Abstract
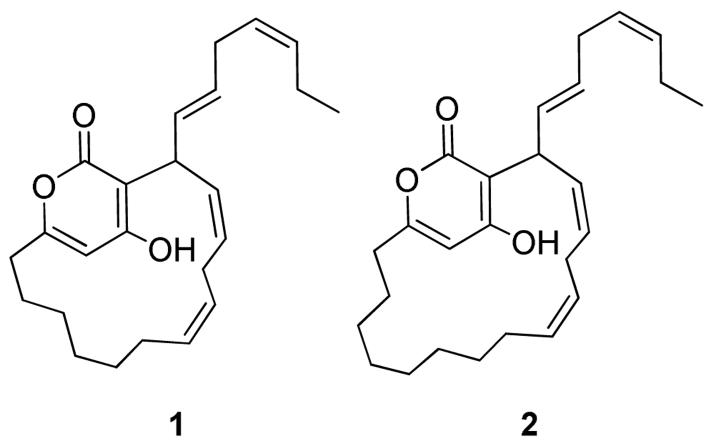
Two novel α-pyrone macrolides, neurymenolides A (1) and B (2), were isolated from the Fijian red alga Neurymenia fraxinifolia and characterized using a combination of NMR and mass spectral analyses. These molecules represent only the second example of α-pyrone macrolides, with 1 existing as interchanging atropisomers due to restricted rotation about the α-pyrone ring system. Neurymenolide A (1) displayed moderately potent activities against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF).
α-Pyrone-containing natural products have been found in bacteria, fungi, plants, and animals, and exhibit a wide range of biological activities, such as antimicrobial, antineoplastic, and anti-HIV effects.1 Despite the abundance of α-pyrone analogs in nature, only one species, the red alga Phacelocarpus labillardieri, has previously been reported to produce macrocyclic α-pyrones.2,3 Herein we report the discovery of two novel macrocyclic α-pyrones, neurymenolides A (1) and B (2), the first report of natural products from the red alga Neurymenia fraxinifolia (Figure 1).
Figure 1.
Novel natural products from Neurymenia fraxinifolia, neurymenolides A-B (1-2) and natural product (3) from Phacelocarpus labillardieri.3
Extracts of N. fraxinifolia collected from Taveuni, Fiji were first separated by reversed-phase column chromatography guided by growth-inhibitory effects against methicillin-resistant Staphylococcus aureus (MRSA). Following reversed-phase, normal-phase, and chiral high performance liquid chromatography (HPLC), 1 and 2 were isolated.4
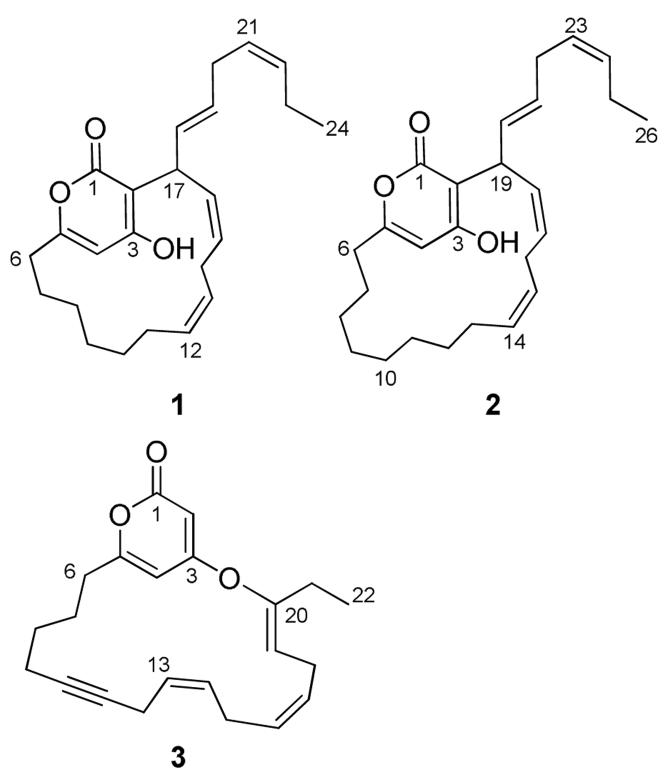
