Abstract
Despite the harmful effects of fetal alcohol exposure, some pregnant women continue to drink alcohol. Thus, it is imperative to pursue safe, effective treatments for children with fetal alcohol spectrum disorders. Using an animal model, our laboratory has demonstrated that choline, an essential nutrient, effectively reduces the severity of some fetal alcohol effects, even when administered after the ethanol insult is complete. The present study investigated whether there is a critical developmental period when choline is most effective in attenuating ethanol’s teratogenic effects. Sprague-Dawley rats were exposed to 5.25 g/kg/day ethanol during the third trimester equivalent brain growth spurt (postnatal days (PD) 4–9) via intubation. A non-intubation control group and a sham intubation control group were included. Following ethanol exposure, pups received subcutaneous injections of saline vehicle or choline chloride (100 mg/kg/day) from PD 11–20, PD 21–30, or PD 11–30. Beginning on PD 45, subjects were tested on a Morris water maze spatial learning task. Performance of both the ethanol-exposed group that did not receive choline and the ethanol-exposed group treated with choline from PD 21–30 was significantly impaired compared to controls during acquisition of the Morris water maze task. Performance of ethanol-exposed groups treated with choline from PD 11–20 or PD 11–30 was intermediate, not differing significantly from any other groups. However, during the probe trial, ethanol exposure produced significant deficits in spatial memory which were mitigated by all choline treatments, regardless of the timing of administration. These findings suggest that choline’s therapeutic window may be very large, or spans across the two developmental periods examined in this study. Importantly, these findings indicate that choline supplementation may effectively reduce some alcohol-related learning impairments, even when administered in later childhood.
Keywords: fetal alcohol, ethanol, treatment, spatial learning, Morris maze
1. Introduction
Fetal alcohol spectrum disorders (FASD) is a term used to describe the wide range of physical, neurological, and behavioral alterations associated with prenatal exposure to ethanol (NIAAA, 2000; Riley and McGee, 2005; Sokol et al., 2003). Alcohol-related disruptions in central nervous system (CNS) development constitute the most devastating consequences (see Spadoni et al., 2007 for review), with damage to areas such as the cerebellum (O'Hare et al., 2005; Sowell et al., 1996), corpus callosum (Bookstein et al., 2007; Riley et al., 1995; Sowell et al., 2008a; Sowell et al., 2001), caudate (Mattson et al., 1996), cortex (Sowell et al., 2008b) and hippocampus (Autti-Ramo et al., 2002; Riikonen et al., 1999). Ethanol-induced neuropathology leads to a variety of behavioral problems, including hyperactivity, attention deficits, motor dysfunction, impairments in language and social skills, and learning deficits (Coles et al., 1997; Kelly et al., 2000; Mattson et al., 2001; Riley and McGee, 2005; Roebuck et al., 1999). Although FASDs are preventable, many pregnant women still drink alcohol and FASDs continue to constitute a serious health concern throughout the world (Warren et al., 2001). In the U.S. alone, it is estimated that 1 in 100 live births exhibit at least some adverse effects of prenatal alcohol exposure (Sampson et al., 1997). Thus, it is imperative that potential interventions/treatments for FASD be identified.
Numerous animal studies have shown that pharmacological manipulations may effectively block ethanol’s teratogenic effects (Bonthius et al., 2003; Chen et al., 2005; Endres et al., 2005; Ieraci and Herrera, 2006; Thomas et al., 2001; Wilkemeyer et al., 2003; Wilkemeyer et al., 2002). However, to be effective, such experimental therapeutics would have to administered during ethanol exposure and there is concern that such treatments could exert undesirable effects on nontargeted developmental processes. Moreover, the opportunity for treatment may not be available until a child is already born with FASD. Fortunately, both human and animal studies have shown that postnatal environmental manipulations can reduce the severity of some fetal alcohol effects. Animal studies have shown that enriched environment (Hannigan and Berman, 2000; Hannigan et al., 1993), exercise (Christie et al., 2005; Thomas et al., in press), and acrobatic motor training (Klintsova et al., 2000; Klintsova et al., 2002) can reduce alcohol’s behavioral teratogenic effects. Similarly, clinical studies have shown that specialized training can improve social skills (O'Connor et al., 2006), behavioral problems (Kable et al., 2007) and math skills (Kable et al., 2007). These data suggest that the CNS can respond to environmental interventions even after a developmental alcohol insult.
We have shown that choline supplementation may serve as an effective dietary intervention to reduce the severity of alcohol’s adverse effects on behavioral development. Choline is an essential nutrient that is critical for brain development and function (Zeisel, 2006a). A growing literature illustrates that pre- and/or early postnatal choline supplementation in otherwise typically developing rats leads to long-lasting morphological (Li et al., 2004; Loy et al., 1991; Williams et al., 1998), electrophysiological (Jones et al., 1999; Pyapali et al., 1998), and neurochemical (Blusztajn et al., 1998; Cermak et al., 1999; Cermak et al., 1998; Coutcher et al., 1992; Meck et al., 1989; Montoya et al., 2000) changes in the CNS that contribute to long-lasting cognitive enhancements (McCann et al., 2006; Meck and Williams, 1997; Meck et al., 1988; Meck and Williams, 2003). Perinatal choline supplementation can also enhance cognitive improvements to later environmental manipulations, such as enriched environment (Tees, 1999). Using a rat model of fetal alcohol exposure, we found that choline supplementation not only reduces the severity of prenatal alcohol effects when administered concurrently with the alcohol (Thomas et al., submitted for publication), but is also effective when administered postnatally. For example, we first reported that choline supplementation during postnatal days (PD) 2–21 reduced the severity of working memory deficits in adult rats following prenatal alcohol exposure (Thomas et al., 2000). Importantly, these findings illustrated that choline is effective even when administered after ethanol exposure is complete. More recently, it’s been shown that choline supplementation from either PD 4–30 or 10–30 reduces overactivity in the open field (Thomas et al., 2007; Thomas et al., 2004), spatial reversal learning deficits (Thomas et al., 2004), trace eyeblink conditioning deficits (Tran and Thomas, 2007), trace fear conditioning deficits (Wagner and Hunt, 2006), and spatial learning deficits (Thomas et al., 2007) associated with alcohol exposure during the 3rd trimester equivalent (PD 4–9). In all of these studies, behavioral testing occurred after choline treatment had ceased, indicating that early postnatal choline supplementation leads to relatively long-lasting changes in behavior.
