Summary
Background
Hysterectomy and bilateral salpingo-oophorectomy (BSO) is the standard surgery for stage I endometrial cancer. Systematic pelvic lymphadenectomy has been used to establish whether there is extra-uterine disease and as a therapeutic procedure; however, randomised trials need to be done to assess therapeutic efficacy. The ASTEC surgical trial investigated whether pelvic lymphadenectomy could improve survival of women with endometrial cancer.
Methods
From 85 centres in four countries, 1408 women with histologically proven endometrial carcinoma thought preoperatively to be confined to the corpus were randomly allocated by a minimisation method to standard surgery (hysterectomy and BSO, peritoneal washings, and palpation of para-aortic nodes; n=704) or standard surgery plus lymphadenectomy (n=704). The primary outcome measure was overall survival. To control for postsurgical treatment, women with early-stage disease at intermediate or high risk of recurrence were randomised (independent of lymph-node status) into the ASTEC radiotherapy trial. Analysis was by intention to treat. This study is registered, number ISRCTN 16571884.
Findings
After a median follow-up of 37 months (IQR 24–58), 191 women (88 standard surgery group, 103 lymphadenectomy group) had died, with a hazard ratio (HR) of 1·16 (95% CI 0·87–1·54; p=0·31) in favour of standard surgery and an absolute difference in 5-year overall survival of 1% (95% CI −4 to 6). 251 women died or had recurrent disease (107 standard surgery group, 144 lymphadenectomy group), with an HR of 1·35 (1·06–1·73; p=0·017) in favour of standard surgery and an absolute difference in 5-year recurrence-free survival of 6% (1–12). With adjustment for baseline characteristics and pathology details, the HR for overall survival was 1·04 (0·74–1·45; p=0·83) and for recurrence-free survival was 1·25 (0·93–1·66; p=0·14).
Interpretation
Our results show no evidence of benefit in terms of overall or recurrence-free survival for pelvic lymphadenectomy in women with early endometrial cancer. Pelvic lymphadenectomy cannot be recommended as routine procedure for therapeutic purposes outside of clinical trials.
Funding
Medical Research Council and National Cancer Research Network.
Introduction
Endometrial cancer is now the most common gynaecological malignancy in western Europe and North America. About 6400 women are affected every year in the UK,1 81 500 in the European Union,2 and 40 100 women in North America.3 More than 90% of cases occur in women older than 50 years of age, with a median age of 63 years. The incidence in older women (aged 60–69 years) increased in the UK by 19% between 1993 and 2001.1 It is the seventh most common cause of death from cancer in women in western Europe, accounting for 1–2% of all deaths from cancer. Roughly 75% of women survive for 5 years.4 This high survival rate is attributable to most women being diagnosed at an early stage after postmenopausal bleeding.2
At diagnosis, about three-quarters of women have disease confined to the uterine corpus. Standard definitive surgery includes hysterectomy and bilateral salpingo-oophorectomy (BSO). Most tumours are of endometrioid type; other histological types include serous, mucinous, clear cell, and mixed epithelial. Endometrial tumours are graded as well (grade 1), moderately (grade 2), or poorly (grade 3) differentiated, apart from clear cell and serous, which are generally regarded as grade 3. Endometrial cancer spreads beyond the uterus by infiltrating directly through the myometrium, extending into the cervix, and metastasising most often to the pelvic nodes and less frequently directly to the para-aortic nodes. Pelvic lymph-node metastases occur in about 10% of women with clinical stage I (ie, confined to the corpus) endometrial cancer.5,6 Within stage I disease, 3–5% of women with well differentiated tumours and superficial myometrial invasion will have lymph-node involvement. This proportion rises to roughly 20% of women with poorly differentiated tumours and deep myometrial invasion.6
In Europe, traditional management of women with stage I disease has consisted of surgery, which is typically combined with adjuvant radiotherapy for women whose pathological features suggest an increased risk of nodal metastases. Tumour type, grade, and depth of myometrial invasion are key prognostic factors for recurrence, and are used to assess risk of recurrence and need for adjuvant treatment. A systematic review and meta-analysis of 1770 patients from four randomised trials7 and data from the ASTEC/EN.5 radiotherapy trial8 show that adjuvant radiotherapy results in a small reduction in risk of isolated pelvic recurrence (2·9%), but no evidence that it affects overall or disease-specific survival.
Since 1988, the International Federation of Gynaecology and Obstetrics (FIGO) classification of stage of endometrial cancer has required a full systematic pelvic and para-aortic lymphadenectomy.9 Some propose that adjuvant radiotherapy can be avoided and treatment morbidity reduced when lymphadenectomy shows no indication of disease in the nodes. However, evidence is scarce of a therapeutic benefit for lymphadenectomy in terms of survival. Lymphadenectomy is undertaken widely in North America and Australia, and data from non-randomised studies and case series, which have shown an association between lymphadenectomy and increased survival, lend support to the procedure.6,10,11 Other observational studies, however, have not shown any such benefit.12 ASTEC (A Study in the Treatment of Endometrial Cancer) was designed to assess the therapeutic benefit of lymphadenectomy in endometrial cancer, independent of the effect of adjuvant radiotherapy.
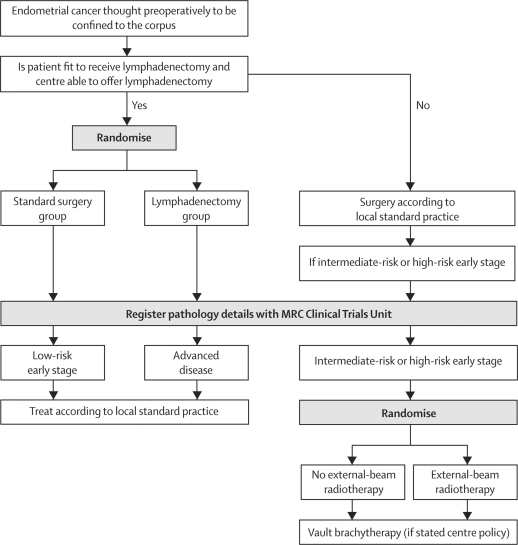
ASTEC consisted of two trials with separate randomisations that were designed to answer a surgical and a radiotherapy question (figure 1). The surgical trial investigated whether pelvic lymphadenectomy could improve survival of women with endometrial cancer, which was thought preoperatively to be confined to the corpus. The radiotherapy trial addressed whether adjuvant external-beam radiotherapy (EBRT) could improve survival of women with intermediate-risk and high-risk early-stage endometrial cancer. In this Article we report the results of the surgical randomisation.
Figure 1.
ASTEC trial design
Methods
Study design and women
We undertook a randomised controlled trial between July 1, 1998, and March 31, 2005 in 85 centres in four countries (UK, South Africa, Poland, and New Zealand). We included women with histologically proven endometrial carcinoma that was thought preoperatively to be confined to the corpus, and those who were able to undergo both systematic lymphadenectomy and EBRT. Women who had a CT or MRI scan suggesting node enlargement were not excluded from randomisation. Specialist gynaecological surgeons who were experienced in pelvic lymphadenectomy undertook all surgical procedures. The same surgeon, who was specified before randomisation, had to undertake surgery irrespective of whether the woman was randomly assigned to the standard surgery group alone or the group with systematic lymphadenectomy. All women provided written, informed consent at initial recruitment to both the surgical and radiotherapy randomisation, in the event that they would be eligible for the radiotherapy trial. We obtained ethics approval for the trial from the North West Multi-centre Regional Ethics Committee (UK).
Interventions
We randomly allocated eligible women to the standard surgery group or lymphadenectomy group. Women in the standard surgery group had a hysterectomy and BSO, peritoneal washings, and palpation of para-aortic nodes. Nodes that were suspicious could be sampled if the surgeon believed it to be in the woman's best interest. Women in the lymphadenectomy group had standard surgery plus a systematic dissection of the iliac and obturator nodes. If the nodes could not be dissected thoroughly because of obesity or anaesthetic concern, sampling of suspect nodes was recommended and para-aortic node sampling was at the discretion of the surgeon.
