Abstract
BACKGROUND
Alcohol intoxication impairs innate immune responses to bacterial pneumonia, including neutrophil influx. Lipopolysaccharide (LPS)-induced chemokine (LIX or CXCL5) is a recently described chemokine produced by type II alveolar epithelial (AE2) cells which facilitates neutrophil recruitment. The effect of acute alcohol intoxication on AE2 cell expression of LIX is unknown.
METHODS
C57BL/6 mice were given an intraperitoneal (i.p.) injection of ethanol (4 g/kg) or saline 30 min prior to intratracheal (i.t.) injection with 10 μg E. coli LPS. In vitro stimulation of primary AE2 cells or murine AE2 cell line MLE-12 was performed with LPS and tumor necrosis factor-alpha (TNF-α).
RESULTS
LIX protein is readily detectable in the lung but not plasma following LPS administration, demonstrating “compartmentalization” of this chemokine during pulmonary challenge. In contrast to the CXC chemokines keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2), which are abundantly expressed in both lung tissue and alveolar macrophages, LIX expression is largely confined to the lung parenchyma. Compared to controls, intoxicated animals show a decrease in LIX and neutrophil number in bronchoalveolar lavage (BAL) fluid following LPS challenge. Ethanol inhibits LIX at the transcriptional level. In vitro studies show that LPS and TNF-αare synergistic in inducing LIX by either primary AE2 or MLE-12 cells. Acute ethanol exposure potently and dose-dependently inhibits LIX expression by AE2 cells. Activation of nuclear factor-κB (NF-κB) is critical to LIX expression in MLE-12 cells, and acute ethanol treatment interferes with early activation of this pathway as evidenced by impairing phosphorylation of p65 (RelA). Inhibition of p38 mitogen-activated protein kinase (MAPK) signaling, but not ERK1/2 activity, in MLE-12 cells by acute alcohol is likely an important cause of decreased LIX expression during challenge.
CONCLUSIONS
These data demonstrate direct suppression of AE2 cell innate immune function by ethanol and add to our understanding of the mechanisms by which acute intoxication impairs the lung’s response to microbial challenge.
Keywords: ALCOHOL, LIX, CXCL5, CHEMOKINES, LIPOPOLYSACCHARIDE, LUNG, ALVEOLAR EPITHELIUM, NF-κB
Introduction
The lung is routinely exposed to pathogenic bacteria. Successful pulmonary host defense requires the coordinated recruitment of appropriate effector cells into infected tissue for microbial killing. Alveolar recruitment of neutrophils is an early and critical component of pulmonary innate immunity and is mediated by the expression of CXC chemokines containing the Glu-Leu-Arg motif (Mizgerd, 2002). These so-called ELR+ CXC chemokines bind CXCR2 found on neutrophils. The most-characterized murine ELR+ CXC chemokines are keratinocyte cell-derived chemokine (KC or CXCL1), macrophage inflammatory protein-2 (MIP-2 or CXCL2/3), and more recently, lipopolysaccharide (LPS)-induced CXC chemokine (LIX or CXCL5). LIX is expressed by type II alveolar epithelial (AE2) cells in response to airway LPS challenge and is necessary for normal pulmonary neutrophil recruitment in this model (Jeyaseelan et al., 2004).
While chronic alcohol abuse has long been associated with an increased risk of pneumonia (Osler, 1905), it is now appreciated that acute intoxication, without a history of alcoholism, is also a risk factor for bacterial pneumonia (Ruiz et al., 1999). The effect of acute alcohol intoxication on pulmonary neutrophil recruitment and function during infection has been well-studied (Nelson et al., 1989a; Zhang et al., 2007). It is now appreciated that the alveolar epithelium plays an important role in the lung’s innate immune response, including neutrophil recruitment (Mason, 2006). However, little work has focused on the effect of acute ethanol on alveolar epithelial cell function in the early inflammatory response to bacterial challenge. Our current study evaluates the effect of acute alcohol intoxication on LPS-induced pulmonary expression of LIX, a chemokine abundantly expressed by AE2 cells. Here we show that acute ethanol intoxication inhibits pulmonary LIX production and alveolar neutrophil influx. AE2 cell release of LIX is critically dependent upon intact nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (MAPK) signaling, and alcohol inhibits activation of these pathways. The relatively low dose (25 mM) of ethanol required to inhibit these cell functions suggests AE2 cells are quite sensitive to ethanol, and their dysfunction may be a significant consequence of acute intoxication.
Materials and Methods
Mice
Specific pathogen-free male wild-type C57BL/6 mice (Charles River Laboratories; Wilmington, MA) were used at 6 to 8 weeks of age. All mice were housed in the LSUHSC vivarium and treated in accordance with institutional guidelines. Mice were provided with food and water adlibitum and received 12-hr light/dark cycles. All procedures were approved by the LSUHSC Animal Care and Use Committee.