One important question is whether there are critical periods during which choline is most effective in mitigating ethanol’s adverse effects on behavioral development. Indeed, the effects of choline supplementation among rats not exposed to alcohol have been shown to depend on the timing of administration. Choline supplementation during gestation produces more robust long-lasting improvements in visuospatial memory compared to postnatal choline supplementation (from PD 1–24), although the combination of pre- and postnatal choline supplementation is more effective than either period alone (Meck et al., 1989). Meck et al (2008) further investigated critical periods of choline sensitivity among otherwise typically developing rats, administering choline for various periods throughout gestation and up to PD 75. They found that choline led to long-lasting improvements in spatial memory, as well as increased hippocampal dendritic spine densities, only when administered from GD 12–17 or PD 16–30. However, to date, no studies have examined the effects of varying developmental timing of choline administration among subjects exposed to alcohol during development.
In the present experiment, rat pups were exposed to ethanol during a period of brain development equivalent to the third trimester brain growth spurt. This period of brain development occurs postnatally in rats, from PD 4–9 (Dobbing and Sands, 1979). Subjects were subsequently treated with choline (choline chloride 100 mg/kg/day via s.c. injection) during one of two developmental phases following alcohol exposure: PD 11–20 (EtOH c/s) or PD 21–30 (EtOH s/c), or a combination of both periods (PD 11–30) (EtOH c/c). Ethanol-treated subjects that did not receive choline (EtOH s/s), sham intubated (SHAM s/s), and nonintubated controls (NC s/s) were included and injected with saline from PD 11–30. From PD 45–50, subjects were tested on a standard spatial learning version of the Morris maze, where the location of a hidden escape platform within a tank of opaque water remained constant throughout training for each subject and had to be found using extramaze spatial cues. Twenty-four hours after training, subjects were tested on a probe trial to measure spatial memory. Finally, performance on a visible platform was measured to determine if other variables (i.e. motivation, motor, sensory) influenced spatial learning performance.
2. Results
Body Growth
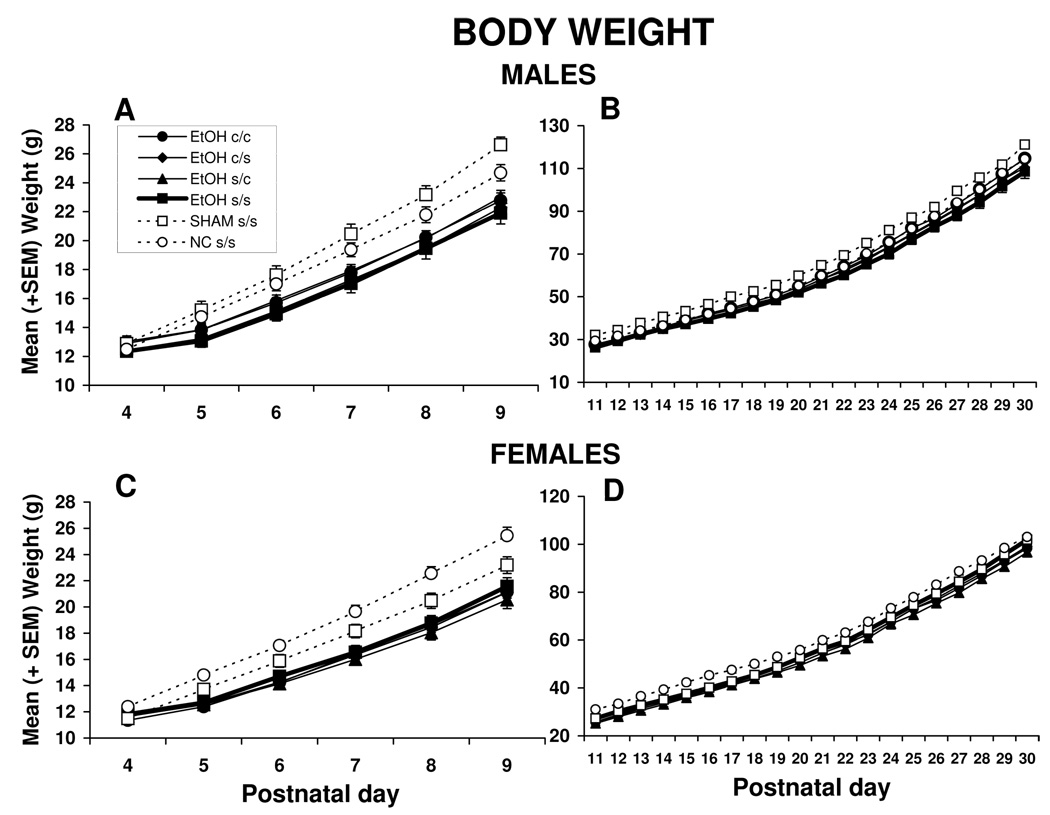
Body weight data are shown in Figure 1. Male and female data from the ethanol exposure period, PD 4–9, were analyzed separately (panels A and C). There were no significant differences in body weight among groups at PD 4. Beginning on PD 5, ethanol-exposed subjects lagged in growth, producing a significant main effect of treatment [F(5,105) = 11.5, p<0.001], as well as an interaction of day by treatment [F(25,525) = 15.4, p<0.001]. In addition, body weights of the male subjects increased in comparison to the females, resulting in a day by sex interaction [F(5,525) = 2.9, p<0.01] and a main effect of sex [F(1,105) = 18.7, p<0.001]. Subjects of both sexes gained weight over time, producing a main effect of day [F(5,525) = 4938, p<0.001]. There was also a significant day by sex by treatment interaction [F(25,525) = 1.9, p<0.01], due to faster growth among SHAM s/s controls in males and NC s/s controls in females.
Figure 1.
Body weights for males and females over treatment days. Panels A and C show growth during the ethanol treatment period, whereas Panels B and D show growth during the choline treatment period. Ethanol-exposed subjects lagged in growth compared to controls (NC s/s and SHAM s/s) beginning on PD 5, but caught up by PD 30. There were no significant effects of choline on body growth.
