Abstract
Among a range of cognitive deficits, human cocaine addicts display increased impulsivity and decreased performance monitoring. In order to establish an animal model that can be used to study the underlying neurobiology of these deficits associated with addiction, we have developed a touch screen based Stop Signal Response Task for rhesus monkeys. This task is essentially identical to the clinically used Stop Signal Task employed for diagnostic and research purposes. In this task, impulsivity is reflected in the amount of time needed to inhibit a response after it has been initiated, the Stop Signal Response Time (SSRT). Performance monitoring is reflected by the slowing of response times following Stop trials (Post-Stop Slowing, PSS). Herein we report on the task structure, the staged methods for training animals to perform the task, and a comparison of performance values for control and cocaine experienced animals. Relative to controls, monkeys that had self-administered cocaine, followed by 18 months abstinence, displayed increased impulsivity (increased SSRT values), and decreased performance monitoring (decreased PSS values). Our results are consistent with human data, and thereby establish an ideal animal model for studying the etiology and underlying neurobiology of cocaine-induced impulse control and performance monitoring deficits.
Keywords: Rhesus Monkey, Macaque, Addiction, Cocaine, Cognition, Impulsivity, Performance Monitoring, Anterior Cingulate Cortex, Prefrontal Cortex
1. Introduction
Elevated impulsivity (Fillmore and Rush, 2002; Jentsch and Taylor, 1999; Li et al., 2006; Moeller et al., 2002) and other cognitive control deficits (Aron and Paulus, 2007; Li et al., 2006) accompany cocaine dependence, as well as other types of addictions. Because impulsivity is a predictor of negative treatment outcome (Patkar et al., 2004), understanding its underlying neurobiology and etiology is critically important for advancing treatment. It continues to be emphasized in the clinical literature that the role of preexisting traits of cognitive and behavioral control deficits, versus consequence of drug exposure, is unclear (Aron and Paulus, 2007). Therefore an animal model that can be used in longitudinal studies of the consequences of cocaine self-administration would be of significant value. Motoric impulsivity and other cognitive deficits linked to addiction are intimately associated with cortical dysfunction (Aron and Paulus, 2007; Jentsch and Taylor, 1999). Monkeys are an ideal animal model for studying cortical dysfunction because of their phylogenetic proximity to humans, resulting in highly concordant cortical development and neuroanatomical circuitry (Croxson et al., 2005). Also, the acute effects of cocaine on cerebral metabolism and chronic effects of cocaine on dopaminergic systems in monkeys and humans are similar, whereas both differ substantially from the rat (Bradberry, 2007). Monkeys are also better able to learn complex cognitive tasks that closely parallel those used clinically. Because of these characteristics, it is important to develop procedures for use in a non-human primate model to examine the critically important question of whether cocaine self-administration by itself results in elevated motoric impulsivity. To do this, we developed a touch screen-based Stop Signal Task in rhesus monkeys and evaluated performance differences between monkeys with a cocaine self-administration history and controls.
The Stop Signal Task is a clinically employed tool for diagnosis of impulse control disorders (Aron and Poldrack, 2005). This task measures motoric impulsivity by evaluating the time required to inhibit a response after it has been intiated, the Stop Signal Response Time (SSRT), which is elevated in cocaine users (Fillmore and Rush, 2002; Li et al., 2006). Performance monitoring is reflected behaviorally by an increase in response time (RT) on trials following conflict trials (Li et al., 2006; Ridderinkhof et al., 2004). The parameter that reflects this in the Stop Signal Task is the difference in RT before and after a Stop trial (Post-Stop trial Slowing, PSS). Performance monitoring deficits are reflected in cocaine users by decreased PSS values (Li et al., 2006), which is consistent with an extensive literature suggesting impaired function of prefrontal regions such as the anterior cingulate cortex (Garavan and Stout, 2005; Hester et al., 2007; Li et al., 2008) believed to help mediate performance monitoring (Emeric et al., 2007).
