Abstract
A new Ginkgo biloba extract P8A (TTL), 70% enriched with terpene trilactones, prevents Aβ1-42 induced inhibition of long-term potentiation in the CA1 region of mouse hippocampal slices. This neuroprotective effect is attributed in large part to ginkgolide J that completely replicates the effect of the extract. Ginkgolide J is also capable of inhibiting cell death of rodent hippocampal neurons caused by Aβ1-42. This beneficial and multi-faceted mode of action of the ginkgolide makes it a new and promising lead in designing therapies against Alzheimer’s disease.
Keywords: Gingko Biloba, Long-term potentiation, Amyloid beta, Cell Death
Introduction
Extracts of the leaves and bark of the Ginkgo tree (Ginkgo biloba) have been used to enhance memory in Asian traditional medicine for centuries. Globally, standardized extracts of Ginkgo leaves are currently the largest selling herbal supplements. Two large studies performed in the United States and in Europe respectively are currently evaluating the efficacy of the Gingko derivative EGB-761 against AD [10,51]. In spite of this long experience, the number of well-controlled studies on the efficacy of these extract is limited and its effect in neurodegenerative disorders characterized by dementia, such as AD needs to be confirmed [2,11,20,24,31,32,34,38,40,46,54].
Synaptic dysfunction is likely to occur at early stages of Alzheimer’s disease (AD) [29]. Low levels of oligomeric amyloid-β (Aβ) alter mechanisms underlying the excitatory response at single synapses producing synaptic dysfunction before synapse loss, cell death, and a complex series of events including inflammation, deposition of Aβ in senile plaques and within the walls of the cerebral microvasculature, and appearance of neurofibrillary tangles [42]. An understanding of the effects of Ginkgo biloba and its derivatives on Aβ-induced synaptic dysfunction may provide the basis for rational treatment of dementia with these compounds.
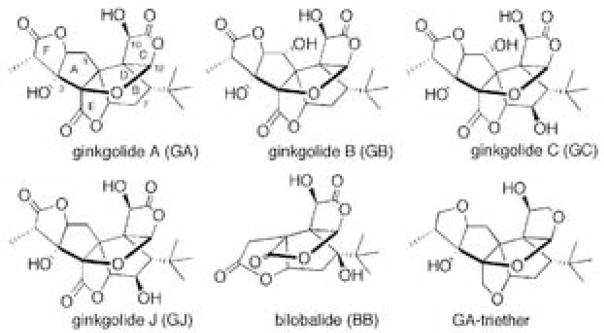
A recent review of the literature finds that Ginkgo biloba extracts affect a variety of systems in the brain and that there is a need for further research on the effects Ginkgo biloba on learning and memory, while questioning whether these results suggest a specific action as compared to a more general effect [14]. The multiplicity of effects of Ginkgo biloba extracts on the brain could well be the result of the complex chemical composition of the extracts. The widely used Ginkgo biloba extract, EGb-761, contains 6–7% terpene trilactones and 27% flavonoids, compounds with anti-oxidant activity, as well as a variety of other materials. While oxidative changes have been reported in AD [1,28,35,37,39,45], behavioral studies on APP transgenic mice show improvement in spatial memory in ginkgo treated mice in spite of unaltered amyloid levels and increasing levels of protein oxidation as compared to wild-type controls [47]. Moreover, one of the terpene trilactone components, GA, has also been found to alleviate the paralysis, reduced chemotaxic behavior and 5-HT hypersensitivity in a Caenorhabditis elegans (C. elegans) transgenic model of AD [55]. These findings point to the terpene trilactones – the ginkgolides and bilobalide (Figure 1) as the active compounds in ginkgo biloba extracts.
Figure 1.
Structures of native terpene trilactones from Ginkgo biloba extract and a reduced derivative, GA-triether.
In the studies reported here, we have examined the ability of Ginkgo biloba extracts enriched 10 fold in terpene trilactones (P8A, also known as TTL) as compared to standardized commercial extracts such as Bioginkgo 7/27 ® from Pharmanex, for example, as well as the individual ginkgolides, bilobalide and a synthetic ginkgolide derivative (Figure 1) to reverse the Aβ-induced inhibition of long-term potentiation (LTP) in the CA1 hippocampal region and to block Aβ-induced cell death. LTP is an electrophysiological correlate of memory storage and is strongly inhibited by Aβ [7,9,23,52,53]. Micromolar concentrations of the enriched terpene trilactone extract P8A and the individual ginkgolide GJ completely blocked the detrimental effect of Aβ on LTP. In addition, both P8A and GJ blocked Aβ-induced cell death. These results point to a rational physiological basis for the use of GJ and/or its derivatives in the treatment of dementia.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich. Aβ1-42 was purchased from American peptide. Bioginkgo 7/27® was a gift from Pharmanex, Inc. Ginkgo derivatives were synthesized as described below.
Production of 70% enriched terpene trilactone fraction (P8A)
In brief, a commercial extract of Ginkgo biloba leaves (Bioginkgo 7/27 ®) was boiled with 3% hydrogen peroxide to prevent the formation of emulsions that hindered efficiency of subsequent extractions. This was followed by extraction with ethyl acetate, washing with basic solutions, and charcoal filtration yielding an off-white powder with terpene trilactone content of approximately 70% [26].