EtOH s/s = ethanol-exposed, saline treated; EtOH c/s = ethanol-exposed, choline from PD 11–20, saline from PD 21–30; EtOH s/c = ethanol-exposed, saline from PD 11–20, choline from PD 21–30; EtOH c/c = ethanol-exposed, choline from PD 11–30, SHAM s/s = sham control; NC s/s = nonintubated control.
During the choline treatment periods [(PD 11–20, 21–30 and 11–30)], ethanol-exposed subjects began to catch up in weight. As seen in Figure 1 B and D, all subjects gained weight each day [F(19,1995) = 12884, p<0.001]. Among males, the SHAM s/s subjects weighed significantly more than all other groups, and among females, the NC s/s controls weighed significantly more than all other groups. These differences contributed to an interaction of treatment by sex [F(5,105) = 3.0, p<0.05] and a main effect of treatment [F(5,105) = 5.3, p<0.001], with the control subjects weighing more than the ethanol-treated groups. Male subjects grew faster than females during this period of treatment, producing a main effect of sex [F(1,105) = 44.8, p<0.001] and a sex x day interaction [F(19,1995) = 90.6, p<0.001]. By PD 30, there were no longer treatment effects in body weight. Importantly, at no time were significant weight differences observed between the choline-treated and vehicle-treated ethanol subjects.
Blood Alcohol Levels
Mean ± SEM blood alcohol concentrations were 320 ± 5 mg/dl for ethanol-exposed subjects. It should be noted that eight blood samples were lost during a power outage and not used in the analysis.
Morris Water Maze
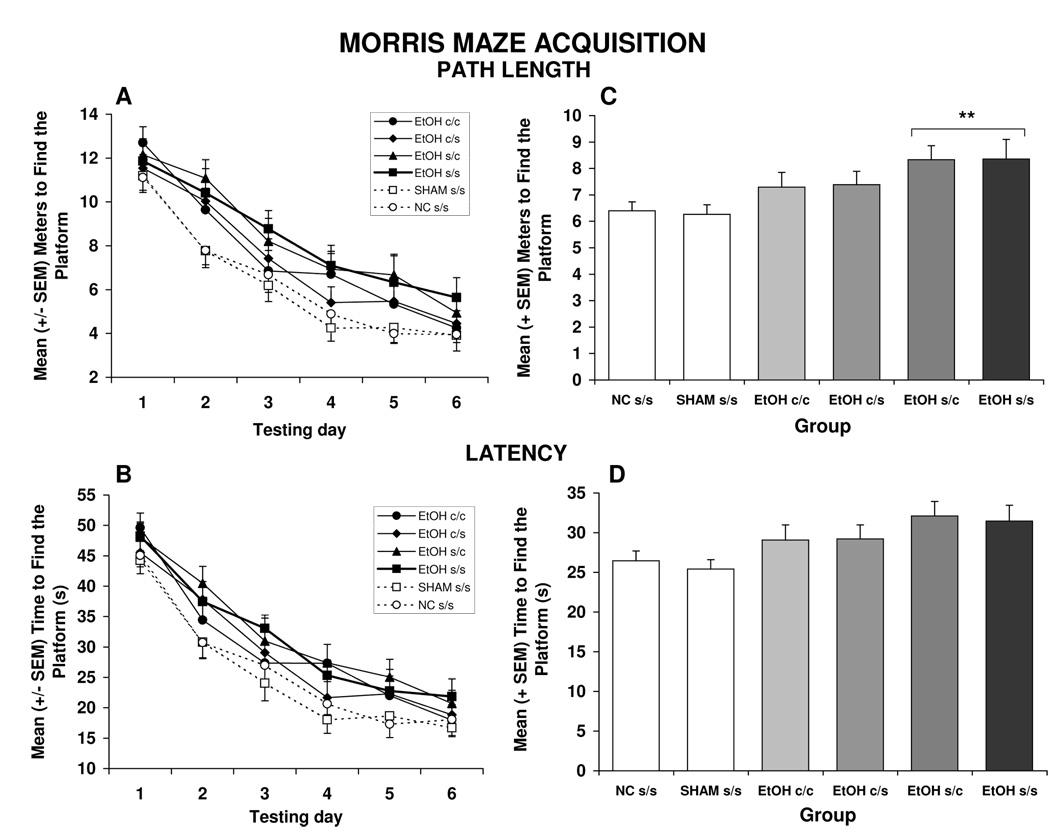
Acquisition of the Morris maze was impaired by ethanol exposure, an effect that was not significantly attenuated by choline treatment. Three subjects were not included in the analyses because of experimenter error (1 SHAM s/s, 1 EtOH c/c and 1 EtOH c/s). Ethanol-exposed subjects treated with vehicle (EtOH s/s) or with choline from PD 21–30 (EtOH s/c) were significantly impaired during the acquisition phase of the Morris water maze task. As shown in Figure 2A and 2C, EtOH s/s, and EtOH s/c subjects took significantly longer path lengths to find the escape platform compared to controls, producing a significant treatment effect [F(5,102) = 2.5, p<0.05]. In contrast, the performance of groups treated with choline from PD 11–20, the EtOH c/c and EtOH c/s groups, did not differ significantly from that of controls, but also did not differ significantly from the EtOH s/s and EtOH s/c groups either. There was also a main effect of sex [F(1,102) = 5.6, p<0.05], as males swam shorter routes to the escape platform compared to females, but there were no interactions of treatment by sex (data not shown). Finally, there was an interaction of day by trial [F(15,1530) = 1.8, p<0.05] and main effects of day [F(5,510) = 103.2, p<0.001] and trial [F(3,306) = 54.8, p< 0.001], as performance of subjects improved across days and trials. This improvement was greatest during the first days of training.
Figure 2.
Path length (A) and latency (B) to find the hidden platform during Morris maze acquisition. Performance of all groups improved over days. When collapsed across days (C), EtOH s/s and EtOH s/c subjects took significantly longer path lengths to find the platform compared to controls, whereas performance of EtOH c/s and EtOH c/c subjects was intermediate, not differing significantly from controls or other ethanol-exposed groups. Treatment effects were not significant on the latency to find the hidden platform (panel D shows group effects collapsed across days).
