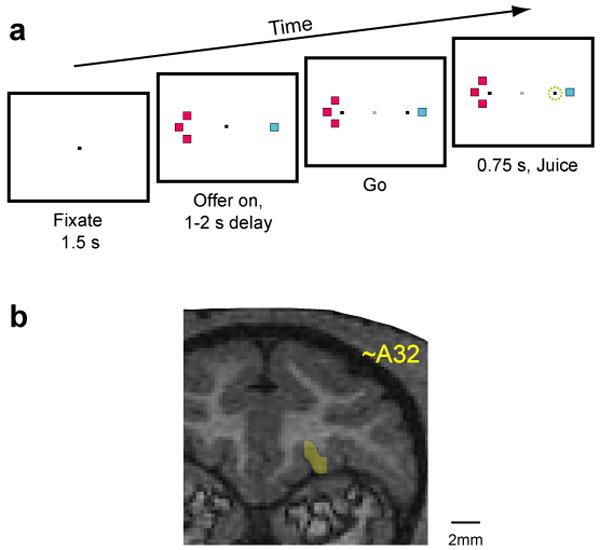
Experimental design. a. Trial structure. The offers were represented by sets of colored squares on a computer monitor and monkeys indicated their choice by making an eye movement. At the beginning of each trial, the monkey fixated a spot (0.2° of visual angle) in the center of the monitor. Two sets of squares appeared on opposite sides of the fixation point (7° to the left and to the right of the fixation point; offer). Different colors of the squares indicated different juice types and the number of squares indicated the juice amount. After a randomly variable delay (1–2s), two saccade targets (0.2° of visual angle) appeared near the offers (‘go’ signal). The monkey indicated its choice and maintained fixation on the saccade target for 0.75s before juice delivery (juice). The trial was aborted if the monkey broke fixation before the ‘go’. Trials were separated by a 1.5 s inter-trial interval, and center fixation was imposed within 1°. b. Recording region. Based on the MRI and on the patterns of white and grey matter encountered by the electrodes, we tentatively identified the recording region with area 13m.
Choice patterns and value transitivity
The experimental design and data analysis were based on the following assumption. If a monkey is offered the choice between a quantity qX of juice X and a quantity qY of juice Y, its choice only depends on the ratio qY/qX. In the language of economic theory, this corresponds to the assumption of “linear indifference curves”. In essence, this amounts to assuming that the relationship between the juice quantity and the assigned value (the “value function”) is the same for different juices (up to a scaling factor). Several elements suggest that the assumption was warranted in our experiments (Supplement, p.S1).
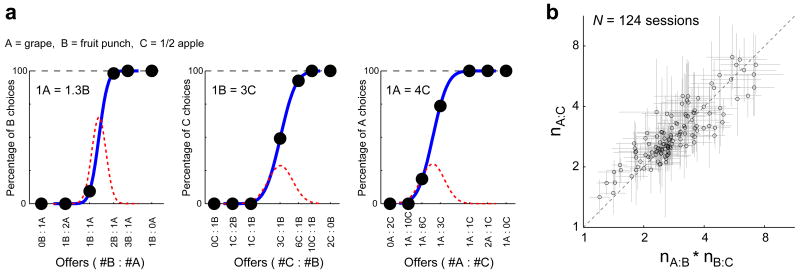
For any given juice pair, the quantities of the two juices offered to the monkey varied from trial to trial. We thus obtained in each session three choice patterns corresponding to the three juice pairs. We illustrate the behavior recorded in a representative session, in which the monkey was offered grape juice (A), fruit punch (B), and diluted apple juice (C) (Fig. 2a). The three panels show separately the choice patterns recorded for juice pairs A:B, B:C and C:A. In the first panel, the x-axis represents the offer type, and different offer types are ordered by the ratio of qB/qA, where qA and qB are the quantities of juices A and B offered to the monkey. The y-axis represents the percentage of trials in which the monkey chose juice B. Analogously, in the second and third panel, the y-axis represents the percentage of trials the monkey chose, respectively, juice C and juice A. For each juice pair, we fit the choice pattern with a sigmoid, from which we obtained an estimate for the relative value of the two juices (see Methods). The relative value corresponds to the indifference point—the ratio of quantities for which the monkey would choose either juice equally often. We indicate with V(x) the value of x, and with nX:Y the relative value of juices X and Y, such that V(X)=nX:YV(Y). For the session illustrated (Fig. 2a), the sigmoid fits provide the relative values V(A)=1.3V(B), V(B)=3.0V(C) and V(A)=4.0V(C). Notably, these values satisfy the relationship 1.3*3≈4. In other words, monkey's choices in this session satisfy the condition of “value transitivity”.
Figure 2.
Analysis of choice patterns. a. One session. The three panels refer to the three juice pairs (trials A:B, B:C and C:A). The x-axes represent different offer types and the y-axes represent the percentage of trials the monkey chose, respectively, juice B, juice C and juice A. Black dots are data points, blue continuous lines are fitted sigmoids, and red dashed lines are the underlying normal distributions. For each fitted sigmoid, the mean and standard deviation of the normal distribution provide an estimate and error of measure for the log relative value (see Methods). The three relative values (top left in each panel) were each computed from the corresponding sigmoid. b. All sessions. Each data point in the scatter plot represents one session and all sessions are shown. For each session, gray error bars represent the errors of measure (s.d.). The diagonal dashed line corresponds to nA:C = nA:B * nB:C. Relative values measured in any given session satisfy transitivity unless they are significantly removed from this line.