2. Materials and Methods
2.1. Subjects
Six adult Rhesus macaques (three male, three female) that were obtained as a single cohort were used in the present study. All procedures were in accord with the Principles of Laboratory Animal Care (NIH publication no. 86-23, revised 1996). For all behavioral procedures, animals were placed in a behavioral chair (Primate Products, Redwood City, CA) using standard pole and collar methods. All animals were initially trained to lever press for food pellets under an FR1 lever response schedule of reinforcement using a visual discriminative stimulus (a light on the panel) as previously described (Bradberry et al., 2000). After establishment of food self-administration, the two animals (one male, one female) that went on to chronically self-administer cocaine had a vascular access port placed mid-scapula from which a catheter extended subcutaneously to either a femoral or internal jugular vein (Wojnicki et al., 1994). Two of the control animals (13, 16, one male and one female) also had vascular access ports placed, providing a control for non-specific effects of the surgery. The cocaine animals underwent 24 months of progressive ratio self-administration studies designed to compare the reinforcer efficacy of cocaine and cocaethylene, a cocaine metabolite with equivalent dopaminergic properties as cocaine as well as local anesthetic effects (Tokuno et al., 2004), but with decreased potency at the noradrenergic and serotonergic transporters (Bradberry et al., 1993; Elsworth et al., 1993; Iyer et al., 1995). Mean total cumulative intake was 360 mg/kg cocaine, and 52 mg/kg cocaethylene (mass of the molar equivalent of cocaine hydrochloride). Individual values: Animal 15: 362 mg/kg cocaine; 90 mg/kg cocaethylene; Animal 19: 359 mg/kg cocaine; 13 mg/kg cocaethylene. Control animal 16 had originally been slated for cocaine self-administration, and had several initial training sessions at a unit dose of 0.1 mg/kg, with a total cumulative consumption of 7 mg/kg cocaine, prior to surgical removal of the vascular access port due to complications. This took place approx. four and one half years prior to Stop Task testing. Because of the very low cumulative exposure to cocaine relative to animals 15 and 19 (7 versus 360 mg/kg), and the evidence that cognitive deficits are linked to cumulative exposure (Bolla et al., 1999; Di Sclafani et al., 2002), we believe it is appropriate to place animal 16 in the control group. Both of the chronic cocaine animals had not received cocaine for 18 months prior to Stop Task testing.
Initial touch screen training has been described in a publication available online (Liu et al., in press). That report detailed evidence of temporal cortical dysfunction (poor stimulus discrimination learning) also displayed by the two chronic cocaine animals used in the present report, compared to 3 of the 4 control animals used in the present report. It also detailed the complete drug and behavioral histories of those five animals. Animal 16 was not a part of that study. Her behavioral history is one of not being used for any behavioral procedures except occasional chairing and target training from the time of catheter removal, until touch screen training began six months prior to completing the Stop Task.
2.2. Apparatus
Animals were trained to use a touch screen and tested on the Stop Task in a sound-attenuated chamber (Eckel Industries, Ontario, Canada, model AB4240, custom manufactured with integral mounting plates in the rear wall) fitted with a 40 W house light and white noise generator. A 15 inch touch screen (30.0 cm wide X 22.5 cm high, Elo systems CarrollTouch, Menlo Park, CA) that utilizes a grid of infrared sensors just off the surface was used to monitor touches. The touch screen was rear mounted to a ¼ inch (0.6 cm) aluminum panel attached to the mounting plates with 3/8 inch (1.0 cm) X 16 bolts inside 4 inch (10.1 cm) lengths of ½ inch (1.3 cm) copper pipe to offset the panel from the rear wall. A Crist Liquid Reward System (Crist Instrument Co, Hagerstown, MD, Model 5-RLD-E1v) was used to deliver water reward. E-prime (Psychology Software Tools, Pittsburgh, PA) was used for all schedules of stimulus presentation and response recording, with a dedicated Windows based PC for each testing chamber.
2.3. Staged training for Stop Task
Animals were trained 5 days a week and were water regulated (from midday Sunday to Friday afternoon) with ad lib water over the weekend. They were supplemented with water in the afternoons to maintain adequate physiological needs (25 ml/kg/day). Training for the Stop Task took placed in a series of stages. Throughout training and testing, monkeys performed one session per day with 200 trials, five days per week.