** = significantly different from SHAM s/s and NC s/s control groups
Similar to the path length, improvement in performance during acquisition was evident in the latency to find the platform, producing significant effects of day [F(5,510) = 122.5, p<0.001] and trial [F(3,306) = 64.5, p<0.01] (see Figure 2B and 2D). Although both control groups tended to have shorter latencies than the ethanol-exposed groups, treatment effects failed to reach significance [F(5,102) = 2.0, p<0.1]. Interestingly, when data from the EtOH s/s group and controls were analyzed without the choline groups, there was a significant ethanol effect [F(1,49) = 520.3, p<0.05], suggesting that there were mild ethanol-related deficits. Additionally, a trial by sex interaction [F(3,15) = 2.6, p<0.05] was evident as there were modest sex differences in the latency patterns from trial to trial.
Neither ethanol exposure nor choline treatment had significant effects on swimming speed [F(5,102) = 1.4, p<0.2] (data not shown). Female subjects swam faster than males [F(1,102) = 11.8, p<0.001]. Additionally, swim speed generally decreased over days [F(5,510) = 17.9, p<0.001], and trials [F(3,306) = 25.9, p<0.001]. As training progressed, the subjects’ speed on each of the four daily trials decreased, resulting in a day by trial interaction [F(15,1530) = 2.9, p<0.01].
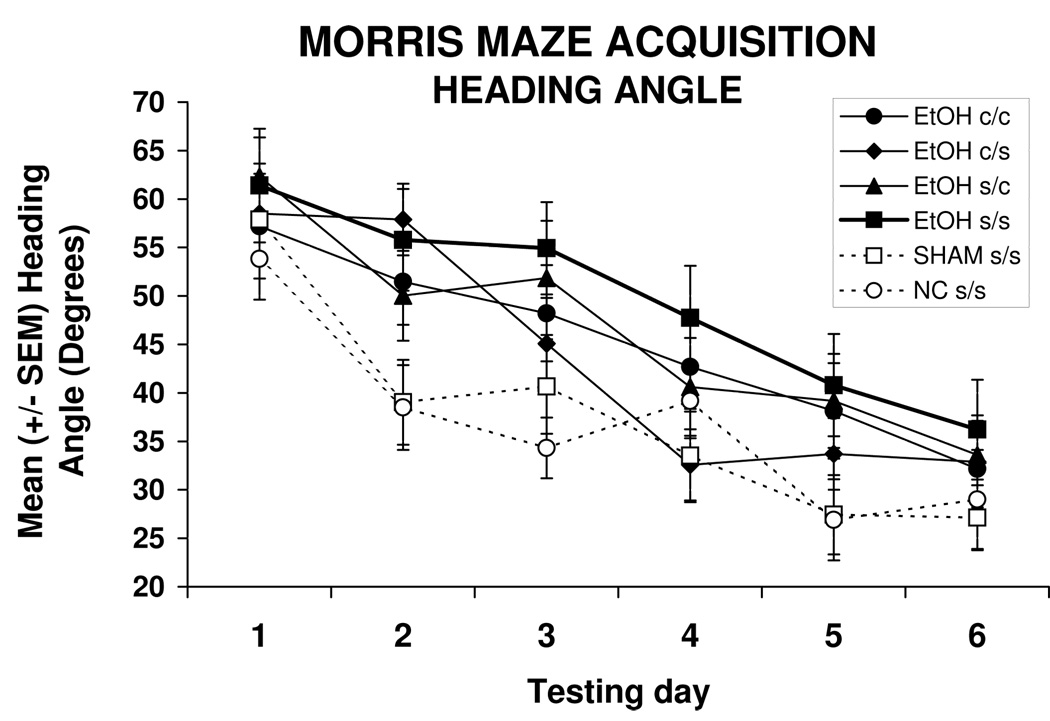
Heading angle represents the relationship of the subject’s initial chosen path compared to the path that would lead directly to the hidden target platform. The ethanol-exposed subjects treated with vehicle (EtOH s/s) or treated with choline from PD 21–30 (EtOH s/c) demonstrated significantly larger heading angles than controls, producing a main effect of treatment [F(5,102) = 2.5, p<0.05]. Similar to the path length data, follow-up analyses indicated that heading angles of EtOH c/c and EtOH c/s subjects were intermediate, not differing significantly from any other groups (see Figure 3). Heading angles decreased over days [F(5,510) = 36.6, p<0.001] and with each successive trial [F(3,306) = 10.9, p<0.001].
Figure 3.
The heading angles of the EtOH s/s and EtOH s/c groups were significantly greater than those of NC s/s and SHAM s/s controls, indicating that ethanol-exposed subjects that did not get choline during the early period (PD 11–20) were less accurate in their spatial navigation.