Transitivity is a fundamental trait of economic choice behavior25,26. Given three options X, Y and Z, if an individual prefers X to Y, and if she prefers Y to Z, she ought to prefer X to Z (preference transitivity). Analogously, if an individual is indifferent between X and Y, and if she is indifferent between Y and Z, she ought to be indifferent between X and Z (indifference transitivity). The importance of transitivity for economic theory cannot be overstated. For example, economic value cannot be defined unless choices satisfy transitivity25,26. Monkeys' choices in our experiments generally satisfied two conditions (Fig. 2). First, monkeys generally had strict economic preferences (i.e., for offer types away from the indifference point, data points were close to 0% or 100%). Second, monkeys' preferences generally satisfied transitivity. Indeed, in 121/124 (98%) of our sessions monkeys preferred 1A to 1B, 1B to 1C and 1A to 1C. Furthermore, choice patterns usually were strictly increasing. In other words, for any n and m such that n>m, if the monkey preferred 1A to nB, it also preferred 1A to mB. This implies preference transitivity, because for n>m monkeys generally prefer nB to mB.
Under the assumption of linear indifference curves, indifference transitivity is satisfied if the following relationship holds statistically true: nA:B*nB:C=nA:C. We refer to this condition as “value transitivity”. As noted above, in the session illustrated (Fig. 2a), monkeys' choices satisfied value transitivity because 1.3*3≈4. Monkeys' choices in our experiments satisfied value transitivity in general. In the scatter plot (Fig. 2b), the x-axis represents the product nA:B*nB:C, the y-axis represents nA:C, and each data point represents one of the 124 sessions in our data set. In accord with value transitivity, data lie close to the identity diagonal. Even using a liberal criterion to identify transitivity violations (z-test, p<0.1; see Methods), measured relative values satisfy value transitivity in 122 (98%) cases. Using a slightly less liberal criterion (z-test, p<0.05), measured relative values satisfy value transitivity in all 124 sessions.
In summary, monkeys in our experiments assigned to different juices values that satisfied transitivity. One important implication of value transitivity is that we could measure quantities of the three juices on a common value scale—for example in units of V(C) (see Methods).
Neuronal data base and single-juice-pair responses
We analyzed the activity of 557 OFC neurons recorded while two monkeys engaged in this task. Cell activity did generally not depend on either the spatial configuration of visual stimuli or the direction of the eye movement (replicating previous results22); we thus collapsed data across these dimensions. We analyzed neuronal responses in seven time windows: 0.5s pre-offer (a control time window); 0.5s post-offer; late delay (0.5–1.0s after the offer); 0.5s pre-go; reaction time (RT; from ‘go’ to saccade); 0.5s pre-juice; and 0.5s post-juice. Neuronal responses recorded in different time windows likely differ for their functional significance. However, the results of this study (in particular, menu invariance) held similarly true in all time windows. In the following sections, we will thus describe the results pooling together neuronal responses from different time windows. However, we will also report the results broken down by time window (Tables 1 and 3).
Table 1.
Population summary (N=557 cells). Results obtained pooling trials with the three juice pairs. The first column indicates the number of cells modulated by the offer type in each time window (ANOVA, p<0.001). Of the 557 cells in our data set, 351 (63%) passed the ANOVA criterion in at least one time window. The three columns on the right indicate the number of responses classified as encoding each of the three variables. Since OFC responses are typically menu invariant, this classification is based on simple regressions of neuronal responses onto different variables. A variable “explains” a response if the slope of the regression differs significantly from zero (p<0.05); responses explained by more than one variable are assigned to the variable with the highest R2. Responses that pass the ANOVA criterion but are not explained by any variable (48/1,019=5%, unclassified) do not appear in the three columns on the right.
| offer value | chosen value | taste | ||
|---|---|---|---|---|
| 1 | pre-offer | 0 | 0 | 1 |
| 208 | post-offer | 75 | 80 | 51 |
| 163 | late delay | 43 | 47 | 60 |
| 103 | pre-go | 35 | 32 | 29 |
| 71 | RT | 18 | 27 | 17 |
| 239 | pre-juice | 46 | 58 | 127 |
| 234 | post-juice | 51 | 61 | 113 |
| 1,019 | total | |||
| 351 | at least one | |||
Table 3.
Analysis of menu dependent encoding (ANCOVA), by time window. Same results summarized in Table 2, broken down by time window. Top. Numbers correspond to the number of significant effects due to the three factors [variable] (var), [juice pair] (pair) and [variable × juice pair] (int). Bottom. Same data reported as percentages (normalized by time window). Menu invariance is particularly clear in the most salient time windows (post-offer, pre-juice and post-juice), where responses are more frequent and modulation is higher.
| Number of Significant Effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| offer value | chosen value | taste | |||||||
| var | pair | int | var | pair | int | var | pair | int | |
| post-offer | 62 | 8 | 1 | 80 | 5 | 3 | 31 | 2 | 0 |
| late delay | 38 | 6 | 2 | 37 | 4 | 0 | 32 | 8 | 0 |
| pre-go | 15 | 4 | 0 | 33 | 2 | 2 | 12 | 5 | 0 |
| RT | 8 | 4 | 2 | 25 | 2 | 1 | 8 | 2 | 1 |
| pre-juice | 38 | 4 | 1 | 53 | 3 | 4 | 99 | 6 | 4 |
| post-juice | 45 | 2 | 0 | 63 | 4 | 3 | 88 | 4 | 2 |
| Percentage of Significant Effects | |||||||||
| offer value | chosen value | taste | |||||||
| var | pair | int | var | pair | int | var | pair | int | |
| post-offer | 87 | 11 | 1 | 91 | 6 | 3 | 94 | 6 | 0 |
| late delay | 83 | 13 | 4 | 90 | 10 | 0 | 80 | 20 | 0 |
| pre-go | 79 | 21 | 0 | 89 | 5 | 5 | 71 | 29 | 0 |
| RT | 57 | 29 | 14 | 88 | 7 | 4 | 73 | 18 | 9 |
| pre-juice | 88 | 9 | 2 | 88 | 5 | 7 | 91 | 6 | 4 |
| post-juice | 96 | 4 | 0 | 90 | 6 | 4 | 94 | 4 | 2 |
In the analysis of neuronal activity, we first considered for each cell the activity recorded with each juice pair separately. We defined a “response” as the activity of one neuron in one time window. Pooling time windows, we identified 1,660 responses significantly modulated by the offer type (ANOVA, p<0.001). We analyzed this data set using the same procedures employed in our previous study22, and we defined 19 variables that OFC responses might potentially encode (Supplement, p.S2). Each response was linearly regressed separately on each variable and we identified the variables that best explained this population. The results obtained with this new data set closely replicated our previous findings22: single-juice-pair OFC responses encoded one of three variables: offer value, chosen value and taste (Supplement, p.S2).
