Summary
Background
Early endometrial cancer with low-risk pathological features can be successfully treated by surgery alone. External beam radiotherapy added to surgery has been investigated in several small trials, which have mainly included women at intermediate risk of recurrence. In these trials, postoperative radiotherapy has been shown to reduce the risk of isolated local recurrence but there is no evidence that it improves recurrence-free or overall survival. We report the findings from the ASTEC and EN.5 trials, which investigated adjuvant external beam radiotherapy in women with early-stage disease and pathological features suggestive of intermediate or high risk of recurrence and death from endometrial cancer.
Methods
Between July, 1996, and March, 2005, 905 (789 ASTEC, 116 EN.5) women with intermediate-risk or high-risk early-stage disease from 112 centres in seven countries (UK, Canada, Poland, Norway, New Zealand, Australia, USA) were randomly assigned after surgery to observation (453) or to external beam radiotherapy (452). A target dose of 40–46 Gy in 20–25 daily fractions to the pelvis, treating five times a week, was specified. Primary outcome measure was overall survival, and all analyses were by intention to treat. These trials were registered ISRCTN 16571884 (ASTEC) and NCT 00002807 (EN.5).
Findings
After a median follow-up of 58 months, 135 women (68 observation, 67 external beam radiotherapy) had died. There was no evidence that overall survival with external beam radiotherapy was better than observation, hazard ratio 1·05 (95% CI 0·75–1·48; p=0·77). 5-year overall survival was 84% in both groups. Combining data from ASTEC and EN.5 in a meta-analysis of trials confirmed that there was no benefit in terms of overall survival (hazard ratio 1·04; 95% CI 0·84–1·29) and can reliably exclude an absolute benefit of external beam radiotherapy at 5 years of more than 3%. With brachytherapy used in 53% of women in ASTEC/EN.5, the local recurrence rate in the observation group at 5 years was 6·1%.
Interpretation
Adjuvant external beam radiotherapy cannot be recommended as part of routine treatment for women with intermediate-risk or high-risk early-stage endometrial cancer with the aim of improving survival. The absolute benefit of external beam radiotherapy in preventing isolated local recurrence is small and is not without toxicity.
Funding
Medical Research Council, National Cancer Research Network, National Cancer Institute of Canada, with funds from the Canadian Cancer Society.
Introduction
After surgery for endometrial cancer, external beam radiotherapy has been offered to women with early disease (International Federation of Gynecology and Obstetrics [FIGO] stage I and IIA) whose pathological features indicate an increased likelihood of nodal metastases at diagnosis and who might therefore benefit from adjuvant treatment. These pathological features have included histological type, grade, and depth of myometrial invasion. There are national and international variations in the definitions of intermediate and high risk, the types of women offered adjuvant radiotherapy as part of routine clinical practice, and the range of stage I and IIA women entered into clinical trials. In addition to external beam radiotherapy, local vaginal radiotherapy (brachytherapy) is also used to prevent cancer recurrence.
EN.5 and ASTEC were set up as individual trials to investigate the benefit or otherwise of postoperative adjuvant pelvic radiotherapy in women with early endometrial cancer and pathological features suggestive of intermediate or high risk of recurrence and death. The EN.5 trial of the National Cancer Institute of Canada (NCIC) Clinical Trials Group started in 1996, but could not recruit sufficient patient numbers to complete the study as it was originally envisaged. In 1998, the UK Medical Research Council (MRC) launched ASTEC, and invited the NCIC Clinical Trials Group to plan a prospective combination of the EN.5 data with those of ASTEC. ASTEC/EN.5 therefore consists of two trials with separate randomisations combined to make one intergroup trial.
A recent systematic review and meta-analysis1 identified four completed randomised trials2–5 which assessed the benefit of adjuvant external beam radiotherapy in women with early endometrial cancer. Data for 1770 women (including 258 deaths) in these trials showed that adjuvant radiotherapy reduces the risk of pelvic recurrence, but there was no evidence that it improves overall survival.1 The ASTEC and EN.5 trials with 905 women (and 135 deaths) provide additional data, particularly for high-risk women within stage I disease, who were under-represented in the earlier trials, and could allow smaller, clinically important differences to be reliably detected.
Methods
Participants
Eligible women had histologically confirmed endometrial cancer, macroscopically confined to the uterine corpus (FIGO stage I) or endocervical glands (IIA), with pathological features suggestive of an intermediate or high risk of recurrence including: FIGO stage IA and IB grade 3; IC all grades; papillary serous; or clear cell histology all stages and grades (table 1).6 Lymphadenectomy as part of surgical staging was not a requirement for randomisation. Positive para-aortic nodes were viewed as indicative of unrecognised macroscopic disease and were an exclusion to randomisation. Pelvic lymph nodes could be negative or not examined; women with positive pelvic lymph nodes were eligible for ASTEC but not for EN.5. Peritoneal cytology could be negative, positive, or not done. Women had to be fit to receive external beam radiotherapy and all women gave written, informed consent. Women in the ASTEC surgical trial gave consent for both surgical and radiotherapy randomisations before surgery. Ethics approval for the trial was obtained from the North West Multi Centre Regional Ethics Committee in the UK and each participating institution in Canada.
Table 1.
ASTEC/EN.5 FIGO stage and tumour grade entry criteria, intermediate-risk or high-risk classification, and patient distribution
| G1 | G2 | G3 | Papillary serous/clear cell | |
|---|---|---|---|---|
| IA | 1 (<1%) | 1 (<1%) | 8 (1%)* | 15 (2%)† |
| IB | 1 (<1%) | 5 (1%) | 99 (11%)* | 48 (5%)† |
| IC | 213 (24%)* | 337 (37%)* | 100 (11%)† | 27 (3%)† |
| IIA | 9 (1%)* | 19 (2%)* | 6 (1%)† | 3 (<1%)† |
| IIB‡ | 2 (<1%) | 0 | 0 | 1 (<1%) |
Data are number (%). Left column is FIGO stage.
Intermediate subgroup. All others would be deemed low risk.
High-risk subgroup.
IIB patients were not eligible but those randomised were included in the high-risk subgroup.