Stage 1
Here, the monkeys were taught to touch a solid green circle to get a reward and to withhold their response when a solid red circle was on the screen. This was accomplished using alternating 10 trial blocks of Go and Stop trials. For the Go trials, a green circle (5.6 cm diameter) was presented at the center of the touch screen, and the animal was rewarded for accurately touching it (correct trial). The green circle stayed on the screen until the animal touched the screen. But the reward was only given if the touch was accurately placed on the circle. On Stop trials, the red circle (5.6 cm diameter) was presented at the center of the touchscreen for 1.5 sec, and withholding the response was counted as a correct response. On both trial types, monkeys got a reward (0.07 ml/kg of water) for a correct response and no reward plus a 5 sec timeout for an incorrect response. The normal inter-trial interval was 2 s. When accuracy for both the Go trials and the Stop trials was ≥ 0.8 for 3 continuous sessions, the monkeys were moved to the next stage. The mean number of sessions to accomplish stage 1 for the control animals was 12.0 ± 2 (SEM); for the cocaine animals it was 9 ± 1.
Stage 2
This stage was same as Stage 1 except that Go and Stop trials were randomly intermixed. When monkeys met the criteria that accuracies for both go trials and stop trials were >=0.8 for continuous 3 sessions, they were moved the next stage. The mean number of sessions to accomplish stage 2 for the controls was 5. ± 1.4; for the cocaine animals it was 5.5 ± 1.5.
Stage 3
Here, the monkeys were introduced to larger rewards for faster responses. This is important because the Stop Signal Task is dependent on the prepotent nature of a rapid response. In order to bias for fast responding, a quicker response led to more water reward by manipulating the reward solenoid valve open time as follows: Water amount (ml) = -0.08299 + 1.8347 * body mass (kg) – (0.083) / (1 + 0.04 * Go RT (msec)). The reward amount for withholding response on stop trials remained 0.07 ml/kg water. Touching the area outside the green circle on Go trials or touching the screen on Stop trials led to no reward and a 5 s timeout. The inter-trial interval was 2 s. When monkeys met the criterion of success indicated in stage 1, they progressed to the next stage. The mean number of sessions to accomplish stage 3 for the controls was 4.8 ± 1.4; for the cocaine animals it was 16.5 ± 13.5.
Stage 4
For additional biasing toward quick response on Go trials, a limited hold of 1.5 s was introduced for Go trials. The criteria to move to the next stage for this program were accuracies for both Go and Stop trials ≥ 0.8 for 3 successive sessions and mean Go RT for these 3 sessions was ≤ 600 ms. One animal (cocaine treated subject 15) failed to reach this criteria but achieved a stable RT of 657 msec. The mean number of sessions to accomplish stage 4 for the controls was 16.0 ± 2; the cocaine animal that reached criterion took 16 sessions.
Stage 5
At this point, a “hold stimulus” was introduced to begin each trial. This is important in that it defines a fixed hand position for each trial, and indicates the animal is attending to the screen. Pressing and holding a blue rectangle for a defined time (the “hold” time) initiated a Go or Stop trial, randomly distributed at 50% each. The hold time started at 200 ms and gradually increased to a random 400, 800, 1200, 1600, or 2000 ms. If the accuracies for both the Go and Stop trials at the random 400 – 2000 ms hold time were ≥ 0.8 for 3 continuous sessions and the mean Go RT for these 3 sessions was ≤ 600 ms, the monkeys were moved to the Stop Signal Task testing. The mean number of sessions to accomplish stage 5 for the controls was 16 ± 2.1; for the cocaine animals it was 27 ± 12.