Classification is consistent across juice pairs
We next examined whether the neuronal representation of value in OFC depends on the menu. We considered two possible types of menu dependence: “classification conflict” and “menu dependent encoding.” A case of classification conflict would be that of a neuron encoding different variables depending on the juice pair. For example, one cell could encode offer value A when the monkey chooses between A and B and offer value B when the monkey chooses between B and C (this putative cell would always encode the value of the preferred juice). A case of menu dependent encoding would be that of a neuron that encodes always the same variable, but such that the linear relationship between the cell activity and the variable depends on the particular juice pair. As described below, our analyses argue against both these hypotheses.
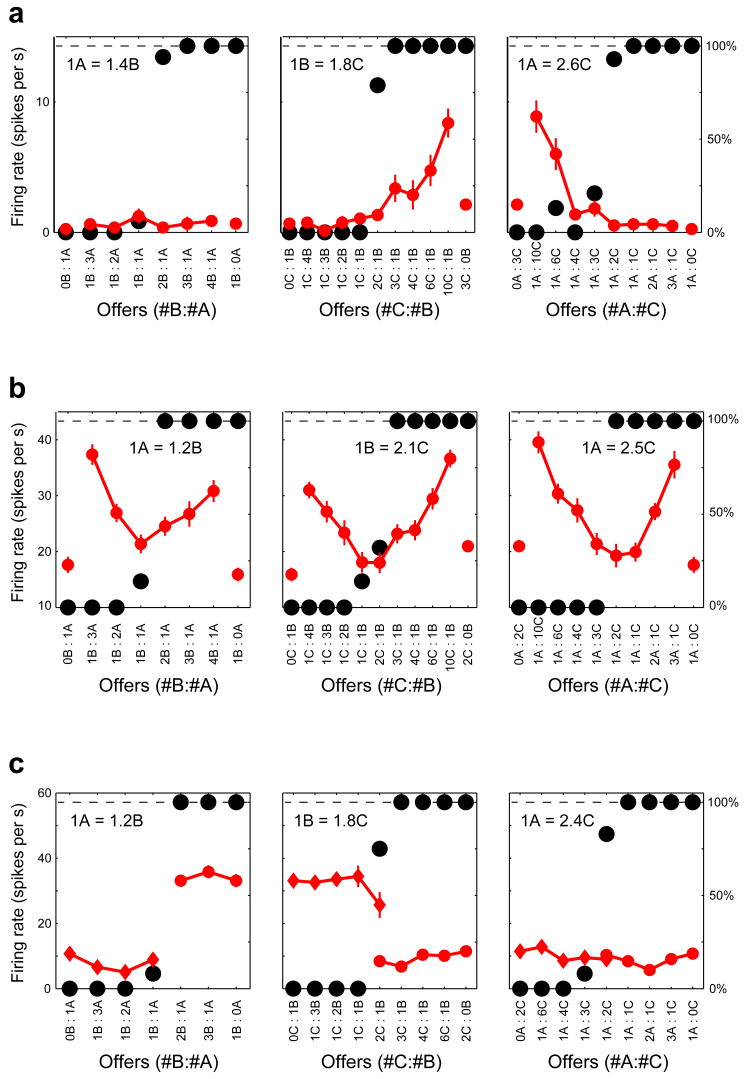
We illustrate three cases of classification consistency (Fig. 3). One neuron (Fig. 3a) encodes offer value C, independently of whether the monkey chooses between C and B or between C and A; the activity of the neuron is low and not modulated when the monkey chooses between A and B. Another neuron (Fig. 3b) encodes chosen value for each of the three juice pairs. Finally, another neuron (Fig. 3c) encodes taste B, independently of whether the monkey chooses juice B against juice A or against juice C; the activity of the neuron is low and not modulated when the monkey chooses between A and C. These examples suggest that the variable encoded by individual OFC neurons does not depend on the juice pair. Indeed, across a population of 760 relevant instances (i.e., cases in which at least one of the three single-juice-pair responses passed the ANOVA criterion), classification conflicts were significantly less frequent than would be expected by chance (p<10−6, bootstrap analysis; Supplement, p.S2). A specific analysis also showed that instances in which offer value or taste responses reflected the preference ranking as opposed to the identity of the encoded juice were very rare (4/760; Supplement, p.S3).
Figure 3.
Responses of three OFC neurons. a. Response encoding offer value C independently of the juice pair. The three panels refer to trials A:B, B:C and C:A. In each panel, black symbols represent the behavioral choice pattern and red symbols represent the neuronal activity (±s.e.m.). Relative values (top left) are computed assuming transitivity (see Methods). b. Response encoding chosen value independently of the juice pair. Same conventions as in (a). c. Response encoding taste B independently of the juice pair. Same conventions as in (a). Here we separated trials depending on the chosen juice.
