Abstract
Background
Progressive resistance exercise training (PRT) improves physical functioning in patients with HIV infection. Creatine supplementation can augment the benefits derived from training in athletes and improve muscle function in patients with muscle wasting. The objective of this study was to determine whether creatine supplementation augments the effects of PRT on muscle strength, energetics, and body composition in HIV-infected patients.
Methodology/Principal Findings
This is a randomized, double blind, placebo-controlled, clinical research center-based, outpatient study in San Francisco. 40 HIV–positive men (20 creatine, 20 placebo) enrolled in a 14-week study. Subjects were randomly assigned to receive creatine monohydrate or placebo for 14 weeks. Treatment began with a loading dose of 20 g/day or an equivalent number of placebo capsules for 5 days, followed by maintenance dosing of 4.8 g/day or placebo. Beginning at week 2 and continuing to week 14, all subjects underwent thrice-weekly supervised resistance exercise while continuing on the assigned study medication (with repeated 6-week cycles of loading and maintenance). The main outcome measurements included muscle strength (one repetition maximum), energetics (31P magnetic resonance spectroscopy), composition and size (magnetic resonance imaging), as well as total body composition (dual-energy X-ray absorptiometry). Thirty-three subjects completed the study (17 creatine, 16 placebo). Strength increased in all 8 muscle groups studied following PRT, but this increase was not augmented by creatine supplementation (average increase 44 vs. 42%, difference 2%, 95% CI −9.5% to 13.9%) in creatine and placebo, respectively). There were no differences between groups in changes in muscle energetics. Thigh muscle cross-sectional area increased following resistance exercise, with no additive effect of creatine. Lean body mass (LBM) increased to a significantly greater extent with creatine.
Conclusions / Significance
Resistance exercise improved muscle size, strength and function in HIV-infected men. While creatine supplementation produced a greater increase in LBM, it did not augment the robust increase in strength derived from PRT.
Trial Registration
ClinicalTrials.gov NCT00484627
Introduction
In people with HIV infection and other chronic diseases, maintenance or augmentation of muscle mass is important for preserving functional status and forestalling disease progression [1]. Progressive resistance exercise training (PRT), either alone or in combination with aerobic exercise training, can increase muscle mass and improve physical performance in persons with HIV infection [2]–[10]. Many individuals employ so-called ergogenic aids, including substances such as anabolic steroids and growth hormone, to facilitate muscle accrual, and to enhance the anabolic effect of PRT on body composition with a view to both functional and aesthetic improvements [11], [12]. Creatine monohydrate is a nutritional supplement that has been shown to enhance short-term energy availability during intense exercise and to improve recovery between intense exercise bouts [13], [14]. Based on such findings, it is being used by competitive and recreational athletes as an ergogenic aid to improve their training efficiency. Although in some populations creatine supplementation has been shown to improve isotonic strength [15], torque [16] and power [17] other studies have failed to show any such effects [18]–[21].
To date, there have been no specific studies of the efficacy of the medical use of creatine supplementation in people with HIV infection. Further, the effects of creatine supplementation in combination with PRT on muscle energetics (e.g. mitochondrial function, resistance to fatigue, or intramuscular fat content) are not known in the HIV population, even though these are considered the primary benefits of creatine supplementation. The present study was designed to test the hypothesis that the medical use of creatine supplementation would augment increases in muscle strength derived from PRT in HIV-positive adults. The functional impact and potential mechanisms of these strength gains were also assessed.
Methods
Protocol
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1.
Ethics
The study was approved by the Committees on Human Research at the University of California San Francisco and the San Francisco VA Medical Center. All subjects gave written informed consent.
Participants
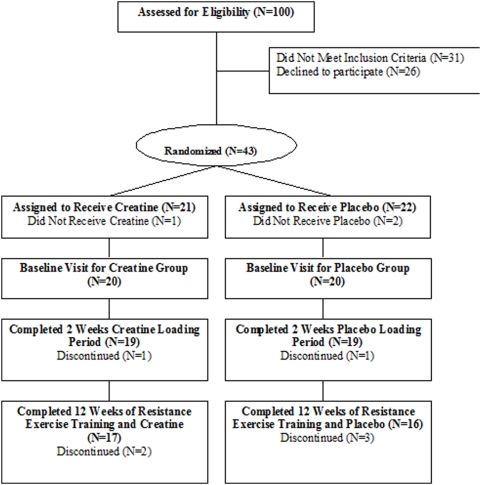
Forty-three clinically-stable, sedentary HIV-positive subjects (42 men, 1 woman) living in the San Francisco Bay area were recruited by our study coordinators and studied between August 2001 and January 2004 (Figure 1). Subjects using antiretroviral therapy (ART) were required to be on stable regimens for at least 30 days before enrollment and were asked to remain so during the study. Subjects who had received no ART for the preceding 30 days and had no plans to initiate therapy during the study were also eligible.
Figure 1. CONSORT Diagram showing the disposition of subjects randomized to receive creatine monohydrate or an equivalent number of placebo capsules for 14 weeks.