2.4. Stop Signal Task structure
For the Stop Signal Task, we used a staircase procedure and formulation of parameters developed by Logan and colleagues (Logan, 1994), and used by others for clinical (Aron and Poldrack, 2005; Fillmore and Rush, 2002; Li et al., 2008; Li et al., 2006) and rodent preclinical studies (Feola et al., 2000). Each session of the stop task consisted of 200 trials: 150 Go trials and 50 Stop trials, randomly interposed. A diagram of each trial type is presented in Fig 1. Both trial types started with a blue rectangle (5.0 cm wide X 3.8 cm high; centered horizontally, with midpoint 7.0 cm from the bottom of the touch screen) which the animal had to touch and hold to initiate a trial. After a touch to the blue rectangle was maintained for a time randomly chosen from 400, 800, 1200, 1600, or 2000 ms, the hold stimulus was offset, and the Go stimulus (green circle) was presented. On the Go trials, the animal had 1.5 sec to touch the Go stimulus, with reward amount determined as indicated in Stage 3 above.
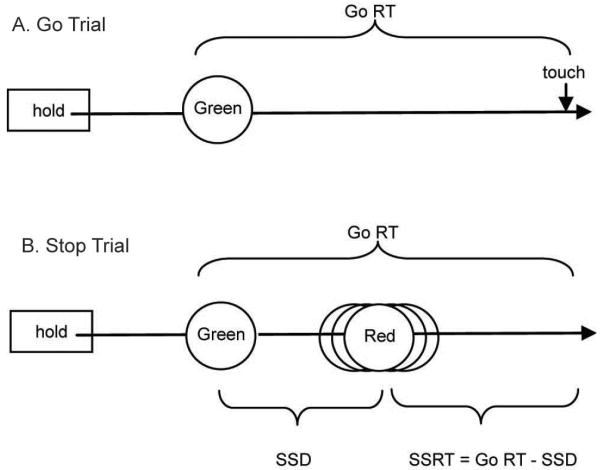
Fig 1.
Sequence of events in Stop Signal Task. All trials began with a hold rectangle on the screen for a variable duration, after which the rectangle was offset and the Go Stimulus (Green circle) appeared. A) On Go trials, the Go stimulus remained unchanged, and had to be touched quickly for maximal reward. B) On Stop trials, the circle changed to red (Stop stimulus) after a variable delay (SSD) and the response had to be withheld to receive reward. A successful stop caused the SSD to increase by 20 msec on the next stop trial; a failed stop caused the SSD to decrease by 20 msec on the next stop trial.
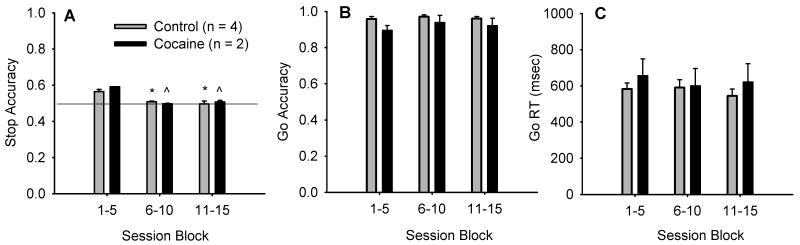
On Stop trials, the green circle changed color to red after a certain time (Stop Signal Delay, SSD). The SSD started at zero on the first session, and then afterwards at the mean SSD from the previous session. Trial to trial, the SSD increased 20 ms on the next stop trial after a correct response on a Stop trial and decreased 20 ms on the next Stop trial after an incorrect response. This staircase procedure results in a mean SSD from the session that corresponds to the average time at which an individual would fail half the stop trials. A 50% error rate was consistently obtained for all individuals after the performance was stabilized as shown in Fig 2A.
Fig 2.
Stop Task performance across session block. A) Stop accuracy over sessions. The staircase procedure for adjusting the SSD resulted in consistent performance of 50 % correct on stop trials in all animals in session 6-15 and there was no significant difference in Stop accuracy between treatment groups. Shown are group means ± SEM. * p < 0.05, vs session 1-5 of control; ˆ p < 0.05, vs session 1-5 of cocaine. B) Go accuracy over sessions. There was no significant difference in Go accuracy between session 6-10 and session 11-15, or between cocaine and control groups over sessions 6-15. C) Go RT over sessions. There was no significant difference in Go RT between cocaine and control groups and no significant difference in Go RT between session 6-10 and session 11-15.