Alcohol administration and intratracheal injection
Mice received intraperitoneal (i.p.) injection with ethanol [4.0 g/kg; 20% v/v ethanol in sterile phosphate buffered saline (PBS)] or an equivalent volume of PBS. Thirty minutes after i.p. injection, animals were anesthetized with isoflurane for exposure and direct intratracheal (i.t.) administration of 10 μg Escherichia coli LPS (List Biological Laboratories; Campbell, CA). n ≥5 per group for all in vivo experiments.
Blood, lung and bronchoalveolar lavage (BAL) harvest
At designated time points, mice were anesthetized with isoflurane and sacrificed by diaphragmatic interruption. Whole blood was obtained by right ventricular puncture. Plasma was isolated by supernatant aspiration following centrifugation at 10,000g for 1 min. Lungs were removed en bloc and homogenized (Omni GLH, Omni International; Warrenton, VA) in 1 mL of buffer RLT (RNeasy system, Qiagen; Valencia, CA) for RNA isolation or PBS with 0.1% Triton X-100 and a protease inhibitor cocktail (Complete Mini; Roche Diagnostics; Indianapolis, IN) for protein analysis. BAL cells were retrieved by lavage of removed lungs with PBS containing 0.1% glucose. A total of 10 mL lavage was performed in 1-mL aliquots. The supernatant from the initial 1-mL aliquot was used for protein analysis.
Plasma ethanol concentration
Alcohol measurements were made by analysis of 10-μL aliquots of plasma using the AM1 Alcohol Analyzer (Analox Instruments; Lunenburg, MA).
Type-II alveolar epithelial cell isolation and cell culture
Primary AE2 cells were prepared using modification of previously published protocols (Corti, Brody, & Harrison, 1996;Gereke et al., 2007). Briefly, mice were sacrificed and the lungs were perfused with 10 mL PBS via the right ventricle until visually free of blood. 2 mL dispase (BD Biosciences; Bedford, MA) was instilled into the lungs immediately followed by 500 μL 1% low-melt agarose (Sigma-Aldrich; St. Louis, MO). Lungs were removed en bloc, placed in a 4°C bath of PBS for 2 minutes to set the agarose, and then moved to a culture dish containing an additional 2 mL dispase and incubated at room temperature for 45 min. The lungs were then transferred to a culture dish containing 7 mL solution of DMEM and 25 mM HEPES with 350 μg DNase I (Sigma-Aldrich). The lung parenchyma was teased away from the tracheobronchial tree and the resultant suspension was agitated for 10 min at room temperature followed by sequential filtration through 70 μm and 30 μm nylon mesh screens. Cells were incubated in biotinylated anti-CD16/32 (0.65 μg per million cells) and anti-CD45 (1.5 μg per million cells) (eBioscience; San Diego, CA) for 30 minutes at 4°C. Negative selection was performed using streptavidin-coated magnetic beads (CELLectionTM Dynabeads®, Invitrogen; Carlsbad, CA) per the manufacturer’s protocol. Cell purity was at least 85% as validated by pro-surfactant protein C antibody staining (Chemicon AB3786; Temecula, CA). The mouse alveolar epithelial cell line MLE-12 (ATCC #CRL-2110) was also used for in vitro studies. The cells were regularly passed in DMEM/F-12 medium with 1.94 mM L-glutamine adjusted to contain 10 mM HEPES, 5 μg/mL insulin, 10 μg/mL transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM β-estradiol, penicillin, streptomycin, and 2% fetal bovine serum. Cells were seeded at 4 x 105 cells/mL in 96-well plates. In designated alcohol experiments, culture media was replaced with media containing 0, 25, 50, or 100 mM ethanol 90 min prior to stimulation. Ethanol concentrations were maintained by placing plates in dedicated CO2 incubators containing a water bath with ethanol, as previously described (Zhang et al., 2001). In vitro stimulation was performed by adding E. coli LPS at 500 ng/mL final concentration and/or recombinant mouse TNF-α(eBioscience) at 10 ng/mL final concentration. In separate experiments, ERK1/2 inhibitors PD 98059 and U0126, p38 inhibitors SB202190 and SB203580 (InvivoGen; SanDiego, CA), or NF-κB p65 inhibitors SN50 and BAY 11–7082 were used (all inhibitors from Calbiochem; La Jolla, CA except as noted). Pretreatment duration was 90 min for MAPK inhibitors and 60 min for NF-κB inhibitors.
ERK1/2, p38, NF-κB p65 (RelA), and LIX ELISA
Total and phosphorylated ERK1/2 (p44 and p42) and p38 (T180/Y182) concentrations were determined using a fluorometric ELISA (R&D Systems). Total and phosphorylated (S468/S536) NF-κB p65 (RelA) concentrations were determined using a colorimetric cell-based ELISA (Active Motif; Carlsbad, CA). LIX concentrations in cell culture supernatants were determined by colorimetric sandwich ELISA (R&D Systems).
