Abstract
The HNSCC cell line, FaDu was stably transfected with control vector (FaDu) or with plasmid expressing small interfering RNA against EMMPRIN (FaDu/siE). Tumor cells were treated with bevacizumab (0, 25, 50, and 75 ng/mL) in vitro, and then cell counts were performed at 72 hours. For in vivo analysis, tumor cells were xenografted onto the flank of SCID mice, and were treated with 100 μg bevacizumab twice weekly for three weeks. Xenograft samples from the control and treatment groups were analyzed for microvessel density. Escalating doses of bevacizumab had no effect on the growth of tumor cells in vitro (P ≥ 0.086). However, tumor xenografts expressing EMMPRIN responded to bevacizumab treatment (P = 0.0013), whereas the EMMPRIN knockdown cell line did not (P = 0.7942). Immunohistochemical analysis demonstrated that microvascular density was reduced in the treated FaDu tumors (P = 0.005), but not in the FaDu/siE tumors (P = 0.48). Currently there is limited information on biomarkers to predict response to bevacizumab. By demonstrating effectiveness of bevacizumab therapy in tumors that express EMMPRIN, but not in tumors with silenced EMMPRIN expression, this study suggests that EMMPRIN may serve as a biomarker for response to bevacizumab treatment.
Keywords: EMMPRIN, head and neck squamous cell carcinoma, CD147, VEGF, cancer therapy
1. Introduction
Tumor growth beyond 1–2 mm is highly dependant upon the complex process of angiogenesis [1]. Vascularization and tumor growth are stimulated by cytokines elicited by endothelial cells and stromal cells surrounding the tumor, as well as the tumor itself. Vascular endothelial growth factor (VEGF) and other angiogenic growth factors cause endothelial cell proliferation and migration from existing blood vessels [2, 3, 4]. During endothelial migration through the tumors, matrix metalloproteases (MMPs) degrade the extracellular matrix, which then allows for growth of these newly formed vessels as they move into the growing neoplasm [5, 6, 7]. Extracellular matrix metalloprotease inducer (EMMPRIN) has been shown to induce MMPs and is believed to stimulate VEGF expression [5, 8].
Extracellular matrix metalloprotease inducer (EMMPRIN, also known as CD147 or basigin) is a membrane bound glycoprotein found on the surface of tumor cells [9]. EMMPRIN overexpression is exhibited to varying degrees in most tumor types, with head and neck squamous cell carcinoma (HNSCC) having some of the highest levels [10]. Elevated EMMPRIN expression has been shown to correlate with metastasis and tumor progression in tumors of the oral cavity [11] and larynx [12], along with multiple other tumor types [13, 14].
Although the exact mechanisms by which EMMPRIN promotes HNSCC growth is unknown, it has been well established that EMMPRIN promotes expression of matrix metalloproteases (MMPs) and modulates the tumor microenvironment by stimulation of MMP-1, MMP-2 and MMP-3 production by stromal cells [15, 16, 17]. Evidence has recently begun emerging that EMMPRIN promotes neovascularization not only through the elaboration of MMPs, but also by stimulation of VEGF expression [18, 19]. The relationship between EMMPRIN and VEGF has been illustrated in breast cancer [18, 20], hepatocellular carcinoma [19], gastric carcinoma [21], pancreatic carcinoma [22], melanoma [23], and glioblastoma [24]. We have previously demonstrated that EMMPRIN expression in HNSCC increases growth of tumor xenografts and can increase VEGF secretion [25].
Bevacizumab (Avastin; Genentech USA, Inc., San Francisco, CA) is a humanized recombinant IgG antibody that targets and neutralizes VEGF activity, causing inhibition of angiogenesis and tumor growth. It is currently indicated for use in metastatic colorectal cancer [26], non-small cell lung cancer (NSCLC) [27], and metastatic breast cancer [28]. Although there is no good biomarker for determining which patients will respond, bevacizumab is typically used in combination with traditional cytotoxic agents, which improves patient outcomes without overlapping toxicities. Preclinical evidence has recently emerged that bevacizumab may have a role in treatment of head and neck squamous cell carcinoma (HNSCC) [29] and Phase I–II trials are underway. A significant percentage of patients do not respond to bevacizumab. Due to the possibility of significant toxicities and the costs involved with therapy, it is important to identify a marker that would predict which patients will respond to treatment.