Procedures
Eligible women were randomly allocated after surgery to either the observation group with no external beam radiotherapy or systemic treatment until recurrence, or to the external beam radiotherapy group. Radiotherapy was started as soon as possible after wound healing, 6–8 weeks after surgery. In EN.5, a specified date on which radiotherapy would occur (if allocated) was available at randomisation and that date was no later than 12 weeks after surgery. A target dose of 40–46 Gy (45 Gy in EN.5) in 20–25 daily fractions (25 fractions in EN.5) to the pelvis, treating five times a week was specified. Brachytherapy was allowed if the centre's policy was to offer it to all stage I or IIA women irrespective of radiotherapy allocation. In ASTEC, two fractions of 4 Gy at 0·5 cm over 3–7 days at high dose rate or 15 Gy at low dose rate (50 cGy per h) was recommended to the upper third of the vagina. When using the LDR Selectron (Nucletron, Veenendaal, Netherlands) at around 170 cGy per h, a dose of 13·5 Gy at 0·5 cm depth was suggested. In EN.5, brachytherapy was given in accordance with local practice.
Randomisation was done via telephone to the MRC Clinical Trials Unit (ASTEC centres) or to the NCIC Clinical Trials Group (EN.5 centres). Computer randomisation in both trials used a method of minimisation. In ASTEC, minimisation factors were centre, WHO performance status (0–1 vs 2–4), nodes involved (yes vs no), depth of invasion (inner half vs outer half), positive peritoneal cytology (yes vs no), and tumour grade (G1/G2 vs G3). In EN.5, minimisation factors were centre, tumour grade (G1 vs G2 vs G3), surgical staging defined as at least one pelvic lymph node identified (yes vs no), and sexual health assessment (yes vs no). Each centre indicated its brachytherapy policy.
Randomisation was based on local pathology. In ASTEC, the minimum dataset of the Royal College of Pathologists was used as the standard for pathology reporting.7 Surgery and local pathology details were reviewed centrally at the MRC Clinical Trials Unit after randomisation. After randomisation in EN.5, central pathology review, with representative blocks from each patient, was done by two reference pathologists. For consistency, the primary analysis of ASTEC/EN.5 was based on the local pathology report.
The classification of women into intermediate and high risk was on the basis of risk of distant recurrence, previously defined in the GOG99 and PORTEC1 studies.4,5 High risk included all papillary serous and clear cell subtypes, all other subtypes in IC (grade 3), and IIA (grade 3) and all women in stage IIB. Intermediate risk included subtypes other than papillary serous and clear cell histology within stage IA and IB (grade 3), and stage IC and IIA (grade 1 and 2; table 1).
In ASTEC, women were assessed before randomisation, after all postoperative treatment, including brachytherapy and external beam radiotherapy (if given), had been completed (around 3 months after pathology registration and 4 months after surgery), then roughly 6 months after pathology registration. In EN.5, women were assessed 2 months after start of radiotherapy or 4 months after surgery. Follow-up in both trials continued every 3 months for the first year, every 6 months in years 2 and 3, and every year thereafter. Follow-up data included details of endometrial cancer recurrence and treatment, vital status, and short-term and long-term toxicities. Acute toxicity was assessed after completion of radiotherapy in those who received it, and roughly 4 months after completion of surgery in those who did not.
Statistical analysis
The primary outcome measure was overall survival. When the plan for the joint analysis of ASTEC and EN.5 was developed, an overall sample size of 900 women was chosen to observe 130 events (deaths), to detect a 10% improvement in 5-year overall survival from 75% in the observation group versus 85% in the external beam radiotherapy group (hazard ratio 0·56), with a 5% significance level and 90% power. Combined with previous studies GOG993 and PORTEC1,4 this definition would allow detection of a 6–7% absolute difference in overall survival with 90% power. Also, combining ASTEC/EN.5 participants with those from other major studies (specifically GOG99 and PORTEC1) would allow reliable exclusion of an absolute difference in 5-year overall survival of more than 5%, if there was no difference detected, by determining the width of the 95% CI around a hazard ratio of 1·0.
Secondary outcome measures were disease-specific survival, disease-specific recurrence-free survival, isolated loco-regional recurrence, and treatment toxicity. Overall survival was defined as the time from randomisation to death from any cause; women known to be still alive at the time of analysis were censored at the time of their last follow-up. Disease-specific survival was defined as time from randomisation to death from endometrial cancer or death due to treatment; deaths from other causes without disease recurrence were treated as a competing risk. Disease-specific recurrence-free survival was defined as time from randomisation to first appearance of endometrial cancer or death from endometrial cancer or due to treatment. Time to loco-regional recurrence was defined as time from randomisation to vaginal or pelvic recurrence alone, and did not include women who had simultaneous local and distal recurrence or distal recurrence alone. In ASTEC, assessment of cause of death was made by the chief investigator blinded to treatment group, and was classified as treatment-related, disease-related, treatment and disease-related, or other (non-endometrial cancer, non-treatment-related). Competing risk methods were used in the comparisons for disease-specific survival and disease-specific recurrence-free survival.8 The log-rank analysis was obtained indirectly by subtracting the log-rank statistic for non-endometrial cancer deaths from the log-rank statistic for all deaths (the two observed values were subtracted from each other; the two expected values were subtracted from each other; and the two variances were subtracted from each other).
The standard log-rank test was applied for all other time-to-event outcome measures. All comparisons are expressed relative to women in the observation group; a hazard ratio less than 1·0 indicates a decreased risk of the event for women in the external beam radiotherapy group. The absolute difference between the treatment groups with time was modelled using the “stpm” command in Stata for the flexible parametric models of Royston and Parmar.9
Adjusted analyses using the Cox model were done to assess robustness of results. Covariates included the patient characteristics (age, WHO performance status, and whether lymphadenectomy was done) and pathology details (summarised as risk group) at baseline. Predefined subgroups included women grouped by risk of recurrence and whether systematic lymphadenectomy was undertaken as part of initial surgery. Other effects of external beam radiotherapy on primary and secondary outcome measures were assessed in an exploratory manner in subgroups. To test for differences in the relative size of effect in different subgroups, a χ2 test for interaction, or, when appropriate, a χ2 test for trend was used. All analyses were done on an intention-to-treat basis. All analyses on time-to-event outcome measure were stratified by the component of ASTEC versus EN.5. All p values are two-sided.
Established search strategies10 used in a previous meta-analysis1 were updated to identify other randomised trials of external beam radiotherapy in endometrial cancer, and the Cochrane database was searched for systematic reviews. Summary statistics abstracted from the identified studies were used in the analysis.
