Summary
Background
Most malaria deaths occur in rural areas. Rapid progression from illness to death can be interrupted by prompt, effective medication. Antimalarial treatment cannot rescue terminally ill patients but could be effective if given earlier. If patients who cannot be treated orally are several hours from facilities for injections, rectal artesunate can be given before referral and acts rapidly on parasites. We investigated whether this intervention reduced mortality and permanent disability.
Methods
In Bangladesh, Ghana, and Tanzania, patients with suspected severe malaria who could not be treated orally were allocated randomly to a single artesunate (n=8954) or placebo (n=8872) suppository by taking the next numbered box, then referred to clinics at which injections could be given. Those with antimalarial injections or negative blood smears before randomisation were excluded, leaving 12 068 patients (6072 artesunate, 5996 placebo) for analysis. Primary endpoints were mortality, assessed 7–30 days later, and permanent disability, reassessed periodically. All investigators were masked to group assignment. Analysis was by intention to treat. This study is registered in all three countries, numbers ISRCTN83979018, 46343627, and 76987662.
Results
Mortality was 154 of 6072 artesunate versus 177 of 5996 placebo (2·5% vs 3·0%, p=0·1). Two versus 13 (0·03% vs 0·22%, p=0·0020) were permanently disabled; total dead or disabled: 156 versus 190 (2·6% vs 3·2%, p=0·0484). There was no reduction in early mortality (56 vs 51 deaths within 6 h; median 2 h). In patients reaching clinic within 6 h (median 3 h), pre-referral artesunate had no significant effect on death after 6 h or permanent disability (71/4450 [1·6%] vs 82/4426 [1·9%], risk ratio 0·86 [95% CI 0·63–1·18], p=0·35). In patients still not in clinic after more than 6 h, however, half were still not there after more than 15 h, and pre-referral rectal artesunate significantly reduced death or permanent disability (29/1566 [1·9%] vs 57/1519 [3·8%], risk ratio 0·49 [95% CI 0·32–0·77], p=0·0013).
Interpretation
If patients with severe malaria cannot be treated orally and access to injections will take several hours, a single inexpensive artesunate suppository at the time of referral substantially reduces the risk of death or permanent disability.
Funding
UNICEF/UNDP/World Bank Special Programme for Research and Training in Tropical Diseases (WHO/TDR); WHO Global Malaria Programme (WHO/GMP); Sall Family Foundation; the European Union (QLRT-2000-01430); the UK Medical Research Council; USAID; Irish Aid; the Karolinska Institute; and the University of Oxford Clinical Trial Service Unit (CTSU).
Introduction
Death and permanent disability from malaria are avoidable by prompt, effective treatment.1 Malaria can, however, progress from fever to life-threatening disease within hours, and some people who survive severe malaria sustain substantial permanent damage to the CNS.2–5 The only way to give effective antimalarial treatment to patients in the community who cannot be treated orally is to take them to a health-care facility (eg, hospital, dispensary, or other clinic) at which injections can be given. From an isolated rural village, however, reaching such a clinic can take many hours, or even days, by which time the disease may have progressed too far to be treated successfully. An inexpensive artesunate suppository has therefore been developed that can be given rectally at, or near, home by a parent, neighbour, or community health worker when severe malaria is suspected, reducing time to treatment. It could also replace some antimalarial injections in clinics.
Artemisinins act more rapidly than other classes of antimalarial drugs do. Artesunate reduces parasitaemia within hours, acting on both mature and young parasites.6 Hospital-based studies have shown that a single dose of artesunate, given rectally, can provide parasiticidal blood concentrations within 10–20 min, and can halve parasitaemia numbers within 6–12 h.7–9 If a severely ill patient is given rectal artesunate in the community, however, referral to a medical facility is still needed for diagnosis and treatment. For, even in malaria-endemic areas, symptoms that are thought to be due to severe malaria could well be found to be due to another potentially lethal infection (eg, pneumonia) that requires different treatment. Moreover, one pre-referral dose of artesunate is not a complete treatment for severe malaria, and fully curative treatment can best be undertaken at a clinic with facilities for antimalarial injections and supportive care.
Prompt effective treatment of severe malaria should limit or prevent permanently disabling neurological damage,4,5 but treatment of severe disease could prevent death in some patients who already have irreversible brain damage, leaving them alive but permanently disabled. Furthermore, protracted exposure to high doses of lipid-soluble analogues of artesunate has caused neurological damage in animal experiments.10 Hence, the net effects of pre-referral rectal artesunate not only on mortality but also on severe and persistent neurological sequelae needed to be assessed.
We report a community-based placebo-controlled trial of rectal artesunate versus placebo in patients with suspected severe malaria who could not take medication orally and were not at a clinic at which injections could be given. The main objective was to determine whether, in these circumstances, rectal artesunate plus referral to such a clinic reduced mortality and permanent disability compared with rectal placebo plus referral.
Methods
Patients, procedures, and study setting
The study took place in 291 rural villages in Ghana, Tanzania, and Bangladesh. Patients were eligible for randomisation if they were thought by the recruiter to have malaria and could not be treated orally.
Locally resident village recruiters (268 in 142 villages in Africa, 149 in 149 villages in Bangladesh), most with little previous medical knowledge and no research experience, underwent training and recruited patients in their communities on the basis of clinical symptoms. Recruiters were visited every few days by 74 field supervisors throughout the study (Aug 3, 2000–July 30, 2006). For patients meeting eligibility criteria, consent was signed (or fingerprinted) by caretakers and witnessed, a brief entry form was completed, a peripheral blood smear was generally sought by the recruiter for later parasitology, and the next numbered treatment box was used. (Boxes for individuals were packed into block-balanced cartons of eight or four random allocations, computer generated by the Oxford Clinical Trial Service Unit, UK.) Each box contained computer-generated identification labels for forms and any blood slides, and, sealed in a waterproof sachet, one artesunate or placebo suppository of identical appearance. (Bulk artesunate, Abbott, Liestal, Switzerland; encapusaltion, Scherer capsules, Eberbach, Germany; sealing, Scanpharm packaging, Copenhagen, Denmark.) All study staff remained masked to the treatment allocation until the endpoints had been finalised on Sept 19, 2006, and the study was unblinded.
The suppository was inserted into the rectum and the buttocks held together for about 10 min to help prevent expulsion. On successful insertion, the patient was in the study even if the suppository was later expelled. Re-randomisation within 30 days was disallowed (five smear-positive patients: all recovered). After insertion, the patient was to go to a clinic at which injections could be given. Two blood slides were taken in Asia; the extra one accompanied the patient to hospital.