Although many stimuli have been identified that upregulate VEGF expression [30] and VEGF expression has been shown to predict poor outcomes in cancer patients [31, 32], levels of VEGF stimulators and levels of VEGF itself have failed to predict which patients will respond [33]. Although the relationship between EMMPRIN expression and VEGF levels has been demonstrated, to our knowledge no one has shown that EMMPRIN mediated VEGF expression affects efficacy of anti-VEGF tumor therapy. In this study, we investigate how EMMPRIN expression affects VEGF targeted therapy of head and neck squamous cell carcinoma xenografts using in vivo and in vitro models.
2. Materials and methods
2.1. Cells and culture conditions
FaDu (ATCC, Manassas,VA) cells were maintained in Dulbecco’s modified Eagle medium (DMEM; Mediatech, Manassas,VA) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone, Logan, UT) and 1% penicillin-streptomycin solution (10,000 units/mL penicillin and 10,000 μg/mL streptomycin, Mediatech) in a humidified atmosphere containing 5% CO2 at 37°C. Because tumor cells interact with stromal cells in vivo, tumor cells were co-cultured with and without normal dermal fibroblasts to better evaluate EMMPRIN’s role in cell growth. Normal dermal fibroblasts (NDFs) were isolated from primary culture and maintained in DMEM with 10% FBS and antibiotics as described previously [34].
To obtain an EMMPRIN stably silenced cell line, oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA) and cloned into pSilencer 4.1 CMV puro vectors (Ambion Inc., Austin, Texas) as described in the manufacturer’s instruction manual. Vectors containing the inserts were transformed into NovaBlue Singles (EMD Biosciences Inc, La Jolla, CA) competent cells. After PCR and DNA sequencing, confirmed plasmids were propagated and transfected into FaDu cells. Cells resistant to puromycin (1.0μg/ml) selection were analyzed by Western blot for EMMPRIN expression and then selected by flow cytometry. The target sequence selected for EMMPRIN silencing is GAGCTACACATTGAGAACCTG. The expression levels of EMMPRIN were routinely monitored using Western blot analysis using anti-EMMPRIN monoclonal antibody (Zymed Laboratories, Inc., San Francisco, CA). Mouse monoclonal antibody to β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was then used to confirm equal loading.
2.2. Reagents
We used bevacizumab (Avastin, Genentech USA Inc., San Francisco, CA), a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biologic activity of human VEGF. Bevacizumab contains human framework regions and the complementarity-determining regions of a murine antibody that binds to VEGF. Bevacizumab is specific for human VEGF, and has been shown to have no activity against murine VEGF [35].
2.3. Effects of bevacizumab in vitro
To measure the effects of bevacizumab on tumor cell growth and VEGF expression in vitro, tumor cells were seeded at 50,000 per well on a 96 well plate were in DMEM with 0.1% BSA and 1% penicillin/streptomycin. Normal dermal fibroblasts were (NDFs) were seeded at 12,000 per well. All treatments were done in triplicate. Bevacizumab (0, 25, 50, and 75 ng/mL) was added 24 hours after seeding. Media was changed at 48 hours and cells were counted at 72 hours.