In both groups, a vertical incision was recommended, unless a transverse incision was preferred by the surgeon because of gross obesity. Surgery could be undertaken by laparoscopic technique provided that the procedure could be accomplished safely and as thoroughly as an open procedure. The type of surgical access (open or laparoscopic) to be used was specified before randomisation and was therefore independent of the type of surgery allocated. During the trial we monitored the type of incision (vertical or transverse); when we noted an imbalance in the type of incisions being undertaken between the groups, the incision type also had to be specified before randomisation. Thromboprophylaxis and prophylactic antibiotics were prescribed according to local practice.
Procedures
Randomisation was done by a telephone call to the Medical Research Council Clinical Trials Unit (MRC CTU), and we used a method of minimisation. Stratification factors were centre, WHO performance status (0–1 vs 2–4), time since diagnosis (≤ 6 weeks vs >6 weeks), and planned surgical approach (open vs laparoscopic). We registered surgery and pathology details (based on local reporting) with the MRC CTU after surgery. We used the minimum dataset of the Royal College of Pathologists as the standard for pathology reporting.13 Women were classified in three categories: low-risk, early-stage disease (FIGO IA or IB and low grade pathology [G1, G2]); intermediate-risk and high-risk, early-stage disease (FIGO IA or IB with high grade pathology [G3, papillary serous or clear cell], FIGO IC or IIA); and advanced disease—ie, spread beyond the uterine corpus (FIGO stage IIB, IIIA, IIIB, and IV). Pelvic lymph-node status was not taken into account; thus no FIGO stage IIIC category was included.
Further randomisation into the ASTEC radiotherapy trial (comparing EBRT and observation with no EBRT or systemic treatment until recurrence) was required for women with intermediate-risk and high-risk, early-stage disease, including those with positive lymph nodes. Without this second randomisation, differences in postsurgical treatment could have arisen, with women in the standard surgery group either having more radiotherapy (because their lymph-node status was unknown) or less radiotherapy (because they were less likely to have positive lymph nodes identified) than women in the lymphadenectomy group were having. We offered women with low-risk, early-stage disease and women with advanced disease further treatment according to standard practice.
We assessed women before randomisation and surgery, 3 months after randomisation, then every 3 months in the first year, every 6 months in years 2 and 3, and every year thereafter. Follow-up data collected included details of endometrial cancer recurrence and treatment, vital status, and short-term and long-term toxic effects. The primary outcome measure was overall survival. Secondary outcome measures were recurrence-free survival, adverse effects from treatment, and disease-specific survival.
Statistical analysis
We planned to randomise 1400 women to detect an improvement in 5-year overall survival from 80% in the standard surgery group to 90% in the lymphadenectomy group (hazard ratio [HR] 0·47) with 5% significance level and 90% power. We allowed for non-compliance of roughly 20% in the sample size calculations to take account of women who would have no lymph nodes removed or have lymph node sampling only in the lymphadenectomy group.
We compared Kaplan-Meier curves for all time-to-event outcome measures with the standard (non-stratified) log-rank test. We defined overall survival as the time from randomisation to death from any cause; women who were known to be still alive at the time of the analysis were censored at the time of their last follow-up. We defined recurrence-free survival as the time from randomisation to first reappearance of endometrial cancer or death from any cause; women who were known to be alive and without recurrent disease at the time of analysis were censored at the time of their last follow-up. Disease-specific survival was defined as time from randomisation to death from endometrial cancer or death due to treatment. To compare disease-specific survival we undertook a competing risk analysis by subtracting the log-rank statistic for non-endometrial cancer deaths from the log-rank statistic for all deaths (ie, the two observed values were subtracted from each other, the two expected values were subtracted from each other, and the two variances were subtracted from each other).14
The Chief Investigator, blinded to treatment group, made the assessment of cause of death, and classified it as treatment related, disease related, treatment and disease related, or other (non-endometrial cancer, non-treatment related). All deaths within 30 days of surgery were classified as treatment related irrespective of their cause. All comparisons are expressed relative to women in the standard surgery group; therefore an HR less than 1·0 indicates a decreased risk of the event for women in the lymphadenectomy group. We modelled the absolute difference between the treatment groups using the flexible parametric models of Royston and Parmar.15
Although unadjusted HRs are presented for all outcome measures, we also calculated an adjusted HR with the Cox model for disease-specific, overall, and recurrence-free survival. Covariates included the characteristics: age (as a continuous variable), WHO performance status (0, 1, 2, 3, or 4), weeks between diagnosis and randomisation (≤6 weeks vs >6 weeks), surgical technique intended (open vs laparoscopic), and type of incision (vertical vs Pfannestiel vs other transverse, two dummy variables used); and pathology details: extent of tumour (confined vs spread), histology (endometrioid/adenocarcinoma vs other), depth of invasion (inner half of myometrium vs endometrium only, outer half of myometrium vs endometrium only), and differentiation (grade 1, 2, or 3). We assessed any benefits of lymphadenectomy on survival in an exploratory manner in subgroups. Predefined subgroups included grouping centres according to the median number of lymph nodes removed (<10, 10–14, ≥15) in the lymphadenectomy group, and grouping women by risk of recurrence: low risk of recurrence, intermediate and high risk of recurrence, and advanced disease (according to eligibility for the radiotherapy randomisation). To test for differences in the relative size of effect in different subgroups, we used a χ2 test for interaction, or, when appropriate, a χ2 test for trend. All analyses were done on an intention-to-treat basis. All p values are two-sided. Analyses were undertaken with SAS System (version 9.10), apart from the flexible parametric models for which we used stata (version 9.1). This study is registered, number ISRCTN 16571884.
Role of the funding source
The sponsor of the study reviewed and approved the trial design, and the execution was overseen by an independent data monitoring committee and independent trial steering committee. The sponsor of the study had no role in data collection, data analysis, data interpretation, or writing of the report. The writing committee had full access to all the data in the study, and the trial management group had final responsibility for the decision to submit for publication.
Results
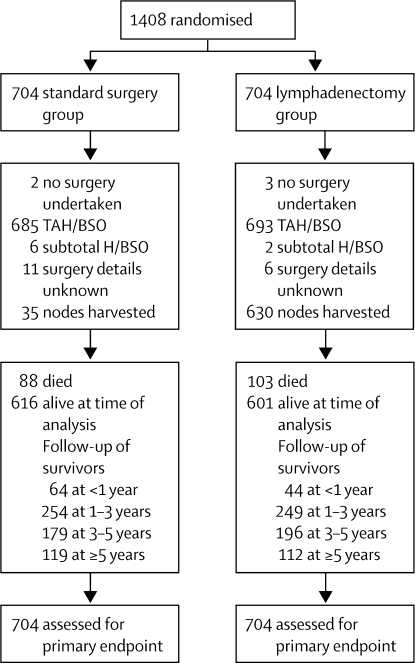
1408 women were randomly assigned: 704 to the standard surgery group and 704 to the lymphadenectomy group (figure 2). Patient characteristics at randomisation were generally much the same between the two groups (table 1).
Figure 2.
Profile of ASTEC surgical trial
We did not collect logs of patients assessed for eligibility. TAH=total abdominal hysterectomy. BSO=bilateral salpingo-oophorectomy. H=hysterectomy.
Table 1.