The three African study sites were all in high malaria transmission areas, and they recruited only young children aged 6–72 months. The Asian study site was in a low unstable transmission area and recruited older patients as well. The main difference between the African and Asian settings was, however, in the rapidity and nature of clinical care. In Africa, many patients were still not in clinic more than 6 h after randomisation, the first health facility for injections was often just a dispensary, and injection on arrival was usually intramuscular. In Asia, the first facility was a hospital offering study patients free, immediate admission and treatment at any hour, and special arrangements had been made to ensure free reliable availability and prompt use of intravenous quinine and other appropriate treatment, including broad-spectrum antibiotics and supportive care. Hence, patients in Bangladesh went to hospital rapidly and mortality was low; time to arrival was typically a couple of hours after randomisation and time to discharge was typically about 3 days.
Before the study began in a village, it was explained to leaders, traditional healers, and the community, usually culminating in a large public village meeting at which the individual consent form, use of placebo, and importance of proceeding to the referral clinic were discussed in detail, and the trained local village recruiters were introduced. International monitors verified that selected villagers did understand the study. The trial was approved by national ethics committees and the WHO ethics committee, and was reviewed regularly by an independent data monitoring committee.
Outcomes
Primary endpoints were mortality and permanent disability. Mortality was to be assessed (usually at home) 7–30 days after randomisation. Patients had to be reliably reported as dead or seen alive. For the few (141/17 826 [1%]) for whom neither was possible, an apparently reliable report (usually from a family member or near neighbour) of recovery was acceptable. Only eight (<0·1%) participants, all allocated placebo, were completely lost; all are assumed to have recovered and survived. At the 7–30 day follow-up, a questionnaire about possible neurological damage in survivors was administered. Patients with new problems that might be an indicator of neurological damage were assessed by a study clinician whose decision was final as to whether there was substantial damage from the index episode or its treatment. Only patients with clinically confirmed neurological damage were followed up further. A study clinician revisited them periodically until the symptoms resolved, the patient died, or the study ended. Classification as resolved, resolving, or persistent was made before unblinding.
There was, by definition, no overlap between 7–30 day mortality and neurological damage (although a few later deaths were attributed to such damage). For patients with either endpoint, a study clinician recorded narratives of the main events before and after randomisation, including time to reach clinic and time to death, on the basis of any clinical records plus a structured interview with the family. A blinded endpoint review committee (ERC) finalised time to reach clinic (ie, time to reach a facility at which injections could be given), time to death, and which few endpoints were definitely not due to the index episode of malaria.
Quality assurance
Cartons of boxes were replaced if nearly empty, and the number of treatments used was checked against the entry forms completed; any discrepancies were investigated. Only one box (placebo) remained unaccounted for; we assumed it was used and that that placebo-allocated patient recovered. At intervals throughout the trial, field samples of study capsules were blindly analysed quantitatively, showing no loss of potency or errors of packaging. As a further check for errors of packaging for the study drug for older patients, all artesunate or placebo 400 mg capsules remaining at the end of the study were blindly analysed qualitatively, again showing no errors.
Parasites were counted on Giemsa-stained slides until at least 200 white cells or 500 parasites had been counted. Parasite density per μL was taken as 8000 times the ratio of asexual parasites to white cells. A slide was declared negative only if no parasites were seen after examination of at least 500 white cells.
Statistical methods
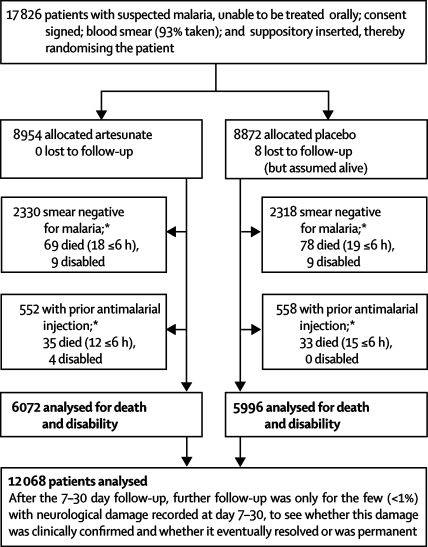
We analysed results with Stata (version 9.2). Proportions were compared by χ2 tests. All p values are two-sided. The main analyses are of mortality by the 7–30 day follow-up and of permanent disability (primary endpoints). The indication for rectal artesunate that the study evaluated (as filed with the US Food and Drug Administration at the start of the study) was for “the initial management of acute malaria…in patients who cannot take medication by mouth and for whom parenteral treatment is not available.” Hence, it was decided before the results were unblinded to exclude patients if a blood smear just before randomisation was reliably classified as having no malaria parasites, or the randomisation form recorded an immediately previous antimalarial injection (figure 1). The analyses are of all other patients into whom a trial capsule was inserted (ie, by intention to treat), even though the capsule was quickly expelled in eight who died later (four artesunate, four placebo).
Figure 1.
Trial profile
Main 7–30 day follow-up was at a median of 14 days after randomisation. *Randomised patients were excluded only if a prerandomisation blood smear was free of malaria parasites (26% of all smears were negative for malaria); or the entry form recorded that an antimalarial drug had already just been injected. In patients with smear-negative disease who were still alive but not injected or in clinic more than 6 h after randomisation, 28/475 artesunate-allocated vs 25/472 placebo-allocated patients died or were disabled.
This study is registered in Bangladesh, Ghana, and Tanzania, numbers ISRCTN83979018, 46343627, and 76987662.
Role of the funding sources
The external funding sources of the study had no role in study design, data collection, data analysis, interpretation, or writing of the report. The corresponding author had full access to the data and final responsibility for submission for publication.
Results
A trial suppository was inserted in 17 826 patients, but after the prespecified exclusions of 4648 with a negative blood smear before randomisation and a further 1110 with an antimalarial injection before randomisation (figure 1), the main analyses are of death or permanent disability in 12 068 patients with malaria, 6072 randomly allocated artesunate and 5996 placebo. The baseline characteristics were well balanced (table 1). Half the patients were in Africa and half in Asia: in both continents Plasmodium falciparum predominated (table 1). Of positive blood smears, 5318 (48·2%) had more than 25 000 parasites per μL (table 1). Overall, 980 (8%) patients were comatose, a further 2434 (20%) had had convulsions, and most others were prostrated by their illness (table 1). All patients in Africa and a third of those in Asia had an assessed age of 6–72 months.
Table 1.