Role of the funding source
The MRC, as funder of ASTEC, reviewed and approved its design. The NCIC Clinical Trials Group reviewed the design of EN.5. The execution, and approval for combination of the trials, was overseen by an independent data monitoring committee and independent trial steering committee. The sponsor of the study had no role in data collection, data analysis, data interpretation, or writing of the report. The writing committee had full access to all the data in the study, and the trial management group had final responsibility for the decision to submit for publication.
Results
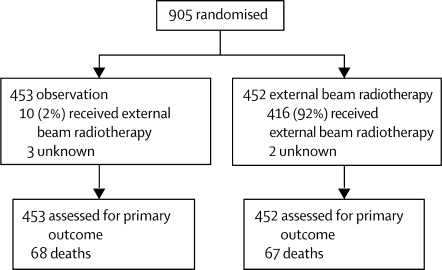
Between July, 1996, and March, 2005, 905 (789 ASTEC, 116 EN.5) women from 112 centres in seven countries (UK, Canada, Poland, Norway, New Zealand, Australia, USA) were randomised: 453 to observation and 452 to external beam radiotherapy (figure 1). Patient characteristics at randomisation including details of surgery and local pathology are shown in table 2. The predominant histological subtype was endometrioid (83%). The baseline data were generally balanced between the two groups, except for a small imbalance in the proportion of high-risk women, with 25% of those in the observation group classified as high risk compared with 20% in the external beam radiotherapy group.
Figure 1.
Trial profile
Table 2.
Pretreatment characteristics, surgery received, and local pathology details reported at randomisation
| Observation (N=453) | External beam radiotherapy (N=452) | ||
|---|---|---|---|
| Age (years) | |||
| Median (range) | 66 (31–88) | 65 (36–88) | |
| WHO/Eastern Cooperative Oncology Group performance status | |||
| 0 | 290 (64%) | 304 (67%) | |
| 1 | 151 (33%) | 136 (30%) | |
| 2 | 10 (2%) | 11 (2%) | |
| 3 | 2 (<1%) | 1 (<1%) | |
| Surgery received | |||
| TAH/BSO | 326 (72%) | 313 (69%) | |
| TAH/BSO/lymphadenectomy | 127 (28%) | 139 (31%) | |
| Histology | |||
| Endometrioid | 372 (83%) | 371 (83%) | |
| Adenocarcinoma not otherwise specified | 14 (3%) | 18 (4%) | |
| Clear cell | 15 (3%) | 7 (2%) | |
| Papillary serous | 23 (5%) | 14 (3%) | |
| Squamous | 4 (1%) | 6 (1%) | |
| Mucinous | 1 (<1%) | 0 | |
| Mixed epithelial high grade | 14 (3%) | 21 (5%) | |
| Mixed epithelial low grade | 2 (<1%) | 2 (<1%) | |
| Other epithelial, other mixed or no details | 4 (1%) | 5 (1%) | |
| Mixed epithelial stromal (ineligible) | 0 | 2 (<1%) | |
| Sarcoma (ineligible) | 1 (<1%) | 1 (<1%) | |
| Unknown | 3 | 5 | |
| Differentiation or grade | |||
| Well (G1) | 107 (24%) | 120 (27%) | |
| Moderate (G2) | 185 (41%) | 180 (40%) | |
| Poor (G3) | 107 (24%) | 107 (24%) | |
| Clear cell/serous papillary/mixed epithelial high grade | 52 (12%) | 42 (9%) | |
| Not applicable (sarcoma and mixed epithelial stromal) | 2 (<1%) | 3 (1%) | |
| Lymphovascular permeation | |||
| Yes | 102 (26%) | 99 (25%) | |
| No | 293 (74%) | 302 (75%) | |
| Not mentioned in the pathology report | 52 | 42 | |
| Unknown | 6 | 9 | |
| Positive peritoneal status | |||
| Yes | 20 (5%) | 15 (3%) | |
| No | 407 (95%) | 415 (97%) | |
| Not done | 24 | 22 | |
| Unknown | 2 | 0 | |
| Nodes present in pathology specimen | |||
| No | 289 (68%) | 265 (63%) | |
| Yes | 137 (32%) | 159 (38%) | |
| Unknown | 27 | 28 | |
| Number of nodes removed | |||
| 1–5 | 51 | 48 | |
| 6–10 | 36 | 36 | |
| 11–15 | 24 | 22 | |
| >15 | 21 | 48 | |
| Unknown | 5 | 5 | |
| Median (range) number of nodes removed | 8 (1–39) | 10 (1–40) | |
| Nodal involvement (if nodes removed) | |||
| No | 132 (96%) | 153 (96%) | |
| Yes | 5 (4%) | 6 (4%) | |
| FIGO stage | |||
| IA (endometrium only) | 11 (2%) | 15 (3%) | |
| IB (<inner half myometrium) | 79 (18%) | 76 (17%) | |
| IC (outer half myometrium) | 336 (75%) | 343 (76%) | |
| IIA (endocervical gland invasion) | 21 (5%) | 16 (4%) | |
| IIB (cervical stromal invasion) | 3 (1%) | 0 | |
| III/IV (spread beyond uterus or cervix) | 0 | 1 (<1%) | |
| Unknown | 3 | 1 | |
| Risk group | |||
| Intermediate risk | 335 (75%) | 358 (80%) | |
| High risk | 113 (25%) | 89 (20%) | |
| Unknown | 5 | 5 | |
TAH/BSO=total abdominal hysterectomy with bilateral salpingo-oopherectomy.
Details of radiotherapy received are summarised in table 3. 92% of women randomised to external beam radiotherapy received it with or without brachytherapy. Median dose was 45 Gy in 25 fractions over 34 days, giving 82% compliance with the planned dose of 40–46 Gy in 20–25 fractions. Compliance with stated brachytherapy policy was 80%. Similar proportions of women in both groups received brachytherapy, with 235 (52%) in the observation group and 242 (54%) in the external beam radiotherapy group. Pretreatment characteristics of women who did and did not receive brachytherapy are shown in the webtable.
Table 3.