Isolation of ginkgolides and bilobalide
The individual terpene trilactones were isolated and characterized as previously described [19,36] and their identity was confirmed by NMR, HRMS and found identical to the reported data. Purity of all compounds was >98% (estimated by 1H NMR). Briefly, Bioginkgo 7/27® extract was washed with ethyl acetate, volatiles were removed under vacuum, and the residue was subjected to column chromatography (silicagel, EtOAc/hexane). BB was separated directly by column chromatography, and purified by washing with Et2O. Fractions containing mixtures of ginkgolides, i.e., GA/GB, GC/GJ with small amounts of GA and GB, were subjected to benzylation with benzyl bromide in the presence of K2CO3 in DMF. Individual compounds, i.e., GA, GJ and benzylated-GB and benzylated-GC were separated by column chromatography, and GB and GC were obtained following the hydrogenation over Pd/C.
Synthesis of GA-triether
GA-triether was prepared according to the published procedure [18], Briefly, GA was reduced with DIBAL-H in THF to the corresponding GA-trilactol, which was isolated as an oil and subsequently converted into GA-triether upon treatment with Et3SiH and BF3·Et2O, to yield the desired compound as a white solid.
Production of β-amyloid oligomers
Oligomeric Aβ1-42 (American Peptide) was prepared according to the methods of Stine et al [48]. Lyophilized Aβ1-42 was allowed to equilibrate at room temperature for 30 min to avoid condensation upon opening the vial. The lyophilized peptide was resuspended in 1,1,1,3,3,3-Hexafluoro-2-Propanol (HFIP; Sigma) to 1 mM using a glass gas-tight Hamilton syringe with a Teflon plunger. HFIP was allowed to evaporate in a fume hood and the resulting clear peptide film was dried under vacuum (6.7 mTorr) in a SpeedVac (Savant Instruments). We stored the dessicated pellet at −20°C. Immediately prior to use, the aliquots were resuspended to 5 mM in dimethylsulfoxide (DMSO) by pipette mixing followed by bath sonication for 10 minutes at 4°C. 5 mM Aβ1-42 in DMSO was diluted to 100 μM in ice-cold cell culture media, immediately vortexed for 30 seconds and incubated at 4°C for 24 hours. The concentration of Aβ was determined based on the amounts of total Aβ content in our preparation including different forms of oligomeric Aβ.
Slice preparation
C57BL/6 mice (3–4 months of age, The Jackson Laboratory, Bar Harbor, ME) were decapitated, and their hippocampi were removed. Transverse hippocampal slices with a thickness of 400 μm were maintained in an interface chamber at 29°C, as previously described [15,52]. They were perfused with perfusion buffer (124.0mM NaCl, 4.4mM KCl, 1.0mM Na2HPO4, 25.0mM NaHCO3, 2.0mM CaCl2, 2.0mM MgSO4, 10mM glucose) continuously bubbled with 95% O2 and 5% CO2 (flow rate 1ml/min in a chamber of 1 ml volume). Slices were permitted to recover for at least 90 minutes before recording.
Electrophysiological Recordings
fEPSPs were recorded from the CA1 region of the hippocampus by placing both the stimulating and the recording electrodes in CA1 stratum radiatum. BST was assayed by plotting the stimulus voltage (V) against slopes of fEPSP to generate input-output relations. For LTP experiments, baseline stimulation was delivered every minute at an intensity that evoked a response approximately 35% of the maximum evoked response. Baseline response was recorded for 15 minutes prior to beginning the experiment to assure stability of the response. LTP was induced using theta-burst stimulation (4 pulses at 100 Hz, with the bursts repeated at 5 Hz and each tetanus including 3 ten-burst trains separated by 15 seconds). P8A, GA, GB, GC, GJ, BB, GA-triether and vehicle in 0.1% DMSO were individually added to the bath solution for 20 min at the same time as Aβ1-42 before inducing LTP.
Hippocampal neuronal cultures
Hippocampal cell cultures were prepared according to the method previously described [17]. Briefly, fetuses at embryonic day 18 (E18) from timed pregnant Sprague-Dawley rats (Taconic Farms) were sacrificed and the hippocampi removed. Neurons were then dissociated, plated at a density of 106 cell/well on 6 well-plates coated with poly-L-lysine (Sigma) and maintained in a defined serum-free medium (95% neurobasal, 2% B-27 supplement, 0.5 mM L-glutamine, 0.6% glucose, 1% penicillin/streptomycin). These cultures are enriched in large pyramidal neurons that constitute the main initial target in AD pathogenesis. Cultures at 5–6 days in vitro (DIV) cells were used for the experiments.
Neuronal cell death assay
Hippocampal cultures were treated by adding 10 μM Aβ1-42 in its oligomeric form with or without P8A, or alternatively with and without each of the individual ginkgolides and bilobalide (GA, GB, GC GJ, BB). Similar to the LTP experiments, these compounds were dissolved in DMSO and added to the culture medium at a ratio of 1:1000 (v/v), yielding a 0.1% DMSO solution. After 24 hrs the number of viable cells was assessed by nuclear counting [49]. In a separate set of experiments viable cells were counted by using the ethidium homodimer/calcein AM combination of vital dyes (LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells; Invitrogen), according to the manufacturer instructions. Values represent mean ± SEM of three consecutive experiments. Each experiment was performed in triplicate.
Statistics
Experiments were performed with the investigator blinded to the treatment the animals received. Data were expressed as mean ± SEM. Electrophysiological results were normalized to the basal values and analyzed by two-way ANOVA. Residual levels of potentiation and neuron cell death experiments were analyzed using Student-Newman-Keuls multiple comparison test. The level of significance was set for p < 0.05.