Baseline characteristics and non-adherence with referral advice, by treatment allocation
|
Artesunate |
Placebo |
||||
|---|---|---|---|---|---|
| Number of patients entered (N=6072) | Percentage never went to a clinic (7·8% [470/6015]) | Number of patients entered (N=5996) | Percentage never went to a clinic (6·9% [411/5945]) | ||
| Parasite species at baseline | |||||
| Africa | 3041 (50%) | 13·6% (407/2999) | 2999 (50%) | 12·5% (370/2960) | |
| P falciparum only | 2532 | 12·2% (305/2502) | 2476 | 11·0% (269/2449) | |
| P ovale only | 2 | .. | 1 | .. | |
| P malariae only | 5 | .. | 4 | .. | |
| No blood slide* | 502 | 20·8% (102/490) | 518 | 19·8% (100/506) | |
| Asia | 3031 (50%) | 2·1% (63/3016) | 2997 (50%) | 1·4% (41/2985) | |
| P falciparum only | 2369 | 1·9% (44/2356) | 2335 | 1·4% (33/2325) | |
| P falciparum and P vivax | 60 | .. | 61 | .. | |
| P vivax only | 598 | 2·7% (16/597) | 597 | 1·0% (6/597) | |
| No blood slide | 4 | .. | 4 | .. | |
| Parasite density per μL | |||||
| <5000 | 1456 (26%) | 7·4% (107/1442) | 1370 (25%) | 6·7% (91/1353) | |
| 5000–25 000 | 1404 (25%) | 6·5% (91/1398) | 1492 (27%) | 6·1% (91/1487) | |
| >25 000 | 2706 (49%) | 6·3% (168/2681) | 2612 (48%) | 4·9% (127/2595) | |
| Parasitology unknown* | 506 | 21·1% (104/494) | 522 | 20·0% (102/510) | |
| Clinical status at entry† | |||||
| Coma | 497 (8%) | 7·9% (37/467) | 483 (8%) | 8·1% (37/458) | |
| Repeated convulsions | 1248 (21%) | 6·3% (78/1237) | 1186 (20%) | 6·0% (71/1174) | |
| Other | 4327 (71%) | 8·2% (355/4311) | 4327 (72%) | 7·0% (303/4313) | |
| Gender | |||||
| Female | 2747 (45%) | 8·3% (225/2719) | 2757 (46%) | 7·7% (211/2732) | |
| Male | 3324 (55%) | 7·4% (244/3295) | 3238 (54%) | 6·2% (199/3212) | |
| Unknown | 1 | .. | 1 | .. | |
| Artesunate dose tested and age | |||||
| 100 mg (age 6–72 months‡) | 4063 (67%) | 10·6% (427/4016) | 3987 (66%) | 9·8% (385/3941) | |
| Handeni, Tanzania, 2002–06 | 726 | 18·9% (133/704) | 737 | 18·3% (131/716) | |
| Kilosa, Tanzania, 2000–06 | 1170§ | 18·7% (217/1159) | 1169 | 16·5% (192/1163) | |
| Navrongo, Ghana, 2000–04 | 1145 | 5·0% (57/1136) | 1093 | 4·4% (47/1081) | |
| Chittagong, Bangladesh, 2000–04 | 1022 | 2·0% (20/1017) | 988§ | 1·5% (15/981) | |
| 400 mg (age >72 months; Chittagong only, 2000–04) | 2009 (33%) | 2·2% (43/1999) | 2009 (34%) | 1·3% (26/2004) | |
| 6–14 years | 605 | 1·5% (9/602) | 618 | 1·5% (9/614) | |
| 15–24 years | 654 | 2·0% (13/652) | 636 | 0·9% (6/636) | |
| ≥25 years | 750 | 2·8% (21/745) | 755 | 1·5% (11/754) | |
Since early death can prevent arrival at clinic, non-adherence (percentage who never went to a clinic at which treatment could be injected) was calculated only in those surviving more than 6 h.
At one site (Handeni), blood smears were not collected during most of the trial.
Defined by a question about coma at three study sites and a question about altered consciousness at Handeni: risks were comparable.
Apparent age 6–60 months in Tanzania, 6–72 months elsewhere; overall, the mean age was 20 months for those randomly assigned to the 100 mg dose of artesunate.
Only two case report forms were missing, one from each group; the child allocated to artesunate recovered fully and the analyses assume both did.
Randomisation was by successful rectal insertion of the trial capsule containing artesunate or placebo. Almost all Asian patients then went to hospital, arriving shortly after randomisation, but a substantial minority in Africa never went to a clinic (table 1), or arrived after a delay of several hours. The main reasons given for delay were that randomisation took place too late in the day for travel, or that it took time for the parents to gather funds for the trip or treatment. Artesunate had only a slight effect on attendance; in those who survived more than 6 h, the proportions who never reached clinic were 7·8% artesunate and 6·9% placebo (p=0·03 in an analysis stratified for study site; table 1). Mean duration of hospital stay (available only in Asia) was 3·13 days with artesunate versus 3·20 days with placebo (SD 0·94; p=0·004).
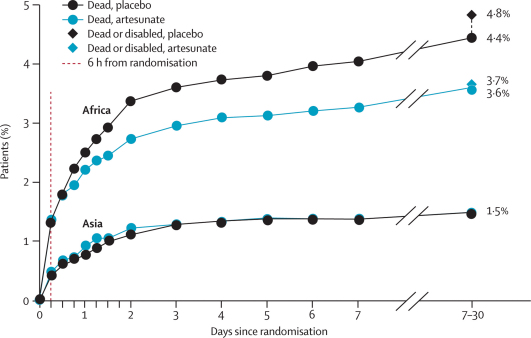
Figure 2 shows, for Africa and Asia separately, treatment-specific mortality during the first 7 days after randomisation, and at the 7–30 day follow-up (which took place, on average, at day 14). (CNS damage that resulted in permanent disability was considered comparable in importance with death, but CNS damage that eventually resolved was not.) In Asia, where patients reached effective hospital treatment quickly, mortality was low and seemed to be unaffected by rectal artesunate. In Africa, where delays were often longer and pre-referral rectal artesunate had a longer time in which to act alone, it appeared to improve outcome (figure 2).
Figure 2.
Death by time since randomisation and death or permanent disability at the 7–30 day follow-up
The main aim of pre-referral rectal treatment in this study was to bring forward by some hours the control of parasitaemia. For patients who reached a clinic (or died) within a few hours of randomisation, only a short time was gained by pre-referral treatment, but for patients who took longer to reach a clinic, many hours could be gained. Table 2 subdivides the deaths both by time to death and by the time taken to reach clinic, and provides results separately for Africa, Asia, and all study sites.
Table 2.