Radiotherapy received
| Observation (N=453) | External beam radiotherapy (N=452) | |
|---|---|---|
| External beam radiotherapy only | 3 (1%) | 184 (41%) |
| External beam radiotherapy plus brachytherapy | 7 (2%) | 232 (52%) |
| Brachytherapy | 228 (51%) | 10 (2%) |
| None | 212 (47%) | 24 (5%) |
| Unknown | 3 | 2 |
Adjuvant treatment for endometrial cancer before disease recurrence was given to a small proportion of women. In ASTEC, 40 (5%, 19 external beam radiotherapy, 21 observation) women received hormone therapy and seven (1%, five external beam radiotherapy and two observation) received other treatment.
Reported toxicity is summarised in table 4. Acute toxicity after completion of all surgery and radiotherapy was greater in the external beam radiotherapy group than with observation (any toxicity: 121 women [27%] observation vs 258 women [57%] external beam radiotherapy; severe or life-threatening toxicity: three [<1%] observation vs 14 [3%] external beam radiotherapy). Late toxicity, predominantly gastrointestinal or urogenital, was also more commonly reported after external beam radiotherapy (any late toxicity 202 women [45%] observation vs 274 [61%] external beam radiotherapy; severe toxicity 15 [3%] observation vs 30 [7%] external beam radiotherapy). Four women (1%), all of whom received external beam radiotherapy, were reported with life-threatening toxicity.
Table 4.
Toxicity
| Observation (N=453) | External beam radiotherapy (N=452) | ||
|---|---|---|---|
| Acute toxicity | |||
| Any acute toxicity experienced | |||
| No | 329 (73%) | 191 (43%) | |
| Yes | 121 (27%) | 258 (57%) | |
| Unknown | 3 | 3 | |
| Worst score of acute toxicity | |||
| Mild | 77 (17%) | 143 (32%) | |
| Moderate | 38 (8%) | 100 (22%) | |
| Severe or life threatening | 3 (<1%) | 14 (3%) | |
| Unknown | 3 | 1 | |
| Late toxicity | |||
| Any late toxicity experienced | |||
| No | 251 (55%) | 178 (39%) | |
| Yes | 202 (45%) | 274 (61%) | |
| Worst score of late toxicity | |||
| Mild | 110 (24%) | 135 (30%) | |
| Moderate | 71 (16%) | 99 (22%) | |
| Severe | 15 (3%) | 30 (7%) | |
| Life threatening | 0 | 4 (1%) | |
| Unknown | 6 | 6 | |
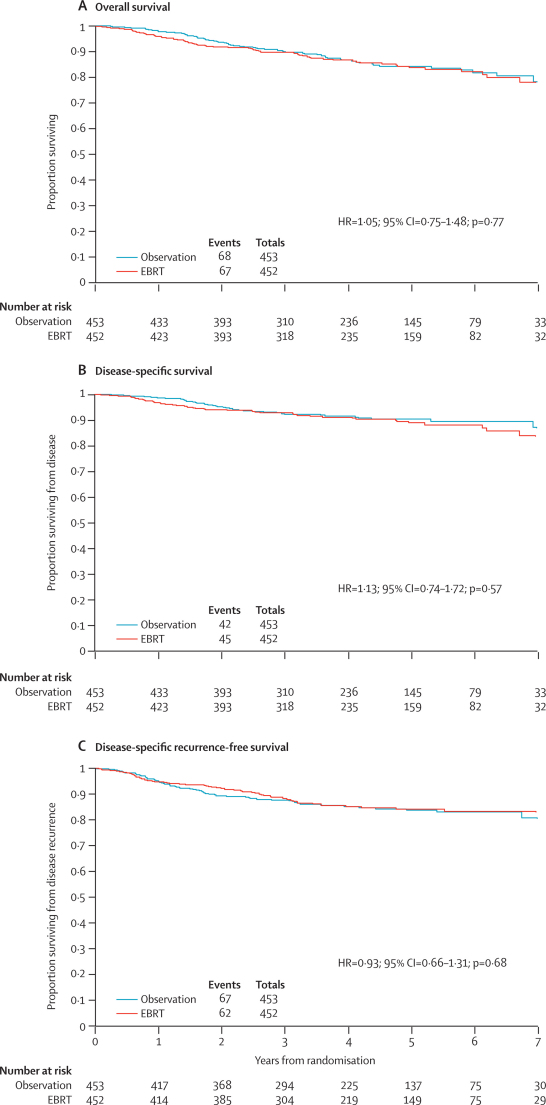
A summary of events is shown in table 5 and a summary of the primary comparisons with 5 year rates in table 6. Kaplan-Meier plots for the two treatment groups are shown in figure 2. At the time of analysis, with a median follow up of 58 months, 135 (15%; 68 observation, 67 external beam radiotherapy) women had died. Overall survival curves showed no evidence of a difference between the two groups with a hazard ratio of 1·05 (95% CI 0·75–1·48; p=0·77, figure 2A). The 5-year overall survival was 84%.
Table 5.
Summary of events
| Observation (N=453) | External beam radiotherapy (N=452) | Total (N=905) | ||
|---|---|---|---|---|
| Overall survival | ||||
| Alive | 385 (85%) | 385 (85%) | 770 (85%) | |
| Dead | 68 (15%) | 67 (15%) | 135 (15%) | |
| Cause of death | ||||
| Disease related | 39 | 41 | 80 | |
| Treatment related | 0 | 1 | 1 | |
| Disease and treatment | 1 | 1 | 2 | |
| Other | 28* | 22 | 50 | |
| Unknown | 0 | 2 | 2 | |
| Recurrence-free survival | ||||
| No recurrence or death | 360 (79%) | 368 (81%) | 728 (80%) | |
| Recurrence only | 25 (5%) | 17 (4%) | 42 (5%) | |
| Death only | 27 (6%) | 30 (7%) | 57 (6%) | |
| Recurrence and death | 41 (9%) | 37 (8%) | 78 (9%) | |
| Disease recurrence | ||||
| No | 387 (85%) | 398 (88%) | 785 (87%) | |
| Yes | 66 (15%) | 54 (12%) | 120 (13%) | |
| Site of first recurrence | ||||
| Isolated vaginal or pelvic intial recurrence | ||||
| Local/vaginal | 17 | 7 | 24 | |
| Pelvic | 12 | 5 | 17 | |
| Local vaginal plus pelvic | 0 | 1 | 1 | |
| Distant with or without local recurrence | ||||
| Local/vaginal and distant | 3 | 1 | 4 | |
| Pelvic and distant | 3 | 6 | 9 | |
| Distant | 31 | 34 | 65 | |
Two patients with disease recurrence before death are included as events in disease-specific and disease-specific recurrence-free survival analyses.