Results
Our previous studies have shown that the inhibition of LTP by Aβ1-42 oligomers can be reversed by treatment with the phosphodiesterase 4 (PDE4) inhibitor rolipram [15,52]. Recent studies have revealed that the Ginkgo biloba extract, EGb-761, exerts PDE4 inhibitory activity with a pharmacological profile similar to that of rolipram [6,41]. The possibility that Ginkgo biloba extracts might also rescue LTP inhibition by Aβ1-42 oligomers prompted us to test them on hippocampal slices. Based on a prior study suggesting that the effect of ginkgo extracts was likely to be mediated by the ginkgolides rather than by a decrease in oxidative damage mediated by the flavonoids [47], we decided to test a new Ginkgo extract (P8A) that is approximately 10-fold enriched in terpene trilactones and contains bilobalide and the four ginkgolides (GA, GB, GC, GJ) extracted from the leaves of the plant [26].
As previously reported [7,9,23,52,53], treatment of hippocampal slices with 200 nM Aβ1-42 oligomers depressed LTP in the CA1 area to about 50% of control values within 20 min of exposure (Figure 2). Treatment with P8A at 200 μg/ml at the same time as 200 nM Aβ1-42 was able to restore LTP levels to control values (Figure 2). Neither Aβ nor P8A alone affected baseline transmission in experiments where no tetanus was applied (data not shown).
Figure 2. Aβ-induced LTP impairment in the CA1 region of hippocampal slices and its reversal by P8A.
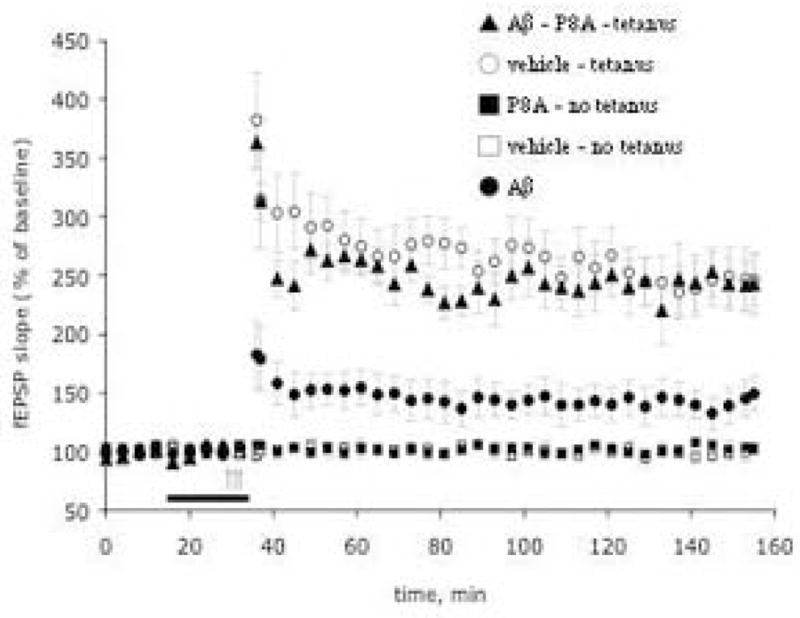
P8A reverses Aβ-induced LTP impairment in the CA1 region of hippocampal slices (n = 8 slices from 8 mice for P8A, 6 slices from 6 mice for Aβ, and 6 slices from 6 mice for vehicle). Both Aβ and P8A did not affect baseline transmission in experiments where no tetanus was applied (n = 3 slices from 3 mice both for P8A, Aβ and vehicle). The horizontal bar indicates the period during which Aβ (200 nM) and P8A (200 μg/ml) were added to the bath solution. The arrows indicate the time at which the theta-burst stimulation was applied in this and the following figures. Every fourth recording point is shown for clarity in this and the following figures. Values represent mean ± SEM (Two-way ANOVA F(1, 12)=10.64, P<0.01 for P8A + Aβ vs. Aβ alone).
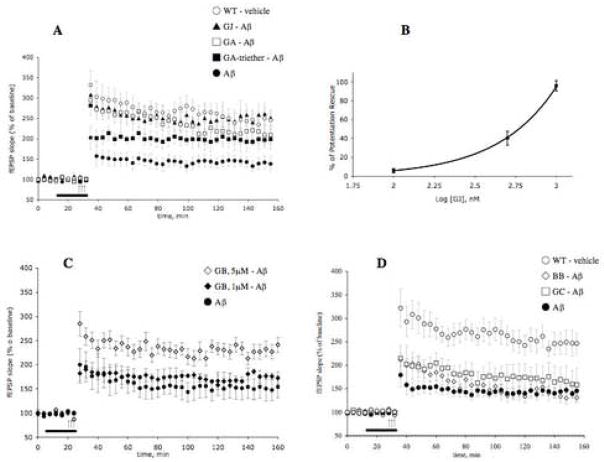
Given the heterogeneity of molecules present in the mixture as described above, we next attempted to identify the one(s) responsible for the LTP rescue. In the same experimental setting, we co-treated slices with 200 nM Aβ1-42 oligomers and each one of the ginkgolides. As shown in Figure 3a, GJ was the only compound that at a concentration of 1 μM was capable of completely reproducing the activity of P8A and reversing Aβ-induced LTP impairment in the CA1 region of hippocampal slices. The efficacy of GA and GA-triether was less than that of GJ, especially in case of GA-triether for the first 60 min after the tetanus. A dose-response curve for GJ indicated that it had its maximal effect on the rescue of LTP at a concentration of 1 μM (Figure 3b). A similar concentration (1 μM) of the ginkgolide GB did not rescue LTP impairment in slices treated with Aβ for 20 min prior to LTP induction (Figure 3c). However when we increased the concentration of GB to 5 μM, the compound fully reversed the LTP impairment (Figure 3c). Both GC and the bilobalide BB at 1 μM failed to rescue Aβ-induced LTP impairment in the same experimental paradigm used for the other ginkgolides (Figure 3d). None of the compounds alone (GJ, GA, GA-triether, GB, GC, or BB) affected baseline transmission in experiments where no tetanus was applied (data not shown). The results of electrophysiological experiments are summarized in Figure 4, as average amounts of potentiation during the last 10 min of the recording. It is evident that GJ is the most potent ginkgolide, completely mimicking the activity of the enriched extract.