Effects of trial treatment on early and later mortality and on permanent disability in Africa (three study sites), Asia (one study site), and all four study sites
|
Africa (three study sites) |
Asia (one study site) |
All four study sites |
|||||
|---|---|---|---|---|---|---|---|
| Artesunate (N=3041) | Placebo (N=2999) | Artesunate (N=3031) | Placebo (N=2997) | Artesunate (N=6072) | Placebo (N=5996) | Significance | |
| Death by 7–30 day follow-up* | |||||||
| Death in 0–6 h (at a median of 2 h) | 42 | 39 | 14 | 12 | 56 | 51 | NS |
| Reached clinic in 0–6 h (∼3 h†), died after hour 6 | 42 | 47 | 29 | 28 | 71 | 75 | NS |
| Still not in clinic at 6 h (∼15 h†), died after hour 6 | 25 | 47 | 2 | 4 | 27 | 51 | p=0·0039 |
| Alive at 7–30 day follow-up, but with permanent disability‡ | |||||||
| CNS HIV or CNS tuberculosis | 1 | 0 | 0 | 1 | 1 | 1 | NS |
| Sequelae of cerebral malaria | 1 | 12 | 0 | 0 | 1 | 12 | p=0·0020 |
| Overall | |||||||
| Death/permanent disability | 111 (3·6%) | 145 (4·8%) | 45 (1·5%) | 45 (1·5%) | 156 (2·6%) | 190 (3·2%) | p=0·0484 |
NS=not significant.
Seven vs seven of the deaths (all in Africa) could not have been affected by the trial capsule (four vs four patients: capsule expelled intact and not re-inserted, plus three vs three patients: death attributed blindly by endpoint review committee to a disease other than malaria).
Median time (for those with an adverse outcome) to arrival at clinic, or prior death (ie, death without arrival at clinic).
One vs one permanent disability could not have been affected by trial capsule (one HIV CNS disease in Africa, one tuberculosis meningitis in Asia; both died after 7–30 day follow-up). All other cases of permanent disability were from CNS malaria in children in Africa, and all were severe; five, all in the placebo group, died after 7–30 day follow-up.
In the overall results there was no significant effect of pre-referral rectal artesunate on the numbers of deaths within 6 h of randomisation (56 artesunate vs 51 placebo; median time to death only 2 h [IQR 1–3]), or on the numbers of later deaths in those who reached clinic within 0–6 h (71 artesunate vs 75 placebo; median time to clinic only 3 h [IQR 1–4]). In patients who had still not reached clinic after more than 6 h, about half still had not done so after more than 15 h, giving the trial treatment allocation long enough to have a substantial effect on the malaria parasites. In these patients, pre-referral rectal artesunate reduced mortality significantly (27 artesunate vs 51 placebo deaths; p=0·0039). All these 78 deaths were of young children (age 6–72 months, median 17 months [IQR 10–24]), and 72 (92%) were in Africa.
Table 2 also shows the numbers who, although still alive at the 7–30 day follow-up, were permanently disabled by other diseases or by neurological sequelae of the original episode of malaria (or its treatment). One patient given artesunate versus 12 given placebo had permanent disability from cerebral malaria (p=0·0020, all children in Africa). Of these children, five eventually died after the 7–30 day visit (0 artesunate vs five placebo) and eight still had severe CNS damage that was considered permanent when last assessed. 19 given artesunate vs 12 given placebo had CNS damage that resolved (p=0·2). Of those with any clinician-confirmed malarial CNS damage, therefore, the proportions that resolved were 19 of 20 for artesunate versus 12 of 24 for placebo (p=0·0037 for difference).
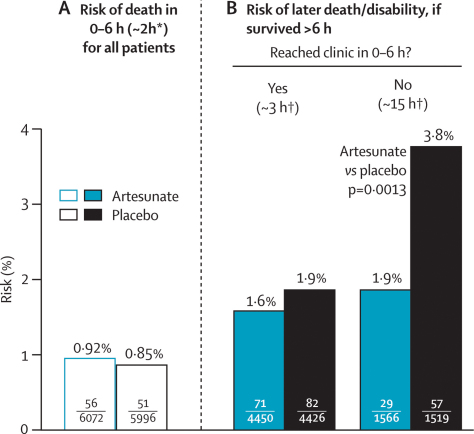
Table 3 and figure 3 both combine the results for death and permanent disability from table 2, subdividing them by time to death and by time taken to reach clinic, and relating them to the estimated numbers at risk. As in a previous trial of artesunate versus quinine,11 there was no apparent effect of artesunate on early mortality (within 0–6 h; figure 3). About three-quarters of patients who survived more than 6 h reached a clinic within 0–6 h; among them, the median time taken to reach clinic was about 3 h, and artesunate did not have a significant effect on outcome (71 vs 82 [1·6% vs 1·9%] dead after 6 h or permanently disabled; risk ratio 0·86 [95% CI 0·63–1·18]; p=0·35). Artesunate did, however, have a significant effect on outcome in the quarter of patients who were still not in clinic more than 6 h after randomisation. For them, the probability of death or permanent disability was only 1·9% with rectal artesunate versus 3·8% with placebo (29 artesunate vs 57 placebo dead or permanently disabled; risk ratio 0·49 [95% CI 0·32–0·77]; p=0·0013).
Table 3.
Effects of trial treatment (artesunate or placebo) on death or permanent disability, subdivided by study site and time taken to reach clinic
|
Risk of death in 0–6 h (median 2 h) for all patients |
Risk of later death/disability (if survived >6 h)*Reached clinic in 0–6 h? |
|||||
|---|---|---|---|---|---|---|
| Artesunate | Placebo |
Yes (∼3 h†) |
No (∼15 h‡) |
|||
| Artesunate | Placebo | Artesunate | Placebo | |||
| Africa (age 6–72 months) | ||||||
| Handeni, Tanzania | 22/726 | 21/737 | 15/286 | 17/292 | 17/418 | 33/424 |
| Kilosa, Tanzania | 11/1170 | 6/1169 | 8/542 | 11/539 | 8/617 | 14/624 |
| Navrongo, Ghana | 9/1145 | 12/1093 | 19/816 | 26/798 | 2/320 | 5/283 |
| All in Africa | 42/3041 (1·4%) | 39/2999 (1·3%) | 42/1644 (2·6%) | 54/1629 (3·3%) | 27/1355 (2·0%) | 52/1331 (3·9%) |
| Chittagong, Asia (by age) | ||||||
| 6–72 months | 5/1022 | 7/988 | 7/947 | 19/918 | 2/70 | 5/63 |
| Older child/adult | 9/2009 | 5/2009 | 22/1859 | 9/1879 | 0/141 | 0/125 |
| All in Asia | 14/3031 (0·5%) | 12/2997 (0·4%) | 29/2806 (1·0%) | 28/2797 (1·0%) | 2/211 (0·9%) | 5/188 (2·7%) |
| Total | ||||||
| Africa and Asia | 56/6072 (0·94%) | 51/5996 (0·85%) | 71/4450 (1·6%) | 82/4426 (1·9%) | 29/1566 (1·9%) | 57/1519 (3·8%) |
| Relative risk (95% CI) | 1·10 (0·75–1·61) | 0·86(0·63–1·18) | 0·49(0·32–0·77) | |||
| p value§ | 0·61 | 0·35 | 0·0013 | |||
Denominators=numbers surviving more than 6 h after entry, subdivided by whether patient reached clinic in 0–6 h. Time to clinic was recorded in all who died or had neurological damage; otherwise, it was recorded routinely only in Kilosa and Navrongo. For those who did not die in Handeni and Chittagong, it was recorded whether they reached a clinic. For this table it is assumed that, if they did die, the proportions doing so in 0-6 h were 50% in Handeni and 95% in Chittagong.