2.4. Animal models
Severe combined immunodeficient (SCID) male mice, age 4–6 weeks (Charles River Laboratories, Wilmington, MA and NCI-Frederick, Frederick, MD) were obtained and housed in accordance with The University of Alabama’s Institutional Animal Care and Use Committee (IACUC) guidelines. FaDu cells transfected with the control vector or FaDu cells carrying the EMMPRIN silencing construct (FaDu/siE) were used to generate subcutaneous flank tumors. Mice were divided into 4 groups: FaDu (n = 4), FaDu + bevacizumab (n = 5), FaDu/siE (n = 4), and FaDu/siE + bevacizumab (n = 8). The variable number of mice per group resulted from inconsistent tumor growth. Once tumors were established to a mean of 23 mm2, treatment with bevacizumab began. Animals received 100 μg bevacizumab (i.p.) biweekly for three weeks. Tumors in all four groups were measured on treatment days; tumor area was calculated by multiplying length and width. Measurements continued for one week beyond treatments, at which time mice were sacrificed according to our IACUC guidelines.
2.5. Immunohistochemistry
Formalin fixed paraffin embedded 5μm tissue sections mounted on Bond-Rite (Richard-Allan Scientific, Kalamazoo, MI) slides were heated to 60° for two hours. After three baths of xylenes and graded alcohols to rehydrate the sections, antigen retrieval was performed with high temperature treatment with pH 10 Tris buffer 0.5M. H2O2, avidin and biotin solutions and 3% goat serum were used to quench peroxidases, block endogenous biotin and block nonspecific binding. Rabbit polyclonal antibody to CD31 (Abcam Inc., Cambridge, MA) was diluted 1:200 and applied to the tissue at room temperature for one hour. The secondary antibody was goat anti-rabbit (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and the label was avidin-HRP (Signet Pathology Systems, Inc., Dedham, MA). After the DAB chromagen (BioGenex Laboratories, Inc., San Ramon, CA) was applied the tissues were counterstained with hematoxylin and the cover slips mounted with Permount.
2.6. Statistical analyses
For FaDu and FaDu/siE tumors treated with bevacizumab, the response to treatment underwent the following analysis: the tumor area data for each animal were transformed to represent % change from untreated tumor, i.e. (average tumor area treated group/average tumor area untreated group). A two-sample t-test was used to compare the two groups across all time points using GraphPad Prism software.
Statistical significance was measured using an unpaired t-test. Data analysis for immunohistochemistry and in vitro cell growth was done using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). P < 0.05 was considered significant in t test analysis.
3. Results
3.1. Silencing EMMPRIN results in decreased cell growth
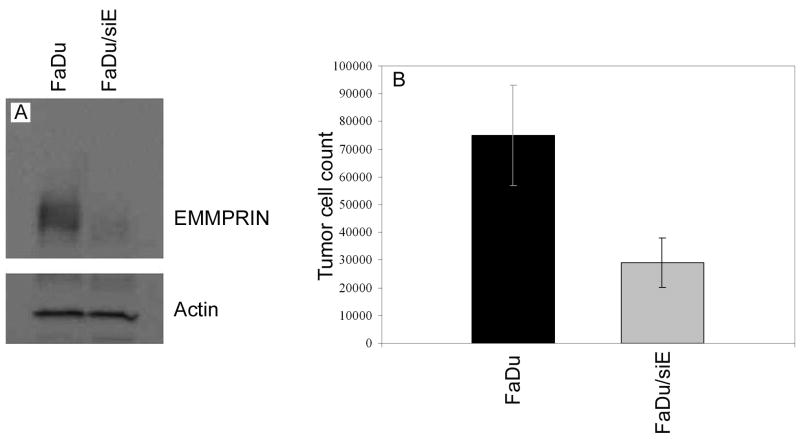
Western blot analysis was performed to verify decreased extracellular matrix metalloprotease inducer (EMMPRIN) expression in the silenced FaDu cell lines (Fig. 1A). Results verified knockdown of EMMPRIN expression in the FaDu/siE cell line and intermediate levels of expression were seen in the control vector transfected line (FaDu). To ensure silencing of EMMPRIN functionality (in cell growth), cells were placed in media both with and without normal dermal fibroblasts (NDFs) and allowed to grow for 72 hours, at which time cells were trypsinized and counted (Fig. 1B). Control vector cells plated with NDFs demonstrated higher growth rates compared to silenced cells (FaDu vs. FaDu/siE, P = 0.0009), whereas the differences seen between cell lines plated without NDFs did not reach significance (P = 0.0861). Though these differences did not reach significance, the apparent trend warrants further investigation.