Baseline characteristics
| Standard surgery (N=704) | Lymphadenectomy (N=704) | ||
|---|---|---|---|
| Age (years) | 63 (36–89) | 63 (34–93) | |
| WHO performance status | |||
| 0 | 520 (74%) | 537 (76%) | |
| 1 | 156 (22%) | 139 (20%) | |
| 2 | 23 (3%) | 22 (3%) | |
| 3 | 4 (1%) | 5 (1%) | |
| 4 | 1 (<1%) | 1 (<1%) | |
| Time between diagnosis and randomisation (weeks) | 4 (0–16) | 4 (0–26) | |
| ≤6 weeks | 576 (82%) | 588 (84%) | |
| >6 weeks | 128 (18%) | 116 (16%) | |
| Surgical technique intended | |||
| Open | 650 (92%) | 659 (94%) | |
| Laparoscopic | 54 (8%) | 45 (6%) | |
| Body-mass index | 29 (16–79) | 29 (10–69) | |
| Unknown | 161 | 177 | |
Data are median (range) or number (%) unless otherwise specified.
Table 2 summarises the pathology findings of women who were confirmed as having endometrial cancer at surgery. Most women (80%) had endometrioid endometrial cancer (545/681 [80%] standard surgery group, 541/684 [79%] lymphadenectomy group). Slightly more women had worse prognosis tumours with respect to histology (clear cell and papillary serous) and greater depth of invasion (10% more involvement of the outer half of the myometrium) in the lymphadenectomy group than in the standard surgery group (table 2).
Table 2.
Pathology details
| Standard surgery (N=683)* | Lymphadenectomy (N=686)* | ||
|---|---|---|---|
| Extent of tumour | |||
| Confined to corpus uteri | 553 (81%) | 538 (79%) | |
| Spread beyond corpus uteri | 128 (19%) | 146 (21%) | |
| Extension to endocervical glands | 33 (27%) | 34 (24%) | |
| Extension to cervical stroma | 53 (43%) | 57 (40%) | |
| Extension beyond uterus | 38 (31%) | 52 (36%) | |
| Unknown | 4 | 3 | |
| Unknown | 2 | 2 | |
| Histology | |||
| Endometriod | 545 (80%) | 541 (79%) | |
| Adenocarcinoma NOS | 46 (7%) | 37 (5%) | |
| Clear cell | 10 (1%) | 17 (2%) | |
| Papillary serous | 21 (3%) | 32 (5%) | |
| Squamous | 6 (1%) | 5 (1%) | |
| Mucinous | 1 (<1%) | 4 (1%) | |
| Mixed epithelial stromal | 7 (1%) | 8 (1%) | |
| Sarcoma | 10 (1%) | 9 (1%) | |
| Other epithelial | 4 (1%) | 6 (1%) | |
| Mixed epithelial | 31 (5%) | 25 (4%) | |
| Unknown | 2 | 2 | |
| Differentiation or grade | |||
| Well (G1) | 225 (33%) | 213 (31%) | |
| Moderate (G2) | 300 (44%) | 290 (43%) | |
| Poor (G3)† | 139 (20%) | 158 (23%) | |
| Not applicable‡ | 16 (2%) | 16 (2%) | |
| Unknown | 3 | 9 | |
| Depth of invasion | |||
| Endometrium only | 96 (14%) | 89 (13%) | |
| Inner half of myometrium | 369 (55%) | 310 (46%) | |
| Outer half of myometrium | 212 (31%) | 274 (41%) | |
| Unknown | 6 | 13 | |
| Lymphovascular permeation | |||
| Present | 125 (19%) | 140 (22%) | |
| Not present | 407 (63%) | 377 (59%) | |
| Not stated | 111 (17%) | 127 (20%) | |
| Unknown | 40 | 42 | |
| Nodal involvement (if nodes harvested) | |||
| Yes | 9 (27%) | 54 (9%) | |
| No | 23 (72%) | 560 (91%) | |
| Unknown | 0 | 1 | |
| Number of involved nodes | |||
| 1 | 5 (56%) | 28 (52%) | |
| 2 | 3 (33%) | 12 (22%) | |
| 3 | 0 | 6 (11%) | |
| 4 | 0 | 2 (4%) | |
| 5 | 1 (11%) | 4 (7%) | |
| 6 | 0 | 2 (4%) | |
| Position of involved nodes | |||
| Unilateral | 6 (67%) | 31 (58%) | |
| Bilateral | 2 (22%) | 19 (36%) | |
| Para-aortic | 1 (11%) | 3 (6%) | |
| Unknown | 0 | 1 | |
| FIGO stage§ | |||
| IA | 88 (13%) | 84 (12%) | |
| IB | 318 (47%) | 261 (39%) | |
| IC | 147 (22%) | 187 (28%) | |
| IIA | 33 (5%) | 34 (5%) | |
| IIB | 53 (8%) | 57 (8%) | |
| III/IV | 38 (6%) | 52 (8%) | |
| Unknown | 6 | 11 | |
Data are number (%) or number. NOS=not otherwise specified.
Excludes patients whose pathology details did not confirm endometrial cancer: 39 women (21 standard surgery group, 18 lymphadenectomy group) who had no other tumour in the surgical specimen; atypical hyperplasia; or cervical, ovarian, or colorectal cancer.
Including clear cell and serous papillary.
Sarcoma and mixed epithelial sarcoma.
FIGO IIIC is not included here. Women with positive lymph nodes are classified irrespective of nodal status.
Almost all women in both groups had a total abdominal hysterectomy and BSO (table 3). In the lymphadenectomy group, 58 (8%) women had no nodes removed because of anaesthetic concerns (n=22), obvious extra-uterine disease (12), obesity (9), withdrawal at patient request (9), or unknown reasons (6). We obtained the number of lymph nodes removed from the pathology report. In the lymphadenectomy group, 72 (12%) had one to four nodes removed and 396 (65%) women had ten or more removed (median 12 nodes). In the standard surgery group, of the 35 (5%) women who had any nodes removed (as was allowed within the protocol at the surgeon's discretion), the median node count was two, and only four women had ten or more removed. In the standard surgery group, surgeons mainly took biopsy samples of suspect nodes, which accounted for the difference in the proportion of positive nodes in the standard surgery group compared with the lymphadenectomy group.
Table 3.
Surgery details
| Standard surgery (N=702)* | Lymphadenectomy (N=701)* | ||
|---|---|---|---|
| Surgery received | |||
| Total abdominal hysterectomy/BSO | 685 (99%) | 693 (99%) | |
| Subtotal hysterectomy/BSO | 6 (1%) | 2 (<1%) | |
| Unknown | 11 | 6 | |
| Nodes harvested | |||
| Yes | 35 (5%) | 630 (92%) | |
| No | 652 (95%) | 58 (8%) | |
| Unknown | 15 | 13 | |
| Number of nodes harvested | |||
| 1–4 | 26 (76%) | 72 (12%) | |
| 5–9 | 4 (12%) | 142 (23%) | |
| 10–14 | 1 (3%) | 153 (25%) | |
| >14 | 3 (9%) | 243 (40%) | |
| Unknown | 1 | 20 | |
| Median (range) | 2 (1–27) | 12 (1–59) | |
| Required blood transfusion | |||
| Yes | 30 (4%) | 39 (6%) | |
| Unknown | 18 | 12 | |
| Number of units | |||
| 1 | 2 (7%) | 1 (3%) | |
| 2 | 13 (45%) | 21 (54%) | |
| 3 | 6 (21%) | 5 (13%) | |
| 4 | 6 (21%) | 9 (23%) | |
| ≥5 | 2 (7%) | 3 (8%) | |
| Unknown | 1 | 0 | |
| Median (range) | 2 (1–6) | 2 (1–7) | |
| Length of operation (min)† | |||
| ≤60 | 336 (52%) | 83 (13%) | |
| 60–90 | 220 (34%) | 285 (44%) | |
| 90–120 | 69 (11%) | 190 (29%) | |
| >120 | 25 (4%) | 94 (14%) | |
| Unknown | 52 | 49 | |
| Median (range) | 60 (10–255) | 90 (10–390) | |
| Number of postoperative days in hospital | |||
| Median (range) | 6 (2–120) | 6 (2–106) | |
| Unknown | 22 | 23 | |
| Surgical technique used | |||
| Laparoscopic | 42 (6%) | 45 (6%) | |
| Open | 647 (94%) | 648 (94%) | |
| Vertical incision | 287 (45%) | 384 (60%) | |
| Pfannenstiel | 311 (49%) | 208 (32%) | |
| Other transverse | 43 (7%) | 49 (8%) | |
| Unknown | 6 | 7 | |
| Unknown | 13 | 8 | |
Data are number (%) unless otherwise specified. BSO=bilateral salpingo-oophorectomy.