Probe Trial
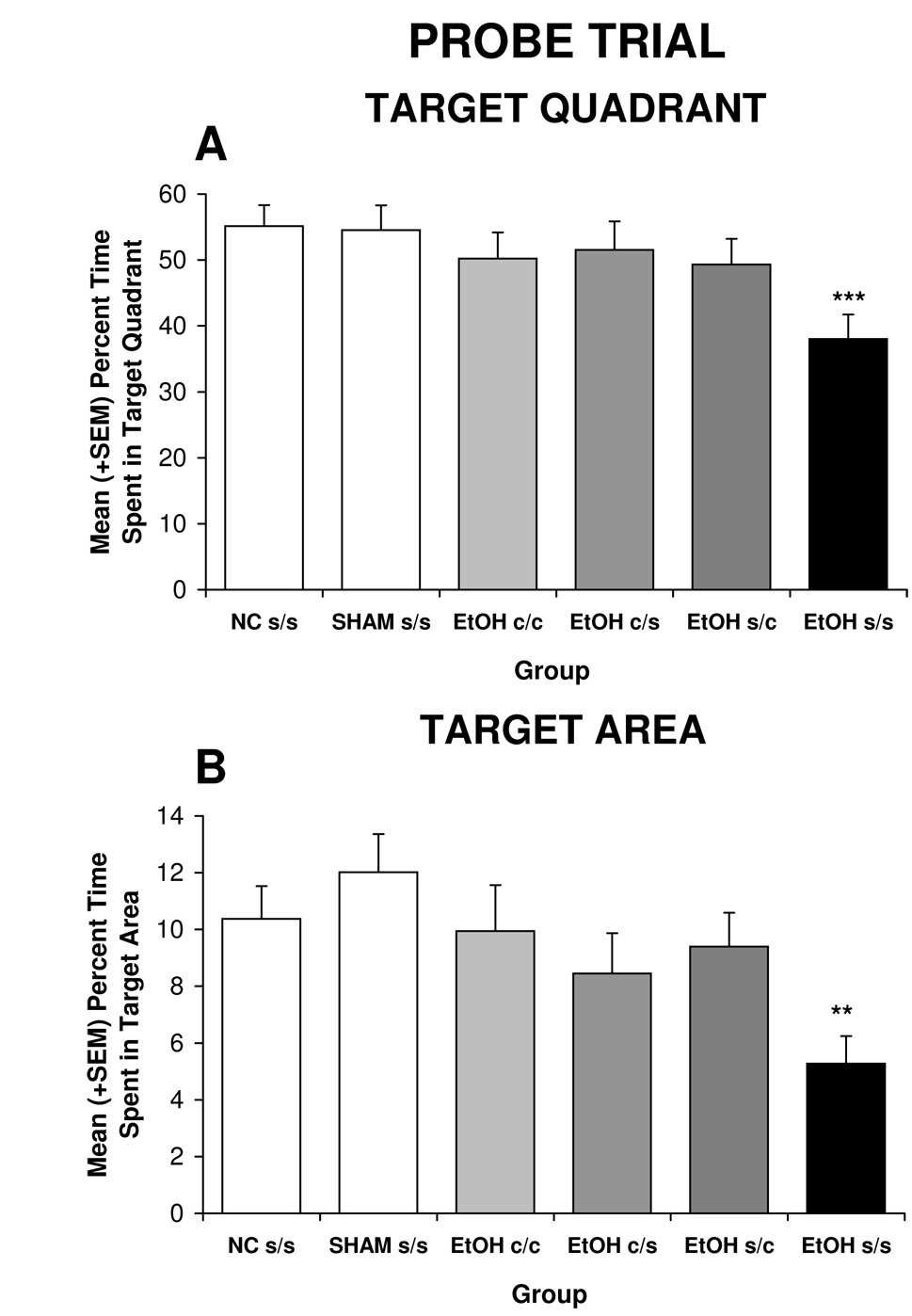
In contrast to acquisition, both early (PD 11–20) and later (PD 21–30) choline treatment significantly attenuated ethanol’s adverse effects on probe trial performance. Figure 4A shows the significant effect of treatment on mean time spent in the target quadrant [F(5,102) = 2.4, p<.05]. Post-hoc analyses illustrated that EtOH s/s subjects spent significantly less time in the target quadrant compared to all other groups. In fact, performance of ethanol-exposed subjects treated with choline during either developmental period was not significantly different from that of controls. There were no significant effects of treatment in time spent in any of the other quadrants (data not shown).
Figure 4.
Time spent in the target quadrant (A) and target area (B) during the probe trial. EtOH s/s subjects were significantly impaired in spatial memory compared to all other groups.
** = significantly different from all groups except EtOH c/s; *** = significantly different from all other groups
Similar effects were seen with time spent in the target area (three times the target diameter). There was a significant effect of treatment on the time spent close to the platform [F (5,102) = 2.6, p<0.05]. EtOH s/s subjects spent less time near the target compared to all groups except for the EtOH c/s group, which failed to differ significantly from any other group. In fact, the choline-treated ethanol groups spent almost twice as much time in the target area compared to the EtOH s/s group (see Figure 4B).
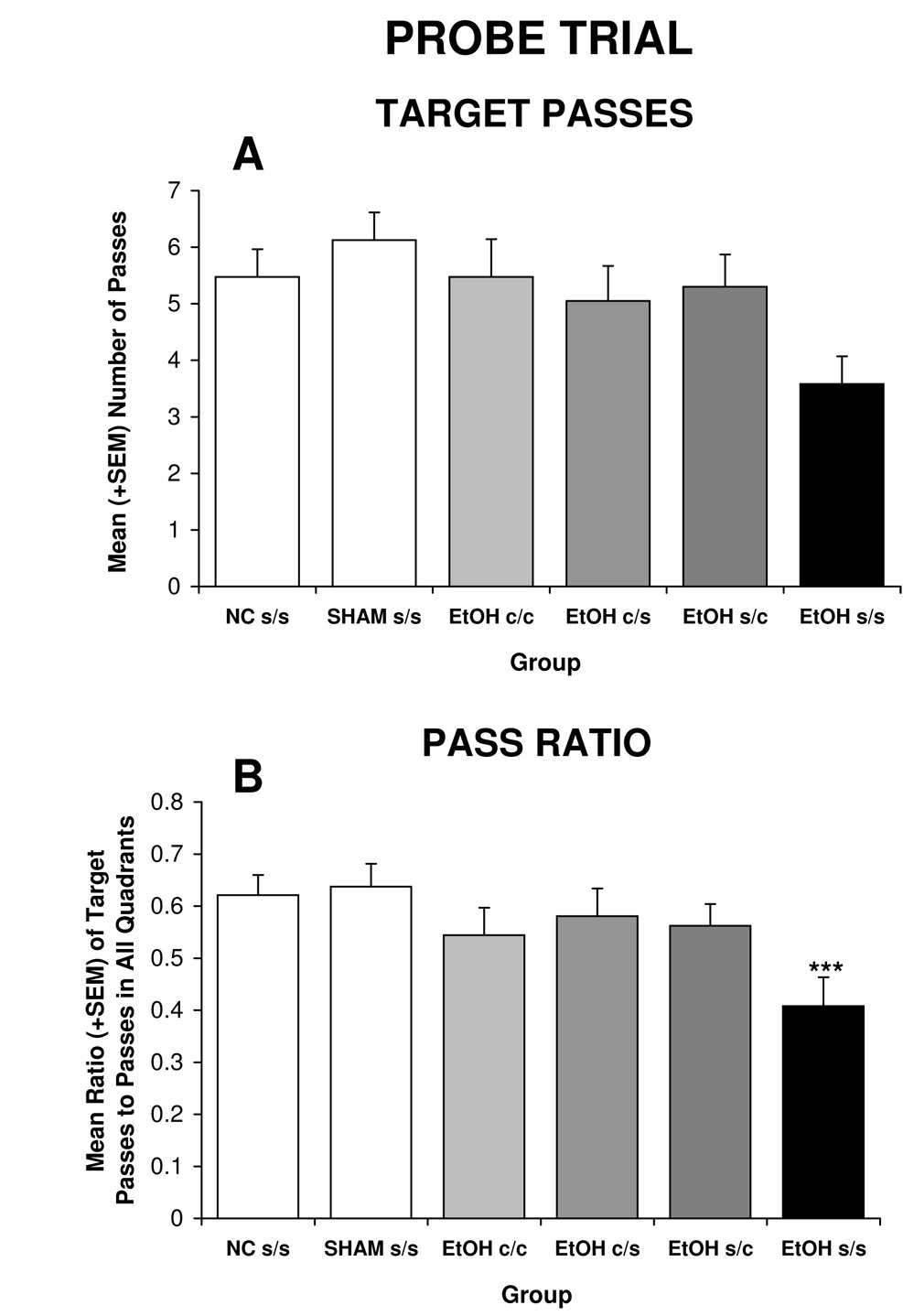
The pattern of passes through the target area closely resembles that of the other probe measures (see Figure 5A), although effects of treatment failed to reach statistical significance [F(5,102) = 2.1, p<0.08]. When examining the ratio of passes through the target area to passes through all four quadrant areas, the treatment effect was significant [F(5,102) = 2.6, p<0.05] (Figure 5B). Ethanol exposure significantly reduced the ratio of passes through the target area, an effect that was mitigated by all choline treatments.
Figure 5.
Ethanol exposure did not significantly influence the number of target passes (A), but did significantly reduce the ratio of passes through the target compared to other quadrant targets (B). Choline administration during any of the time periods significantly mitigated ethanol’s effects.
*** = significantly different from all other groups
Visible Platform
There were no main effects of treatment or sex on any variables during the visible platform task (data not shown). Thus, the ethanol-related deficits observed during the spatial learning component are unlikely to be due to sensory, motivational, or motor differences.
3. Discussion
This study is the first demonstration that as few as 10 days of postnatal choline supplementation can reduce the severity of developmental alcohol effects. Alcohol exposure during the third trimester equivalent produced significant deficits in both the acquisition and probe phases of the Morris water maze spatial learning task, consistent with previous reports (Berman and Hannigan, 2000; Goodlett and Johnson, 1997; Kelly et al., 1988). Although choline did not significantly reduce ethanol-related deficits during acquisition, choline supplementation during either the early (PD 11–20) or late (PD 21–30) phase significantly mitigated ethanol-induced deficits during the probe trial. These data illustrate that choline can effectively reduce some of the consequences of a developmental alcohol insult, even when administration is initiated 10 days after ethanol exposure.