Neuronal encoding is invariant for changes of menu
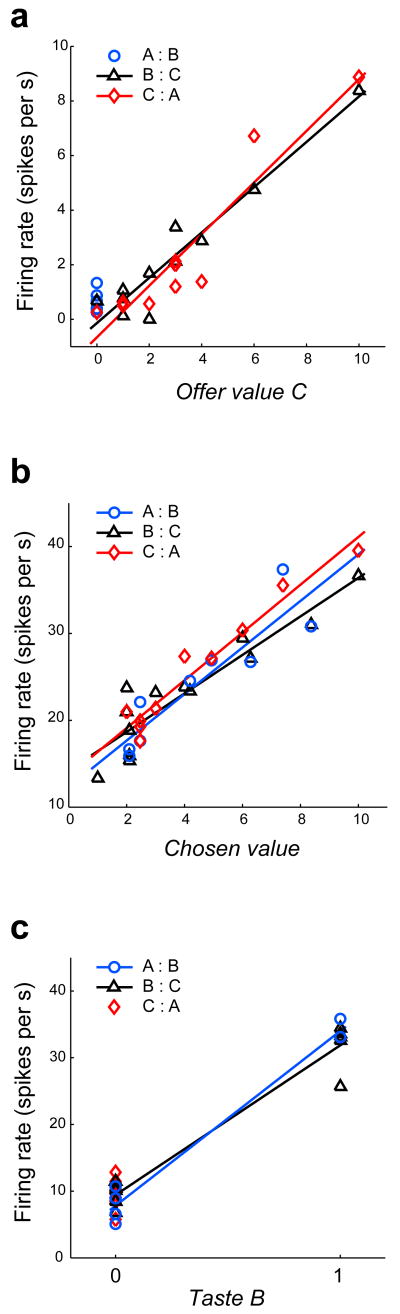
According to our definition, a neuronal response “encodes” one variable if there is a linear relationship between the cell activity and that variable22. If the coefficients of the linear relationship depend on the juice pair, the encoding is menu dependent. Conversely, if the coefficients do not depend on the juice pair, the encoding is menu invariant. This latter situation was typically observed in OFC. Figure 4a–c illustrates the same three neuronal responses shown in Figure 3a–c. Each panel represents one response (one neuron), and the firing rate (y-axis) is plotted, respectively, against variables offer value C, chosen value and taste B (x-axis). For each neuron, different symbols and colors refer to the three juice pairs. For each neuron, the encoding would be menu dependent if the regression lines differed significantly from one another, either for their intercepts or for their slopes. To the contrary, in each panel, regression lines are very similar from one another, indicating that the encoding is menu invariant. A formal statistical test required an analysis of co-variance (ANCOVA), as follows.
Figure 4.
Menu invariance. a. Same neuronal response as in Fig. 3a, combining data from the three juice pairs. The firing rate (y-axis) is plotted against variable offer value C, and different symbols and colors refer to the three juice pairs (blue circles for A:B, black triangles for B:C, and red diamonds for C:A). Each symbol represents one trial type. The regression lines are obtained from a full-model analysis of covariance; no regression line is plotted in for A:B trials because the data points are collapsed on the x-axis (offer value C = 0). b. Same neuronal response as in Fig. 3b (y-axis) plotted against variable chosen value (x-axis). The chosen value is expressed in units of V(C). All conventions as in (a). c. Same neuronal response as in Fig. 3c (y-axis) plotted against variable taste B (x-axis). All conventions as in (a). All regression lines (a-c) are obtained from an analysis of covariance (ANCOVA).
Pooling trials with the three juice pairs, we restricted the analysis to responses significantly modulated by the trial type (p<0.001, ANOVA). In total, 1,019 responses from different time windows satisfied this criterion (Table 1). The subsequent analysis was restricted to this population.
We defined 7 variables: offer value A, offer value B, offer value C, chosen value, taste A, taste B, and taste C (see Methods). We then analyzed each response with an ANCOVA, using one of the 7 variables as the predictor and grouping data by the juice pair. We computed the full linear model, including the three factors [variable], [juice pair], and [variable × juice pair] interaction, and we obtained the R2. For each response, we repeated this analysis separately with each of the 7 variables, and we identified the variable encoded by the response as the one that provided the highest R2. Focusing now on that particular variable, we established whether each of the three factors [variable], [juice pair] and [variable × juice pair] provided a significant contribution to the explained variance (p<0.01). For example, referring to the three regression lines in Figure 4b, the factor [variable] would be significant if a single regression line (not shown) of all the data points had a non-zero slope; the factor [juice pair] would be significant if the three intercepts differed significantly from one another; the interaction [variable × juice pair] would be significant if the three slopes differed significantly from one another. In this case (Fig. 4b), the factor [variable] is significant (p<10–12), but the two other factors are not (p>0.25).
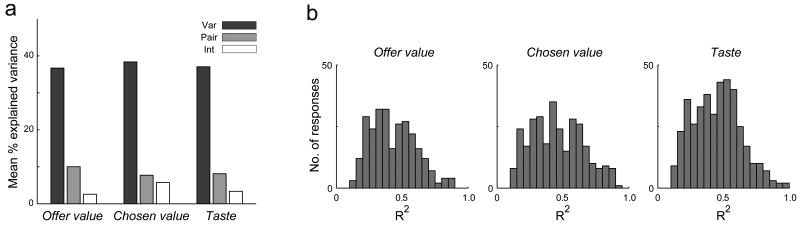
Results were consistent across the neuronal population. Analyzing each of the 1,019 responses with the ANCOVA, we obtained a total of 868 significant effects across the three factors. Of these, 767 (88%) were provided by the factor [variable]. In contrast, factors [juice pair] and [variable × juice pair] rarely yielded significant effects (respectively, 9% and 3%; Table 2). The measure of explained variance corroborated this result, as the factor [variable] typically accounted for most of the explained variance (Fig. 5).