Fig. 1.
Extracellular matrix metalloprotease inducer (EMMPRIN) expression in transfected FaDu cell lines. (A) Western blot analysis confirms that EMMPRIN expression was reduced in the FaDu/siE cell lines, whereas control vector transfected cells (FaDu) expressed intermediate basal levels of EMMPRIN. Equal protein loading was confirmed with β-actin. (B) To confirm that EMMPRIN functionality was suppressed as well, tumor cells were plated with and without normal dermal fibroblasts (NDFs). After 72 hours, control vector cells plated with NDFs demonstrated higher growth rates compared to silenced cells (P = 0.0009).
3.2. Bevacizumab does not effect tumor cell growth in vitro
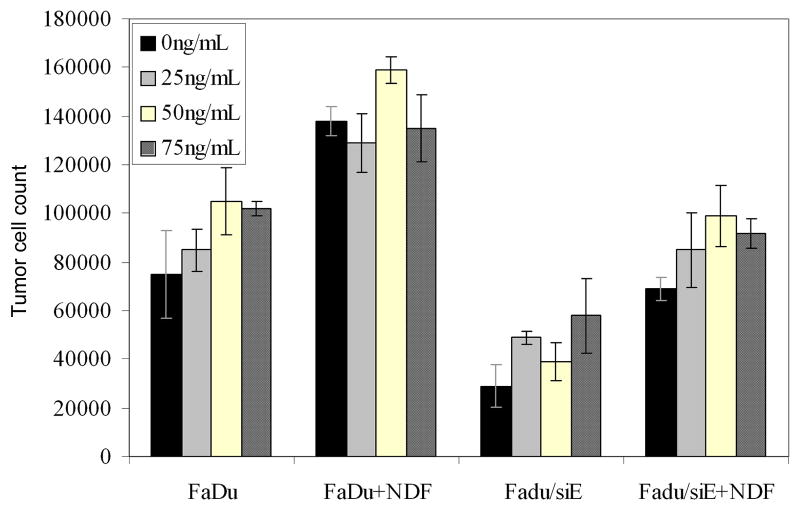
To measure the effects of bevacizumab on tumor cell growth in vitro, FaDu and FaDu/siE cells were seeded with and without normal dermal fibroblasts (NDFs) in a 96 well plate and treated with 0, 25, 50, and 75 ng/mL bevacizumab. After 72 hours, tumor cells were counted. Bevacizumab treatment had no effect on the growth rate of either of the cell lines (P ≥ 0.086, Fig. 2).
Fig. 2.
Bevacizumab has no effect on tumor cells in vitro. Tumor cells from the FaDu and FaDu/siE cell lines were plated with and without normal dermal fibroblasts were treated with 0, 25, 50 and 75 ng/mL of bevacizumab. After 72 hours, cells were trypsinized and counted. Bevacizumab had no effect on cell growth, regardless of EMMPRIN expression (P ≥ 0.086).