Because developmental timing of administration did not influence choline’s ability to reduce alcohol-related deficits on the probe trial, the developmental window for choline to be effective is either quite large, or spans between the PD 11–20 and PD 21–30 time periods. It is interesting, and somewhat surprising, to note that there were not additive effects when choline supplementation occurred from PD 11–30. This may be due to a ceiling effect, but more likely suggests that there is not a strict temporal window for choline to be effective, at least on some behavioral measures. That being said, the findings during Morris maze acquisition are less clear. During acquisition, EtOH s/c subjects were significantly impaired, with performance levels similar to EtOH s/s subjects. In contrast, acquisition of EtOH c/s subjects was intermediate, not differing significantly from that of controls, but also not differing significantly from that of EtOH s/s subjects. Although not statistically significant, these data suggest that choline supplementation during the earlier period (PD 11–20) may have additional beneficial effects. Testing on a wider range of behavioral domains will further elucidate whether the effects of choline depend on critical developmental periods and whether these vary, dependent on behavioral task.
Although we have yet to elucidate the neural targets of choline supplementation in ethanol-exposed subjects, given the role of the hippocampus in spatial learning and the known vulnerability of the developing hippocampus to alcohol-induced damage (Berman and Hannigan, 2000), it is likely that choline is enhancing hippocampal function. Indeed, choline supplementation from PD 16–30 has been shown to increase spine densities in both CA1 pyramidal and dentate gyrus cells in otherwise typically developing rats (Meck et al., 2008). Prenatal choline supplementation also leads to enhanced cholinergic efficiency (Cermak et al., 1999; Cermak et al., 1998), likely due to early metabolic imprinting (Meck and Williams, 2003). Postnatal days 11–20 comprise a stage of developmental immaturity in the hippocampal cholinergic system, characterized by rapid axon growth (Aznavour et al., 2005), synaptogenesis, and massive increases in AChE and ChAT activity (Matthews et al., 1974; Nadler et al., 1974). However, during this phase, cholinergic development remains incomplete, and enzyme levels are considerably lower than the levels found in adult rats. It is not until the second stage, from PD 21–30, that the cholinergic systems reach maturity. At this point, ChAT activity is mature and the majority of axon growth and synaptogenesis is complete (Nadler et al., 1974). Given the present results, it is likely that choline may influence cholinergic development throughout this early postnatal period; it would be of further interest to determine if choline is still effective even after PD 30.
Critical periods for the beneficial effects of choline supplementation among control subjects have been reported, with GD 12–17 and PD 16–30 being periods when choline leads to long-lasting enhancements in spatial memory as well as morphological changes in the hippocampus (Meck et al., 2008). GD 12–17 is a period of rapid neuronal and glial proliferation that includes neurogenesis of cholinergic basal forebrain cells, whereas PD 16–30 corresponds to a period marked by differentiation, synaptogenesis and myelination, that includes the maturation of cholinergic functioning in the hippocampus, as discussed above. Benefits of choline were not evident when administered during PD 1–15 or after PD 30 (Meck et al., 2008). However, it is likely that the critical windows are different for the compromised brain. Indeed, choline supplementation improves behavioral outcome in rats exposed to alcohol during development, even without significantly affecting performance in controls (Thomas et al., 2004; Tran and Thomas, 2007), suggesting that the damaged or dysfunctional CNS may respond differently to choline supplementation compared to a typically developing CNS. In fact, Holmes et al. (Holmes et al., 2002) reported that choline supplementation reduces the severity of hippocampal damage induced by seizures, even when administered after PD 35. Acetylcholine can enhance plasticity in the hippocampus and cortex, and this may contribute to protection against insults as well as recovery from damage (Conner et al., 2005).