For those who reached clinic in 0–6 h and then died after hour 6, median time to arrival was 2 h in Chittagong and 4 h in Africa.
For those still not in clinic after more than 6 h who died, the median time to reach clinic (or to death without reaching clinic) was 15 h.
Relative risk (95% CI) and p value for artesunate versus placebo.
Figure 3.
Effects of trial treatment on early mortality and, subdivided by time taken to reach clinic, later mortality or permanent disability
*Median time to death. †Median time (for those with adverse outcome) to arrival at clinic, or prior death.
Table 3 subdivides these results by study site and, within Asia, by age; the significant treatment effect in the two right-hand columns is accounted for entirely by young children, mostly in Tanzania (where arrival at a dispensary or other clinic at which antimalarial injections could be given often took several hours). Time to reach clinic was recorded for all patients with an adverse outcome and was adjudicated, blind to treatment, by the ERC. Hence, the numerators (29 vs 57 dead or disabled) at the right-hand side of table 3 and figure 3 are reliably known. Any slight uncertainties in the denominators (1566 vs 1519; table 3 footnote) do not materially affect the p value, the two-fold relative risk, or the 95% CI associated with it.
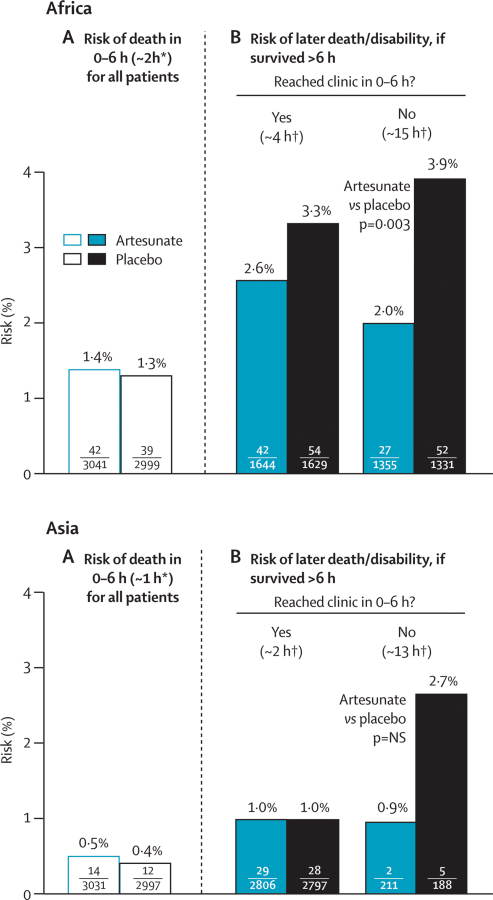
Figure 4 presents the results for Africa and Asia separately; the absolute risks were three times as great in Africa, partly because all patients were young children and almost all parasites were P falciparum. Perhaps more importantly, patients in Africa generally took longer to reach effective treatment, whereas those in Asia quickly got intravenous quinine and hospital care. Even in patients who reached a clinic within 0–6 h, the median time to arrival was about 4 h in Africa and only 2 h in Asia, and the probability of an adverse outcome was about 3% in Africa (42 artesunate vs 54 placebo) and only about 1% in Asia (29 artesunate vs 28 placebo).
Figure 4.
Effects of trial treatment in Africa and in Asia
NS=not significant. *Median time to death. †Median time (for those with adverse outcome) to arrival at clinic, or prior death.
Although these results from Asia (29 vs 28: figure 4) suggest little difference between artesunate and placebo in patients who reach effective treatment rapidly, subdivision by age suggests a favourable effect in young children (seven vs 19) but an adverse result in older patients (22 vs nine). This apparent interaction with age is significant (p=0·0025), but it might nevertheless represent just a chance irregularity in circumstances in which treatment had too little time to have any material effect on outcome in either age range. The mean time spent in hospital in Asia (artesunate vs placebo) was 3·00 versus 3·11 days in young children and 3·20 versus 3·24 days in older patients (p=0·22 for interaction).
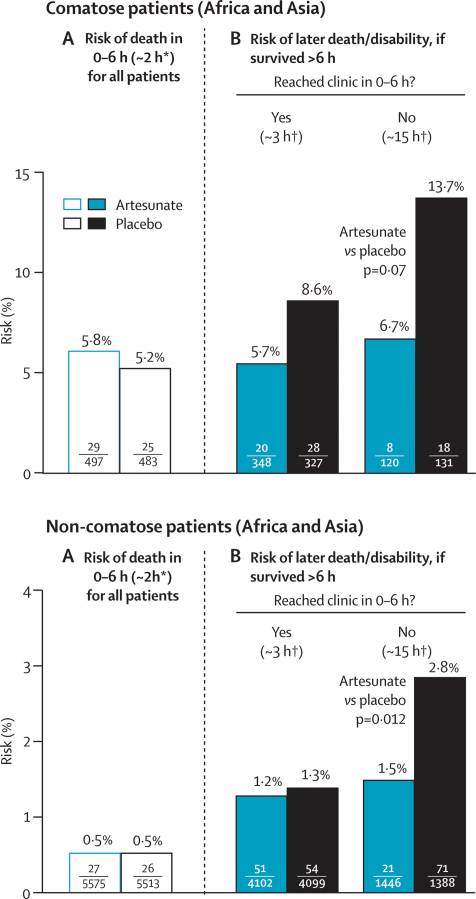
Figure 5 subdivides by coma the results for death or permanent disability. Of the early deaths within 6 h after randomisation, half (54/107) were in the 8% of patients who were already comatose. The trial treatment had no apparent effect on this early mortality. This finding does not necessarily mean, however, that other comatose patients could not benefit from such treatment. In patients still not in clinic more than 6 h after randomisation, the absolute benefit seemed to be substantially greater for those in coma when randomised (6·7% artesunate vs 13·7% placebo, p=0·07) than for those not (1·5% vs 2·8%, p=0·01), but the numbers in coma are too small for this result to be reliable. Results for those who were not in coma (and, by subtraction from table 3, for those in coma) are given in more detail in table 4.
