Abstract
A large Stokes shift dye, composed of water-solubility and near-infrared feature, was developed for multichannel imaging applications.
The development of near-infrared (NIR) dyes is a rate-limiting step in any potential advancement and translation of in vivo optical molecular imaging. Since scattering and autofluorescence decrease as wavelength increases, NIR fluorescent dyes offer a considerable advantage over blue-shift dyes. Specially, because the fluorescent labels associated with NIR emission wavelengths can penetrate tissue deeper than those associated with blue emission wavelengths, imaging within the NIR window provides enormous potential for non-invasive in vivo imaging applications. Several NIR fluorescent dyes based on cyanine scaffolding have demonstrated great promise for in vivo imaging of several biological targets such as somastostatin receptors,1 and proteases.2-5
In recent years, there has been increasing interest in imaging multiple biological events simultaneously in one environment. One approach to realizing this goal is the use of optical reporters which emit photons that are readily distinguishable both from their own excitation photons and from the emission photons of other reporters.6 For example, quantum dots, with their large Stokes shift, are a preferred optical contrasting agent in many such applications. On the basis of this concept involving multichannel imaging that utilizes large Stokes shift molecules, we hypothesized that NIR organic dyes with a large Stokes shift would have similar applicability. Furthermore, since organic dyes have lower steric hindrance and toxicity than quantum dots, we conjecture that large Stokes shift organic dyes may provide superior sources of optical contrast in biological studies.
In this report, we discuss the development of a large Stokes shift dye and quantification of its fluorescent intensity for application in multichannel imaging. The synthesis begins with the condensation of disulfonated indole 1 and the iminium adduct 2 in the presence of sodium acetate and ethanol. This provides the dye intermediate 3 which has also been reported by others7,8 (Scheme 1). This compound contains four sulfonate groups, thus enabling its isolation from other less water-soluble by-products, such as aniline, from the elimination process or the product of semi-condensation (this intermediate absorbs at λmax = 650 nm) with a decent yield. We observed that if the final dye product has distinct polarity when compared to the by-products, the purification process is simple and could be performed on a flash column for large scale operation. Otherwise, we are required to use HPLC to isolate the product, as seen in the case NIR820-II9 (Fig. 1).
Scheme 1.
Synthesis of a large Stokes shift and conjugatable near-infrared dye.
Fig. 1.
Previous generations of NIR820 dyes.
Due to high polarity, compound 3 precipitated out in ethanol as soon as the starting material completely disappeared, to afford the expected product with good yield (39%). Other by-products can be removed by repeatedly washing the product mixture with methanol.
The nucleophilic substitution (SNR1) at the central vinylogous halide carbon (C(sp2)–X) of compound 3 by an amine group from aminodecanoic acid 4 at an elevated temperature provides the final product 4-Sulfonir with good yield (73%). Our motivation for accomplishing this reaction is twofold. First, the generation of a carboxylic functional group creates a site for the bioconjugation of targeted molecules. Secondly, a large Stokes shift is formed as a result of substitution with a amino group at the central methine moiety which contributes to the intramolecular charge transfer (ICT) process.10,11 Interestingly, we learned that this reaction is conveniently monitored by a color change in the reaction solution. Specifically, the completion of the reaction is indicated by a conversion of color from green to blue, which corresponds to a hypsochromic shift of the dye from the initial wavelength at 790 nm to 600 nm. It is worth emphasizing that this reaction does not occur at room temperature. This eliminates concerns regarding the reaction of the NIR820 and NIR820-II at central chloride by amine-containing biomolecules.
The quantum yield and molar extinction coefficient of 4-Sulfonir is 37% and 1.0 × 105 M−1 cm−1, respectively, as measured against indocyanine green (supporting information†). Experiences with the previous generations of NIR dyes suggest that the sulfonate groups cause the enhanced water solubility, thus reducing the effect of hydrophobic-mediated quenching-effect. In the previous works, we reported that the quantum yield of the second generation NIR820-II is nearly double that of the first generation, NIR820, because the former is more water-soluble9 (Fig. 1). As we have found with the previous dyes, the absorbance bands of 4-Sulfonir are created by the electronic transition of the π electrons across the polymethine bridge. The Stokes shift of this family of cyclic heptamethinylated cyanine dyes can be tuned for imaging purposes depending on where we derivatize the functional groups for bioconjugation. For instance, if the indole ring system is functionalized as seen in NIR820 and NIR820-II, the Stokes shift is ∼30 nm (spectra of NIR820, Fig. 2). However, the Stokes shift expands to 140 nm when the functional group is on the methine bridge. It is noteworthy that the absorbance and emission profiles of 4-Sulfonir are asymmetric, indicating the characteristic geometrical change in the emitting and absorbing states.12
Fig. 2.
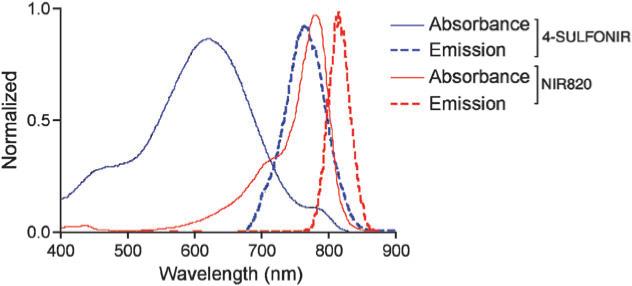
Spectral characteristics of the developed NIR dyes.