Choline may also work through additional mechanisms. Choline is a precursor to major constituents of cell membranes, phosphatidylcholine and sphingomyelin, and can therefore influence the structural integrity and signaling functions of cell membranes (Zeisel, 2006b; Zeisel and Blusztajn, 1994). Indeed, developmental alcohol exposure can alter the phospholipid profile in the hippocampus (Wen and Kim, 2004) and lead to alterations in white matter in various CNS regions (Sowell et al., 2008a). Choline also serves as a methyl donor, influencing the methionine-homocysteine cycle (Zeisel and Niculescu, 2006) and influencing DNA methylation and subsequent gene expression (Kovacheva et al., 2007; Niculescu et al., 2006). Thus, choline may be acting via sites outside of its role as a precursor to acetylcholine, although the mechanisms likely depend on the timing of administration.
The possibility that choline supplementation enhances cognitive abilities even when administered during later stages of development and long after ethanol exposure is complete has important implications when considering the viability of choline as an effective treatment agent in children with FASD. Diagnoses of FASD are not often made directly after birth, but only become evident over time. The present data suggest that choline may be effective even when administered in later childhood while the CNS is still developing, and that behavioral benefits are evident even after choline treatment has ceased. These findings suggest that dietary choline supplementation shows promise as a potential treatment for FASD.
4. Experimental Procedures
Subjects
Subjects were 114 Sprague-Dawley rats (7–11 subjects/ treatment /sex), offspring of animals bred at the San Diego State University Animal Care Facility. Multiparous dams were housed with males overnight, and the presence of a seminal plug in the morning marked gestational day (GD) 0. Pregnant females were then singly housed with food and water ad libitum on a 12:12-hr light/dark schedule with lights on at 0600. On postnatal day (PD) 1 (GD 23), litters were pseudorandomly culled to 10 pups (5 males and 5 females when possible). All procedures included in this study were approved by the SDSU IACUC and are in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Treatment Design
On PD 4, pups were randomly assigned to one of six treatment groups: four ethanol-treated and two control groups. The four ethanol-treated groups (EtOH) received ethanol (5.25 g/kg/day) via oral intubation (11.9% v/v, twice a day, 2 hours apart) from PD 4–9 (a period of rapid brain development that occurs during the third trimester in humans), followed by two additional oral intubations of milk formula 2 hours apart each day (see Goodlett and Johnson, 1997 for details). A sham intubated (SHAM s/s) and non-intubated control (NC s/s) group were also included. Following ethanol treatment, subjects received s.c. injections of choline chloride dissolved in saline (100 mg/kg/day) on PD 11–20 (EtOH c/s), 21–30 (EtOH s/c) or 11–30 (EtOH c/c). One ethanol-treated group (EtOH s/s) and the controls were injected with saline from PD 11–30. When not receiving choline, subjects were injected with saline vehicle, so all subjects received s.c. injections from PD 11–30. To control for litter effects, no more than one subject per sex within the litter was assigned to each treatment. In addition, during treatments, all groups were separated from the dam for equivalent periods of time.
All subjects were weighed daily during treatment and periodically thereafter to monitor body growth. On PD 10, the day after the final intubations, all subjects were assigned a numerical paw code through subcutaneous injections of India ink that would allow experimenters to be blind to treatment condition during behavioral testing. All subjects were housed in standard cages with their dam until weaning on PD 21.
Blood Alcohol Concentration
On PD 6, 1.5 hours following the second ethanol administration, 20 microliters of blood were collected from the subjects via tail clippings to determine peak blood alcohol concentrations. Blood samples were analyzed with the Analox Alcohol Analyzer (Model AMI; Analox Instruments; Lunenburg, MA).
Morris Water Maze
Beginning on PD 45, subjects were tested on a standard spatial learning version of the Morris maze. The testing apparatus consisted of a circular white tank (174 cm diameter) filled with 26°C water. A clear Plexiglas escape platform (10 cm diameter) was submerged 1.25 inches below the surface and powdered milk was added to the water so that the platform was not visible. The room was replete with visuospatial cues (e.g. posters, sink, shelves, video monitors, a stepladder, hoses), which remained static throughout the testing trials. The escape platform was located in the center of one of the four quadrants; the position of the platform was counterbalanced across treatment groups and remained constant each day for each subject.
Subjects were tested for four trials each day for six consecutive days. During each trial, subjects were placed in the tank and allowed to find the hidden platform. To prevent the use of motor strategies, starting location was varied from trial to trial. When the subject reached the escape platform, they remained on the platform for 10 sec. If the subject was unable to locate the platform within 60 s, the experimenter prodded the subject to the platform where they remained for 10 s. Path length and latency to find the platform, as well as heading angle (the angle between the initial swimming direction and location of the platform), served as performance measures. Twenty-four hours after the last day of training, subjects were tested on a probe trial, during which the escape platform was removed and the time spent in the target quadrant, target disc area (3 times the diameter of the platform), as well as passes through the target area, were measured for a 60s trial.
On PD 52 and 53, the subjects’ ability to swim to a visible platform was determined. During the visible platform test, a darkly colored platform rose 1.5 inches above the water. All extra-maze cues were obscured by plain, white curtains. This phase helped to determine whether any other performance measures, such as sensory, motor or motivational factors might contribute to possible group differences.
Data Analyses
Data were analyzed by ANOVAS (p’s<0.05) using SPSS software. Data were analyzed with treatment and sex as between-subject factors. Day served as a within-subject repeated measure for body weight, whereas both day and trial served as within-subject variables for all of the Morris maze acquisition measures. Post hoc comparisons were obtained with Neuman-Keuls analyses (p<0.05).