Real-time reverse transcription and polymerase chain reaction (RT-PCR)
Total RNA from BAL cells and lung homogenates was isolated using RNeasy mini kit (Qiagen). RNA (10 ng) was subjected to two-step RT-PCR using Taqman® linear hydrolysis chemistry on the iCycler thermocycler (Bio-Rad; Hercules, CA). Sequences for LIX are as follows (forward primer, reverse primer, probe): 5′-AGC TGC CCC TTC CTC AGT C -3′, 5′-TTT CTT TAT CAC AGG AGC TTC TGG -3′, 5′-6-FAM/AGC TAT GAC TTC CAC CGT AGG GCA CTG T/3BHQ-1 -3′. Data were normalized to 18s ribosomal RNA content (also determined by real-time RT-PCR) and are expressed as fold induction over baseline expression.
Statistical analysis
Comparisons between different treatment groups were made using Student’s t-test for simple pair-wise comparisons. For data not normally distributed, values were log10 transformed prior to analysis. Differences between treatment groups were accepted as significant when p<0.05.
Results
Lung LIX expression and neutrophil influx following intratracheal LPS
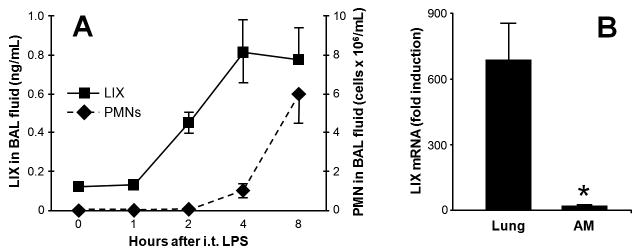
To determine the kinetics of pulmonary LIX expression, we challenged male C57BL/6 with i.t. injection of 10 μg LPS. Fig. 1A shows the onset of LIX protein expression and neutrophil recruitment into the alveolar space in this model. Lung transcripts for LIX peak 2 h post-challenge, and mRNA expression during challenge is largely confined to lung parenchymal cells as minimal LIX induction is seen in alveolar macrophages (AM) (Fig. 1B). These findings are consistent with a previous study demonstrating that endotoxin-induced LIX production in the lung is predominantly by AE2 cells (Jeyaseelan et al., 2005).
Figure 1.
Onset of LIX expression and neutrophil recruitment following i.t. LPS challenge (10 }g). C57BL/6 mice were sacrificed at specified time points for BAL. Alveolar LIX protein expression increases after 1 h and peaks 4 h post-challenge. Neutrophil recruitment into the alveolar space quickly increases following the peak of LIX expression (A). LIX mRNA expression (peak level 2 h post-LPS) is largely confined to lung parenchymal cells, as minimal induction is seen in alveolar macrophages (AM) (B). * p<0.05 vs. lung tissue.
Pulmonary LIX expression is inhibited by acute alcohol administration
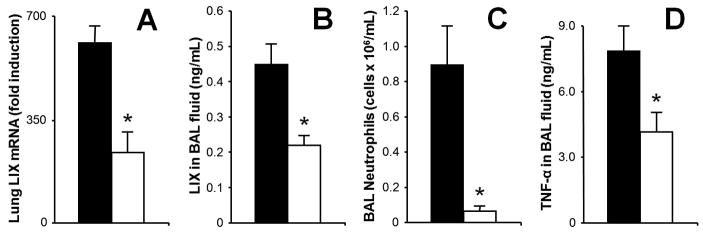
Our laboratory has previously shown that acute intoxication impairs pulmonary neutrophil chemokine induction, including KC and MIP-2 in mice (Happel et al., 2007), and cytokine-induced neutrophil chemoattractant (CINC; the rat orthologue of murine KC) and MIP-2 in rats (Boe et al., 2001;Quinton et al., 2005) during microbial challenge. Because LIX is a more recently described chemokine whose expression (in contrast to KC and MIP-2) appears limited to the lung parenchyma, we examined whether ethanol would affect this chemokine as well. 30 min pre-treatment with i.p. ethanol inhibits lung LIX mRNA and protein expression in BAL fluid at time points which represent their peak expression following i.t. LPS-challenge (Fig. 2A and 2B). At 4 h, alveolar neutrophil recruitment is significantly reduced (Fig. 2C). The BAL concentration of TNF-αfollowing i.t. LPS is also significantly impaired by acute intoxication (Fig. 2D), an observation consistent with a prior report using a similar model (D'Souza El-Guindy, de Villiers, & Doherty, 2007). Plasma ethanol levels at 90 min and 4 h post-i.t. LPS were 669.93 ± 10.05 mg/dL and 218.66 ± 18.54 mg/dL, respectively.
Figure 2.
LPS-induced LIX, TNF-αexpression and neutrophil recruitment are attenuated during acute intoxication. C57BL/6 mice were given i.p. injection of PBS (black bars) or ethanol (4.0g/kg, open bars) followed by 10 }g i.t. LPS challenge 30 min later. Ethanol intoxication inhibits both lung LIX mRNA transcription at 2 h (A) and protein expression at 4 h (B) following LPS challenge, times which represent their peaks of expression. 4 h neutrophil recruitment into alveolar space is significantly impaired during intoxication (C) as is 90 min TNF-αin BAL fluid (D). * p<0.05 vs. non-intoxicated mice.