In light of the unique large Stokes shift of 4-Sulfonir, we speculated that the dye could be used in parallel with other NIR dyes for imaging two molecular events simultaneously in one target. To this end we attempted to define the imaging parameters. The fluorescent signal of 4-Sulfonir and NIR820 were determined in their respective excitation/emission wavelength using the Maestro™ imaging system (Cambridge Research and Instrumentation Inc.) and were analyzed quantitatively using our in-house developed program run in Matlab. When the dyes were excited in the NIR channel (700−850 nm), NIR820 emitted a significant stronger fluorescent signal (emision photons were collected from 780−950 nm) than did 4-Sulfonir (Fig. 3A). In contrast, when the excitation channel was switched to visible light (500−620 nm), under the same settings, the 4-Sulfonir illuminated with remarkable intensity in the NIR region (Fig. 3C). The data were subsequently imported into Matlab for analysis using the trapezoidal integration function to calculate the total signal intensity of each pixel over the emission range to yield an associated matrix of values corresponding to the area under the curve of each pixel's spectra (detailed analysis is available in the supporting information†). Quantitative analysis revealed that when excited in the NIR range, the NIR820 emitted about 90% of the total fluorescent signal (4.1 × 108 arbitrary intensity units), while, the emission of 4-Sulfonir accounted for only 10% (3.4 × 107 arbitrary intensity units) of the total signal (Fig. 3B). To monitor an event linked to 4-Sulfonir, the same process was repeated with excitation in the visible range. In this domain, 4-Sulfonir emitted approximately 80% of the total signal (2.7 × 108 arbitrary intensity units) while the NIR820 emitted around 20% (6.3 × 107 arbitrary intensity units) of the total signal (Fig. 3D). From these results, we concluded that 4-Sulfonir can be used in parallel with other NIR dyes for multichannel imaging.
Fig. 3.

Differential imaging of two near-infrared dyes in one environment gating at different excitation channels (A) and quantitative analysis of the fluorescence intensity of the dyes pertaining to the detection of two distinct events.
In summary, we have demonstrated the synthesis of a stable, water-soluble, and bioconjugatable NIR dye with a remarkable improvement in quantum yield. The synthesis of this dye is cost-effective and time-efficient. Additionally, we also show the proof-of-principle for using this large Stokes shift dye for multichannel imaging with collection in the NIR spectrum. Current efforts in our laboratory are being directed toward applying this new dye for imaging the activities of proteases in a tumor microenvironment within a living system. Finally, it is noteworthy that during the course of work we found that the remarkable stability of 4-Sulfonir constitutes a significant advantage over other dyes, in as much as it supports convenience in our work by eliminating the concern of decay being caused by exposure to light or room temperature.
Supplementary Material
Acknowledgments
This work was supported by a grant from NIA (AG02636601 A1) and an internal funding from the Department of Radiology, Vanderbilt School of Medicine to W. P.
Footnotes
Electronic supplementary information (ESI) available: experimental information including characterization and quantitative analysis procedure.
Notes and references
- 1.Achilefu S, Dorshow RB, Bugaj JE, Rajagopalan R. Invest. Radiol. 2000;35:479–485. doi: 10.1097/00004424-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Blum G, Degenfeld VG, Merchant MJ, Blau HM, Bogyo M. Nat. Chem. Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 3.Pham W, Choi Y, Weissleder R, Tung CH. Bioconjugate Chem. 2004;15:1403–1407. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- 4.Tung CH, Bredow S, Mahmood U, Weissleder R. Bioconjugate Chem. 1999;10:892–896. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 5.Tung CH, Mahmood U, Bredow S, Weissleder R. Cancer Res. 2000;60:4953–4958. [PubMed] [Google Scholar]
- 6.Nida DL, Rahman MS, Carlson KD, Richards-Kortum R, Follen M. Gynecol. Oncol. 2005;99:S89–94. doi: 10.1016/j.ygyno.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Hilderbrand SA, Kelly KA, Weissleder R, Tung CH. Bioconjugate Chem. 2005;16:1275–1281. doi: 10.1021/bc0501799. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Mason JC, Achilefu S. J. Org. Chem. 2006;71:7862–7865. doi: 10.1021/jo061284u. [DOI] [PubMed] [Google Scholar]
- 9.Pham W, Medarova Z, Moore A. Bioconjugate Chem. 2005;16:735–740. doi: 10.1021/bc049700+. [DOI] [PubMed] [Google Scholar]
- 10.Kiyose K, Kojima H, Urano Y, Nagano T. J. Am. Chem. Soc. 2006;128:6548–6549. doi: 10.1021/ja060399c. [DOI] [PubMed] [Google Scholar]
- 11.Peng X, Song F, Lu E, Wang Y, Zhou W, Fan J, Gao Y. J. Am. Chem. Soc. 2005;127:4170–4171. doi: 10.1021/ja043413z. [DOI] [PubMed] [Google Scholar]
- 12.Saha SK, Dogra SK. J. Photochem. Photobiol., A. 1997;110:257–266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