Abbreviations
- FASD
fetal alcohol spectrum disorders
- PD
postnatal day
- GD
gestational day
- EtOH s/s
ethanol-exposed subjects treated with saline from PD 11–30
- EtOH c/s
ethanol-exposed subjects treated with choline from PD 11–20 and saline from PD 21–30
- EtOH s/c
ethanol-exposed subjects treated with saline from PD 11–20 and choline from PD 21–30
- SHAM s/s
sham intubation control group treated with saline from PD 11–30
- NC s/s
nonintubated control group treated with saline from PD 11–30
- CNS
central nervous system
- AchE
acetylcholinesterase
- ChAT
choline acetyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Watkins KC, Descarries L. Postnatal development of the cholinergic innervation in the dorsal hippocampus of rat: Quantitative light and electron microscopic immunocytochemical study. J Comp Neurol. 2005;486:61–75. doi: 10.1002/cne.20501. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: implications for cholinergic neurotransmission. J Physiol Paris. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Pantazis NJ. FGF-2, NGF and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain Res Dev Brain Res. 2003;140:15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Connor PD, Huggins JE, Barr HM, Pimentel KD, Streissguth AP. Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res. 2007;31:868–879. doi: 10.1111/j.1530-0277.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Blusztajn JK, Meck WH, Williams CL, Fitzgerald CM, Rosene DL, Loy R. Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. Dev Neurosci. 1999;21:94–104. doi: 10.1159/000017371. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. Faseb J. 1998;12:349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Chen SY, Charness ME, Wilkemeyer MF, Sulik KK. Peptide-mediated protection from ethanol-induced neural tube defects. Dev Neurosci. 2005;27:13–19. doi: 10.1159/000084528. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Coutcher JB, Cawley G, Wecker L. Dietary choline supplementation increases the density of nicotine binding sites in rat brain. J Pharmacol Exp Ther. 1992;262:1128–1132. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Endres M, Toso L, Roberson R, Park J, Abebe D, Poggi S, Spong CY. Prevention of alcohol-induced developmental delays and learning abnormalities in a model of fetal alcohol syndrome. Am J Obstet Gynecol. 2005;193:1028–1034. doi: 10.1016/j.ajog.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Berman RF. Amelioration of fetal alcohol-related neurodevelopmental disorders in rats: exploring pharmacological and environmental treatments. Neurotoxicol Teratol. 2000;22:103–111. doi: 10.1016/s0892-0362(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Berman RF, Zajac CS. Environmental enrichment and the behavioral effects of prenatal exposure to alcohol in rats. Neurotoxicol Teratol. 1993;15:261–266. doi: 10.1016/0892-0362(93)90007-b. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48:3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Med. 2006;3:e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Taddeo E. Socio-cognitive habilitation using the math interactive learning experience program for alcohol-affected children. Alcoholism: Clinical and Experimental Research. 2007;31:1425–2434. doi: 10.1111/j.1530-0277.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res. 1988;27:247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Goodlett CR, Greenough WT. Therapeutic motor training ameliorates cerebellar effects of postnatal binge alcohol. Neurotoxicol Teratol. 2000;22:125–132. doi: 10.1016/s0892-0362(99)00052-5. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Scamra C, Hoffman M, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002;937:83–93. doi: 10.1016/s0006-8993(02)02492-7. [DOI] [PubMed] [Google Scholar]
- Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem. 2007;282:31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, Swartzwelder HS. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. J Neurophysiol. 2004;91:1545–1555. doi: 10.1152/jn.00785.2003. [DOI] [PubMed] [Google Scholar]
- Loy R, Heyer D, Williams CL, Meck WH. Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Adv Exp Med Biol. 1991;295:373–382. doi: 10.1007/978-1-4757-0145-6_21. [DOI] [PubMed] [Google Scholar]
- Matthews DA, Nadler JV, Lynch GS, Cotman CW. Development of cholinergic innervation in the hippocampal formation of the rat. I. Histochemical demonstration of acetylcholinesterase activity. Dev Biol. 1974;36:130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Frontiers in Integrative Neuroscience. 2008;1:1–11. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res. 2000;123:25–32. doi: 10.1016/s0165-3806(00)00075-4. [DOI] [PubMed] [Google Scholar]
- NIAAA. 10th Special Report to US Congress on Alcohol and Health. Washington, DC: National Institute of Health; 2000. [Google Scholar]
- Nadler JV, Matthews DA, Cotman CW, Lynch GS. Development of cholinergic innervation in the hippocampal formation of the rat. II. Quantitative changes in choline acetyltransferase and acetylcholinesterase activities. Dev Biol. 1974;36:142–154. doi: 10.1016/0012-1606(74)90197-3. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ, Frankel F, Paley B, Schonfeld AM, Carpenter E, Laugeson EA, Marquardt R. A controlled social skills training for children with fetal alcohol spectrum disorders. J Consult Clin Psychol. 2006;74:639–648. doi: 10.1037/0022-006X.74.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev Med Child Neurol. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 2007;31:239–245. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Fleming Sl, Riley EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res. 2001;25:764–773. [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Miura Sather T, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. doi: 10.1037/a0013271. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T, Thomas JD. Perinatal choline supplementation mitigates trace eyeblink conditioning deficits associated with 3rd trimester alcohol exposure in rats. San Diego, CA: Society for Neuroscience; 2007. [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Warren KR, Calhoun FJ, May PA, Viljoen DL, Li TK, Tanaka H, Marinicheva GS, Robinson LK, Mundle G. Fetal alcohol syndrome: an international perspective. Alcohol Clin Exp Res. 2001;25:202S–206S. doi: 10.1097/00000374-200105051-00033. [DOI] [PubMed] [Google Scholar]
- Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer MF, Chen SY, Menkari CE, Brenneman DE, Sulik KK, Charness ME. Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci U S A. 2003;100:8543–8548. doi: 10.1073/pnas.1331636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkemeyer MF, Menkari CE, Spong CY, Charness ME. Peptide antagonists of ethanol inhibition of l1-mediated cell-cell adhesion. J Pharm Exp Ther. 2002;303:110–116. doi: 10.1124/jpet.102.036277. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. J Pediatr. 2006;149:S131–S136. doi: 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]