Figure 5.
Effects of trial treatment if comatose and if not comatose
*Median time to death. †Median time (for those with adverse outcome) to arrival at clinic, or prior death.
Table 4.
Non-comatose patients only: effects of trial treatment (artesunate or placebo) on death or permanent disability, subdivided by time taken to reach clinic
|
Risk of death in 0–6 h (median 2 h) for all patients |
Risk of later death/disability (if survived >6 h)*Reached clinic in 0–6 h? |
||||||
|---|---|---|---|---|---|---|---|
| Artesunate | Placebo |
Yes (∼3 h†) |
No (∼15 h‡) |
||||
| Artesunate |
Placebo |
Artesunate |
Placebo |
||||
| Patients not in coma, by site | |||||||
| Africa (age 6–72 months) | |||||||
| Handeni, Tanzania | 11/625 | 10/629 | 12/246 | 14/255 | 11/368 | 23/364 | |
| Kilosa, Tanzania | 7/1153 | 5/1148 | 8/535 | 9/534 | 8/611 | 9/609 | |
| Navrongo, Ghana | 3/974 | 6/942 | 12/696 | 14/692 | 2/275 | 3/244 | |
| All in Africa | 21/2752 (0·8%) | 21/2719 (0·8%) | 32/1477 (2·2%) | 37/1481 (2·5%) | 21/1254 (1·7%) | 35/1217 (2·9%) | |
| Chittagong, Asia (by age) | |||||||
| 6–72 months | 2/934 | 1/901 | 4/870 | 13/842 | 0/62 | 4/58 | |
| Older aged/adult | 4/1889 | 4/1893 | §15/1755 | §4/1776 | 0/130 | 0/113 | |
| All in Asia | 6/2823 (0·2%) | 5/2794 (0·2%) | 19/2625 (0·7%) | 17/2618 (0·6%) | 0/192 (0%) | 4/171 (2·3%) | |
| All patients not in coma | |||||||
| Total, Africa and Asia | 27/5575 (0·5%) | 26/5513 (0·5%) | 51/4102 (1·2%) | 54/4099 (1·3%) | 21/1446 (1·5%) | 39/1388 (2·8%) | |
| p value¶ | 1·0 | 0·76 | 0·0121 | ||||
Corresponding numbers for patients who were in coma at entry can be obtained by subtracting this table from table 3.
Denominators=numbers surviving more than 6 h after entry, subdivided by whether patient reached clinic in 0–6 h. Time to clinic was recorded in all who died or had neurological damage; otherwise, it was recorded routinely only in Kilosa and Navrongo. For those who did not die in Handeni and Chittagong, it was recorded whether they reached a clinic. For this table it is assumed that, if they did die, the proportions doing so in 0–6 h were 50% in Handeni and 95% in Chittagong.
For those who reached clinic in 0–6 h and then died after hour 6, median time to arrival was 2 h in Chittagong and 4 h in Africa.
For those still not in clinic after more than 6 h who died, the median time to reach clinic (or to death without reaching clinic) was 15 h.
Includes six artesunate and one placebo patients who were treated rapidly in hospital, left prematurely against medical advice, then died several days later. (One comatose artesunate-allocated adult patient did likewise).
p value for artesunate versus placebo.
In Asia, quinine was given intravenously, but children in Africa had intramuscular injections. The intramuscular injection site was not systematically recorded, but in Tanzania (not Ghana) it was often the buttock, and in eight cases this caused serious sciatic nerve damage (three artesunate vs five placebo, although the trial treatment is unlikely to have affected this risk). All cases resolved without causing permanent disability, but some took many months to do so. If we assume that about 2000 of the children in Tanzania received injections into the buttock, then their absolute risk of serious sciatic nerve damage from this was about 0·4% (which could have been avoided by use of a different intramuscular injection site).
Discussion
Death from malaria reflects delay in administration of effective antimalarial treatment. Our results provide strong evidence that if patients with severe malaria cannot be treated orally and referral is likely to take several hours, an immediate rectal dose of artesunate before referral substantially reduces the risk of death or permanent disability. This main finding is based on only about 3000 of the 18 000 patients originally recruited (since some 5000 had a negative blood smear, a further 1000 had just been injected with an antimalarial drug [figure 1], and three-quarters of the remaining 12 000 quickly reached a clinic at which such an injection could be given [figure 3]).
The time that would be taken to reach clinic appeared to be a key determinant of the importance of pre-referral treatment. Rectal artesunate takes 6–12 h to produce much change in the parasite count,7–9 and, as expected,11 there was no apparent reduction in early mortality. Although artesunate kills parasites in a wider range of the parasite life cycle than quinine does, there was no significant additional effect of the trial treatment on outcome in patients who were already at, or only a few hours away from, a clinic at which they could be given quinine. The lower confidence limit of 0·63 for the risk ratio among such patients means, however, that even in these circumstances inclusion of an initial rectal dose of artesunate could be of some real value.
The risk reduction was statistically definite only in the 3000 patients who were still not at such a clinic after more than 6 h; half of these patients were still not there after more than 15 h, giving the trial treatment enough time to affect the progression of the disease substantially and thereby achieve a highly significant reduction in death or permanent disability. The adverse outcomes contributing to this significant result were all among young children, mostly in Africa, but that does not mean that the results apply only to children, or only to Africa. The importance of prompt, effective treatment with artesunate could well be greater in young children than in older patients, however, since acute malaria can progress so rapidly in young children that gaining extra hours might be particularly important.2
Presumably, the longer the time to clinic, the greater the importance of pre-referral treatment. We note that the cutoff of 6 h in our analyses was not prespecified, and that this trial should not be taken as showing that pre-referral artesunate is of substantial benefit only if the time to clinic will be more than exactly 6 h.
Age-specific analyses were not prespecified in the original trial protocol but the data analysis plan agreed before unblinding specified that the main analysis would consider young children (assessed age 6–72 months, randomised between 100 mg artesunate and placebo) separately from older patients (school-aged children or adults, randomised between 400 mg artesunate and placebo). This separation would not materially affect the key result among patients still not at clinic more than 6 h after randomisation, since all with an adverse outcome were young children (table 3). Likewise, it would not affect the results in Africa, where all were young children.