Five women (two standard surgery group, three lymphadenectomy group) were unable to complete surgery.
Defined as “from knife to skin”.
Women in both groups were managed in a similar way with respect to thromboprophylaxis, use of prophylactic antibiotics, type of anaesthetic, requirement for blood transfusion, and number of days spent in hospital (table 3). The median length of operation was 50% longer when lymphadenectomy was done (table 3). The proportion of women having laparoscopic procedures was similar in both groups (table 3). More women in the standard surgery group had a transverse incision rather than a vertical incision compared with the lymphadenectomy group. The risk of developing short-term major surgical complications was generally low in both groups but more women in the lymphadenectomy group than in the standard surgery group developed specific complications of: ileus (18 [3%] vs eight [1%]), deep-vein thrombosis (six [1%] vs one [0·1%]), lymphocyst (ten [1%] vs two [0·3%]), and major wound dehiscence (ten [1%] vs two [0·3%]).
Table 4 summarises use of adjuvant radiotherapy for all randomised women. Similar proportions of women in both groups received postoperative radiotherapy. Of those who had radiotherapy, most had a combination of EBRT and brachytherapy (table 4). A few women with low-risk early-stage disease received radiotherapy and a smaller proportion received EBRT with or without brachytherapy (table 5). In the intermediate-risk and high-risk early-stage group, 137 (56%) and 138 (53%) women in the standard surgery and lymphadenectomy groups, respectively, received postoperative radiotherapy; 102 (42%) and 98 (37%), respectively, received EBRT with or without brachytherapy. About half the women in this subgroup received EBRT as part of the ASTEC radiotherapy trial (46/102 standard surgery group and 51/98 lymphadenectomy group). In the advanced disease group, a higher proportion of women in the standard surgery group than the lymphadenectomy group had radiotherapy and EBRT with or without brachytherapy (table 5).
Table 4.
Adjuvant radiotherapy received (within 3 months after surgery)
| Standard surgery (N=704) | Lymphadenectomy (N=704) | ||
|---|---|---|---|
| Radiotherapy received | 227 (33%) | 228 (33%) | |
| External beam +/− brachytherapy | 173 (25%) | 165 (23%) | |
| Brachytherapy only | 54 (8%) | 63 (9%) | |
| Radiotherapy not received | 471 (67%) | 469 (67%) | |
| Unknown | 6 | 7 | |
Table 5.
Adjuvant radiotherapy received (within 3 months after surgery), nodes removed, nodal status, and external-beam radiotherapy (EBRT) received in node-positive patients by risk group defined according to ASTEC radiotherapy eligibility
| Standard surgery (N=683)* | Lymphadenectomy (N=686)* | ||
|---|---|---|---|
| Low-risk early-stage endometrial cancer | |||
| Total | 330 (49%) | 282 (42%) | |
| Radiotherapy received | |||
| Yes | 22 (7%) | 24 (9%) | |
| No | 305 (93%) | 257 (91%) | |
| Unknown | 3 | 1 | |
| External beam +/− brachytherapy | 12 (4%) | 9 (3%) | |
| Brachytherapy only | 10 (3%) | 15 (5%) | |
| Nodes removed | |||
| Yes | 8 (2%) | 255 (92%) | |
| No | 314 (98%) | 22 (8%) | |
| Unknown | 8 | 5 | |
| Median number of nodes (range) | 2 (1–3) | 12 (1–59) | |
| Nodal involvement | |||
| Yes (received EBRT) | 1 (1) | 6 (2) | |
| No | 7 | 249 | |
| Intermediate-risk and high-risk early-stage endometrial cancer | |||
| Total | 243 (36%) | 264 (39%) | |
| Radiotherapy received | |||
| Yes | 137 (56%) | 138 (53%) | |
| No | 106 (44%) | 124 (47%) | |
| Unknown | 0 | 2 | |
| External beam +/− brachytherapy | 102 (42%) | 98 (37%) | |
| Brachytherapy only | 35 (14%) | 40 (15%) | |
| Nodes removed | |||
| Yes | 14 (6%) | 244 (93%) | |
| No | 226 (94%) | 17 (7%) | |
| Unknown | 3 | 3 | |
| Median number of nodes (range) | 1 (1–16) | 12 (1–51) | |
| Nodal involvement | |||
| Yes (received EBRT) | 4 (1) | 21 (12) | |
| No | 10 | 233 | |
| Advanced endometrial cancer | |||
| Total | 103 (15%) | 124 (19%) | |
| Radiotherapy received | |||
| Yes | 62 (61%) | 64 (53%) | |
| No | 40 (39%) | 56 (47%) | |
| Unknown | 1 | 4 | |
| External beam +/− brachytherapy | 55 (54%) | 55 (46%) | |
| Brachytherapy only | 7 (7%) | 8 (7%) | |
| Nodes removed | |||
| Yes | 10 (10%) | 103 (85%) | |
| No | 91 (90%) | 18 (15%) | |
| Unknown | 2 | 3 | |
| Median number of nodes | 2 (1–6) | 13 (1–38) | |
| Nodal involvement | |||
| Yes (received EBRT) | 4 (0) | 25 (8) | |
| No | 6 | 78 | |
| Unknown, unable to classify risk group | |||
| Total | 7 | 16 | |
Excludes patients whose pathology details did not confirm endometrial cancer.
A small proportion of women in both groups received other anticancer treatment during follow-up before disease recurrence, including chemotherapy (31 standard surgery group; 26 lymphadenectomy group) and progestogens (15 standard surgery group; 17 lymphadenectomy group).
Within the treatment groups, the median number of nodes removed was similar in the three subgroups of low-risk early-stage, intermediate-risk and high-risk early-stage, and advanced disease (table 5). Within these subgroups, the proportion of women with nodes removed who had positive nodes in the lymphadenectomy group was 2·4% (six of 255 women), 9% (21 of 244), and 24% (25 of 103), respectively.
After adjuvant treatment, including postoperative radiotherapy in participants who received it, more women in the lymphadenectomy group than in standard surgery group reported moderate or severe morbidity or treatment-related complications (119/684 [17%] vs 85/686 [12%]). Of the 26 women (two standard surgery group; 24 lymphadenectomy group) who had lymphoedema, eight received EBRT (all in lymphadenectomy group). In the lymphadenectomy group we noted no clear evidence of an association between EBRT and lymphoedema (relative risk 1·57 [95% CI 0·66–3·74]; p=0·28).