Exclusion criteria included regular resistance exercise training, use of anabolic hormones and other putative ergogenic aids (e.g. amino acids, protein supplements, β-agonists), serum creatinine >1.5 mg/dl; history of renal disease; creatine kinase (CK) >1.5 times the upper limit of normal (ULN); hemoglobin <8.5 g/dl; liver transaminase or lactate dehydrogenase levels ≥5×ULN; uncontrolled diarrhea, nausea, or vomiting; impaired oral food intake; untreated hypogonadism; pharmacologic use of anabolic or immune modulating therapies; systemic infection within 30 days; history of heart disease; and current pregnancy or lactation. Subjects on stable testosterone replacement for ≥6 months were eligible.
Sample Size
Sample size calculations were based on other studies with similar design and allow for a detection of standardized effect sizes of 0.9 with 80% power to detect changes in intramuscular phosphocreatine (PCr) levels.
Randomization
Subjects were randomized, in a blinded 1∶1 fashion, to receive identical-appearing capsules containing either pure creatine monohydrate or placebo (both provided by Jarrow Universal Herbs Inc., Union City, CA) for 14 weeks (Figure 1). A computer-generated randomization list was prepared by a statistical consultant and given directly to the research pharmacy at San Francisco General Hospital (SFGH), where the capsules were bottled and dispensed. All data were collected and analyzed without knowledge of treatment assignment.
Three subjects (1 creatine, 2 placebo), including the one woman, withdrew after randomization but before baseline testing or initiation of treatment and are thus excluded from the analysis.
Intervention
Subjects began with a loading dose of 20 g/day (divided among four doses) or an equivalent number of placebo capsules for 5 days, followed by maintenance dosing of 4.8 g/day (in two 2.4 g doses) or placebo. Maintenance dosing was interrupted briefly for additional 5-day loading cycles at the beginning of weeks 7 and 13. This dosing regimen is similar to those employed in several recent studies (as reviewed by Nissen [13]). Subjects were given a non-caffeinated fruit juice beverage containing 20 g of simple carbohydrate to take with each dose of study medication [22].
Training Regimen
After two weeks, all subjects began a 12-week program of supervised PRT, while continuing on their assigned study medication. Training was performed using a Hoist 5000 Multi-Gym Fitness System (San Diego, CA). Subjects exercised for 1½ hours 3 days weekly for 12 weeks. The training included ankle dorsiflexion, ankle plantar flexion, ankle plantar flexion with bent knee, leg press, leg curls, pec decks chest exercises, tricep pushdown, bicep curl and three types of abdominal crunches. Strength in each muscle group was assessed by the one repetition maximum (1RM) test [23] except in the rectus abdominus, in which the number of sit-ups performed in 30 seconds was recorded. Each training session included 4 sets of 8 repetitions at 80% of the 1RM for each exercise. The 1RM for each exercise was reassessed once every two weeks and training intensity adjusted to maintain 80% of the 1RM. All measurements were performed after two days of rest in absence of any symptoms of muscle soreness or fatigue.
Objectives
The primary aim was to compare the changes in muscle strength (1RM, sit-ups and MVC) from week 0 to week 14 in the two treatment groups. Secondary aims were to compare changes in muscle size, composition, energetics and fatigue, as well as body composition and biochemistry.
Outcome Measurements
Subjects were studied in the General Clinical Research Center (GCRC) at SFGH and in the Magnetic Resonance Unit at the San Francisco VA Medical Center. Height and weight were recorded with subjects wearing a hospital gown. Measurements of muscle strength, size, composition, energetics and fatigue, as well as body weight and composition and serum biochemistries, were made at baseline, after two weeks of treatment with creatine or placebo (before PRT began), and again after 12 weeks of PRT (study week 14). Safety was monitored throughout the study.
Muscle Energetics and Fatigue
Non-invasive measures of intracellular phosphocreatine (PCr), inorganic phosphorous (Pi), and pH were obtained from the dorsiflexor muscle of the right ankle by 31P magnetic resonance spectroscopy (MRS) using a 30-cm bore 1.9 T superconducting Oxford magnet, as described in detail elsewhere [24], [25]. Subjects performed two contraction protocols on the same day, separated by 20 minutes of rest. The first protocol consisted of a 15-second maximum voluntary contraction (MVC), which was used to determine the muscle's capacity for oxidative phosphorylation [24] reported as the half-time (T1/2) of PCr recovery following the contraction. The recovery analysis indicated that our MRS system can detect PCr changes of 0.5 mM. The second protocol was used to assess muscle function during high-intensity, fatiguing conditions. Muscle force and energetics were measured simultaneously during 36 consecutive MVCs over 6 minutes, one every 10 seconds. The fatigue profile was calculated as described by Karatzaferi et al [26].
Dorsiflexor Strength
Isometric MVC force was recorded during ankle dorsiflexion while the leg was positioned in the magnet for the MRS studies [24], [25] The highest force from the 3 MVCs was used to quantify dorsiflexion strength [24] and scale performance during the aforementioned fatigue task.