Figure 3. Effect of individual ginkgolides and bilobalide on Aβ-induced LTP impairment in CA1 region of hippocampal slices.
A) Summary graph showing that 20 min treatment with 1 μM GJ, GA and GA-triether rescues LTP impairment in slices treated with 200 nM Aβ1-42 for 20 min prior to LTP induction (n = 9 slices from 8 mice for GJ + Aβ, 8 slices from 8 mice for GA + Aβ and 8 slices from 7 mice for GA-triether + Aβ, 6 slices from 6 mice for Aβ alone). Similar concentrations of GJ, GA and GA-triether did not affect baseline transmission in experiments where no tetanus was applied (n = 4 slices from 4 mice for each group; data not shown). Two way ANOVA: F(1, 13)=12.08, P<0.01 for GJ + Aβ vs. Aβ alone; F(1, 12)=10.02, P<0.01 for GA + Aβ vs. Aβalone; F(1, 12) = 5.83, P<0.05 for GA-triether + Aβ vs. Aβ alone). B) A dose-response curve for GJ effect on Aβ-induced LTP impairment indicates that GJ has its maximal beneficial effect at 1 μM (n = 12 slices from 12 mice for each point). Nonlinear regression analysis was used to fit curves for different concentrations using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). C) Summary graph showing that 20 min treatment with 5 μM (but not 1 μM) GB re-establishes normal LTP in slices treated with 200 nM Aβ1-42 for 20 min prior to LTP induction (n = 12 slices from 10 mice for 5 μM and 8 slices from 8 mice for 1 μM). Both 5 and 1 μM concentrations GB did not affect baseline transmission in experiments where no tetanus was applied (n = 4 slices from 4 mice for each group; data not shown). Two way ANOVA: F(1, 16)=9.47, P<0.01 for 5 μM GB + Aβ vs. Aβ alone; and F(1, 12)=1.85, P>0.05 for 1 μM GB + Aβ vs. Aβ. Alone. D) Summary graph showing that 20 min treatment with the ginkgolides GC as well as the bilobalide BB does not rescue LTP impairment in slices treated with 200 nM Aβ for 20 min prior to LTP induction (n = 9 slices from 7 mice for GC, and 7 slices from 7 mice for BB). Both GC and BB did not affect baseline transmission in experiments where no tetanus was applied (n = 4 for each group; data not shown). The horizontal bar indicates the period during which these drugs were added to the bath solution. Experiments were interleaved with each other. Two way ANOVA: F(1, 13)=0.98, P>0.05 for GC + Aβ vs. Aβ alone and F(1, 11)=0.78, P>0.05 for BB + Aβ vs. Aβ alone. Every fourth recording point is shown for clarity.
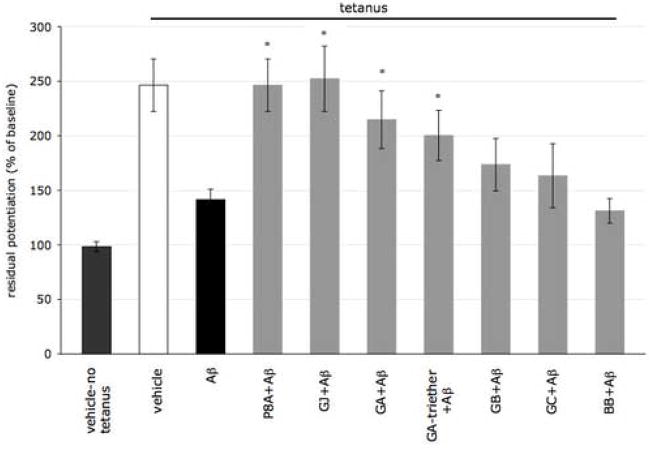
Figure 4. Residual potentiation during the last 10 min, following administration of 1 μM ginkgo derivatives.
Residual levels of potentiation were averaged during the last 10 min of LTP. P8A, GJ, GA and GA-triether rescued the LTP impairment (Student-Newman-Keuls multiple comparison test, p<0.05 for Aβ vs P8A + Aβ, Aβ vs GJ + Aβ, Aβ vs GA + Aβ, and Aβ vs GA-triether + Aβ, *). Vehicle-no tetanus = 100.0% ± 4.69. Tetanus + vehicle = 246.32 ± 24.0. Tetanus + Aβ = 148.85% ± 9.5. Tetanus + P8A = 246.33% ± 24.1. Tetanus + GJ = 252.25% ± 29.9. Tetanus + GA = 214.72% ± 26.4. Tetanus + GA-triether = 200.23% ± 22.9. Tetanus + GB = 173.45% ± 24.0. Tetanus + GC = 163.31% ± 29.3. Tetanus + BB = 131.09% ± 11.3.