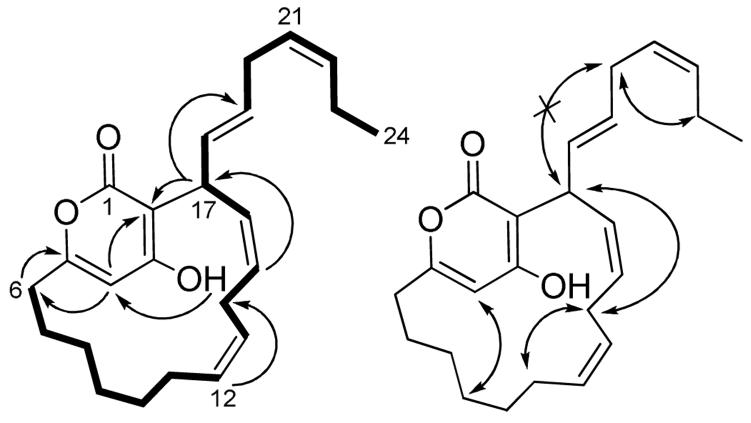
Neurymenolide A (1) displayed an [M+H]+ m/z of 369.2428 by HRESIMS, suggesting a molecular formula of C24H33O3. Analysis of 1H, 13C, DEPT NMR, and IR spectra indicated six carbon-carbon double bonds, one carbonyl, and based upon the index of hydrogen deficiency, two rings (Table 1). Three quaternary carbons displayed 13C NMR chemical shifts at 164.7-165.1 ppm, supporting the presence of one ester group (IR 1680 cm-1) and two aromatic carbinol carbons, accounting for all three oxygen atoms. These data and UV spectrophotometric properties of 1 (λmax = 295 nm) were consistent with the literature on hydroxyl-substituted α-pyrones.3,5 HMBC correlations from the aromatic hydroxyl proton (δ 6.48) to C-3 (δ 164.7) and C-4 (δ 101.4), as well as correlations from H-4 (δ 5.81) to C-2 (δ 103.9), C-6 (δ 33.5), and C-1 (δ 165.1) and/or C-5 (δ 165.1), confirmed the hydroxy-substituted α-pyrone ring (Figure 2; Supporting Information). Additional 2D NMR spectral data led to the identification of a macrocyclic ring connected via the pyrone system by C-5-C-6 and C-2-C-17 bonds, with H-17 (δ 4.55) coupled to C-2 and H-6 coupled to C-5 in the HMBC spectrum (Figure 2). Double bonds within the macrocycle were assigned at Δ12,13 (δ 131.0, 126.6) and Δ15,16 (δ 135.2, 127.0) based upon COSY correlations between olefinic protons and adjacent methylenes (Figure 2). Finally, the unsaturated 7-carbon aliphatic chain at C-17 was established through COSY and HMBC correlations, terminating with Me-24 (δ 14.2).
Table 1.
13C and 1H NMR spectral data for neurymenolides A-B (1-2) (500 MHz; in CDCl3).