AE2 LIX expression is inhibited by acute ethanol treatment
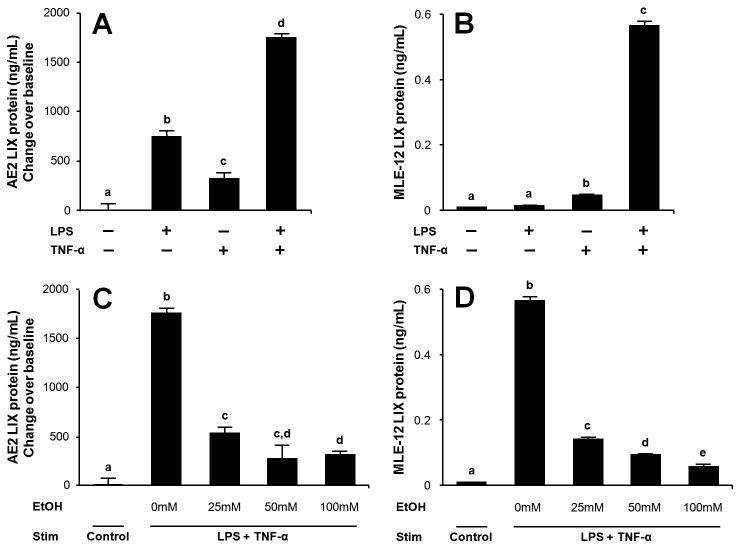
Because AE2 cells are known to secrete LIX during in vivo endotoxin challenge, we investigated whether ethanol affects LIX production by these cells. Primary isolated AE2 cells were rested overnight, media changed, and stimulated with LPS, TNF-α, or both stimuli for 16 h. Primary murine AE2 cells isolates spontaneously produce LIX (approximately 1ng/mL after 16 h culture) in the absence of LPS or TNF-α. Either stimulus alone increases LIX expression, and the combination stimulus shows a mildly synergistic effect on LIX induction (Fig. 3A). 90 min pre-treatment with ethanol suppresses LIX induction (Fig. 3C). We also tested the effect of ethanol on MLE-12 cells, a line derived from primary murine AE2 cells which displays similar morphology and function as primary cells (Ohinata et al., 2005;Tong et al., 2006;Wikenheiser et al., 1993). MLE-12 cells do not spontaneously release LIX but show a markedly synergistic LIX response to combined LPS and TNF-αtreatment (Fig. 3B). A substantial alcohol dose-dependent inhibition of chemokine release is also observed in MLE-12 cells, where an approximately 75% decrease occurs in the 25 mM ethanol exposure group (Fig. 3D).
Figure 3.
Primary AE2 cell and MLE-12 cell LIX expression is suppressed by acute alcohol exposure. Cells were harvested, allowed to rest overnight, and stimulated for 16 hr with LPS (500 ng/mL) or TNF-α(10 ng/mL) alone or in combination. Either stimulus alone is sufficient for LIX expression in primary AE2 cells, whereas combined stimulation results in maximal LIX expression (A). In MLE-12 cells, combination stimulation induces a robust LIX response (B). 90-min ethanol pretreatment followed by LPS/TNF-αstimulation shows that alcohol dose-dependently suppresses LIX expression in primary AE2 cells (C) and MgLE-12 cells (D). Letters indicate statistically significant differences (p<0.05).
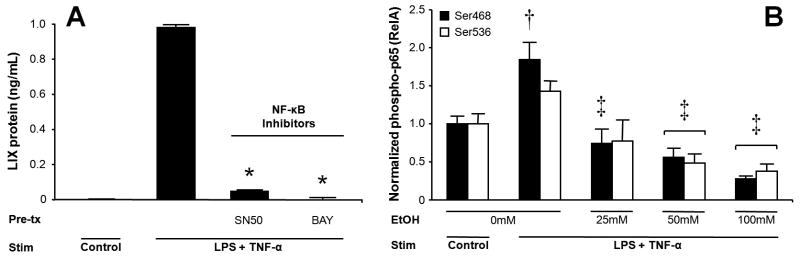
Acute ethanol exposure suppresses activation of NF-κB p65 (RelA), which is critical for LIX expression in MLE-12 cells
Prior work suggests NF-κB signaling is necessary for LIX expression in rat cardiomyocytes in response to oxidative stress (Chandrasekar, Smith, & Freeman, 2001). An inhibitory effect of acute ethanol on NF-κB activation has been shown in monocytes (Mandrekar, Catalano, & Szabo, 1999), murine peritoneal cells (Pruett et al., 2004), and the RAW 264.7 cells (Mandrekar et al., 2006). We therefore examined whether NF-κB is important to alveolar epithelial cell LIX expression, and what, if any, effect acute ethanol exposure had on NF-κB activation in these cells. Fig. 4A shows that MLE-12 LIX release is critically dependent on intact NF-κB signaling. During stimulation, acute alcohol treatment significantly reduces phosphorylation of the major trans-activating NF-κB domain p65 (RelA) at critical Ser468 and Ser536 residues, reflecting a substantial and dose-dependent decrease in the level of active NF-κB in the presence of ethanol (Fig. 4B).