The primary measure of impulsivity is the SSRT. This was determined for each session as: SSRT = mean Go RT – mean SSD, as shown on Fig 1. The primary measure of performance monitoring is the Post-Stop trial Slowing (PSS). This was determined as a mean of the individual values of: PSS = Go RT of the first Go trial following a stop trial – Go RT of the last Go trial prior to that stop trial.
2.5. Data analysis
For evaluating general performance on the Stop Signal Task, the mean accuracies of Go trials (Go accuracy) and Stop trials (Stop accuracy) and the mean Go RT for correct Go trials for session 1-5, session 6-10 and session 11-15 were calculated. A two-way repeated measure ANOVA was used to evaluate effects of session block (session 1-5, 6-10, or 11-15), treatment (cocaine or control) and session*treatment interaction on mean Go accuracy, mean Stop accuracy and mean Go RT. A Multiple Comparison Procedures (Tukey Test) was used to compare group differences in the event of a significant main effect. Because Stop accuracy took several sessions to stabilize at 50%, we calculated the mean SSRT and PSS of each subjects for session 6-15 and compared these values between control and cocaine groups using a t test.
During data analysis, a session was not used if an animal did not finish all 200 trials of a session in 90 minutes, or if Go trial accuracy was below 80%. Both of those conditions would indicate the animal was not engaged sufficiently with the task on that day. In the cocaine group, one session from animal 19 and one from animal 15 were eliminated because they were not completed, and one from animal 15 was eliminated because Go accuracy was less than 80%. In the control group, two sessions were eliminated because of a failure to finish from animal 16, and one from animal 14. The subsequent session was substituted in the event a session was eliminated based on the above criteria.
3. Results
3.1. Stop Signal Task general performance
For Stop accuracy (Fig. 2A), there were a main effect of session block (F(2,8) = 15.1, p = 0.002), no main effect of treatment (P(1,4) = 5.00, p = 0.089) and no significant session*treatment interaction (F(2,8) = 0.612, p = 0.566). Pairwise Multiple Comparison Procedures (Tukey Test) showed that the Stop accuracy for session 1-5 were significantly higher than that for session 6-10 and session 11-15 with both cocaine group and control group. The higher Stop accuracy for the first block resulted from an initial setting of zero for SSD, which then adjusted by only 20 msec with each successful Stop trial. For Go accuracy (Fig. 2B), there was a main effect of session block (F(2,8) = 9.739, p = 0.007, no main effect of treatment (F(1,4) = 2.721, p = 0.174) and no significant session*treatment interaction (F(2,8) = 3.44, p = 0.083). Pairwise Multiple Comparison Procedures (Tukey Test) showed that within monkeys with cocaine use history, the go accuracy for session 1-5 was significantly lower than that for session 6-10. Go accuracies for session 6-10 and for session 11-15 were not significantly different for both groups.
For Go RT (Fig.2C), there was no main effect of session (F(2,8) = 3.289, p = 0.091) or treatment (F(1,4) = 0.495, p = 0.520) and no significant session*treatment interaction (F(2,8) = 3.406, p = 0.085).
3.2. Motoric impulsivity and Performance monitoring
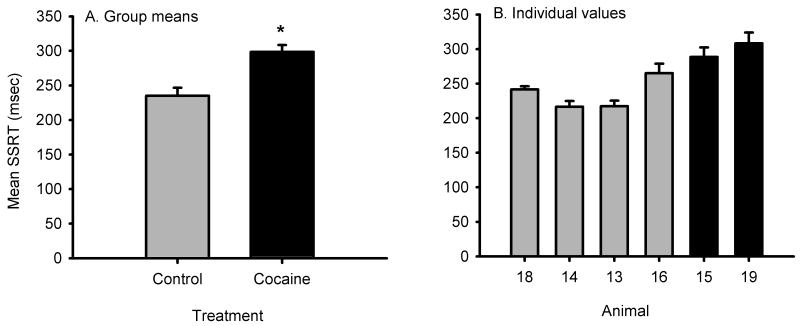
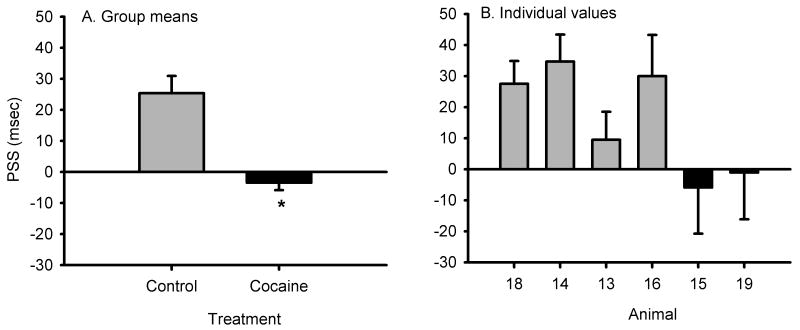
Because performance for both cocaine and control groups was stable from session 6-15, we calculated the mean SSRT and PSS values for sessions 6-15 for treatment group comparison. The mean SSRT of session 6-15 for the monkeys with cocaine use history was significantly higher than that for the control monkeys (p < 0.05, Fig. 3). The mean PSS of session 6-15 for the monkeys with cocaine use history was significantly smaller than that for the control monkeys (p < 0.05, Fig. 6).