Table 6.
Summary of comparisons for time to event outcome measures
| Total events |
Unadjusted |
5-year rate |
Absolute difference in 5-year rate (95% CI) |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Observation | EBRT | Hazard ratio (95% CI) | p value | |||
| Overall survival | 135 | 1·05 (0·75 to 1·48) | 0·77 | 83·9% | 83·5% | 0·4%(−5·0% to 5·7%) | 1·19 (0·85–1·68) | 0·31 |
| Disease-specific survival | 87 | 1·13 (0·74 to 1·72) | 0·57 | 89·9% | 88·5% | 1·4%(−3·2% to 5·9%) | 1·26 (0·83 to 1·94) | 0·28 |
| Disease-specific recurrence-free survival | 129 | 0·93 (0·66 to 1·31) | 0·68 | 84·7% | 85·3% | 0·6%(−4·4% to 5·7%) | 0·99 (0·70 to 1·40) | 0·95 |
*Adjusted for age (continuous variable), WHO (0,1,2,3), risk group (intermediate vs high), and lymphadenectomy (yes, no). EBRT=external beam radiotherapy.
Figure 2.
Kaplan-Meier plots for outcome measure
EBRT=external beam radiotherapy.
A proportion of women did not die from endometrial cancer and had no disease recurrence before death. 48 (36%) deaths (26 observation, 22 external beam radiotherapy) were classified as non-disease and non-treatment-related, whereas 87 (64%; 42 observation, 45 external beam radiotherapy) women died from disease or treatment (including two women in the observation group with disease recurrence before death, table 5). An analysis which treated the non-disease, non-treatment-related deaths as a competing risk showed a hazard ratio of 1·13 (95% CI 0·74–1·72; p=0·57, figure 2B), although inevitably the confidence intervals are wider with fewer events in this analysis. The 5-year disease specific survival for all women was 90% in the observation and 89% in the external beam radiotherapy group.
At the time of analysis, 129 women (67 observation, 62 external beam radiotherapy) had disease recurrence or had died from endometrial cancer. Kaplan-Meier plots for disease-specific recurrence-free survival are shown in figure 2C. Competing risk analysis gave a hazard ratio of 0·93 (95% CI 0·66–1·31, p=0·68). The 5-year disease-specific recurrence-free survival was 84·7% in the observation group and 85·3% in the external beam radiotherapy group, with no evidence of difference on disease specific recurrence-free survival between the two groups.
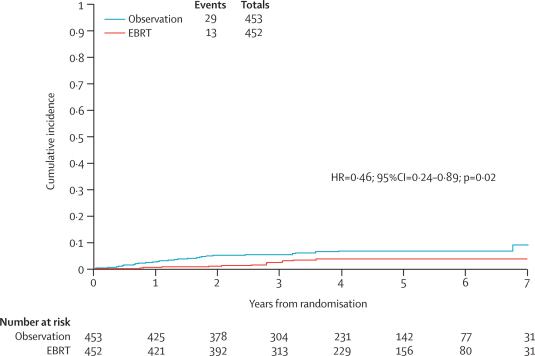
Site of recurrence is shown in table 5. The cumulative incidence of isolated vaginal or pelvic initial recurrence curves are shown in figure 3. The hazard ratio for isolated vaginal or pelvic recurrence-free survival was 0·46 (95% CI 0·24–0·89, p=0·02) with a 5-year cumulative incidence rate of 6·1% in the observation group and 3·2% in the external beam radiotherapy group, an absolute difference of 2·9% (95% CI <0·1%–5·9%). Only 35% (42 of 120) of the total recurrences were isolated local recurrence, and although external beam radiotherapy seems to improve local control, this analysis excluded most women (78; 37 observation, 41 external beam radiotherapy) who had local and distant recurrence or distant recurrence alone.
Figure 3.
Isolated vaginal or pelvic initial recurrence
EBRT=external beam radiotherapy.
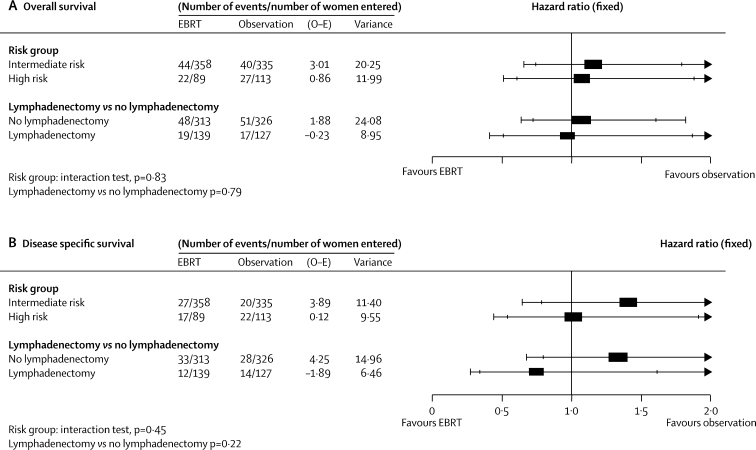
We also did analyses of overall survival, disease-specific survival, and disease-specific recurrence-free survival, adjusting for baseline characteristics using the Cox model (table 6). The results were similar to the unadjusted analysis. There was no evidence of a difference in results according to randomising group (MRC Clinical Trials Unit vs NCIC). For the analyses of risk group interactions, exploratory analyses on overall survival and disease-specific survival are shown in figure 4. Disease-specific recurrence-free survival (chosen as the most sensitive because of number of events, and least confounded by deaths from causes other than endometrial cancer) was used to explore outcomes in women defined as high and intermediate risk. The effect of risk group on this outcome measure is shown in the webfigure. The hazard ratio of 2·71 (95% CI 1·88–3·90; p<0·0001) shows that women defined as high risk in ASTEC/EN.5 do have a significantly increased risk of disease-specific recurrence-free survival compared with those classified as intermediate risk (77 events intermediate risk, 50 events high risk). 5-year disease-specific recurrence-free survival for women at intermediate risk was 88·8% compared with 73·7% for high risk (absolute difference at 5 years 15·1% [8·1–22·0%]). Exploratory interaction analyses of the effect of external beam radiotherapy in groups defined as intermediate or high risk, and in those women who had lymphadenectomy or no lymphadenectomy as part of primary surgery, are shown in figure 4. There is no evidence that the effect of external beam radiotherapy is different in subgroups of women defined as intermediate risk and high risk (test for interaction for overall survival p=0·83, for disease-specific survival p=0·45). There is no quality evidence that the effect of external beam radiotherapy is different in women who have had lymphadenectomy as part of primary surgery (test for interaction for overall survival=0·79, for disease-specific survival p=0·22).