In Asia, however, 57 patients (29 artesunate vs 28 placebo; figure 4) reached hospital within 0–6 h, should have received intravenous quinine promptly (at a median of about 2 h after randomisation), and then died after the 6th hour. Subdivision of these deaths by age found apparently opposite effects in young children and older patients in Chittagong: seven versus 19 in young children (in whom such a large benefit from 100 mg artesunate is unlikely, in view of the results in figure 4 for African children) and 22 versus nine in older patients (in whom direct hazard from 400 mg artesunate is unlikely, in view of the significant benefits of artesunate over quinine in a large multicentre trial11 among Asian patients, many in Chittagong). Drug packaging reversal was excluded by post-trial analyses of the remaining 400 mg capsules.
Pre-referral treatment of older patients might have made a few patients less likely to complete hospital treatment (table 4), but had little overall effect on the mean duration of hospital stay. It could not have materially affected treatment-seeking behaviour in those in Asia who reached hospital within 6 h, because their median time to arrival was only 2 h. If substantial benefit and hazard are both unlikely, then the most trustworthy evidence is probably that from the overall results, not subdivided by age (figures 3 and 4).
The number that need to be treated to avoid one death depends on the type of patient. In patients who took several hours to reach clinic, pre-referral rectal artesunate reduced the risk of death or permanent disability by about half in the particular circumstances of this study. The analyses, however, excluded patients with suspected severe malaria who had a negative blood smear, in whom no benefit of artesunate was expected (or observed; figure 1). If used without a rapid diagnostic test, the absolute benefit would depend on the proportion of suspected malaria that really is malaria. Since only about three-quarters of all blood smears in this study were positive, the absolute gain among all patients with suspected severe malaria who were several hours from clinic would be only about 1·4% (ie, three-quarters of 1·9%). The absolute gain in patients who actually have severe malaria depends, however, on their absolute risk (eg, figure 5). In those still not in clinic after more than 6 h, the background risks of death or permanent disability in young children varied substantially between the four study sites (from 2% to 8%, with an overall mean of 4%). When the background risk is higher than in any of these study sites and referral even more difficult, the absolute benefit would be considerably greater than 1·4%, and could well be much greater than 2%. A 100 mg rectal artesunate capsule costs only about US$0·10–0·15 to manufacture, so in such circumstances it could be very cost effective.
The main concern about any type of community treatment of suspected severe malaria is that it could interfere with referral and proper clinical management. One dose of artesunate will not cure severe malaria, and widespread use of rectal artesunate without further consolidation treatment that is curative could favour the development of resistance.12,13 Referral remains important both to complete the treatment of malaria and to diagnose any other underlying life-threatening infection. (Even in the present study with trained recruiters, a quarter [of whom 3% died: figure 1] did not have malaria.) For referral, appropriate health-care facilities need to be functioning reliably and accessible.
The ideal is for appropriate oral artesunate combination treatment to be affordably available14 and used in time to prevent uncomplicated disease from becoming severe. But, for the foreseeable future, there will be many patients in the community with suspected severe malaria who could, if treated earlier, have been managed orally, but can no longer be treated orally and need immediate help. This study shows that referral alone is not enough (unless the patient reaches medical care very quickly) and that rectal artesunate is acceptable, effective, and safe. Moreover, it could also be used in clinics, reducing the need for injections. Indeed, widespread use in clinics might facilitate widespread use in the community.
While the study was in progress, compliance with the advice to go immediately to a clinic was remarkably high both in Africa and in Asia,15–17 partly because there was a recruiter in every village and communities were continually educated about malaria. Additionally, patients in Bangladesh were given a blood slide to take with them to hospital, and on arrival they were to be admitted and treated immediately free of charge with intravenous quinine, broad spectrum antibiotics, and good supportive care. Hence, in the 149 Bangladesh study areas there was an increase in adherence to referral advice from 12% just before the trial18 to 98% during the trial, a median delay of only about 2 h between randomisation and reaching effective treatment in hospital and, overall, only 1·5% mortality (and no permanent disability). This shows, importantly, that accessible clinics and good organisation within villages and within hospitals can greatly reduce malaria mortality and morbidity even without pre-referral treatment.
Acknowledgments
Acknowledgments
We thank the hundreds of villages and thousands of parents or guardians who agreed to take part in this trial; the 417 village recruiters and 74 field supervisors; the local clinics and hospitals in Bangladesh, Ghana, and Tanzania; and the facilities of the Bangladesh Institute of Tropical and Infectious Diseases, the Ghanaian Ministry of Health, and the Tanzanian National Institute of Medical Research. The study was supported financially by the UNICEF/UNDP/World Bank Special Programme for Research and Training in Tropical Diseases (WHO/TDR), the Global Malaria Programme (WHO/GMP), the Sall Family Foundation, the European Union (QLRT-2000-01430), the UK Medical Research Council, USAID, Irish Aid, the Karolinska Institute, and the University of Oxford Clinical Trial Service Unit (CTSU). NJW is a Wellcome Trust Principal Fellow.
Contributors
The authors accept full responsibility for the overall content of this report, and the study director (MG) and study monitors (IR and JF) are its guarantors. The study was initially designed by members of the WHO Task Force on Severe Malaria (PF, NJW, SK, FB, PO, IR, MG) and the CTSU (R Collins, RP, A Young); the principal investigators then adapted the design locally and conducted the trial. All investigators discussed the unblinded results; MG then drafted this report with RP, PF, and NJW, and it was reviewed, revised, and agreed by all authors.