Fig 3.
Comparison of motoric impulsivity (SSRT) values from chronic cocaine and control monkeys. A) Group means were compared using an unpaired t test, t(4) = 3.63, p = 0.022. B) Values from individual animals. All indications of variance are SEM. Error bars for individual animals correspond to variance of the ten session mean for that individual.
3.2. Comparison of monkey performance with clinical measures
Table 1 demonstrates that there is good agreement between performance values obtained with control and cocaine exposed groups of monkeys and humans on the Stop Task. (The version of the Stop Task used by Fillmore and Rush differed slightly in that it used several fixed stop signal delays as opposed to a tracking procedure used in the present report and in both Li et al. studies.) The comparison indicates increased impulsivity and decreased performance monitoring in both species following long term cocaine use, and thus provides support for the use of this task for translational studies of these phenomena.
Table 1.
Comparison of monkey and human Stop Task Performance
| control | cocaine | |||||
|---|---|---|---|---|---|---|
| Reference | Go RT | SSRT | PSS | Go RT | SSRT | PSS |
| Fillmore and Rush (2002) | 591 ± 5 | 286 ± 3 | NR | 579 ± 5 | 350 ± 5* | NR |
| Li et al., (2006) | 520 ± 9 | 210 ± 3 | 26 ± 2 | 523 ± 16 | 223 ± 5* | 17 ± 2* |
| Li et al., (2008) | 524 ± 23 | 200 ± 7 | NR | 565 ± 33 | 213 ± 9 | NR |
| this study (monkey) | 568 ± 40 | 235 ± 12 | 25 ± 6 | 610 ± 99 | 299 ± 105* | -3 ± 2* |
Significantly different from control values within species, p < 0.05. Human values adapted from Li et al., 2006;
NR - Not reported
4. Discussion
This report establishes a procedure for a touch screen-based Stop Signal Task for use in rhesus monkeys. Using this task, we also demonstrate in preliminary studies that animals that chronically self-administered cocaine (and to a much lesser extent cocaethylene) displayed elevated SSRT and decreased PSS values.
The Stop Signal Task in the present study used a staircase procedure to continuously adjust the SSD. As expected, the stop accuracy of monkeys was close to 0.5 and was not significantly different across sessions after the monkey's performance was stabilized (session 6-15). The mean Go accuracy and RT were also stable over session 6-15.
We observed that monkeys with cocaine use history displayed higher SSRT and lower PSS values than control monkeys while there were no significant differences in Stop accuracy, Go accuracy or Go RT between the two groups. Overall, these results obtained with monkeys agree well with clinical studies of cocaine users employing the Stop Task (Fillmore and Rush, 2002; Li et al., 2006), with significant differences seen between control and cocaine experienced monkeys and humans on SSRT and PSS, and no differences in Go RT, or accuracy on either Go or Stop trials.