Figure 4.
Effect of external beam radiotherapy on subgroups defined as at high and intermediate risk of recurrence and on women who had lymphadenectomy or no lymphadenectomy as part of initial surgery
EBRT=external beam radiotherapy. O–E=observed minus expected. Outer bars=99% CI. Inner bars=95% CI.
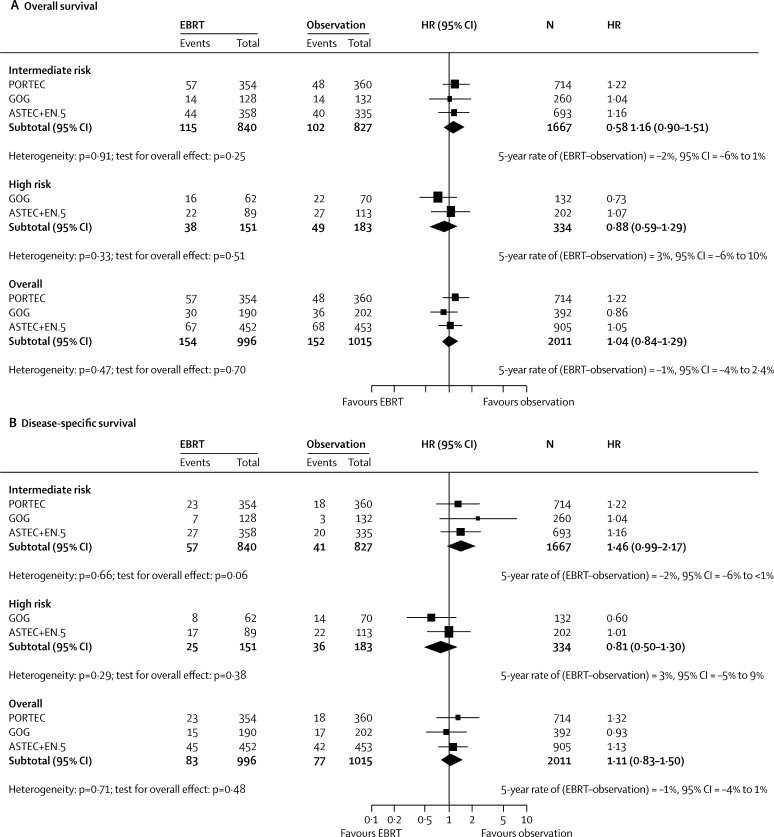
A Cochrane systematic review and meta-analysis published in 2007 identified three published trials (Aalders et al,2 GOG99,4 and PORTEC15) and one unpublished trial (Soderini and Sardi,3 with 123 women). No further trials have been identified. The main aim of updating the published review (figure 5) was to assess the effect of radiotherapy in intermediate-risk and high-risk early-stage disease. The Aalders trial2 was excluded because it was undertaken between 1968 and 1974, before the introduction of FIGO staging. The Soderini trial was excluded because it was published in abstract form only with no definitive time-to-event data. The updated meta-analysis of the effect of external beam radiotherapy on overall survival, including ASTEC/EN.5 results, gives a hazard ratio of 1·04 (95% CI 0·84–1·29; p=0·38). The pooled results effectively rule out an absolute benefit of more than a 3% increase in overall survival from adjuvant pelvic radiotherapy.
Figure 5.
Meta-analysis
EBRT=external beam radiotherapy. HR=hazard ratio.
Discussion
The ASTEC/EN.5 trial has shown no evidence of a benefit for external beam radiotherapy for early endometrial cancer at intermediate or high risk of recurrence, in terms of overall, disease-specific, and disease-specific recurrence-free survival. Combining these findings with data from other trials, we can exclude even a very small benefit of radiotherapy on overall survival. Adjuvant external beam radiotherapy did result in a small reduction in isolated local recurrence, but this analysis only included women who had local recurrence alone, and ignores 65% of women who had local and distant recurrence at the same time, or distant recurrence alone. The small reduction in isolated local recurrence does not translate into an effect on overall or recurrence-free survival. Additionally, the isolated local recurrence rate without external beam radiotherapy (and with brachytherapy) is small (6·1%). With clear evidence from ASTEC/EN.5 that adjuvant external beam radiotherapy is associated with more acute and long-term toxicity than observation with or without brachytherapy, adjuvant radiotherapy after surgery to achieve isolated local control is not justified as the treatment of choice.
We are aware that there was no central pathology review of specimens in ASTEC. However, all pathologists in participating centres were given guidance to ensure that there was consistency across centres in the determination of high-risk pathology. All pathology reports were reviewed centrally to ensure that the histological description matched the diagnosis. PORTEC1 and GOG99 also relied on local pathology data for analyses.
The first trial of external beam radiotherapy in early endometrial cancer included all stage I women.1 Subsequent trials selected women with intermediate-risk or high-risk features who were thought more likely to benefit from adjuvant radiotherapy. All women in the GOG99 trial had surgical staging, and women with positive lymph nodes were excluded. 30% of women in PORTEC1 were IB grade 2, a low-risk group considered ineligible for ASTEC/EN.5. Neither PORTEC1 nor GOG99 included women with papillary serous or clear cell histology because they were deemed to be at high risk of recurrence. Although there is no standard definition of intermediate-risk or high-risk endometrial cancer, a consensus has emerged that women with papillary serous or clear cell endometrial cancer, and women with all other histological subtypes who are stage IC with high-grade tumours, are at high risk of recurrence. Despite the negative trials, this subgroup had been thought to benefit from external beam radiotherapy; however, this theory is not supported by data from ASTEC/EN.5, with over 200 women randomised in this high-risk category.
The number of women in ASTEC/EN.5 who had lymphadenectomy as part of primary surgery was small. Therefore, drawing firm conclusions about any possible interaction of lymphadenectomy and postoperative adjuvant external radiotherapy is difficult.