| 1 |
2 |
|||
|---|---|---|---|---|
| no. | δ 13C | δ 1H(JH,H) | δ 13C | δ 1H(JH,H) |
| 1 | 165.1 | - | 165.0 | - |
| 2 | 103.9 | - | 103.8 | - |
| 3 | 164.7 | - | 164.3 | - |
| 4 | 101.4 | 5.81s | 101.2 | 5.77s |
| 5 | 165.1 | - | 165.0 | - |
| 6 | 33.5 | 2.30ddd (14, 11, 3.3), 2.61ddd (14, 10,3.1) | 33.3 | 2.26ddd (15, 11, 3.2), 2.63ddd (15, 113.4) |
| 7 | 25.6 | 1.57m, 1.86m | 24.6 | 1.49m, 1.82m |
| 8 | 26.9 | 1.24m, 1.34m | 25.2 | 1.15m, 1.22m |
| 9 | 27.7 | 1.18m, 1.35m | 27.1 | 1.08m, 1.25m |
| 10 | 27.1 | 1.07m, 1.15m | 29.7 | 1.24m, 1.30m |
| 11 | 26.6 | 1.76m, 1.83m | 28.7 | 1.18m, 1.23m |
| 12 | 131.0 | 5.23m | 26.7 | 1.20m, 1.27m |
| 13 | 126.6 | 5.21m | 27.6 | 1.88m, 2.01m |
| 14 | 27.0 | 2.52br d (17), 2.85m | 131.3 | 5.34m |
| 15 | 135.2 | 5.65m | 126.4 | 5.32m |
| 16 | 127.0 | 5.64m | 26.8 | 2.63m, 2.94m |
| 17 | 36.5 | 4.55br s | 134.9 | 5.60m |
| 18 | 129.4 | 5.61m | 127.0 | 5.67m |
| 19 | 129.9 | 5.61m | 36.2 | 4.61br s |
| 20 | 30.0 | 2.77m | 129.9 | 5.62m |
| 21 | 125.9 | 5.30dt(11, 7.2) | 130.4 | 5.62m |
| 22 | 132.9 | 5.42dt(11, 7.2) | 35.3 | 2.71m |
| 23 | 20.5 | 2.00p (15, 7.4) | 126.8 | 5.33m |
| 24 | 14.2 | 0.93t (7.4) | 133.5 | 5.43m |
| 25 | - | - | 25.5 | 1.99m |
| 26 | - | - | 13.8 | 0.94t (7.5) |
| OH | - | 6.48br s | - | 6.40br s |
Figure 2.
Key COSY (bold) and HMBC (arrow) correlations established the macrocyclic α-pyrone system of neurymenolide A (1). NOE correlations (double-headed arrows) established the stereochemistry of the double bonds in 1.
Due to substantial chemical shift overlap in the olefinic region of the 1H NMR spectrum of 1, E/Z stereochemistry could not be assigned using J couplings. Instead, ROESY NMR spectral data for well resolved allylic protons were used (Figure 2). NOEs were observed between H-11b (δ 1.83) and both H-14s (δ 2.52, 2.85), suggesting a Z configuration at Δ12,13. Similarly, correlations between both H-14s and H-17 implied a Z configuration at Δ15,16. No NOEs were observed between H-17 and H2-20 (δ 2.77), suggesting an E configuration at Δ18,19. Finally, NOEs between H2-20 and H2-23 (δ 2.00) were evident, supporting a Z configuration at Δ21,22. We were unable to assign the stereochemistry of chiral C-17; thus at present, the absolute stereochemistry of 1 is unknown.
High-resolution mass spectral data indicated that neurymenolide B (2) possessed two additional methylene units relative to 1, displaying an [M+H]+ m/z 397.2765, consistent with a molecular formula of C26H37O3. Comparison of 1H and 13C NMR spectral data of 2 with that of 1 suggested that the α-pyrone and linear aliphatic systems were identical. The two extra methylenes were assigned in the macrocyclic ring, based on a combination of COSY and HMBC correlations (Supporting Information). Equivalent NOEs were observed for 1 and 2, suggesting that the E/Z stereochemistry is identical for both natural products of N. fraxinifolia.