3.3. Silencing EMMPRIN inhibits the effects of bevacizumab in vivo
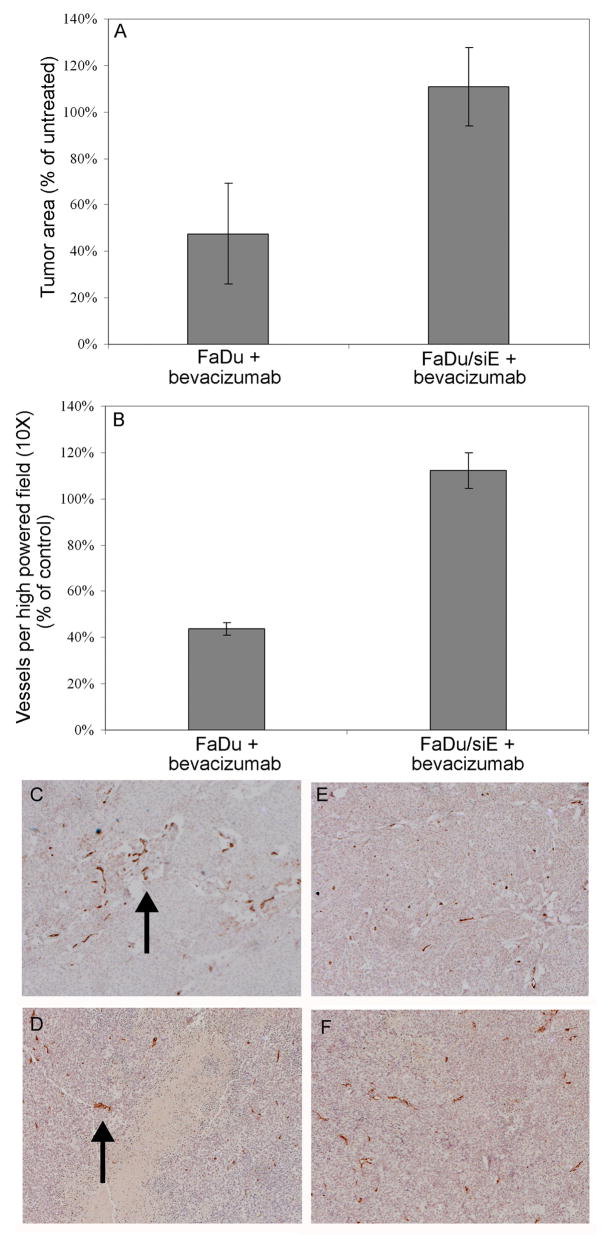
To determine the role of EMMPRIN in VEGF mediated tumor growth, mice xenografted with FaDu and FaDu/siE cells were treated with and without bevacizumab (100 μg i.p./biweekly) for three weeks. Tumors were allowed to reach a mean size of 23 mm2 prior to initiating treatment. At the end of treatment, the FaDu tumors treated with bevacizumab were 53% smaller than untreated control (Fig. 3A; P = 0.0013). Average tumor size in the FaDu/siE group treated with anti-VEGF antibody did not differ from the untreated control (P = 0.7942).
Fig. 3.
EMMPRIN expression required for bevacizumab response in vivo. (A) bevacizumab was effective in treating HNSCC xenografts in EMMPRIN expressing FaDu tumors (P = 0.0013), but response was not seen in tumors with knockdown EMMPRIN expression (FaDu/siE, P = 0.7942). Analysis of xenograft samples by immunohistochemistry staining for CD31 (B) revealed that bevacizumab decreased microvascular density of tumors that expressed EMMPRIN (P = 0.005), but had no effect on the vascularity of FaDu/siE tumors (P = 0.48). Immunohistochemistry of CD31 expression by FaDu control tumors (C) and tumors treated with bevacizumab (D) compared to FaDu/siE control (E) and treated (F) tumors. Data are normalized as percentage of untreated controls. Arrows indicate vascularity.
3.4. Reduced microvascularization in treated FaDu tumors
To investigate the effects of anti-VEGF therapy on vascularization, xenografts of each tumor line treated with bevacizumab were analyzed for microvessel density (CD31). The percentage of cells staining positively for CD31 in treated FaDu xenografts was 44% of untreated control FaDu xenografts (Fig. 3B; P = 0.005). No statistical difference was found between the treated and untreated FaDu/siE tumors (P = 0.48).
4. Discussion
We have demonstrated that xenografted tumors expressing EMMPRIN responded to anti-VEGF therapy, while those without EMMPRIN did not. Although VEGF is widely expressed in most tumor types, including HNSCC, there are still many patients that do not respond to anti-VEGF therapy [36, 37]. Studies to date have failed to demonstrate a correlation between levels of plasma angiogenic factors (such as VEGF or basic fibroblast growth factor (bFGF)) and response to bevacizumab [33]. Determining which patients will respond could help guide therapy, which could increase success rates and decrease costs and toxicity related to unsuccessful therapy.