Though the literature suggests that the PDE4 inhibitory activity in Ginkgo extracts is associated with the flavanoids [6,21], we assayed both P8A and each of the ginkgolides for their ability to inhibit PDE4. There was a trace of activity in P8A and none in any of the individual ginkgolides (data not shown) suggesting that the effects studied here are independent of PDE4.
Cell death is a feature of AD [12], probably occurring temporally after synaptic dysfunction. It requires higher concentrations of Aβ and more prolonged Aβ exposures than those responsible for synaptic dysfunction [49]. Therefore, in a parallel set of experiments we tested the ability of P8A, the ginkgolides GJ, GA, GB, GC and bilobalide (BB) to protect against cell death induced by 10 μM oligomeric Aβ1-42 applied for 24 hrs. In a first set of experiments the number of viable cells was assessed by nuclear counting [49]. After 24 hour exposure to 10 μM oligomeric Aβ1-42 only 48.5% ± 2.4 of cultured hippocampal cells survived. When cotreated for 24 hours with 10 μM oligomeric Aβ1-42 with either 50 μg/ml P8A or 1 μM GJ, hippocampal cultures displayed a percentage of surviving cells of 76.0% ± 7.9 and 70.7% ± 1.4, respectively. A higher concentration of GJ (5 μM) completely prevented Aβ toxicity (data not shown). No improvement in the number of surviving cells was noted with any of the other ginkgolides or with bilobalide BB even at higher concentrations (data not shown). None of these substances affected neuronal viability when added alone. These results were confirmed when the number of viable cells was assessed with the ethidium homodimer/calcein AM combination of vital dye assay [Aβ alone: 42,7% ± 3.5; P8A (1 μM) 74.6% ± 4.2; GJ (1 μM) 64.2% ± 1.1; GA (1 μM) 47.1% ± 2.4; GB (1 μM) 44.6% ± 2.7; GC (1 μM) 47.3% ± 3.6; Student-Newman-Keuls multiple comparison test, p<0.001 for Aβ vs P8A + Aβ and for Aβ vs GJ + Aβ; data not shown].
Thus, these results differ from those obtained in the electrophysiological experiments and allow us to conclude that: a) GJ, GA, GA-tri-ether and GB are capable of blocking, at least in part, the Aβ1-42 induced damage to synaptic plasticity, likely acting on the same molecular pathway; b) GJ is more effective than the other three Ginkgo derivatives; c) GJ can prevent cell death induction by higher concentrations of Aβ1-42, suggesting that this ginkgolide may act also on an alternative pathway as a consequence of its differences in chemical structure from those of GA and GB.
Discussion
Since its introduction in the Western pharmacopeia, the ginkgo biloba leaf extract EGb-761 has been the object of a plethora of studies, both in vivo and in vitro. The results appear to indicate that the extracts could be beneficial in several conditions ranging from cerebral and peripheral vascular insufficiency, to retinopathy, to depression, to finally the most debated one, age-related cognitive decline or dementia (reviewed in [3,13,27,33]). The literature on this subject is crowded with studies whose results gave rise to anecdotal reports of the standardized mixture as a panacea capable of treating a broad range of ailments. As a consequence ginkgo and EGb-761 have been relegated to the realm of phytomedicines possessing dubious beneficial effects on health and looked upon with skepticism by the much of the medical community. Recent well-designed animal [47,55] and human studies [2,32,34] have shown that EGb-761, a complex mixture, can improve the cognitive performance of AD transgenic mice as well as the cognitive performance of people with early AD. In the studies reported here, we have attempted to analyze the effect of individual terpene trilactones (ginkgolides and bilobalide) in a system that is known to be affected by Aβ and is probably connected to early synaptic and cognitive changes following amyloid elevation – hippocampal LTP. It is important to stress that the results of our study for the first time show that GJ is a ginkgolide with high potential for preventing synaptic damage induced by oligomeric Aβ1-42. This finding is consistent with the observation that GJ has been shown to alleviate Aβ-induced paralysis in the transgenic C. elegans [55]. Finally, this discovery is particularly relevant in light of the observation that synaptic dysfunction is highly correlated with the severity of dementia in AD [29].
In a previous study it has been shown that GB but not GA suppressed the K+-evoked acetylcholine release in rat hippocampal slices treated with the Aβfragment(25–35) [25]. Although we have found that higher concentrations of GB re-establish normal LTP and therefore this aspect of our work is consistent with these studies, GA in our experimental paradigm was also able to re-establish normal LTP. Thus, with regard to GA there is an apparent conflict with these previous studies. However, it is hard to compare the two experimental paradigms. Indeed, it has been demonstrated that Aβ peptides with less than 40 amino acids which do not form conducting pores in black lipid bilayer films and in intact cell membranes, are not neurotoxic in cultured hippocampal neurons [44]. Thus, it is possible that the discrepancy with our data is due the different form of Aβ utilized. It is important to note that the 42 amino acid oligomers of Aβ are widely held to naturally occurring neurotoxic form.
Our studies have also shown that GJ, but not the remaining gingkolides or bilobalide, might be beneficial against another important feature of AD - Aβ-induced cell death - further underlying the potential of GJ as therapeutic agent for AD. This finding is consistent with the observation that EGb-761 protects cultured neurons from Aβ-induced toxicity [4,56]. However, these results are at variance with previous studies showing a blockade of cell death with both GA and GB [5]. The difference may be due to the fact that primary rodent hippocampal neurons were used in our studies while the other study used the SY5Y human neuroblastoma cell line or a model of cell death in which cortical neurons were exposed to Aβ and microglia. In future studies we will test the hypothesis that GJ interferes with caspase activation as it has been shown that caspases play a critical role in apoptosis initiated by Aβ toxicity [50]. Additionally, we will determine whether GJ interferes with mechanisms of glutamate-induced toxicity that have been found to be involved in cell death by Aβ elevation [30]. Nevertheless, the effect of GJ on cell death is consistent with its beneficial effect on synaptic dysfunction.