Table 2.
Analysis of menu dependent encoding (ANCOVA), pooling time windows. Number and percentage of significant effects due, for each variable (columns), to the three factors (rows).
| offer value | chosen value | taste | Total | Percent | |
|---|---|---|---|---|---|
| variable | 206 | 291 | 270 | 767 | 88 |
| juice pair | 28 | 20 | 27 | 75 | 9 |
| interaction | 6 | 13 | 7 | 26 | 3 |
Figure 5.
Explained variance. a. Analysis of covariance. The plot shows percentage of variance explained by the three factors [variable] (var), [juice pair] (pair) and [variable × juice pair] (int), separately for the three response types. The percentage of explained variance is averaged across responses, pooling time windows. b. Linear fit. Since OFC responses are typically menu invariant, we can estimate the variance explained by the encoded variable with a simple linear regression. The histogram shows for different R2 (x-axis) the number of corresponding responses (y-axis), separately for the three response types. The medians of the three distributions are, respectively, 0.42, 0.44 and 0.46.
Notably, menu invariance held true for all response types and in all time windows (Table 3). For example, for responses encoding the offer value in the post-offer time window, significant effects provided by the factor [variable] were 62/71 (87%). Similarly, restricting the analysis to responses encoding offer value B or taste B, significant effects provided by the factor [variable] were 86/105 (82%). In conclusion, neuronal responses in OFC were typically invariant for changes of menu.
Discussion
In summary, by interleaving trials with different juice pairs, we observed that monkeys assign to three beverages values that respect transitivity. Replicating our previous results22, we found that single-juice-pair responses in OFC encode three variables: offer value, chosen value and taste. The main result of this study is that neuronal responses in OFC are invariant for changes of menu. In other words, the activity of neurons encoding the value or taste of one particular juice typically does not depend on what other juices are available at the same time.
Neurons examined here do not encode or reflect the relative preference (i.e., the ordinal ranking) of the offered juices. So how can these results be reconciled with previous observations24 (T&S)? One possibility is that T&S's recordings were from a different brain region. Consistent with this hypothesis, the percentage of task-related neurons in their population (<10% in any time window) was small compared to our estimate (>35% in post-offer, pre-juice and post-juice time windows; 63% pooling time windows; Table 1). Another possibility is that T&S's observations critically depended on the fact that trials were presented in blocks. In principle, a block design could affect neuronal responses in multiple ways. For example, in their study, ‘A:B’ blocks could be considered high-value blocks whereas ‘B:C’ blocks could be considered low-value blocks. The observation of T&S thus suggests that the activity of OFC neurons might adapt to the behavioral condition—i.e., to the general behavioral context defined across many trials (see Introduction). In this view, the changes in neuronal activity observed by T&S were not due to menu dependence, but rather to a slowly adapting neuronal representation. Similar adaptation phenomena might also take place in other brain regions27-29.
If the population of OFC neurons examined here were found to undergo analogous adaptation, that would suggest the following hypothesis. In any given behavioral condition, OFC neurons encode value in a menu invariant way; however, OFC neurons adapt flexibly to different behavioral conditions and thus maintain high value sensitivity. Further work is necessary to test this hypothesis.
Response types, menu invariance and transitivity
To this point, we described transitivity as a trait of choice behavior and menu invariance as a trait of neuronal responses in OFC. In fact, the concepts of transitivity and menu invariance are intimately related both at the behavioral level and at the neuronal level. At the behavioral level, human and animal economic choices typically satisfy transitivity3,8,30-33. However, transitivity violations can sometimes be observed34-37. When they occur, transitivity violations are due to preferences that vary depending on the menu36,38,39. In other words, at the behavioral level, menu invariant values imply transitive preferences. An analogous implication holds true at the neuronal level. Indeed, a neuronal representation of value reflects transitivity if it is stable and invariant for changes of menu (Supplement, p.S3). In this light, we shall now discuss the implications of menu invariance for different neuronal response types in OFC.
Taste responses are binary responses reflecting the chosen juice independently of its amount. Because they do not encode value, transitivity does not apply. Conversely, menu invariance indicates that taste responses reflect the identity of the chosen juice as opposed to its preference ranking. Notably, we label these as taste responses because gustatory activity was previously reported in this area17. However, we use this label somewhat loosely. Indeed, as they appear before juice delivery (ref.[22] and Table 1), taste responses are not purely sensory, at least not in a traditional sense (i.e., they may also represent the expectation of one particular juice). Furthermore, taste responses could in fact encode a more complex impression such as flavor.
Chosen value responses were previously shown to encode the subjective value monkeys assign to the juice they choose to consume22. These responses encode value per se, as opposed to any physical property of the juice. Chosen value responses thus embody two defining properties of economic value—that value is subjective, and that value is a common unit for qualitatively different goods3,23. Menu invariance implies that chosen value responses also embody the other defining trait of economic value—transitivity. One open question is whether and how chosen value responses contribute to the choice process.