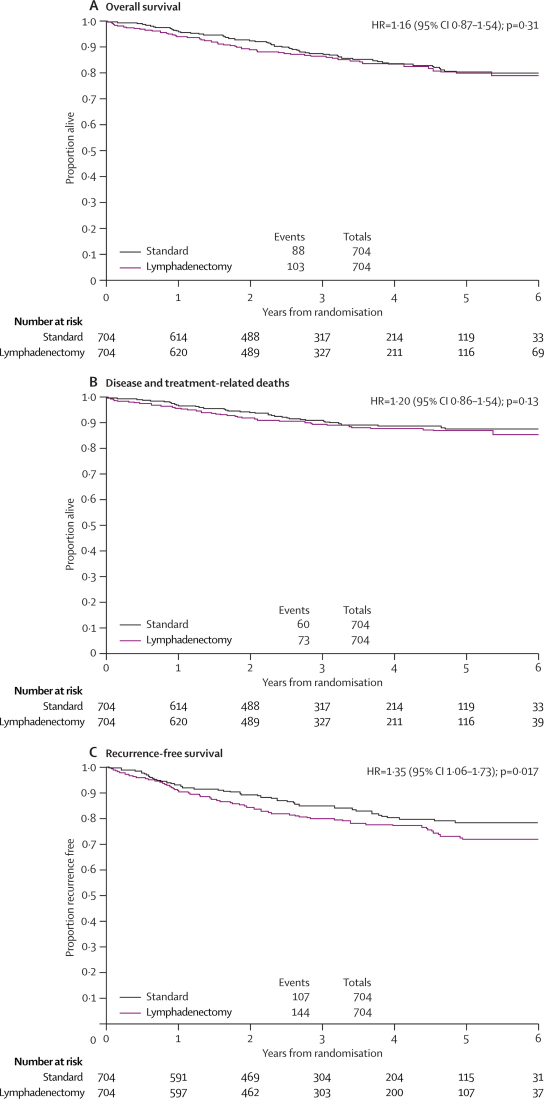
By May, 2006, with a median follow up of 37 months (IQR 24–58 months), 191 (14%) women had died (table 6). Overall survival curves showed no evidence of a difference between the two groups (figure 3).
Table 6.
Cause of death
| Standard surgery (N=704) | Lymphadenectomy (N=704) | |
|---|---|---|
| Total deaths | 88 (13%) | 103 (15%) |
| Disease related | 56 (65%) | 64 (63%) |
| Treatment related* | 4 (5%) | 7 (7%) |
| Disease and treatment related* | 0 | 2 (2%) |
| Not disease or treatment related | 26 (30%) | 28 (28%) |
| Unknown | 2 | 2 |
Details of treatment related, and disease and treatment related, cause of death: standard surgery group—renal failure (n=1), perforated ulcer (1), pulmonary oedema (1), bowel ischaemia (1); lymphadenectomy group—pulmonary embolism (2), perforated duodenal ulcer (1), perforated diverticular disease (1), bowel obstruction (1), bowel infarction (1), deep-vein thrombosis and infection (1), aspiration (1), no details (1).
Figure 3.
Overall survival (A), disease and treatment-related deaths (B), and recurrence-free survival (C) by treatment group
Deaths were classified as treatment related, or disease and treatment related, in four (0·6%) women in the standard surgery group and nine (1·3%) in the lymphadenectomy group (table 6). A third of the deaths were not related to treatment or disease (26/88 deaths in standard surgery group and 28/103 in lymphadenectomy group). 54 deaths were classified as non-disease and non-treatment related (table 6). We therefore undertook a competing risk analysis that takes this factor into account. An analysis of disease or treatment-related deaths again showed an HR in favour of the standard surgery group (figure 3), although inevitably the confidence intervals were wider since there were fewer events in this analysis.
173 (12%) women had had disease recurrence (75 standard surgery group, 98 lymphadenectomy group), and 251 (18%) had died or had recurrence of disease (107 standard surgery group, 144 lymphadenectomy group) at the time of analysis. Table 7 summarises the site of recurrence.
Table 7.
Site of recurrence
| Standard surgery (N=704) | Lymphadenectomy (N=704) | |
|---|---|---|
| Total recurrences | 75 (11%) | 98 (14%) |
| Local/vaginal | 18 (25%) | 24 (27%) |
| Pelvic | 11 (15%) | 10 (11%) |
| Distal | 38 (53%) | 49 (54%) |
| Local/vaginal and distal | 0 | 3 (3%) |
| Pelvic and distal | 5 (7%) | 4 (4%) |
| Unknown | 3 | 8 |
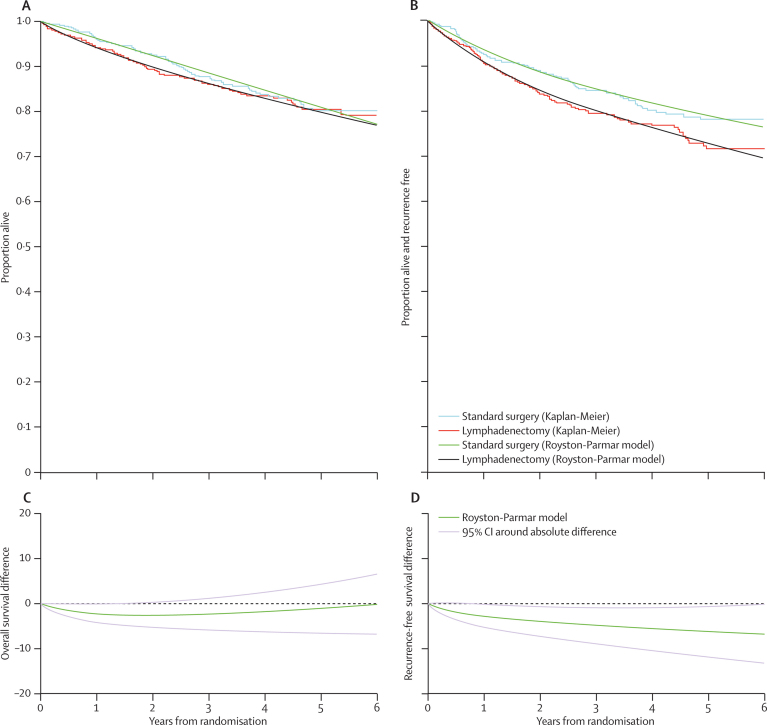
Kaplan-Meier curves for recurrence-free survival showed a conventionally significant benefit for the standard surgery group (figure 3). Figure 4 shows the Kaplan-Meier plots for overall survival and recurrence-free survival together with the model curves from fitting the Royston-Parmar parametric model, as well as the absolute difference between the treatment groups over time. The absolute difference in 5-year overall survival estimated from these curves was a 1% (95% CI −4·0 to 6) improvement in favour of standard surgery (figure 4). The 5-year overall survival was 81% (95% CI 77–85) in the standard surgery group and 80% (76–84) in the lymphadenectomy group. The absolute difference in 5-year recurrence-free survival was 6% (1–12) in favour of standard surgery (figure 4). 5-year recurrence-free survival was 79% (75–83) in the standard surgery group and 73% (69–77) in the lymphadenectomy group. Over time, the absolute difference in overall survival between the two groups remained close to zero, whereas the absolute difference for recurrence-free survival increased over time in favour of standard surgery (figure 4).
Figure 4.
Kaplan-Meier plots for the two treatment groups for overall and recurrence-free survival together with the model curves from fitting the Royston-Parmar parametric model
A and B show Kaplan-Meier estimates, with Royston-Parmar parametric model fitted. C and D show the absolute difference over time (95% CI) in survival from Royston-Parmar parametric model.
Despite randomisation of a large number of women, we recorded some differences in the pretreatment characteristics with respect to histological features that suggested women in the standard surgery group might be at lower risk of recurrence than were those in the lymphadenectomy group. Results of analyses adjusting for these imbalances (table 8) gave an HR closer to 1·0 for both overall survival and recurrence-free survival, and did not suggest a benefit with lymphadenectomy for overall survival (adjusted HR 1·04 [95% CI 0·74–1·45]; p=0·83) or for recurrence-free survival (1·25 [0·93–1·66]; p=0·14), with the point estimate for recurrence-free survival still in favour of standard surgery.
Table 8.