Our study of ginkgolides stemmed from reports that indicated that EGb-761 possesses a PDE4 inhibitory activity pharmacologically similar to that of rolipram, a drug that we have previously shown to be able to prevent LTP impairment and memory loss in a transgenic model of AD [16]. However, we could detect PDE4 inhibitory activity only with EGb-761 and not in the purified ginkgolides (data not shown). Interestingly, EGb-761 as well as GJ and GA reduced Aβ oligomerization in the transgenic C. elegans [55]. Thus, one plausible explanation for part of the beneficial effect of our compounds is that they reduce Aβ oligomerization re-establishing normal synaptic function and cell viability [23,53].
Gingko biloba is widely used as a memory enhancer in healthy people [8] and has been reported to enhance normal memory in young animals [43]. Do individual gingkolides have an effect on normal synaptic plasticity? Previous studies have shown that both GJ and GA enhance short-term potentiation in rat hippocampal slices [22]. Interestingly, it has also been shown that GB reduces LTP when the synapse is primed by a previous high-frequency stimulation capable of inducing short-term potentiation, whereas GJ does not change LTP in the same experimental paradigm [22]. No studies have been performed without priming.
We then tried to understand whether the compounds have moieties that share similar biological activity. Only 1 μM triethyl-GA was able to reproduce the protective activity of GA and GJ These results demonstrate a very fine balance between the structure of the ginkgolide and its biological potential, and leads to preliminary structure-activity relationships: (1) the cage-like structure is required, as BB showed no activity; (2) the methylene group at the 1- position is essential for biological activity, i.e., GA, GJ and “GA-triether” lack the 1-OH and exhibit activity, whereas GB and GC both with 1-OH-group are inactive or active only at higher concentration. (Note that GB and GJ are regional isomers, yet the specific position of the hydroxyl-group determines the potency); (3) the presence of the hydroxyl-group at the 7-position does not seem to be crucial; (4) lactone-groups of native ginkgolides are important but not essential, as “GA-triether” is still biologically active. Since only GJ shows neuroprotective properties in our studies, it is likely that the neuroprotective effects are mediated by a pathway different from that mediating synaptic effects. It also suggests that combinations of ginkgolides or their synthetic derivatives might be used in preventing memory loss and cognitive decline in AD and related dementias by targeting different aspects of the disease process.
In summary, we have shown the effects of the enriched Ginkgo biloba extract P8A as well as natural and modified ginkgolides and bilobalide on two systems that are known to be affected by Aβ and thought to be related to AD – hippocampal LTP and Aβ induced cell death. The enriched extract can completely prevent the detrimental effect of Aβ on LTP. Of great interest is also the additional neuroprotective effect of GJ. Furthermore, analysis of the similarities between ginkgolides has allowed us to identify a derivative, triethyl-GA which shares the same effect and may prove to be the first compound of a new generation of drugs with higher selectivity and specificity in treating synaptic dysfunction induced by oligomeric Aβ1-42. Our results show clearly that at least some of the biological effects of Ginkgo biloba extracts can be attributed to the individual terpene trilactones and that use of these agents or their derivatives might lead to more effective therapy. While the commercial Ginkgo biloba extracts are of very low toxicity and the individual ginkgolides showed no acute toxicity at the concentrations used in our experiments, it still remains to be determined whether the pure ginkgolides are toxic or not in animals when used at potentially therapeutic levels for long periods of time. In the future we aim at further elucidating the mechanisms involved in the observed phenomena and at developing more selective drugs capable of achieving the same effect at lower concentrations and minimum toxicity.
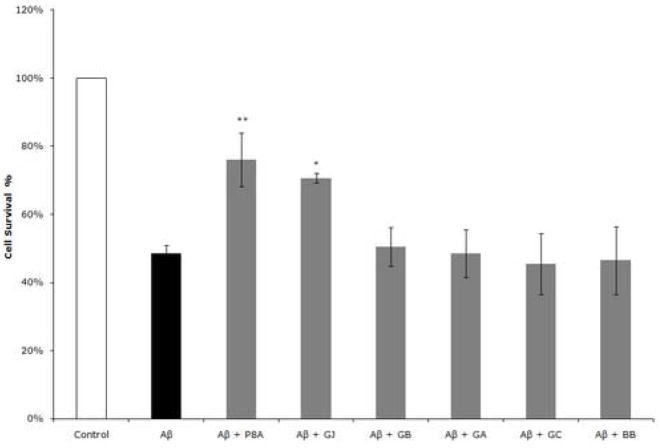
Figure 5. P8A and GJ protect cultured hippocampal neurons treated with oligomeric Aβ peptide against cell death.
Hippocampal cultures were treated by adding 10 μM Aβ1-42 in its oligomeric form with or without P8A at 50 μg/ml, or alternatively each one of the ginkgolides (GA, GJ) at a concentration of 1 μM. After 24 hrs the number of viable cells was assessed using cell nuclei counting. Aβ induced death of ~50% of the cell population. Both the P8A and GJ, but no GA or any of the other ginkgolides were consistently able to prevent cell death (Student-Newman-Keuls multiple comparison test, p<0.01 for Aβ vs P8A + Aβ, **; p<0.05 for Aβ vs GJ + Aβ, *). CTRL = Control, 100%. Aβ = 48.5% ± 2.4. P8A + Aβ = 76.0 ± 7.9. GJ + Aβ = 70.7 ± 1.4. GA + Aβ = 50.5 ± 5.7. GB ± Aβ = 48.5 ± 7.0. GC ± Aβ = 45.5 ± 9.0. BB ± Aβ = 46.5 ± 10.0. Values represent mean ± SEM of three consecutive experiments. Each experiment was performed in triplicate.