Clinical studies of drug users reveal a pattern of cognitive deficits manifested at the levels of inhibitory control, executive functioning and decision-making (Aron and Paulus, 2007; Bolla et al., 1998; Garavan and Stout, 2005; Goldstein and Volkow, 2002). Similar to our findings, clinical patterns of other types of cognitive deficits in drug users are evident even after years of abstinence (Ersche et al., 2006). The Stop Task is well suited to study this pattern of deficits. Elevated SSRT values reflect increased motoric impulsivity. SSRT values also correlate significantly with trait impulsivity (Cools et al., 2005) which is higher in cocaine (Moeller et al., 2002) and amphetamine users (Clark et al., 2006). Importantly, poorer cognitive performance and impulsivity are predictors of poorer clinical outcome (Aharonovich et al., 2006; Aharonovich et al., 2003; Patkar et al., 2004). Thus, an increased understanding of how cognitive deficits arise has potential clinical benefit. It continues to be emphasized in the clinical literature that the role of preexisting traits of cognitive and behavioral control deficits, versus consequence of drug exposure, is unclear (Aron and Paulus, 2007). Our findings are consistent with an effect of chronic cocaine use per se to increase impulsivity and decrease performance monitoring, frequent features of psychostimulant addiction (Aron and Paulus, 2007). They also validate an animal model for understanding the underlying neurobiology of a characteristic associated with addiction. It is a limitation of the current study that only two animals that had chronically self-administered cocaine were available for comparison with the four animal control group. However, group differences were statistically significant, and the unique nature of the long term exposure to cocaine, coupled with the duration of abstinence (18 months) prior to characterization on the Stop Task makes this a uniquely relevant comparison between groups for evaluating the potential of the Task for translational research. The promise of the current studies is that given the high concordance between the monkey and human effects of cocaine exposure, invasive studies in monkeys may better reveal the neurobiology of impulsivity in addiction, and hence, potential clinical targets.
Fig 4.
Comparison of performance monitoring (Post Stop trial Slowing, PSS) values from chronic cocaine and control monkeys. A) Group means were compared using an unpaired t test, t(4) = 3.44, p = 0.026. B) Values from the individual animals.
Acknowledgments
This work was supported by grant support from the National Institute of Drug Abuse DA10331 and the Office of Research and Development Medical Research Service, Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug & Alcohol Dependence. 2003;71:207–11. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102 1:33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–9. doi: 10.1176/jnp.10.3.280. [Review] [82 refs] [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. Journal of Neuropsychiatry & Clinical Neurosciences. 1999;11:361–9. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 2007;191:705–17. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–83. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem. 1993;60:1429–35. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–22. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30:1362–73. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–66. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–71. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Jatlow P, Roth RH. Serotonin involvement in cocaine sensitization: clues from studies with cocaine analogs. Drug Development Research. 1993;30:189–200. [Google Scholar]
- Emeric EE, Brown JW, Leslie MW, Pouget P, Stuphorn V, Schall JD. Performance Monitoring Local Field Potentials in the Medial Frontal Cortex of Primates: Anterior Cingulate Cortex. J Neurophysiol. 2007 doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–47. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behavioral Neuroscience. 2000;114:838–48. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-Error Behavior in Active Cocaine Users: Poor Awareness of Errors in the Presence of Intact Performance Adjustments. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Iyer RN, Nobiletti JB, Jatlow PI, Bradberry CW. Cocaine and cocaethylene: effects on extracellular dopamine in the primate. Psychopharmacol. 1995;120:150–5. doi: 10.1007/BF02246187. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90. doi: 10.1007/pl00005483. [Review] [240 refs] [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–12. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Sampson AR, Wei Z, Bradberry CW. Evidence of Temporal Cortical Dysfunction in Rhesus Monkeys Following Chronic Cocaine Self-Administration. Cerebral Cortex. doi: 10.1093/cercor/bhm236. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action:A users' guide to the stop signal paradigm. In: Dagenbach TH, Carr D, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–11. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–22. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Tokuno HA, Bradberry CW, Everill B, Agulian SK, Wilkes S, Baldwin RM, Tamagnan GD, Kocsis JD. Local anesthetic effects of cocaethylene and isopropylcocaine on rat peripheral nerves. Brain Research. 2004;996:159–67. doi: 10.1016/j.brainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44:491–4. [PubMed] [Google Scholar]