HPLC purification of 1 using several different stationary phases consistently resulted in two peaks, each of which split again into the same two peaks when reanalyzed using the same HPLC column. NMR spectral and optical rotation data were identical for material collected from each of these peaks, which led to the hypothesis that 1 consists of quickly interchanging rotational isomers (atropisomers). Synthetic reports of related compounds suggest planar chirality due to the restricted rotation about the α-pyrone ring.6 Because 1 contains a stereogenic carbon (C-17), these two atropisomers would be expected to behave as diastereomers. To further explore this hypothesis, 1 was acetylated to yield 3-O-acetylneurymenolide A, separated by HPLC, and then analyzed by 1H NMR spectroscopy. The added bulk of the acyl group slowed the rotation about the α-pyrone enough to yield two distinguishable sets of 1H chemical shifts and two HPLC peaks that equilibrated over the course of several hours, rather than the minute time-scale conversion observed for atropisomers of 1 (Supporting Information). Rotational isomerization was not observed with 2, which is reasonable given the larger macrocyclic ring size of 2 relative to 1.
The two unique macrolides described in this study each represent new carbon skeletons and only the second example of macrocyclic α-pyrones occurring in nature. Macrocyclic pyrones isolated from Phacelocarpus labillardieri (e.g., 3; Figure 1) are connected through an ether bond at C-3, unlike the neurymenolides. However, all of these macrocyclic pyrones would be expected to share a common biogenesis. Given the similar chemistry of Neurymenia fraxinifolia and P. labillardieri, it is interesting to note that the two red algal species are only distantly related, sharing the class Florideophyceae. This is the first report of chemistry from the red alga N. fraxinifolia, although its family, Rhodomelaceae, is well studied.7
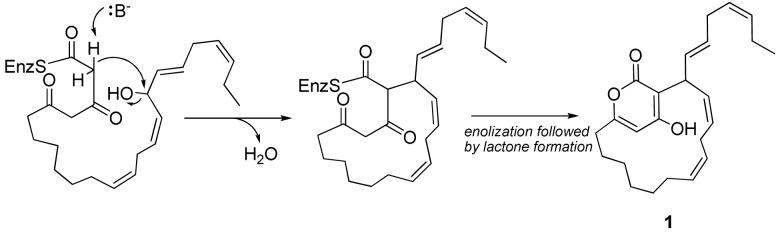
The biosynthesis of the neurymenolides is expected to occur by successive condensations of malonate, beginning at the aliphatic chain terminus and ending at the α-pyrone carbonyl. These natural products may be formed by a mixed polyketide/fatty acid biosynthetic pathway8, with previous such examples reported involving both prokaryotes and eukaryotes.9,10 One could envision the neurymenolides arising from diketide extention of eicosapentaenoic acid or docosahexaenoic acid, with macrocyclization promoted by an enolate attack with loss of water (Scheme 1); however, it is unclear whether the macrocyclization or the α-pyrone formation occurs first. The E configuration at Δ18,19 could potentially occur through a free radical process common to eicosanoids.8
Scheme 1.
Proposed biosynthesis of neurymenolide A (1) from a putative polyketide-extended eicosapentaenoic acid-derived precursor.
Neurymenolide A (1) exhibited moderately potent activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) (IC50 of 2.1 μM and 4.5 μM, respectively, Table 2). Moderate in vitro cytotoxicity against DU4475 breast tumor cells was also observed for 1 (IC50 of 3.9 μM), as well as moderate to weak activity against 11 other tumor cell lines (IC50 values ranging from 5.4 to 28 μM). Neurymenolide B (2) was slightly less active against MRSA and considerably less active in all other pharmacological assays (Table 2), suggesting that the size or conformational restriction of the macrocyclic ring is important for biological activity. 3-O-acetylneurymenolide A (mixed atropisomers) was also less active against MRSA (IC50 of 11 μM), signifying the importance of the aromatic hydroxyl group for antibacterial activity.
Table 2.