Acknowledgments
This work has been supported by the grants from the US National Institute of Health (GM-MHO68817 and NS15076), and by a grant from Itochu Corporation (Tokyo).
Footnotes
Disclosure Statement: Vitolo, O., Jaracz, S., Nakanishi, K., Arancio, O., and Shelanski, M.L. disclose the following patent applications: “Ginkgolide compounds, compositions and extracts, and uses thereof” (PCT/US2005/009417) and “Method for Isolating Terpene Trilactones (Ginkgolides Bilobalide) from Leaves and Pharmaceutical Powders of Ginkgo Biloba” (U.S. Patents 10/194,089, 10/615,346 and related applications).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. Journal of neurochemistry. 2000;74(6):2520–7. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrieu S, Gillette S, Amouyal K, Nourhashemi F, Reynish E, Ousset PJ, Albarede JL, Vellas B, Grandjean H. Association of Alzheimer’s disease onset with ginkgo biloba and other symptomatic cognitive treatments in a population of women aged 75 years and older from the EPIDOS study. The journals of gerontology. 2003;58(4):372–7. doi: 10.1093/gerona/58.4.m372. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett H, Eperjesi F. An ideal ocular nutritional supplement? Ophthalmic Physiol Opt. 2004;24(4):339–49. doi: 10.1111/j.1475-1313.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 4.Bastianetto S, Zheng WH, Quirion R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C. Journal of neurochemistry. 2000;74(6):2268–77. doi: 10.1046/j.1471-4159.2000.0742268.x. [DOI] [PubMed] [Google Scholar]
- 5.Bate C, Salmona M, Williams A. Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1–42. J Neuroinflammation. 2004;1(1):4. doi: 10.1186/1742-2094-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos-Toimil M, Lugnier C, Droy-Lefaix MT, Takeda K. Inhibition of type 4 phosphodiesterase by rolipram and Ginkgo biloba extract (EGb 761) decreases agonist-induced rises in internal calcium in human endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(9):E34–40. doi: 10.1161/01.atv.20.9.e34. [DOI] [PubMed] [Google Scholar]
- 7.Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. Journal of neuroscience research. 2000;60(1):65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Crews WD, Jr, Harrison DW, Griffin ML, Falwell KD, Crist T, Longest L, Hehemann L, Rey ST. The neuropsychological efficacy of ginkgo preparations in healthy and congnitively intact adults. HerbalGram. 2005;67:43–67. [Google Scholar]
- 9.Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8(15):3213–7. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- 10.DeKosky ST, Fitzpatrick A, Ives DG, Saxton J, Williamson J, Lopez OL, Burke G, Fried L, Kuller LH, Robbins J, Tracy R, Woolard N, Dunn L, Kronmal R, Nahin R, Furberg C. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemporary clinical trials. 2006;27(3):238–53. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, Mohs RC, Thal LJ, Whitehouse PJ, DeKosky ST, Cummings JL. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154–66. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 12.Francis P. Targeting cell death in dementia. Alzheimer disease and associated disorders. 2006;20(2 Suppl 1):S3–7. doi: 10.1097/01.wad.0000213803.82058.46. [DOI] [PubMed] [Google Scholar]
- 13.Gertz HJ, Kiefer M. Review about Ginkgo biloba special extract EGb 761 (Ginkgo) Current pharmaceutical design. 2004;10(3):261–4. doi: 10.2174/1381612043386437. [DOI] [PubMed] [Google Scholar]
- 14.Gold PE, Cahill L, Wenk GL. The lowdown on Ginkgo biloba. Scientific American. 2003;288(4):86–91. doi: 10.1038/scientificamerican0403-86. [DOI] [PubMed] [Google Scholar]
- 15.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. The Journal of clinical investigation. 2004;114(11):1624–34. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model following rolipram treatment. J Clin Invest. 2004;114:1624–34. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. Cambridge: MIT Press; 1998. [Google Scholar]
- 18.Ishii H, Dzyuba SV, Nakanishi K. Lactone-free ginkgolides via regioselective DIBAL-H reduction. Organic & biomolecular chemistry. 2005;3(19):3471–2. doi: 10.1039/b509129b. [DOI] [PubMed] [Google Scholar]
- 19.Jaracz S, Malik S, Nakanishi K. Isolation of ginkgolides A, B, C, J and bilobalide from G. biloba extracts. Phytochemistry. 2004;65(21):2897–902. doi: 10.1016/j.phytochem.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Kanowski S, Herrmann WM, Stephan K, Wierich W, Horr R. Proof of efficacy of the ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry. 1996;29(2):47–56. doi: 10.1055/s-2007-979544. [DOI] [PubMed] [Google Scholar]
- 21.Ko WC, Shih CM, Lai YH, Chen JH, Huang HL. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochemical pharmacology. 2004;68(10):2087–94. doi: 10.1016/j.bcp.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Kondratskaya EL, Pankratov YV, Lalo UV, Chatterjee SS, Krishtal OA. Inhibition of hippocampal LTP by ginkgolide B is mediated by its blocking action on PAF rather than glycine receptors. Neurochemistry international. 2004;44(3):171–7. doi: 10.1016/s0197-0186(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 23.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bars PL, Kieser M, Itil KZ. A 26-week analysis of a double-blind, placebo-controlled trial of the ginkgo biloba extract EGb 761 in dementia. Dementia and geriatric cognitive disorders. 2000;11(4):230–7. doi: 10.1159/000017242. [DOI] [PubMed] [Google Scholar]
- 25.Lee TF, Chen CF, Wang LC. Effect of ginkgolides on beta-amyloid-suppressed acetylocholine release from rat hippocampal slices. Phytother Res. 2004;18(7):556–60. doi: 10.1002/ptr.1493. [DOI] [PubMed] [Google Scholar]