Brachytherapy was not allowed in either PORTEC1 or in GOG99. When the ASTEC and EN.5 trials were designed, there was a known risk of centres offering brachytherapy to women assigned to the observation group, which could have created a bias in the trial. A consensus could not be reached between all collaborating centres as to the use of such treatment, and a third patient randomisation to brachytherapy versus no brachytherapy was felt to be impracticable. Therefore centres were asked to state their brachytherapy policy for all trial participants. Compliance with stated policy was around 80% and, importantly, brachytherapy was given to similar proportions of women in the two randomised groups.
Despite randomising higher-risk group women, the isolated loco-regional recurrence rate in ASTEC/EN.5 (5-year cumulative incidence 6·1% observation vs 3·2% external beam radiotherapy) is similar or lower than that seen in other trials recruiting lower-risk women (GOG99: 4-year cumulative incidence 7% vs 2%. PORTEC1: 5-year actuarial incidence 13·7% vs 4·2%). The ASTEC/EN.5 findings suggest that brachytherapy on its own might be an effective strategy for local control (although not based on a randomised comparison).
The updated meta-analysis including ASTEC/EN.5 results shows no benefit of external beam radiotherapy on overall survival in early-stage endometrial cancer. Previous meta-analyses have attempted to use pooled data to investigate the effect of radiotherapy on different subgroups in trials whose entry criteria included intermediate-risk and high-risk women. Using published data, we could not undertake detailed subgroup analyses on the effect of treatment by subtype and risk category; this could only be done in a meta-analysis with individual patient data. Using stated definitions of risk from the individual trials, there is no evidence of benefit with external beam radiotherapy even within high-risk women. A meta-analysis of individual patient data might be able to refine the risk group classification, but if the main interest is in the high-risk group including stage IC grade 3 tumours, numbers of women would be small (not more than 70 women in GOG99 and 202 women in ASTEC/EN.5), and how much a meta-analysis of individual patient data would add to the evidence base presented in the current paper is questionable.
ASTEC/EN.5 and the other relevant randomised trials show that external beam radiotherapy clearly has a local biological effect, with a small reduction in isolated local recurrence, but the observation group of ASTEC/EN.5 also shows that the use of brachytherapy could reduce the isolated recurrence rate at 5 years to 6·1%. The local effect of external beam radiotherapy is therefore only modest. Considering ASTEC/EN.5 and the other relevant trials together, clearly any local effect does not translate into a benefit in terms of reducing distant recurrence or death from local or distant disease or a benefit in terms of improved disease-specific recurrence-free, disease-specific, or overall survival. One interpretation of the ASTEC/EN.5 data is that the addition of external beam radiotherapy is not necessary and that it is possible to achieve this low level of local recurrence with brachytherapy alone. Brachytherapy is a more convenient treatment than external beam radiotherapy and might be associated with less toxicity. Randomised data from the PORTEC2 trial, which directly compares brachytherapy with external beam radiotherapy, will provide further information. External beam radiotherapy does not seem to have a distant effect and it might be necessary to use systemic treatments in very high-risk women to prevent distant metastases and death from endometrial cancer. In conclusion, adjuvant external beam radiotherapy cannot be recommended as part of routine treatment to improve survival for women with early endometrial cancer at intermediate or high risk of recurrence, and brachytherapy might be preferred for local control.
Acknowledgments
Acknowledgments
Sarah Burdett (Meta-analysis Group at MRC Clinical Trials Unit) who helped with meta-analysis plots. We thank the women who participated in this trial and the research staff at the clinical centres, who helped to recruit women and provide data.
Contributors
Writing committee: P Blake (Royal Marsden Hospital, London, UK); Ann Marie Swart (UCL Centre for Clinical Pharmacology and MRC CTU, London, UK); J Orton (St James's Institute of Oncology, Leeds, UK); H Kitchener (University of Manchester School of Cancer and Imaging Sciences, UK); T Whelan, H Lukka (Juravinski Cancer Centre at Hamilton Health Sciences, Hamilton, Canada); E Eisenhauer, M Bacon, D Tu (NCIC CTG, Queen's University, Kingston, Canada); M K B Parmar, C Amos, C Murray, W Qian (MRC CTU, London, UK).
Trial design: ASTEC: P Blake, H Kitchener, J Sandercock, M K B Parmar. EN.5: K James, T Whelan, H Lukka, M Carey, D Tu.
Chief Clinical Investigators: P Blake (ASTEC); T Whelan, H Lukka (EN.5).
Data analysis: W Qian, C Murray, A M Swart, M K B Parmar, D Tu.
Trial Management Group: C Amos, M Bacon, P Blake, A Branson, CH Buckley, E Eisenhauer, H Kitchener, H Lukka, M K B Parmar, D Tu, W Qian, C W Redman, J Shepherd, A M Swart, T Whelan.
Independent Data Monitoring Committee: G Dunn, P Heintz, J Yarnold (chair).
Trial Steering Committee: P Johnson, M Mason (chair), R Rudd.
MRC Clinical Trials Unit Data Management: C Amos, P Badman, S Begum, N Chadwick, S Collins, A Cradduck, K J Goodall, J Jenkins, K Law, P Mook, J Sandercock, C Goldstein, B Uscinska.
NCIC Clinical Trials Group Data Management: M Bacon, C Goudreau, T Williams, D Soroka, E Elliott.