Pharmacological activities of 1-2.
| antibacterial IC50 (μM) |
anticancer IC50 (μM) |
||||||
|---|---|---|---|---|---|---|---|
| cmpd. | MRSA | VREF | tuberculosis | meana | DU4475b | antimalarial IC50 (μM) | antifungal IC50 (μM)c |
| 1 | 2.1 | 4.5 | >100 | 8.8 | 3.9 | 68 | >600 |
| 2 | 7.8 | 31 | >100 | 39 | 19 | >100 | >600 |
Mean of 12 cell lines (see Supporting Information for details)
breast tumor cell line
Using amphotericin B-resistant Candida albicans
The current study describes structurally novel macrocyclic α-pyrones, establishing the first report of chemistry from the red alga Neurymenia fraxinifolia. Determining 1 to be present as interchanging atropisomers presented an exciting chemical challenge, and the putative polyketide/fatty acid biogenesis of the neurymenolides represents another example of the diversity of the acetogenic pathway. Neurymenolide A (1) shows promise as a novel template for the design and development of new antibiotics.
Supplementary Material
Acknowledgment
This research was supported by the U.S. National Institutes of Health's International Cooperative Biodiversity Groups program (grant U01 TW007401-O1). The authors thank the Roko Tui Cakaudrove and the people of Lavena Village for their hospitality and for permission to collect in the reefs of Taveuni, as well as the government of Fiji for permission to perform research in their territorial waters and for permission to export samples.
Footnotes
Supporting Information Available: Additional acknowledgments, experimental details, 2D NMR (COSY, HMBC, ROESY), 1H and 13C NMR spectra for 1-2, and 1H NMR spectral data for 3-O-acetylneurymenolide A are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.McGlacken GP, Fairlamb IJS. Nat. Prod. Rep. 2005;22:369–385. doi: 10.1039/b416651p. [DOI] [PubMed] [Google Scholar]
- 2.Kazlauskas R, Murphy PT, Wells RJ, Blackman AJ. Aust. J. Chem. 1982;35:113–120. [Google Scholar]
- 3.Shin J, Paul VJ, Fenical W. Tetrahedron Lett. 1986;27:5189–5192. [Google Scholar]
- 4.Neurymenolide A (1): clear oil (2.9 mg, 0.098% plant dry mass); [α]24D -150 (c 0.02 g/100 mL, MeOH); UV (ACN) λmax 295 nm (log ε = 3.39); IR (NaCl) νmax 3350 (br), 3010, 2860, 2660, 1680, 1570, 1440, 1280, 1160, 970 cm-1; 1H and 13C NMR (CDCl3, 500 MHz) data see Table 1; COSY, HMBC, and NOE data, see Supporting Information; HR ESI-MS [M + H]+ m/z 369.2428 (calcd for C24H33O3, 369.2429). Neurymenolide B (2): clear oil (1.0 mg, 0.013% plant dry mass); [α]24D -240 (c 0.02 g/100 mL, MeOH); UV (ACN) λmax 295 nm (log ε = 3.41); 1H and 13C NMR (CDCl3, 500 MHz) data see Table 1; COSY, HMBC, and NOE data, see Supporting Information; HR ESIMS [M + H]+ m/z 397.2756 (calcd for C26H37O3, 397.2743).
- 5.Berson JA, Jones WM, O'Callaghan SLF, O'Callaghan CSJ. J. Am. Chem. Soc. 1956;78:622–623. [Google Scholar]
- 6.Sato M, Uehara F, Sato K, Yamaguchi M, Kabuto C. J. Am. Chem. Soc. 1999;121:8270–8276. [Google Scholar]
- 7.Blunt JW, Copp BR, Hu WP, Murray HGM, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2008;25:35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 8.Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 2nd ed. John Wiley & Sons, Ltd; 2001. pp. 35–120. Chapter 3. [Google Scholar]
- 9.Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J. Science. 2001;293:290–293. doi: 10.1126/science.1059593. [DOI] [PubMed] [Google Scholar]
- 10.Kaulmann U, Hertweck C. Angew. Chem. Int. Ed. 2002;41:1866–1869. doi: 10.1002/1521-3773(20020603)41:11<1866::aid-anie1866>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.