Unadjusted and adjusted analysis using the Cox model for overall survival and recurrence-free survival (deaths from all causes), and for disease-specific survival and recurrence-free disease-specific survival (disease and treatment specific deaths only)
|
Overall survival |
Recurrence-free survival |
Disease-specific survival |
Recurrence-free disease-specific survival |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Unadjusted with full data (n=1408) | 1·16 (0·87–1·54) | 0·31 | 1·35 (1·06–1·73) | 0·017 | 1·21 (0·86–1·70) | 0·28 | 1·46 (1·11–1·23) | 0·01 |
| Adjusted* by covariates (n=1337 with imputation by mean for unknown baseline)† | 1·04 (0·74–1·45) | 0·83 | 1·25 (0·93–1·66) | 0·14 | 1·12 (0·75–1·69) | 0·57 | 1·33 (0·96–1·83) | 0·083 |
HR=hazard ratio.
Adjusted for age (continuous), WHO performance status (0, 1, 2, 3, or 4), weeks between diagnosis and randomisation (≤6 weeks vs >6 weeks), surgical technique intended (open vs laparoscopic), type of incision (vertical vs Pfannenstiel vs other transverse), extent of tumour (confined vs spread), histology (endometrioid/adenocarcinoma vs other), depth of invasion (inner half vs endometrium, outer half vs endometrium), differentiation (grade 1, grade 2, grade 3), and centre (dummy variables and centres with less than five patients being grouped as one new centre).
71 patients were not included (37 standard surgery group, 34 lymphadenectomy group): 39 with no disease and 32 with differentiation not applicable (histology mixed epithelial stromal, sarcoma).
We also undertook analyses of disease-specific survival, adjusting for baseline characteristics with the Cox model (table 8). Results were similar to the unadjusted analysis (table 8). Exploratory interaction analyses did not provide any evidence that the effect of lymphadenectomy on overall survival or recurrence-free survival differed in subgroups defined by age, WHO performance status, and pathological features of endometrial cancer including depth of invasion, histology, or grade of tumour (data not shown). We did a predefined analysis to explore the effect of number of nodes removed for systematic lymph node dissection in an unbiased manner by classifying centres according to the median number of nodes removed in the lymphadenectomy group: less than ten, between ten and 14, and 15 or more nodes (table 9). Our results suggest that, if anything, lymphadenectomy could be associated with a worse outcome the more lymph nodes removed (table 9; p=0·13 for overall survival and p=0·16 for recurrence-free survival). Results of the adjusted analyses by hospitals removing different median numbers of nodes (table 9) lends support to this conclusion.
Table 9.
Unadjusted and adjusted analysis classifying centres by median number of nodes harvested, with the Cox model for overall survival and recurrence-free survival
| Overall survival | Recurrence-free survival | |
|---|---|---|
| Centres with median LN count <10 | ||
| Unadjusted (n=489) | 0·81 (0·50–1·31) | 1·01 (0·67–1·54) |
| Adjusted* (n=481) | 0·54 (0·31–0·95) | 0·72 (0·45–1·16) |
| Centres with median LN count 10–14 | ||
| Unadjusted (n=314) | 1·40 (0·74–2·64) | 1·72 (1·00–2·96) |
| Adjusted* (n=307) | 1·39 (0·67–2·90) | 1·81 (0·99–3·27) |
| Centres with median LN count ≥15 | ||
| Unadjusted (n=553) | 1·57 (1·00–2·45) | 1·71 (1·14–2·56) |
| Adjusted* (n=536) | 1·37 (0·83–2·26) | 1·50 (0·95–2·37) |
Data are hazard ratio (95% CI). LN=lymph node.
Adjusted by covariates (with imputation by mean for unknown baseline) for age (continuous), WHO performance status (0, 1, 2, 3, or 4), weeks between diagnosis and randomisation (≤6 weeks vs >6 weeks), surgical technique intended (open vs laparoscopic), type of incision (vertical vs Pfannenstiel vs other transverse), extent of tumour (confined vs spread), histology (endometrioid/adenocarcinoma vs other), depth of invasion (inner half vs endometrium, outer half vs endometrium), and differentiation (grade 1, grade 2, grade 3).
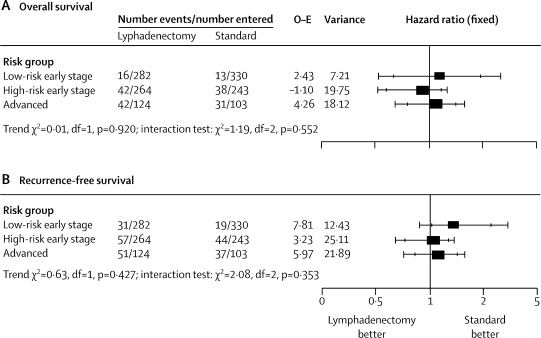
In the subgroups of women classified with low-risk early-stage disease, intermediate-risk and high-risk early-stage disease, and advanced disease (figure 5), we recorded no evidence of a difference in the relative effect of lymphadenectomy versus standard surgery (p=0·55 for overall survival and p=0·35 for recurrence-free survival).
Figure 5.
Effect of lymphadenectomy on overall survival (A) and recurrence-free survival (B) in women in different risk groups of recurrence
O–E=observed minus expected. Outer bars show 99% CI, inner bars show 95% CI.
Discussion
This randomised trial has shown no evidence of a benefit for systematic lymphadenectomy for endometrial cancer in terms of overall, disease-specific, and recurrence-free survival. This study is one of the largest reported surgical gynaecological cancer trials. We undertook searches to identify other published randomised trials of lymphadenectomy in endometrial cancer and searched the Cochrane database for systematic reviews of both randomised and observational studies.14 None was found but a randomised trial (smaller than ASTEC) has just been reported, which confirms that lymphadenectomy is not associated with a survival benefit in endometrial cancer.15
The proportion of women with pelvic-node metastases in ASTEC (9%) was consistent with that in the Gynaecological Oncology Group (GOG) surgical study of disease spread.6 The ASTEC trial design successfully randomised participants to postoperative EBRT independent of lymph-node status, resulting in a balanced proportion of irradiated women in both groups. Lymphadenectomy might otherwise have led to more women categorised as having high-stage disease in the lymphadenectomy group, which would have resulted in more women in this population receiving postoperative EBRT. Thus, any effect in favour of the lymphadenectomy group would have been enhanced. Although more women in the standard surgery group than in the lymphadenectomy group, apart from in the low-risk early-stage subgroup, received EBRT (which might favour standard surgery), differences were not large. Furthermore, a greater proportion of women in the lymphadenectomy group with positive lymph nodes received EBRT (which might favour lymphadenectomy), although numbers were small. However, in view of the results of the systematic review and meta analysis of 1770 women from four randomised trials7 and now data from the ASTEC/EN.5 radiotherapy trial8 showing that even a very small benefit of radiotherapy (more than 3% in 5-year overall survival) can be excluded, this slight imbalance in the use of EBRT is neither statistically nor clinically significant.
Several observational studies have compared outcomes in women who have received systematic lymphadenectomy and those who have not, with some studies supporting lymphadenectomy for all grades of tumour,10,16–18 another supporting it for G3 tumours,12 and others suggesting that benefit depends on the number of lymph nodes removed.17,19,20 However, these studies of treatment benefit should be interpreted with caution since they are prone to bias because of systematic differences in women who do and do not receive lymphadenectomy, including comorbidity and obesity that can be related to poor survival. Additionally, non-randomised studies will always have a higher proportion of node-positive women with occult stage III disease in the group who have not been surgically staged versus those classified as stage I after lymphadenectomy with negative nodes and a reduced risk of recurrence (stage migration). Large cancer registries are invaluable for monitoring trends in cancer incidence and outcomes, for hypothesis generation, and for planning the provision of cancer services, but they are not reliable for assessment of treatment effects. Analysis of large observational cohorts means that small associations could be significant but they can easily arise from very modest bias and should not be misinterpreted as measuring a direct effect of an intervention.