We have previously shown decay of microvessel density and VEGF expression with loss of EMMPRIN [25]. Furthermore, we have shown in HNSCC xenografts that silencing EMMPRIN results in suppression of tumor growth and increased survival [25]. It has also been shown that tumor growth and vascularization can be inhibited by suppressing EMMPRIN expression in both breast cancer [18] and malignant melanoma [23].
Because EMMPRIN modulates VEGF expression, we sought to determine whether levels of EMMPRIN expression would affect response to anti-VEGF therapy. While previous studies have shown correlations between the two molecules and that loss of EMMPRIN does diminish VEGF production [5, 20], it has not been demonstrated if EMMPRIN would affect response to VEGF targeted therapy. In the current study, we found that EMMPRIN expression was required for response to bevacizumab in HNSCC xenografts.
Consistent with the findings of other authors, we found no effect of bevacizumab on tumor cell growth in vitro [29, 38], regardless of EMMPRIN expression. Analysis of the xenograft samples from our treated and untreated mice demonstrated that microvascular density was decreased in tumors that expressed EMMPRIN, but not in those with low EMMPRIN expression. A possible explanation is that without the influence of EMMPRIN, less VEGF is available to stimulate angiogenesis, and therefore bevacizumab is less effective. However, EMMPRIN is certainly not the only factor known to upregulate VEGF expression. A range of stimuli have been shown to upregulate VEGF gene expression, including: epidermal growth factor (EGF), fibroblast growth factor-4, platelet derived growth factor (PDGF), interleukin (IL-1) and IL-6, among others [30]. Furthermore microvessels were still seen within the tumor samples of the FaDu/siE xenografts, but they weren’t affected by treatment with bevacizumab. Although further investigation is required to fully explain these observations, it is possible that upregulation of other vascular growth factors (such as bFGF) is responsible for the vascularization of the FaDu/siE xenografts [39].
From this pilot study of HNSCC xenografts, EMMPRIN appears to be required for tumors to respond to bevacizumab therapy. The precise understanding of how EMMPRIN activity is involved in angiogenesis and anti-VEGF therapy requires further study and has significant implications for cancer treatment. By demonstrating effectiveness of bevacizumab therapy in tumors that express EMMPRIN, but not in tumors with silenced EMMPRIN expression, this study suggests that EMMPRIN levels may aid in determining which patients will respond to bevacizumab treatment.
Acknowledgments
This work was supported by grants from the National Cancer Institute (NCI K08CA102154) and the National Institute of Health (2T32 CA091078-06).
Footnotes
Conflict of Interest Statement: None of the authors have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones CLA. Cancer Biology. In: Longe JL, editor. Gale Encyclopedia of Cancer. Gale; Detroit: 2006. pp. 192–198. [Google Scholar]
- 2.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Davis-Smyth T. The Biology of Vascular Endothelial Growth Factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 4.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–8. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular Matrix Metalloproteinase Inducer Stimulates Tumor Angiogenesis by Elevating Vascular Endothelial Cell Growth Factor and Matrix Metalloproteinases. Cancer Res. 2005;65:3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 6.Moses MA. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180–9. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- 7.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–49. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 8.Zucker S, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J, Foda HD, Tompkins DC, Toole BP. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001;158:1921–8. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9. [PubMed] [Google Scholar]
- 10.Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G, Pantel K. High incidence of EMMPRIN expression in human tumors. Int J Cancer. 2006;119:1800–10. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 11.Bordador LC, Li X, Toole B, Chen B, Regezi J, Zardi L, Hu Y, Ramos DM. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer. 2000;85:347–52. [PubMed] [Google Scholar]
- 12.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003;113:1406–10. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177–84. doi: 10.1016/s0304-3835(00)00485-7. [DOI] [PubMed] [Google Scholar]