Figure 4.
LIX expression by MLE-12 cells is dependent upon NF-κB signaling. 60 min pretreatment with NF-κB inhibitors SN50 or BAY 11–7082 prior to 16-hr LPS/TNF-αstimulation markedly impairs LIX production (A). 90 min ethanol treatment prior to 16-hr LPS/TNF-αstimulation significantly reduces phosphorylation of the major trans-activating NF-κB domain p65 (RelA) at Ser468 and Ser536 residues, reflecting a substantial and ethanol dose-dependent decrease in the level of active NF-κB signaling (B). * p<0.05 vs. stimulated cells in absence of inhibitor. † p<0.05 vs. unstimulated cells. ‡ p<0.05 vs. stimulated cell without ethanol pre-treatment.
MAPK pathways are necessary for LIX expression in LPS-challenged MLE-12 cells
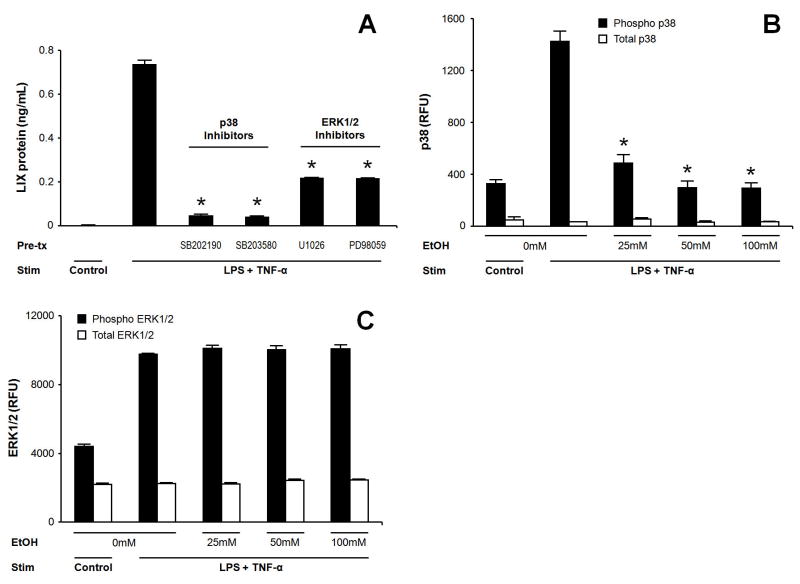
MAPK activity is a critical component of the early inflammatory response in many cell types. Prior work has shown murine AE2 LIX expression is at least partially p38 MAPK dependent, (Jeyaseelan et al., 2005) but no data exist concerning the role of “extracellular” stimulated MAPK (ERK1/2) on alveolar epithelial cell LIX expression. We examined the ERK1/2 and p38 signaling requirement for LIX expression by MLE-12 cells during LPS/TNF-αtreatment. Although LIX expression decreases during blockade of either MAPK pathway, near abolishment of LIX release is seen with p38 inhibition (Fig. 5A).
Figure 5.
MAPK pathway activation is necessary for LIX expression in LPS-challenged MLE-12 cells and shows selective suppression by alcohol exposure. MLE-12 cells were pretreated for 90-min with selective p38 inhibitors (10 μM SB202190 or 0.5 μM SB203580) or ERK1/2 inhibitors (0.1 μM U0126 or 50 μM PD98059) prior to 16-hr LPS/TNF-αstimulation. MAPK blockade decreases LIX expression, with a greater effect of p38 inhibition (A). 90-min ethanol pretreatment prior to 16-hr LPS/TNF-αstimulation inhibits p38 phosphorylation/activation (B), but has no effect on ERK1/2 phosphorylation/activation (C). * p<0.05 vs. stimulated cells in absence of inhibitor or ethanol.
Acute ethanol exposure inhibits activation of p38 but not ERK1/2 in MLE-12 cells
Acute ethanol treatment has been associated with a decrease in MAPK phosphorylation in response to LPS following both in vivo (Goral, Choudhry, & Kovacs, 2004;Kato, Negoro, & Wakabayashi, 2005) and in vitro (Oak et al., 2006) intoxication. Because both p38 and ERK1/2 MAPK pathways appear to play a role in mediating LIX expression in MLE-12 cells, we sought to examine the effect of acute ethanol exposure on MAPK phosphorylation in MLE-12 cells following stimulation with LPS/TNF-α. Ethanol treatment prior to stimulation inhibits p38 phosphorylation but has no effect on ERK1/2 activation (Fig. 5B and 5C).