Offer value responses are interpreted more tentatively, because in this case we cannot distinguish juice value from juice quantity (or from other variables proportional to the juice quantity). We label them offer value responses because lesions to the OFC lead specifically to choice deficits5-12 and because other responses in this area (i.e., chosen value responses) encode value as opposed to quantity. However, more work is necessary to establish whether offer value responses encode value or quantity. If offer value responses encode the juice quantity, menu invariance implies that these responses reflect the identity as opposed to the preference ranking of the encoded juice. Conversely, if offer value responses indeed encode the juice value, menu invariance implies that these responses reflect transitivity. In this respect, it is worth noting that neurons encoding the offer value (in particular, shortly after the offer) could naturally contribute to the choice process, and that menu invariance holds true in all time windows including the post-offer time window. One intriguing possibility is that preference transitivity may be rooted in neuronal menu invariance.
Importantly, the proposal that OFC value-encoding responses (i.e., definitely chosen value responses and possibly offer value responses) reflect transitivity is limited in at least three important ways. First, in some circumstances behavioral preferences can in fact depend on the menu and thus violate transitivity34-39. In such cases, OFC responses might also depend on the menu. Second, while invariant for changes of menu, OFC responses might vary depending on the behavioral condition, as defined above. Although this hypothetical adaptability does not per se imply transitivity violations, whether and how value-encoding responses in OFC reflect transitivity across behavioral conditions remains to be established. Third, further work should verify whether menu invariance holds true when choices involve different types of goods, and different sensory or motor modalities.
Menu invariance might suggest that neurons encoding offer value or taste do not receive information about any juice other than the encoded one. However, it is also possible that a given neuron may adaptively respond to different juices in different behavioral conditions.
Relation with other brain systems
How is economic value computed, and how do value signals in OFC affect various aspects of behavior? These fundamental questions remain largely open. Current anatomical maps4 divide the medial and orbital prefrontal cortices in 22 distinct brain areas, organized in two mostly segregated networks. In the orbital network (including 13m and surrounding areas), anatomical input from all sensory modalities converges with anatomical input from limbic areas such as the amygdala4,40. The orbital network thus seems well placed to compute such a quantity as subjective value. However, this computation may involve multiple brain areas. Future work should thus examine other regions anatomically connected to the area examined here.
Apart from a possible role in economic choice behavior, value-encoding neurons in OFC might inform various other brain systems, including sensory, motor and viscero-motor systems. Through sensory and motor systems, these value signals may contribute to attention and action selection2,41,42. Through the viscero-motor system, these value signals may contribute to the generation and control of emotional and autonomic responses43,44. However, the lack of direct anatomic connections indicates that, at least for motor and viscero-motor systems, these putative modulations are indirect4,40,45.
Interestingly, the representation of value in OFC differs from that found in the lateral intraparietal area (LIP)41,46. In general, neurons in LIP activate when a visual stimulus is placed in their response field and when the monkey plans the corresponding eye movement; LIP responses are enhanced if the value associated with the stimulus or the saccade is higher. Thus for neurons in LIP, value modulates responses that are sensory or motor in nature2. In contrast, neurons in OFC encode economic value per se, independently of visuomotor contingencies22,42. Another critical difference is that the value modulation in area LIP depends on the menu: for any given LIP neuron, the modulation is proportional to the value of the corresponding stimulus/saccade divided by the value sum of all possible stimuli/saccades46, as if reflecting a value weight. In contrast, the representation of value in OFC is invariant for changes of menu.
Conclusions
The behavioral context in which economic choices are made can change from moment to moment (changes of menu) or on a longer time scale (changes of condition). By interleaving trials with different juice pairs, we found that the representation of economic value in OFC is invariant for changes of menu: neuronal responses encoding the value of one particular juice do not depend on what other juice is available at the same time. Neurons in OFC thus encode the economic value in a cardinal (i.e., number-like) sense, not the relative preference (i.e., the ordinal ranking). Moreover, OFC value-encoding neurons reflect transitivity. Whether and how the representation of value in OFC adapts to the behavioral condition remains an important question for future work.
Methods
Experimental design
One male (V, 9.5 Kg) and one female (L, 6.3 Kg) rhesus monkey participated in the experiments. Subjects, experimental setup, surgical procedures, and recording procedures were the same as previously described22. The NIH Guide for the Care and Use of Laboratory Animals and the guidelines of the Harvard Medical School Standing Committee on Animals were strictly followed throughout the experiments.
Under general anesthesia, we implanted a head-restraining device and a recording chamber on the skull of the monkeys, and a scleral eye coil47. We used large oval custom-made chambers (main axes 50×30 mm), centered on stereotaxic coordinates (A30, L0), with the longer axis parallel to a coronal plane. Following surgery, monkeys were given antibiotics (cefazolin, 20 mg kg-1) and analgesics (buprenorphine, 0.005 mg kg-1; flunixin, 1 mg kg-1) for three days. During the experiments, monkeys sat in a monkey chair in a darkened room. Their head was restrained, and their eye position was monitored continuously using a scleral eye-coil system47 (Riverbend Instruments). A computer monitor was placed 57 cm in front of the monkeys, and the behavioral task was controlled by custom-written software.
The trial structure is illustrated (Fig. 1). In each session, we pseudo-randomly interleaved trials with the three juice pairs. For any given offer type, left/right positions were counterbalanced. Typically, 2–4 sessions of 300–600 trials each were run each day. Across sessions, we used a variety of different juices, including high-sugar lemon Kool-Aid®, grape juice, fruit punch (pure or diluted 2/3 with water), apple juice (diluted 1/2 with water), cranberry juice (diluted 1/3 with water), water, peppermint tea, tea, low-sugar agua frescas Kool-Aid®, low-sugar tamarind Kool-Aid®, slightly salted water (0.60 g l-1 or 0.65 g l-1 concentration). We used a total of 23 juice “triplets.”