ASTEC Clinical Centres (investigators):
Aberdeen Royal Infirmary (H Deans, T K Sarkar), Addenbrookes Hospital, Cambridge (L T Tan), Belfast City Hospital incorporating Belvoir Park Hospital (J Clarke, R J McClelland), Bristol Oncology Centre, (V Barley, P Cornes, JD Graham), Broomfield Hospital, Chelmsford (C Goodfellow, S Tahir), Christie Hospital, Manchester, (S Davidson), Churchill Hospital, Oxford (D Cole, B Lavery), Clatterbridge Centre for Oncology, Wirral (A Flavin, K Hayat), Coventry Maternity Hospital (M Hocking, C J R Irwin), Cumberland Infirmary, Carlisle (P Dyson, D Guthrie, J J Nicoll, S E Pearson), Derbyshire Royal infirmary (D Guthrie, M Persic), Derriford Hospital, Plymouth (F N Daniel, D Yiannakis), Essex County Hospital (A Lamont, P Murray, S Tahir), Gloucestershire Hospitals NHS Foundation Trust (A Cook, R Counsell), Great Western Hospital, Swindon (A Horne), Ipswich Hospital (J S Morgan), James Cook University Hospital, South Cleveland (A Rathmell), John Radcliffe Hospital, Oxford (B Lavery), Leicester Royal Infirmary (S Khanna), Maidstone Hospital, Kent (R Jyothirmayi, J Summers), Mount Vernon Hospital, Middlesex (P K Hoskin), Newcastle General Hospital (T Branson), New Cross Hospital, Wolverhampton (A Allerton), Norfolk and Norwich Hospital (M J Ostrowski), North Staffordshire Royal Infirmary (J Scoble), Northern Centre for Cancer Treatment, Newcastle Upon Tyne (T Branson), Nottingham City Hospital (S Chan), Oldchurch Hospital, Romford (M Quigley), Poole Hospital NHS Trust (V Laurence), Princess Royal Hospital, Hull (M J Lind, R Dealey), Queen Elizabeth Hospital, Birmingham (I Fernando, T N Latief, J Mould, A Stevens), Queen Elizabeth the Queen Mother Hospital, Margate (R Jyothirmayi, J Summers), Queen's Hospital, Romford (M Quigley), Royal Marsden Hospital, London (P Blake, C L Harmer, K Harrington), Royal Shrewsbury Hospital (S Oates, R Agrawal, S Awwad), Royal Sussex County Hospital, Brighton (G H Newman), Royal United Hospital, Bath (P Cornes, G Rees), Royal Preston and Sharoe Green Hospital, Preston (A Hindley, G Skailes), St Batholomew's Hospital, London (M Powell), St James' Hospital, Leeds (R A Cooper, C J Orton, R I Rothwell), St. Mary's Hospital, Portsmouth (G Khoury, E M Low), Singleton Hospital, Swansea (P Flynn, O Freites, S El-Sharkawi), United Lincolnshire Hospitals NHS Trust (O A Adeyemi, E M Murray), University College Hospital, London (M McCormack, G Tobias), University Hospital of North Staffordshire, Velindre Hospital, Cardiff (M Adams, L Hanna), Western General Hospital, Edinburgh (V Cowie), Weston Park Hospital, Sheffield (S Pledge, DJ Radstone); Marie Curie Institute, Poland (K Karolewski, M Klimek, P Blecharz); Norwegian Radium Hospital, Norway (M Baekelandt, L Hansen, J Kaern, M Onsrud, H Oksefjell, M Scheistroen, K Sundfoer); Wellington Hospital, New Zealand (D McConnell).
EN.5 Clinical Centres (investigators):
Bliss Murphy Cancer Centre, Newfoundland (P Ghatage); QE II Health Sciences Centre, Nova Scotia (R Grimshaw); Atlantic Health Sciences Corp, New Brunswick (J Carson); Leon Richard Oncology, New Brunswick (S Filice); Centre Hospitalier Sherbrooke, Quebec (P Bessette, A Nabid); CHUQ—Hotel Dieu, Quebec (M Roy); McGill Oncology Montreal, Quebec (M Duclos, L Portelance, L Souhami); Hopital Notre Dame Montreal, Quebec (P Drouin, P Gauthier, J Dubuc, M Jolicoeur, T V Nguyen); Hopital Charles LeMoyne, Quebec (K Chan); Hamilton Health Sciences Cancer Centre, Ontario (C Johanson, E Friedman, M Patel, H Lukka, S Voruganti); Princess Margaret Hospital, Ontario (A Fyles, L Manchul, M Milosevic); London Regional Cancer Centre, Ontario (M Carey, J Radwan, D D'Souza, J Gilchrist, A Hammond); Windsor Regional Cancer Centre (K Schneider); Sudbury Regional Cancer Centre, Ontario (B Lada); Thunder Bay Regional Cancer Centre, Ontario (M Anthes); Allan Blair Cancer Centre, Saskatchewan (P Tai); Tom Baker Cancer Centre, Alberta (P Craighead, K Arthur); Cross Cancer Institute, Alberta (G Dundas); Vancouver Cancer Centre, British Columbia (C Parsons, P Lim); Fraser Valley Cancer Centre, British Columbia (F Wong); Royal Womens Hospital, Australia (M Quinn); SMDC Cancer Centre, Duluth, USA (J Krook).
Conflict of interest statement
We declare that we have no conflict of interest.
Correspondence to: Ann Marie Swart, MRC Clinical Trials Unit, London NW1 2DA, UK ams@ctu.mrc.ac.uk
Web Extra Material
Characteristics of patients who did and did not receive brachytherapy in ASTEC/EN.5
Disease-specific recurrence-free survival by risk group
References
- 1.Kong A, Simera I, Collingwood M, Williams C, Kitchener H, on behalf of Cochrane Gynaecological Cancer Group Adjuvant radiotherapy for stage I endometrial cancer: systematic review and meta-analysis. Ann Oncol. 2007;18:1595–1604. doi: 10.1093/annonc/mdm066. [DOI] [PubMed] [Google Scholar]
- 2.Aalders J, Abeler V, Kolstad P. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- 3.Soderini A, Sardi J. Role of adjuvant radiotherapy in intermediate risk (Ib G2-3-IC) endometriod carcinoma after extended staging surgery. Preliminary reports of a randomised trial. Int J Gynecol Cancer. 2003;13(suppl 1):78. [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Creutzberg CL, van Putten WL, Koper PC. Surgery and postoperative radiotherapy versus surgery alone for patients with stage 1 endometrial carcinoma: multi centre trial. PORTEC study group. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynecol. 1989;96:889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 7.Standards and Minimum Datasets for Reporting Cancers . Minimum dataset for the histopathological reporting of atypical hyperplasia and adenocarcinoma in endometrial biopsy and curettage specimens and for endometrial cancer in hysterectomy specimens. Royal College of Pathologists; 2001. [Google Scholar]
- 8.Early Breast Cancer Trialists' Collaborative Group Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 9.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre C, Clarke M. Identifying randomised trials. In: Egger MSG, Altman DG, editors. Systematic reviews in healthcare: meta-analysis in context. 2nd edn. BMJ Publishing Group; London: 2001. pp. 69–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of patients who did and did not receive brachytherapy in ASTEC/EN.5
Disease-specific recurrence-free survival by risk group