Discussion
Here we show that alcohol impairs expression of the neutrophil chemokine LIX both in vivo and in vitro following acute exposure. This effect is ethanol dose-dependent and likely mediated through impairment of MAPK and NF-κB signaling in the alveolar epithelium. Although much work has previously shown inhibition of chemokines and neutrophil influx into the lungs of acutely intoxicated and infected animals, this is the first demonstration that ethanol directly suppresses alveolar epithelial cell CXC chemokine expression. Because LIX is required for normal pulmonary neutrophil recruitment during local endotoxin challenge (Jeyaseelan et al., 2004), we believe this work is novel in describing one aspect of the impairment alcohol has on the alveolar epithelial cell contribution to innate host defenses.
Recruitment of leukocytes is critical to an effective response during pulmonary infection. As the resident immune cell in the alveolar space, the AM is pivotal in the initiation of the inflammatory response through early phagocytosis and secretion of “alarm” cytokines. TNF-αis a well-described cytokine released by AM which mediates pulmonary host defenses and neutrophil recruitment (Nelson et al., 1989b). Here we confirm previous findings that acute ethanol treatment impairs pulmonary TNF-αexpression during LPS challenge (D'Souza El-Guindy, de Villiers, & Doherty, 2007). Although LPS is known to induce LIX release by AE2 cells (Jeyaseelan et al., 2005), the specific signaling requirements for physiologic LIX expression during airway endotoxin challenge are unknown. Our current work shows LPS and TNF-αare synergistic in inducing release of LIX by AE2 cells. These findings are consistent with prior work showing that TNF-αpotentiates the release of LIX by MC3T3 cells (Ruddy et al., 2004) and the LIX orthologue ENA-78 by A549 cells (Sachse et al., 2005). In the lung, TNF-αmay function as an “amplifier” which is required for maximal AE2 cell LIX expression, in the absence of which LIX expression to LPS is attenuated. This protective mechanism may prevent inappropriate neutrophil recruitment during low-level endotoxin exposure in the alveolar compartment.
Alcohol intoxication disrupts innate immune responses to infection (Happel & Nelson, 2005) and is a well-described clinical modifier for bacterial pneumonia (Schmidt & De, 1972). Our laboratory has previously shown that acute intoxication decreases release of the neutrophil chemokines CINC and MIP-2 in rats as well as KC and MIP-2 in mice, leading to suppression of alveolar neutrophil recruitment following acute alcohol intoxication (Quinton et al., 2005; Boe et al., 2001; Happel et al., 2007)). Unlike KC and MIP-2, LIX is not expressed by leukocytes (Smith & Herschman, 1995) and thus provides an opportunity to study the effect of alcohol on epithelial cell neutrophil chemokine expression, both in vivo and in vitro. In this study, we show suppression of LIX by acute alcohol exposure, thus demonstrating a direct effect on an innate defense function of lung stromal cells. We feel this is an important finding in light of work emphasizing the role of pulmonary epithelial cells in the host response to infection (Mason, 2006; Diamond, Legarda, & Ryan, 2000). The observation of decreased lung LIX mRNA content in the intoxicated group is the result of impaired transcriptional activation, decreased mRNA stability, or both.
The NF-κB family of transcription factors is a central mediator of acute inflammatory genes. Chandrasekar and others have shown a role for NF-κB signaling in LIX production in rodent cardiomyocytes (Chandrasekar, Smith, & Freeman, 2001). Our computational inspection of the murine LIX promoter region suggests NF-κB binding affinity, and our in vitro work confirms alveolar epithelial expression of LIX in response to LPS/TNF-αis critically dependent on NF-κB. Previous work has shown that NF-κB DNA binding activity in LPS-stimulated monocytes is decreased by acute alcohol exposure, and this defect is not the result of impaired IκB degradation (Mandrekar et al., 2006; (Mandrekar et al., 2002). In addition, acute exposure results in decreased nuclear localization of p65 (RelA) without an effect on the non-activating p50 subunit. This results in an increase in the ratio of the inhibitory p50/p50 homodimer to the trans-activating p65/p50 heterodimer in the nucleus. Our current work adds to the existing knowledge of acute alcohol and RelA signaling by demonstrating a defect in phosphorylation of critical serine residues within the RelA protein, an early and necessary step in the NF-κB activation pathway (Brown et al., 1995;Joo & Jetten, 2008). These findings are consistent with the previously reported IκB-independent defect caused by alcohol on RelA nuclear translocation. There are multiple kinase systems responsible for phosphorylation of RelA (Ci et al., 2008;Sun et al., 2008a), and we are currently investigating whether alcohol inhibits these kinase pathways. It is also plausible that alcohol induces phosphatase activity that degrades phospho-RelA, and this possibility is also under study. These findings are the first to extend acute alcohol administration’s derangement of NF-κB signaling to the alveolar epithelium. Given the central role of lung epithelial cell NF-κB signaling in the initiation of pulmonary innate immune responses to infection (Quinton et al., 2007), it is likely that multiple features of innate host defense that occur in the alveolar epithelium are impaired by the effect of alcohol on NF-κB.