No national or international accepted guidelines exist for what is regarded as an adequate systematic lymphadenectomy in terms of node counts, and therefore no specific guidelines were given in the protocol. The number of nodes identified might depend on the physical characteristic of the women, surgical thoroughness, and pathological examination of the tissues. ASTEC succeeded in comparing systematic lymphadenectomy with a more conservative approach of standard surgery with lymphadenectomy in women who only had potentially positive nodes on palpation. The median lymph-node count of 12 in the lymphadenectomy group is broadly similar to that in a large single-institution case series from the USA, in which 11 nodes were removed,16 as well as a study using data from an large observational database in which a median of between seven and 12 nodes were removed.20
We were unable to undertake an unbiased analysis of outcome according to the number of nodes removed for individual patients, since we would have had to break the randomised comparison with the same issues of selection bias (although less stage shift). We were, however, able to do the exploratory analysis that considered standard surgery versus lymphadenectomy in centres which routinely removed different numbers of nodes. Results from this analysis suggested that, if anything, the lymphadenectomy group had poorer outcomes when more nodes were removed than the standard surgery group did. This finding and the trend of a slightly higher recurrence in the lymphadenectomy group is potentially important, although an explanation is not obvious. Although some node sampling occurred in the standard surgery group, only 35 (5%) women had any nodes removed and, of these, 26 women had four or fewer removed. This small amount of non-compliance could have biased the point estimate for the HR very slightly towards 1 (ie, no effect of lymphadenectomy) but the treatment effect would have to have been very large for this small amount of non-compliance to affect the overall result.
One limitation of this study is that the lymphadenectomy specified in the protocol was not comprehensive and did not include all pelvic and para-aortic nodes. At the time ASTEC was conceived, the systematic lymphadenectomy (ie, lymphadenectomy rather than sampling) was considered to be a potentially therapeutic procedure that could be implemented in the range of hospitals where women with endometrial cancer are treated and in the range of women needing it, including those in whom more extensive surgery might be difficult because of lack of surgical access due to obesity. More extensive lymphadenectomy could have had significantly increased treatment-related morbidity and mortality. Data for sentinel nodes have given insight into the totality of the lymph drainage pattern in endometrial cancer and might well direct future surgical research. Improvements in imaging could also make selection of high-risk women easier so that a more extensive lymphadenectomy could be done in the subset of women for whom lymph-node metastases are more likely and a more extensive operation is feasible (those with little comorbidity, including obesity).
Morbidity was low overall, but we noted a substantial increase in the incidence of lymphoedema in the lymphadenectomy group compared with standard surgery. Clinicians might not have noted or reported milder cases, since all reported cases of lymphoedema were moderate and severe.
We acknowledge that although ASTEC is a very large trial, only 191 deaths were recorded. Could a small but important treatment effect have been missed? Recurrence-free survival is the most powerful outcome measure (with 251 events). The lower limit of the 95% CI for the adjusted HR for recurrence-free survival (table 8) of 0·93 translates into a benefit of 1·5% at 5 years (adjusted). Thus we can reliably exclude an improvement in 5-year recurrence-free survival from lymphadenectomy of 1·5% or greater.
ASTEC has important implications for both clinical practice and future trials. The balance of risks and benefits for systematic lymphadenectomy does not favour this intervention, with no clear evidence of benefit in terms of overall or recurrence-free survival and increased risk of lymphoedema. Although the results do not invalidate the use of lymphadenectomy for surgical staging to identify the need for adjuvant treatment, our results suggest that lymphadenectomy in itself has no therapeutic effect and is therefore not justified as a therapeutic procedure in its own right. Some argue that surgical staging allows the most rational use of adjuvant radiotherapy, reserving it for women with proven extrauterine disease. In doing so, long-term effects associated with radiotherapy can be reduced without affecting survival. This argument might be an over simplified view of the evidence, since results of GOG99 study showed that when surgically staged women with negative nodes were randomly assigned, a protective effect of radiotherapy against pelvic recurrence was still recorded although no evidence of a difference in overall survival was noted (incidence of recurrence was 12% in the no radiotherapy group and 3% in the radiotherapy group, relative hazard 0·42 [90% CI 0·25–0·73]; p=0·007).21 Estimated overall 4-year survival was 86% in the no radiotherapy group and 92% for group with radiotherapy (relative hazard 0·86 [90% CI 0·57–1·29]; p=0·56). This finding suggests that the decision regarding adjuvant therapy for intermediate-risk and high-risk disease should be made independently of lymph-node status.
In conclusion, this large randomised trial suggests that unless surgical staging will directly affect adjuvant therapy, routine systematic pelvic lymphadenectomy cannot be recommended in women undergoing primary surgery for stage I endometrial cancer outside of clinical trials. Surgical interventions should be assessed through randomised trials, and surgical staging as part of a management strategy is no exception.
Acknowledgments
Acknowledgments
We thank the women who participated in this trial; the research staff at the clinical centres who helped to recruit women and provide data; and Babak Oskooei for providing statistical input into preliminary analyses. The trial was funded by the MRC through core funding to the MRC CTU.
ASTEC study group
Trial design: H Kitchener, P Blake, J Sandercock, M K B Parmar.
Writing committee: H Kitchener (University of Manchester, School of Cancer and Imaging Sciences, Manchester, UK); A M C Swart (UCL Centre for Clinical Pharmacology and MRC CTU, London, UK); and W Qian, C Amos, M K B Parmar (Cancer Group MRC CTU, London, UK).
Chief clinical investigator: H Kitchener.
Data analysis: W Qian.
Trial management group: C Amos, P Blake, A Branson, C H Buckley, H Kitchener, M K B Parmar, W Qian, CWE Redman, J Shepherd, A M C Swart.
Independent data monitoring committee: G Dunn, P Heintz, J Yarnold (chair).
Trial steering committee: P Johnson, M Mason (chair), R Rudd.
MRC Clinical Trials Unit Data Management: C Amos, P Badman, S Begum, N Chadwick, S Collins, K Goodall, J Jenkins, K Law, P Mook, J Sandercock, C Goldstein, B Uscinska.