Juices were delivered through a three-line juice tube, with each juice line controlled by a separate solenoid valve. We routinely calibrated the juice lines so that the valve-opening times corresponded to the desired multiple of juice quantum. We used quanta of 80 μl and 65 μl for monkeys V and L, respectively.
Neuronal recordings
Multi-electrode neuronal recordings were performed in the same region examined in the previous study22. In monkey V, recordings were centered on stereotaxic coordinates (A32.5, L–9.0) and extended for 6 mm rostro-caudally and 5 mm medio-laterally. In monkey L, recordings were centered on stereotaxic coordinates (A33.5, L8.5) and extended for 6 mm rostro-caudally and 2 mm medio-laterally. Two structural MRI scans (1 mm sections) were made for each animal, before and after implanting the head-post and recording chamber. For the second MRI, mineral-oil filled capillary tubes were placed at known locations in the chamber, to aid in localizing electrode penetrations. We made two MRIs because the second MRI typically has some degree of metal artifact from the titanium plates and screws used to secure the chambers. Sulcal patterns from the two MRIs were superimposed to get a clearer image of the underlying anatomy. On the basis of this image and of the patterns of gray and white matter encountered during penetrations, we tentatively identified the recording region as centered on area 13m [ref.4].
Tungsten electrodes (125 μm diameter, 5±1 MΩ initial impedance, Frederick Haer & Co.) were advanced with custom-built motorized micro-drives (0.5 μm of depth resolution). Typically four electrodes were used each day. Electrodes were usually advanced by pairs (one motor for two electrodes), with the two electrodes placed 1 mm apart. Electrical signals were amplified, bandpass filtered and recorded at 20 kHz (Power 1401, Cambridge Electronic Design). Action potentials were detected online by threshold crossing and waveforms were saved to disk for subsequent analysis. Spike sorting was performed off line semi-manually (Spike 2, Cambridge Electronic Design). We routinely used multiple algorithms, including template matching, clustering on waveform measurements, and principal component analysis. Only neurons that appeared well isolated throughout the recording session and that presented stable waveforms were included in the analysis.
Analysis of choice patterns
The method used to infer relative values is the same that we previously employed for choices between two goods22, generalized to the case of three goods offered pairwise. The approach is similar but not identical to that of Luce48. We generally refer to “relative” values because behavioral analyses allow measuring quantities of different goods on a common value scale defined up to a scaling factor. In other words, values are always expressed in units of some arbitrarily designated good. Our measure of relative value rests on the assumption of linear indifference curves: If a monkey is repeatedly offered the choice between quantities qA and qB of juices A and B (offer qBB:qAA), the rate of “B” choices only depends on the ratio qB/qA (see Supplement, p.S1).
In the analysis, choice patterns recorded for each pair of juices (e.g., A:B) are expressed as a function of log(qB/qA), where qA and qB are, respectively, the quantities of juices A and B offered to the monkey. We then fit the percentage of “B” choices with a normal sigmoid, which is a normal cumulative distribution function of the form . We interpret the underlying Gaussian (which has mean μ and variance σ2) as a probability distribution for the log relative value, and we compute the estimated relative value n̂ = exp (μ̂) (see Fig. 2a).
For each session, we thus obtain the three probability distributions for the log relative values u=log(nA:B), v=log(nB:C) and w=log(nA:C). Testing whether values satisfy transitivity reduces to testing whether the identity u+v=w holds statistically true. Because u, v and w are all normally distributed variables, transitivity violations can be identified with a z-test. As illustrated (Fig. 2b), relative values measured in our experiments rarely violated transitivity.
Since measured values satisfied transitivity, we could measure quantities of the three juices on a unique value scale. The estimated log relative values û, v̂ and ŵ were computed by imposing on the collective probability distribution P(u,v,w) the conditions w=u+v, ∂P/∂u=0 and ∂P/∂v=0. The common value scale was defined up to a scaling factor, and we conventionally expressed values in units of V(C). Except for the variable selection analysis (for which we used the relative values inferred from individual choice patterns), neuronal responses were always analyzed in relation to this common value scale.
Analysis of neuronal data
The 557 neurons analyzed here are an entirely new data set (not previously published). Unless otherwise specified, the analysis was identical to that previously described22. Behavioral and neuronal data were analyzed in Matlab (PC version, MathWorks). We divided trials into “trial types” based on the offer type and the choice. For example, a monkey facing the offer type 3B:1A could choose either 1A or 3B, corresponding to the two trial types (3B:1A, 1A) and (3B:1A, 3B). In the analysis, we included only trial types with two or more trials. The number of trials per trial type thus ranged between 2 and 144 and was typically less than 30 (mean=22.8, median=20). For each trial type, we averaged the activity of each cell across trials separately in each time window. A “response” was defined as the activity of one neuron in one time window as a function of the trial type.
We previously showed that, for the limited quantity range explored in these experiments, the relationship between juice value and value-encoding neuronal responses is roughly linear22. Our analyses were thus based on linear regressions. The procedures and results for the variable selection analysis and for the analysis of classification conflicts are detailed in the Supplement (p.S1–S3).
For the analysis of menu dependent encoding, we defined 7 variables as follows. In any given trial, offer value A, offer value B and offer value C were, respectively, proportional to the quantity of juice A, B and C offered. The chosen value was always proportional to the value chosen by the monkeys, as expressed in the common value scale. Finally, taste A, was proportional to a binary variable equal to 1 when monkeys chose and consumed juice A and equal to 0 otherwise. Variables taste B and taste C were defined analogously for juices B and C. Importantly, the exact proportionality coefficients (i.e., the scale) were in any case irrelevant because the analyses were based on linear regressions.
Supplementary Material
Supplement is attached.