LPS, through TLR4 signaling, causes phosphorylation of ERK1/2, p38, and JNK MAP kinases, and these pathways activate NF-κB (Saklatvala, Dean, & Clark, 2003). Ex vivo inhibition of p38 signaling decreases inflammatory cytokine release from human lung tissue following infection with S. pneumoniae (Xu et al., 2008). Inhibition of the related p38 and JNK kinases has a similar detrimental effect on LIX expression by human corneal epithelial cells (Sun et al., 2008b) and in a murine model of LPS-induced lung injury (Jeyaseelan et al., 2005). Our inhibitor data suggest LIX is at least partially dependent on ERK1/2 signaling in MLE-12 cells, in agreement with previous work using murine cardiac fibroblasts (Lafontant et al., 2006). We did not expect the finding that acute exposure to alcohol (up to 100 mM) would not impede ERK1/2 activation in MLE-12 cells, as our prior study using the MH-S murine AM cell line revealed impaired phosphorylation of this signaling cascade at 50 mM (Happel et al., 2007). Others have also shown that human blood monocyte ERK1/2 activation is alcohol-sensitive (Oak et al., 2006). In contrast, inhibition of p38 MAPK reveals a critical dependence on this pathway for MLE-12 cell LIX production. Similar to previous findings in monocytes (Drechsler et al., 2006), we now show acute alcohol treatment dose-dependently inhibits activation of the p38 kinase system in an alveolar epithelial cell line. This “selectivity” of MAP kinase inhibition in MLE-12 cells argues against a non-specific toxic effect of ethanol in this model system, as does the pronounced p38 inhibition at 25 mM ethanol, a relatively modest concentration corresponding to a blood alcohol content of roughly 0.1%. We conclude that alcohol-sensitive (and insensitive) signaling pathways are differentially expressed and utilized by the various cells types. Because 25 mM ethanol showed substantial inhibition of MAPK and NF-κB activation (in the MLE-12 cells) and impaired LIX release by primary AE2 cells, we conclude that dysfunction of the innate immune response by these cells occurs at physiologically relevant concentrations of ethanol achieved by acute ingestion.
Our current work has several limitations. Although we show ethanol inhibits lung LIX expression, we do not demonstrate, in vivo, that ethanol specifically decreases AE2 LIX expression. Since no other cell type is shown to express LIX in response to LPS challenge (Jeyaseelan et al., 2005), we feel confident our in vivo and in vitro studies implicate a substantial defect in AE2 innate immune function during intoxication. We cannot quantify the relative impact of LIX suppression on the overall defect in neutrophil recruitment into the lung during intoxication and LPS challenge. We and others have shown inhibition of the neutrophil chemokines KC and MIP-2 in lung during alcohol exposure, although not with the marked sensitivity to 25 mM ethanol as seen in vitro with AE2 cells shown herein. Furthermore, our intracellular signaling work was performed on an in vitro cell line model which may not precisely reflect the effects of ethanol in primary cells. MLE-12 cells and primary isolated AE2 cells share a p38 MAP kinase requirement for optimal LIX expression (Jeyaseelan et al., 2005), and, in this study, both show a similar pattern of LIX suppression by alcohol. Hence, we believe that the cell signaling results seen in MLE-12 experiments are likely representative. Lastly, both TLR4 and the TNF-αp55 receptor utilize MAPK and NF-κB pathways. Our signaling work therefore does not identify the effect of alcohol on specific upstream signaling events during combined TNF-αand LPS stimulation, the treatment condition yielding the greatest LIX response in AE2 cells.
In summary, we show acute ethanol intoxication impairs the pulmonary LIX response to intratracheal LPS. AE2 cells are an important source of LIX and express high levels of this chemokine during combined LPS and TNF-αtreatment. Epithelial cell function is sensitive to ethanol treatment, as demonstrated by the impairment in NF-κB activation and selective inhibition of p38 MAPK at low ethanol concentrations. These data extend our understanding of acute intoxication’s effect on alveolar epithelial cell innate immune function during bacterial challenge.