UK centres—Aberdeen Royal Infirmary (M Cruickshank, D E Parkin); Addenbrookes Hospital, Cambridge (R A F Crawford, J Latimer); Bassetlaw Hospital, Worksop (M Michel); Belfast City Hospital incorporating Belvoir Park Hospital (J Clarke, S Dobbs, R J McClelland, J H Price); Birmingham Women's Hospital (K K Chan, C Mann); Bradford Royal Infirmary (R Rand); Brighton General Hospital (A Fish); Bromhead Hospital, Lincoln (M Lamb); Broomfield Hospital, Chelmsford (C Goodfellow, S Tahir); Chelsea and Westminster Hospital, London (J R Smith); Cheltenham General Hospital (R Gornall, R Kerr-Wilson, G R Swingler); Churchill Hospital, Oxford (B A Lavery); City Hospital Birmingham (K K Chan, S Kehoe); Clatterbridge Centre for Oncology, Wirral (A Flavin); Colchester General Hospital (J Eddy); Countess of Chester Hospital, Chester (J Davies-Humphries); Coventry Maternity Hospital (M Hocking, L J Sant-Cassia); Cumberland Infirmary, Carlisle (S Pearson); Derby City General Hospital (R L Chapman, J Hodgkins, I Scott); Derbyshire Royal Infirmary (D Guthrie, M Persic); Derriford Hospital, Plymouth (F N Daniel, D Yiannakis); Doncaster Royal Infirmary (M I Alloub, L Gilbert, M R Heslip); Queen Elizabeth the Queen Mother Hospital, Margate (A Nordin); Edinburgh Royal Infirmary (G Smart, V Cowie); Epsom General Hospital (M Katesmark); Essex County Hospital (P Murray, J Eddy); Gloucestershire Royal Hospital (R Gornall, G R Swingler); Good Hope District Hospital, Sutton Coldfield (C B Finn, M Moloney); Hammersmith Hospital, London (A Farthing, J Hanoch, P W Mason, A McIndoe, W P Soutter); Harold Wood Hospital, Romford (H Tebbutt); Ipswich Hospital (J S Morgan, D Vasey); James Cook University Hospital, South Cleveland (D J Cruickshank, J Nevin); John Radcliffe Hospital, Oxford (S Kehoe, I Z McKenzie); King's Mill Hospital, Nottingham (C Gie); Leicester General Hospital (Q Davies, D Ireland, P Kirwan); Leicester Royal Hospital (Q Davies); Lincoln County Hospital (M Lamb); Liverpool Women's Hospital (R Kingston, J Kirwan, J Herod); LLandough Hospital, Penarth (A Fiander, K Lim); Manor Hospital, Walsall (A C Head); Milton Keynes General Hospital (C B Lynch); New Cross Hospital, Wolverhampton (A J Browning, C Cox, D Murphy); Ninewells Hospital, Dundee (I D Duncan, C Mckenzie); Norfolk and Norwich Hospital (S Crocker, J Nieto); Northern General Hospital, Sheffield (M E L Paterson, J Tidy); Northampton General Hospital (A Duncan); Nottingham City Hospital (S Chan, K M Williamson); Oldchurch Hospital, Romford (A Weekes); Pilgrim Hospital, Boston (O A Adeyemi); Poole Hospital HNS Trust (R Henry, V Laurence, S Dean); Princess Royal Hospital, Hull (D Poole, M J Lind, R Dealey); Queen Elizabeth Hospital, Gateshead (K Godfrey, M M Hatem, A Lopes, J M Monaghan, R Naik); Royal Bournemouth General Hospital (J Evans); Royal Hallamshire Hospital, Sheffield (A Gillespie, M E L Paterson, J Tidy); Royal Marsden Hospital, London (T Ind); Royal Shrewsbury Hospital (J Lane, S Oates, D Redford); Royal Surrey County Hospital, Guilford (M Ford); Royal Sussex County Hospital, Brighton (A Fish, P Larsen-Disney); Royal United Hospital, Bath (N Johnson); Royal Victoria Infirmary, Belfast (A Bolger); Royal Preston Hospital (P Keating, P Martin-Hirsch); Somerset Nuffield Hospital, Taunton (L Richardson); Southmead Hospital, Bristol (J B Murdoch); St Batholomew's Hospital, London (A Jeyarajah); St Georges Hospital, Lincoln (M Lamb); St Helier Hospital, Surrey (N McWhinney); St Mary's Hospital, London (A Farthing, P W Mason); St Mary's Hospital, Manchester (H Kitchener); St Mary's Hospital, Portsmouth (J L Beynon, P Hogston, E M Low, R Woolas); St Michaels Hospital, Bristol (R Anderson, J B Murdoch, P A R Niven); St Pauls Hospital, Cheltenham (R Kerr-Wilson); Staffordshire General Hospital, Stafford (K Chin); Singleton Hospital, Swansea (P Flynn, O Freites); Sussex Oncology (O Freites, A Fish, G H Newman); Taunton and Somerset Hospital (O McNally); The Great Western Hospital, Swindon (J Cullimore); University College Hospital, London (A Olaitan, T Mould); University Hospital of North Staffordshire, Stoke on Trent (V Menon, C W E Redman); University Hospital of North Tees, Cleveland (M George, M H Hatem); University Hospital Wales, Cardiff (A Evans, A Fiander, R Howells, K Lim); Whiston Hospital, Prescot (G Cawdell); Wordsley Hospital, Stourbridge (A P Warwick); Wycombe General Hospital (D Eustace); Yeovil District Hospital (J Giles); Ysbyty Gwynedd Hospital, Bangor (S Leeson).
International centres—Groote Schuur Hospital, South Africa (J Nevin, AL van Wijk); Marie Curie Institute, Poland (K Karolewski, M Klimek, P Blecharz); Wellington Hospital, New Zealand (D McConnell).
Conflict of interest statement
We declare that we have no conflict of interest.
Correspondence to: Ann Marie C Swart, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK ams@ctu.mrc.ac.uk
References
- 1.Cancer Research UK CancerStats: corpus uteri cancer. http://info.cancerresearchuk.org/cancerstats/ (accessed Aug 5, 2008).
- 2.Boyle P, Leon ME, Maisonneuve P, Autier P. Cancer control in women. Update 2003. Int J Gynaecol Obstet. 2003;83(suppl 1):179–202. doi: 10.1016/s0020-7292(03)90121-4. [DOI] [PubMed] [Google Scholar]
- 3.Cancer facts and figures 2008. American Cancer Society; Atlanta: 2006. Available at http://seer.cancer.gov/csr/1975_2005/results_single/sect_01_table.01.pdf (accessed Aug 5, 2008). [Google Scholar]
- 4.Sant M, Aareleid T, Berrino F. EUROCARE-3: survival of cancer patients diagnosed 1990–94—results and commentary. Ann Oncol. 2003;14(suppl 5):v61–118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- 5.Boronow RC, Morrow CP, Creasman WT. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63:825–832. [PubMed] [Google Scholar]
- 6.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(suppl):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Kong A, Simera I, Collingwood M, Williams C, Kitchener H, on behalf of Cochrane Gynaecological Cancer Group Adjuvant radiotherapy for stage I endometrial cancer: systematic review and meta-analysis. Ann Oncol. 2007;18:1595–1604. doi: 10.1093/annonc/mdm066. [DOI] [PubMed] [Google Scholar]
- 8.The ASTEC/EN.5 writing committee. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. [DOI] [PMC free article] [PubMed]
- 9.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96:889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 10.Kilgore LC, Partridge EE, Alvarez RD. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 11.Morrow CP, Bundy BN, Kurman RJ. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 12.Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998;71:340–343. doi: 10.1006/gyno.1998.5254. [DOI] [PubMed] [Google Scholar]
- 13.Wells M. Minimum dataset for the histopathological reporting of atypical hyperplasia and adenocarcinoma in endometrial biopsy and curettage specimens and for endometrial cancer in hysterectomy specimens. Standards and Minimum Datasets for Reporting Cancers. Royal College of Pathologists; London: 2001. [Google Scholar]
- 14.Lefebvre C, Clarke M. Identifying randomised trials. In: Egger MSG, Altman DG, editors. Systematic reviews in healthcare: meta-analysis in context. 2nd edn. BMJ Publishing Group; London: 2001. pp. 69–87. [Google Scholar]
- 15.Panici PB, Basile S, Maneschi F. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 16.Cragun JM, Havrilesky LJ, Calingaert B. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 17.Fanning J. Long-term survival of intermediate risk endometrial cancer (stage IG3, IC, II) treated with full lymphadenectomy and brachytherapy without teletherapy. Gynecol Oncol. 2001;82:371–374. doi: 10.1006/gyno.2001.6276. [DOI] [PubMed] [Google Scholar]
- 18.Mohan DS, Samuels MA, Selim MA. Long-term outcomes of therapeutic pelvic lymphadenectomy for stage I endometrial adenocarcinoma. Gynecol Oncol. 1998;70:165–171. doi: 10.1006/gyno.1998.5098. [DOI] [PubMed] [Google Scholar]
- 19.Lutman CV, Havrilesky LJ, Cragun JM. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol Oncol. 2006;102:92–97. doi: 10.1016/j.ygyno.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Cheung MK, Huh WK. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12333 patients. Cancer. 2006;107:1823–1830. doi: 10.1002/cncr.22185. [DOI] [PubMed] [Google Scholar]
- 21.Keys HM, Roberts JA, Brunetto VL. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]