Study 13 Research Group
Bangladesh—Principal investigator: Abul Faiz. Co-investigators: Emran Bin Yunus, Md Ridwanur Rahman, Md Amir Hossain, Rasheda Samad, A M Bangali, Rafiqul Hassan. Research assistants (in charge of 149 village recruiters): Rajib Palit, Md Sazzad Hossain Chowdhury, Alamgir Rashid Chowdhury, Arman Hossain, Md Golam Kibria, Tafsir Ahmed Chowdhury, A Uye Maung, Nasiruddin Bhuiyan, Sonet Dipta Nath, Ashraful Islam, Ranjan Chowdhury. Field investigators: Ajoy Ghose, M Rashid, Md Badiur Rahman, Narayan Proshad, Dulal C Paul, Ratan Chowdhury. Field supervisors: Anil Baran Paul, Ajit Kumar Bhattacharjee, Baker Siddique, Md Danesh Chowdhury, Abul Kashem, Parimal Sharma, Niranjan Das, Md Yunus, Abul Kashem, Priya Ranjan Paul, Alhaj Nurul Alam, Md Amanul Hoque, Md Ali, Md Nurul Islam, Kallol Barua, Md Soaib, Ranadhir Barua, Manik Chandra Dhar, Md Amanat Ullah, Md Abdul Monaf, Md Syed Siraje, Mamtaz Ahmed, Ashutosh Shil, Nurul Islam, Bangkim Barua, Mong Ting Aung, Farid Alam, Monoranjan Barua, Mahbubul Mawla, M Ibrahim Azad, Manohari Dutta, Siddique Ahmed, Tarun Barua, Anil Kumar Barua, Sunil Kumar Bardan, Suibai Aung Marma, Mayshan Marma, Chaila Mong Chak, Hla Mong Chin Marma, Md Alam, Akter Ahmed, Abul Kalam Azad, Pallab Barua, Akter Hossain, Proshenjit Barua, Amir Ali, Madhu Sudan Dev, Mozaffar Ahmed, Awlad Hossain, Protiva Rani Chy. Data entry clerks: Md Safiqul Mostafa Chowdhury, Md Nizam Uddin, Yeasmin Zahan. Hospital staff: Dolly Biswas, Golapy Kulentunu, Protiva Rani Chowdhury, Habiba Jannat. Laboratory microscopy staff: Nazem Uddin, Eliyas Ahmed.
Ghana—Principal investigator: John Gyapong. Co-investigators: Fred Binka, Christine Clerk, Frank Baiden, Rita Baiden, Seth Owusu Agyei, Nathan Mensah, Abraham Hodgson. Research assistants: Oscar Bangre, Fabian Achana, Samuel Chatio. Field supervisors: (in charge of 45 village recruiters) Roberto Abakeh, Isaac Apuri, James Atintono, Monica Kaba, William Kwarah, Jacob Wedam. Data entry clerks: Louisa Abukari, Clara Awampaga. Laboratory microscopy staff: Abdulai Santama, Bugri Gumah Akalifa, Abdul-Wahab Hamid.
Tanzania—Principal investigator: Marian Warsame. Co-investigator: Andrew Kitua. Kilosa co-investigator: Zakayo Mrango. Handeni co-investigators: Tom Peto, Charles Makasi. Field coordinator: Omari Kimbute. Kilosa field supervisors (in charge of 73 village recruiters): Samuel Mwankusye, Steven Mduma, Joseph Shishira, Mansour H Msabaha, Francis Mulokozi, Goodluck Motta, Jackson Mkwao. Handeni field supervisors (in charge of 150 village recruiters): Elia Msegu, Elias Ndahani, Emmanuel Massawe, Rahim Mohamed, Henerico Ernest, Benson Bundala, Andrew Mkomwa, Daudi Twaha, Daniel Chochole, John Masimba, Revocatus Nyekabora. Kilosa hospital team: Kamuabwa L Rufulenge, Nasemba Njema, Grace Chiduo, Juliana Kitua. Data entry clerks: Avit Kapinga, Irene Kisongele, Nahda Juma. Laboratory microscopy staff: Steven Ngatunga, Fred Clement.
International—Monitoring study conduct (blind to results): Isabela Ribeiro, Jayme Fernandes. Data and Safety Monitoring Committee: Rory Collins (chair), Tim Peto, Lindiwe Makubalo, with Abdel Babiker preparing and presenting unblinded reports. Endpoint Review Committee: Nicholas J White (chair), Tsiri Agbenyega, Melba Gomes.
Conflict of interest statement
NJW and FB (co-chairs) and MAF are on the WHO Antimalarial Treatment Guidelines Committee. WHO employs PO, MW, and MG; CTSU employs RP. All other authors declare that they have no conflict of interest.
References
- 1.for the Disease Control Priorities Project . Global burden of disease and risk factors. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. The World Bank/Oxford University Press; New York: 2006. [Google Scholar]
- 2.Newton CRJC, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux ME. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 4.Carter JA, Mung'ala-Odera V, Neville BGR. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–481. doi: 10.1136/jnnp.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngoungou EB, Dulac O, Poudiougou B. Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, West Africa. Epilepsia. 2006;47:873–879. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 6.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson JA, Agbenyega T, Barnes KI. Population pharmacokinetics of artesunate and dihydroartemisinin following intra-rectal dosing of artesunate in malaria patients. PLoS Med. 2006;3:e444. doi: 10.1371/journal.pmed.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishna S, Planche T, Agbenyega T. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob Agents Chemother. 2001;45:509–516. doi: 10.1128/AAC.45.2.509-516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes KI, Mwenechanya J, Tembo M. Efficacy of rectal artesunate compared with parenteral quinine in initial treatment of moderately severe malaria in African children and adults: a randomised study. Lancet. 2004;363:1598–1605. doi: 10.1016/S0140-6736(04)16203-X. [DOI] [PubMed] [Google Scholar]
- 10.Brewer TG, Peggins JO, Grate SJ. Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg. 1994;88(suppl 1):S33–S36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 11.Dondorp A, Nosten F, Stepniewska K, Day NP, White NJ. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 12.White NJ. Antimalarial drug resistance and mortality in falciparum malaria. Trop Med Int Health. 1999;4:469. doi: 10.1046/j.1365-3156.1999.00435.x. [DOI] [PubMed] [Google Scholar]
- 13.White NJ. Antimalarial resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrow KJ, Panosian CB, Gelband H. Saving lives, buying time: economics of malaria drugs in an age of resistance. National Academy Press; Washington: 2004. [PubMed] [Google Scholar]
- 15.Peterson S, Nsungwa-Sabiiti J, Were W. Coping with paediatric referral—Ugandan parents' experience. Lancet. 2004;363:1955–1956. doi: 10.1016/S0140-6736(04)16411-8. [DOI] [PubMed] [Google Scholar]
- 16.al Fadil SM, Alrahman SH, Cousens S. Integrated Management of Childhood Illness strategy: compliance with referral and follow-up recommendations in Gezira State, Sudan. Bull World Health Organ. 2003;81:708–716. [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury EK, El Arifeen S, Rahman M. Care at first-level facilities for children with severe pneumonia in Bangladesh: a cohort study. Lancet. 2008;372:822–830. doi: 10.1016/S0140-6736(08)61166-6. [DOI] [PubMed] [Google Scholar]
- 18.Yunus EB, Faiz MA, Rahman MR. Study to document pre-admission risk factors for development of severe malaria and the spectrum of it and the outcome in different categories of hospitals in malaria endemic zone of Bangladesh. J Bang Coll Phys Surg. 2004;22:83–88. [Google Scholar]