Acknowledgments
Supported by NIH K08 AA-15163 (KH) and NIH P60 AA-009803 (GB, SN) from the National Institute on Alcohol Abuse and Alcoholism, and NIH R01 HL-091958 (SJ) from the National Heart, Lung, and Blood Institute
References
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184(9):1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103(18):2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- Ci X, Song Y, Zeng F, Zhang X, Li H, Wang X, Cui J, Deng X. Ceftiofur impairs pro-inflammatory cytokine secretion through the inhibition of the activation of NF-kappaB and MAPK. Biochem Biophys Res Commun. 2008;372(1):73–77. doi: 10.1016/j.bbrc.2008.04.170. [DOI] [PubMed] [Google Scholar]
- Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14(4):309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- D'Souza El-Guindy NB, de Villiers WJ, Doherty DE. Acute alcohol intake impairs lung inflammation by changing pro- and anti-inflammatory mediator balance. Alcohol. 2007;41(5):335–345. doi: 10.1016/j.alcohol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, Bach FH, Mandrekar P, Szabo G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol. 2006;177(4):2592–2600. doi: 10.4049/jimmunol.177.4.2592. [DOI] [PubMed] [Google Scholar]
- Gereke M, Grobe L, Prettin S, Kasper M, Deppenmeier S, Gruber AD, Enelow RI, Buer J, Bruder D. Phenotypic alterations in type II alveolar epithelial cells in CD4+ T cell mediated lung inflammation. Respir Res. 2007;8(47) doi: 10.1186/1465-9921-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J Leukoc Biol. 2004;75(3):553–559. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41(5):325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72(12):7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32(6):531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Jetten AM. NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway. J Biol Chem. 2008;283(24):16391–16399. doi: 10.1074/jbc.M800945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Negoro M, Wakabayashi I. Effects of acute ethanol administration on LPS-induced expression of cyclooxygenase-2 and inducible nitric oxide synthase in rat alveolar macrophages. Alcohol Clin Exp Res. 2005;29(12 Suppl):285S–293S. doi: 10.1097/01.alc.0000191809.29775.41. [DOI] [PubMed] [Google Scholar]
- Lafontant PJ, Burns AR, Donnachie E, Haudek SB, Smith CW, Entman ML. Oncostatin M differentially regulates CXC chemokines in mouse cardiac fibroblasts. Am J Physiol Cell Physiol. 2006;291(1):C18–C26. doi: 10.1152/ajpcell.00322.2005. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11(11):1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30(1):135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, Szabo G. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26(11):1609–1614. doi: 10.1097/01.ALC.0000036926.46632.57. [DOI] [PubMed] [Google Scholar]
- Mason RJ. Biology of alveolar type II cells. Respirology. 2006;(11 Suppl):S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14(2):123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989a;160(3):422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Wilson LA, Thompson JJ, Summer WR. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989b;159(2):189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. J Immunol. 2006;176(12):7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Ohinata A, Nagai K, Nomura J, Hashimoto K, Hisatsune A, Miyata T, Isohama Y. Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells. Biochem Biophys Res Commun. 2005;326(3):521–526. doi: 10.1016/j.bbrc.2004.10.216. [DOI] [PubMed] [Google Scholar]
- Osler W. The Principles and Practice of Medicine. Appleton; New York: 1905. [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004;173(4):2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178(3):1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton LJ, Nelson S, Zhang P, Happel KI, Gamble L, Bagby GJ. Effects of systemic and local CXC chemokine administration on the ethanol-induced suppression of pulmonary neutrophil recruitment. Alcohol Clin Exp Res. 2005;29(7):1198–1205. doi: 10.1097/01.alc.0000171927.66130.aa. [DOI] [PubMed] [Google Scholar]
- Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76(1):135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160(3):923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- Sachse F, Ahlers F, Stoll W, Rudack C. Neutrophil chemokines in epithelial inflammatory processes of human tonsils. Clin Exp Immunol. 2005;140(2):293–300. doi: 10.1111/j.1365-2249.2005.02773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J, Dean J, Clark A. Control of the expression of inflammatory response genes. Biochem Soc Symp. 2003;(70):95–106. doi: 10.1042/bss0700095. [DOI] [PubMed] [Google Scholar]
- Schmidt W, De LJ. Causes of death of alcoholics. Q J Stud Alcohol. 1972;33(1):171–185. [PubMed] [Google Scholar]
- Smith JB, Herschman HR. Glucocorticoid-attenuated response genes encode intercellular mediators, including a new C-X-C chemokine. J Biol Chem. 1995;270(28):16756–16765. doi: 10.1074/jbc.270.28.16756. [DOI] [PubMed] [Google Scholar]
- Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008a;294(6):C1586–C1596. doi: 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- Sun Y, Fox T, Adhikary G, Kester M, Pearlman E. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J Leukoc Biol. 2008b;83(6):1512–1521. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Zheng L, Lin L, Li B, Wang D, Li D. Hypoxia-induced mitogenic factor promotes vascular adhesion molecule-1 expression via the PI-3K/Akt-NF-kappaB signaling pathway. Am J Respir Cell Mol Biol. 2006;35(4):444–456. doi: 10.1165/rcmb.2005-0424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA. 1993;90(23):11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Droemann D, Rupp J, Shen H, Wu X, Goldmann T, Hippenstiel S, Zabel P, Dalhoff K. Modulation of the Inflammatory Response to S. pneumoniae in a Model of Acute Lung Tissue Infection. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2007-0328OC. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhong Q, Bagby GJ, Nelson S. Alcohol intoxication inhibits pulmonary S100A8 and S100A9 expression in rats challenged with intratracheal lipopolysaccharide. Alcohol Clin Exp Res. 2007;31(1):113–121. doi: 10.1111/j.1530-0277.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Bagby GJ, Stoltz D, Oliver P, Schwarzenberger PO, Kolls JK. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-alpha production in human monocytic cells. Alcohol Clin Exp Res. 2001;25(3):444–449. [PubMed] [Google Scholar]