- 14.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–8. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 15.Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, Birembaut P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002;19:697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- 16.Braundmeier AG, Fazleabas AT, Lessey BA, Guo H, Toole BP, Nowak RA. Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium. J Clin Endocrinol Metab. 2006;91:2358–65. doi: 10.1210/jc.2005-0601. [DOI] [PubMed] [Google Scholar]
- 17.Dalberg K, Eriksson E, Enberg U, Kjellman M, Backdahl M. Gelatinase A, membrane type 1 matrix metalloproteinase, and extracellular matrix metalloproteinase inducer mRNA expression: correlation with invasive growth of breast cancer. World J Surg. 2000;24:334–40. doi: 10.1007/s002689910053. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–9. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 19.Jia L, Cao J, Wei W, Wang S, Zuo Y, Zhang J. CD147 depletion down-regulates matrix metalloproteinase-11, vascular endothelial growth factor-A expression and the lymphatic metastasis potential of murine hepatocarcinoma Hca-F cells. Int J Biochem Cell Biol. 2007;39:2135–42. doi: 10.1016/j.biocel.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res. 2006;4:371–7. doi: 10.1158/1541-7786.MCR-06-0042. [DOI] [PubMed] [Google Scholar]
- 21.Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95:1371–8. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Erkan M, Abiatari I, Giese NA, Felix K, Kayed H, Buchler MW, Friess H, Kleeff J. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther. 2007;6:218–27. doi: 10.4161/cbt.6.2.3623. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, Wu Y, Li J, Chen M, Chang J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66:11323–30. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 24.Liang Q, Xiong H, Gao G, Xiong K, Wang X, Zhao Z, Zhang H, Li Y. Inhibition of basigin expression in glioblastoma cell line via antisense RNA reduces tumor cell invasion and angiogenesis. Cancer Biol Ther. 2005;4:759–62. doi: 10.4161/cbt.4.7.1828. [DOI] [PubMed] [Google Scholar]
- 25.Newman JR, Bohannon IA, Zhang W, Skipper JB, Grizzle WE, Rosenthal EL. Extracellular Matrix Metalloprotease Inducer Modulates Tumor Cell Growth In Vivo. Arch Otolaryngol Head Neck Surg. 2008 doi: 10.1001/archotol.134.11.1218. Accepted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 27.Di Costanzo F, Mazzoni F, Mela M Micol, Antonuzzo L, Checcacci D, Saggese M, Di Costanzo F. Bevacizumab in non-small cell lung cancer. Drugs. 2008;68:737–46. doi: 10.2165/00003495-200868060-00002. [DOI] [PubMed] [Google Scholar]
- 28.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 29.Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y, Nishimura G, Matsuda H, Tsukuda M. Anti-tumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep. 2007;18:47–51. [PubMed] [Google Scholar]
- 30.Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13:1845–57. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 31.Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, Luh KT, Yang PC, Lee YC. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol. 2001;19:432–41. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 32.Volm M, Koomagi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64–8. doi: 10.1002/(sici)1097-0215(19970220)74:1<64::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Ko AH, Dito E, Schillinger B, Venook AP, Xu Z, Bergsland EK, Wong D, Scott J, Hwang J, Tempero MA. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008 doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal EL, Vidrine DM, Zhang W. Extracellular matrix metalloprotease inducer stimulates fibroblast-mediated tumor growth in vivo. Laryngoscope. 2006;116:1086–92. doi: 10.1097/01.mlg.0000224368.58870.3c. [DOI] [PubMed] [Google Scholar]
- 35.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–61. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 36.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 37.de Gramont A, Van Cutsem E. Investigating the potential of bevacizumab in other indications: metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology. 2005;69(Suppl 3):46–56. doi: 10.1159/000088483. [DOI] [PubMed] [Google Scholar]
- 38.Sandler AB, Johnson DH, Herbst RS. Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res. 2004;10:4258s–4262s. doi: 10.1158/1078-0432.CCR-040023. [DOI] [PubMed] [Google Scholar]
- 39.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]