Acknowledgments
We gratefully acknowledge A. Rustichini for many insightful discussions. We also thank A. Bisin, J. Maunsell, P. Glimcher, and W. Schultz for helpful comments on previous versions of the manuscript. This work was supported by a post-doctoral fellowship from the Harvard Mind/Brain/Behavior Initiative, by a Pathway to Independence Award from the National Institute of Mental Health to C.P.S. (Grant Number K99-MH080852) and by a grant from the National Institute of Neurological Disorders and Stroke to J.A.A. (Grant Number R01-NS41000).
Footnotes
Author Contributions C.P.S. designed the experiment, collected and analyzed the data and wrote the manuscript. J.A.A. assisted in the study and in manuscript preparation.
Author Information The authors declare no competing financial interest.
References
- 1.Fellows LK. The cognitive neuroscience of human decision making: a review and conceptual framework. Behav Cogn Neurosci Rev. 2004;3:159–72. doi: 10.1177/1534582304273251. [DOI] [PubMed] [Google Scholar]
- 2.Glimcher PW, Dorris MC, Bayer HM. Physiological utility theory and the neuroeconomics of choice. Games Econ Behav. 2005;52:213–256. doi: 10.1016/j.geb.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padoa-Schioppa C, Jandolo L, Visalberghi E. Multi-stage mental process for economic choice in capuchins. Cognition. 2006;99:B1–B13. doi: 10.1016/j.cognition.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 5.Pasquier F, Petit H. Frontotemporal dementia: its rediscovery. Eur Neurol. 1997;38:1–6. doi: 10.1159/000112894. [DOI] [PubMed] [Google Scholar]
- 6.Hodges JR. Frontotemporal dementia (Pick's disease): clinical features and assessment. Neurology. 2001;56:S6–10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–8. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb Cortex. 2007;17:2669–74. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- 9.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 10.Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 11.Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant of frontotemporal dementia. Brain. 1999;122(Pt 8):1469–93. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- 12.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27:951–6. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arana FS, et al. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–8. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair K, et al. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci. 2006;26:11379–86. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard TC, et al. Gustatory neural responses in the medial orbitofrontal cortex of the old world monkey. J Neurosci. 2005;25:6047–56. doi: 10.1523/JNEUROSCI.0430-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–81. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 19.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–10. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 20.Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosci. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 21.Roesch MR, Olson CR. Neuronal activity dependent on anticipated and elapsed delay in macaque prefrontal cortex, frontal and supplementary eye fields, and premotor cortex. J Neurophysiol. 2005;94:1469–97. doi: 10.1152/jn.00064.2005. [DOI] [PubMed] [Google Scholar]
- 22.Padoa-Schioppa C, Assad JA. Neurons in orbitofrontal cortex encode economic value. Nature. 2006;441:223–6. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–84. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 25.Kreps DM. A course in microeconomic theory. Vol. 850. Princeton University Press; Princeton, NJ: 1990. [Google Scholar]
- 26.Allingham M. Choice theory: a very short introduction. Oxford University Press; Oxford, UK; New York, NY: 2002. p. 127. [Google Scholar]
- 27.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 28.Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Exp Brain Res. 2005;162:520–5. doi: 10.1007/s00221-005-2223-z. [DOI] [PubMed] [Google Scholar]
- 29.Hosokawa T, Kato K, Inoue M, Mikami A. Neurons in the macaque orbitofrontal cortex code relative preference of both rewarding and aversive outcomes. Neurosci Res. 2007;57:434–45. doi: 10.1016/j.neures.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Logan FA. Decision-making by rats: delay versus amount of reward. J Comp Physiol Psych. 1965;59:1–12. doi: 10.1037/h0021633. [DOI] [PubMed] [Google Scholar]
- 31.Campione JC. Transitivity and choice behavior. J Exp Child Psychol. 1969;7:387–399. doi: 10.1016/0022-0965(69)90001-0. [DOI] [PubMed] [Google Scholar]
- 32.Mazur JE, Coe D. Tests of transitivity in choices between fixed and variable reinforcer delays. J Exp Anal Behav. 1987;47:287–97. doi: 10.1901/jeab.1987.47-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S, Fisman R, Gale D, Kariv S. Consistency and heterogeneity of individual behavior under uncertainty. Am Econ Rev (forthcoming) [Google Scholar]
- 34.Tversky A. The intransitivity of preferences. Psychol Rev. 1969;76:31–48. [Google Scholar]
- 35.Navarick DJ, Fantino E. Transitivity as a property of choice. J Exp Anal Behav. 1972;18:389–401. doi: 10.1901/jeab.1972.18-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafir S. Intransitivity of preferences in honey bees: support for ‘comparative’ evaluation of foraging options. Anim Behav. 1994;48:55–67. [Google Scholar]
- 37.Shafir S. Context-dependent violations of rational choice in honeybees (Apis mellifera) and gray jays (Perisoreus canadensis) Behav Ecol Sociobiol. 2002;51:180–187. [Google Scholar]
- 38.Tversky A, Simonson I. Context-dependent preferences. Management Sciences. 1993;39:117–185. [Google Scholar]
- 39.Grace RC. Violations of transitivity: Implications for a theory of contextual choice. J Exp Anal Behav. 1993;60:185–201. doi: 10.1901/jeab.1993.60-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci. 1999;877:383–96. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 41.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–7. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 42.Padoa-Schioppa C. Orbitofrontal cortex and the computation of economic value. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1401.011. in press. [DOI] [PubMed] [Google Scholar]
- 43.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 45.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 46.Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron. 2004;44:365–78. doi: 10.1016/j.neuron.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–8. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 48.Luce RD. Individual choice behavior; a theoretical analysis. Wiley; New York, NY: 1959. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement is attached.