- 26.Lichtblau D, Berger JM, Nakanishi K. Efficient extraction of ginkgolides and bilobalide from Ginkgo biloba leaves. Journal of natural products. 2002;65(10):1501–4. doi: 10.1021/np0201974. [DOI] [PubMed] [Google Scholar]
- 27.Mahady GB. Ginkgo biloba for the prevention and treatment of cardiovascular disease: a review of the literature. The Journal of cardiovascular nursing. 2002;16(4):21–32. doi: 10.1097/00005082-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain pathology (Zurich, Switzerland) 1999;9(1):133–46. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masliah E. Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol. 1995;10(2):509–19. [PubMed] [Google Scholar]
- 30.Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer’s disease: bad genes and bad habits. J Mol Neurosci. 2001;17(2):205–24. doi: 10.1385/JMN:17:2:205. [DOI] [PubMed] [Google Scholar]
- 31.Maurer K, Ihl R, Dierks T, Frolich L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. Journal of psychiatric research. 1997;31(6):645–55. doi: 10.1016/s0022-3956(97)00022-8. [DOI] [PubMed] [Google Scholar]
- 32.Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13(9):981–5. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- 33.Messina BA. Herbal supplements: Facts and myths--talking to your patients about herbal supplements. J Perianesth Nurs. 2006;21(4):268–78. 79–81. doi: 10.1016/j.jopan.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Mix JA, Crews WD., Jr A double-blind, placebo-controlled, randomized trial of Ginkgo biloba extract EGb 761 in a sample of cognitively intact older adults: neuropsychological findings. Human psychopharmacology. 2002;17(6):267–77. doi: 10.1002/hup.412. [DOI] [PubMed] [Google Scholar]
- 35.Montine TJ, Markesbery WR, Zackert W, Sanchez SC, Roberts LJ, 2nd, Morrow JD. The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of neuritic plaques or neurofibrillary tangles or with APOE genotype in Alzheimer’s disease patients. The American journal of pathology. 1999;155(3):863–8. doi: 10.1016/S0002-9440(10)65185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi K, Jaracz S, Malik S, Ishii H, Dzyuba SV. 2005 [Google Scholar]
- 37.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. Journal of neuropathology and experimental neurology. 2001;60(8):759–67. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 38.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Archives of neurology. 1998;55(11):1409–15. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 39.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21(12):4183–7. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai GS, Shovlin C, Wesnes KA. A double-blind, placebo controlled study of Ginkgo biloba extract (‘tanakan’) in elderly outpatients with mild to moderate memory impairment. Current medical research and opinion. 1991;12(6):350–5. doi: 10.1185/03007999109111504. [DOI] [PubMed] [Google Scholar]
- 41.Saponara R, Bosisio E. Inhibition of cAMP-phosphodiesterase by biflavones of Ginkgo biloba in rat adipose tissue. Journal of natural products. 1998;61(11):1386–7. doi: 10.1021/np970569m. [DOI] [PubMed] [Google Scholar]
- 42.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 43.Shif O, Gillette K, Damkaoutis CM, Carrano C, Robbins SJ, Hoffman JR. Effects of Ginkgo biloba administered after spatial learning on water maze and radial arm maze performance in young adult rats. Pharmacol Biochem Behav. 2006;84(1):17–25. doi: 10.1016/j.pbb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Singer SJ, Dewji NN. Evidence that Perutz’s double-beta-stranded subunit structure for beta-amyloids also applies to their channel-forming structures in membranes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1546–50. doi: 10.1073/pnas.0509892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(23):10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon PR, Adams F, Silver A, Zimmer J, DeVeaux R. Ginkgo for memory enhancement: a randomized controlled trial. Jama. 2002;288(7):835–40. doi: 10.1001/jama.288.7.835. [DOI] [PubMed] [Google Scholar]
- 47.Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184(1):510–20. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 48.Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. The Journal of biological chemistry. 2003;278(13):11612–22. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 49.Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci. 2000;20(4):1386–92. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Troy CM, Shelanski ML. Caspase-2 redux. Cell death and differentiation. 2003;10(1):101–7. doi: 10.1038/sj.cdd.4401175. [DOI] [PubMed] [Google Scholar]
- 51.Vellas B, Andrieu S, Ousset PJ, Ouzid M, Mathiex-Fortunet H. The GuidAge study: Methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761(R) for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology. 2006;67(9 Suppl 3):S6–S11. doi: 10.1212/wnl.67.9_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- 52.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13217–21. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 54.Wettstein A. Cholinesterase inhibitors and Gingko extracts--are they comparable in the treatment of dementia? Comparison of published placebo-controlled efficacy studies of at least six months’ duration. Phytomedicine. 2000;6(6):393–401. [PubMed] [Google Scholar]
- 55.Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26(50):13102–13. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao Z, Drieu K, Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain research. 2001;889(1–2):181–90. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]