Muscle Size and Composition
Proton T1-weighted magnetic resonance imaging (MRI) was used to visualize the cross-sectional area (CSA) of the thigh and calf using a 1.5 T whole body Siemens Magnetom Vision System, as described previously [24]. Data were analyzed using a customized software program (Interactive Data Language Research Systems, Inc., Boulder, CO). The coefficient of variation of muscle CSA measurements was 0.6%.
Body Composition
Whole body and regional fat and lean body mass (LBM) were measured using a Lunar model DPX dual energy x-ray absorptiometer (DEXA) (Madison, WI) [27], [28].
Biochemical Measurements
Fasting blood samples were collected for determination of plasma creatine and lactate and serum creatinine, creatine kinase (CK), lipid, glucose, and insulin levels. Plasma creatine was determined by liquid chromatography – tandem mass spectrometry (LC/MS) using a Surveyor LC interfaced with a TSQ Quantum Ultra triple-stage quadrupole MS (Thermo-Finnigan, San Jose, CA) operated in the positive ion mode using atmospheric pressure chemical ionisation. Quantitation was achieved using selected reaction monitoring of the transitions m/z 114 to m/z 44 for creatine and m/z 117 to m/z 47 for the internal standard. The lower limit of quantitation was 2 µg/mL (18 µmol/L). Intra-day precision ranged from 3.2% to 8.9%, and recovery from 100.3% to 103.4%. Plasma lactate was measured using a YSI STAT2300 analyzer (Yellow Springs, OH), and serum insulin by radioimmunoassay (Linco Research Inc., St. Charles, MO) in the GCRC Core Laboratory. The remaining assays were performed in the SFGH Clinical Laboratories. Insulin resistance was calculated by homeostasis model assessment (HOMA-IR [29]).
Safety
Serum creatinine and CK levels were monitored biweekly. Study medication was suspended for one week if creatinine levels increased to >1.8 mg/dl or CK to >450 U/liter. If, after one week, creatinine and/or CK levels returned to baseline levels, study medication was resumed at half of the initial dosing level and creatinine and/or CK levels were monitored weekly. If creatinine and/or CK levels again exceeded the aforementioned values, study drug was discontinued but subjects continued on study (including exercise). If subjects complained of soreness, the workload for the affected muscle group(s) was reduced until the soreness resolved.
Statistical Analysis
All safety analyses were performed on an intent-to-treat basis in all 40 subjects who underwent baseline testing. Efficacy analyses were performed in the evaluable subset, defined as the number of subjects assigned to each treatment in whom paired results (baseline to week 2, baseline to week 14) were available. The numbers of subjects in whom these results are available are indicated in each table.
Within-group changes from baseline to week 2 and baseline to week 14 were evaluated using paired t-tests. Absolute changes (week 0 to week 2 and week 0 to week 14) in the creatine group were compared with those in the placebo group using unpaired t-tests, and the 95% confidence interval for the differences between mean changes calculated. All statistical analyses were performed using Statview (SAS Institute Inc. Cary, NC). Data are the mean and (SD). Two-tailed p-values <0.05 were considered statistically significant. Non-parametric analyses were also performed and yielded similar results.
Results
Subjects
Of the 40 subjects (20 creatine, 20 placebo) who underwent baseline testing and received at least one dose of study medication, 25% were African-American, 15% Hispanic, and 60% Caucasian. There were no differences between groups in age, racial distribution, BMI, CD4 count, years of known HIV infection, or in the classes of ART used (Table 1).
Table 1. Baseline Characteristics of the Study Population.
| Creatine | Placebo | |
| N | 20 | 20 |
| Age (yrs) | 44 (9) | 44 (8) |
| BMI (kg/m2) | 23.7 (2.6) | 23.7 (2.5) |
| Total body fat (%) | 18.4 (6.7) | 16.0 (5.3) |
| Testosterone (ng/dl) | 725 (295) | 597 (217) |
| Yrs since HIV diagnosis | 10 (7) | 10 (5) |
| CD4 (cells/µl) | 448 (310) | 460 (278) |
| Current antiretroviral therapy: | ||
| PI (%) | 40 | 35 |
| NRTI (%) | 80 | 75 |
| NNRTI (%) | 30 | 30 |
| No Antiretroviral (%) | 20 | 35 |
Subject characteristics at baseline according to the assigned study medication. Data are mean (SD).
Abbreviations: PI, protease inhibitor; NRTI, nucleoside analogue reverse transcriptase inhibitors; NNRTI, non-nucleoside analogue reverse transcriptase inhibitors.
Follow-up and Adherence to Study Treatments
Thirty-three subjects (83%) completed the study. Seven subjects (3 creatine, 4 placebo) withdrew for the following reasons: hypersensitivity reaction (1 creatine), schedule conflicts (2 creatine), family emergency (1 placebo), and non-adherence (missing three consecutive PRT sessions [3 placebo]). Subjects who completed the study attended 95% of their training sessions. Adherence to the treatment regimen was evidenced by the fact that plasma creatine levels increased substantially in all subjects who were receiving creatine at week 14 (see below).
Effects on muscle strength
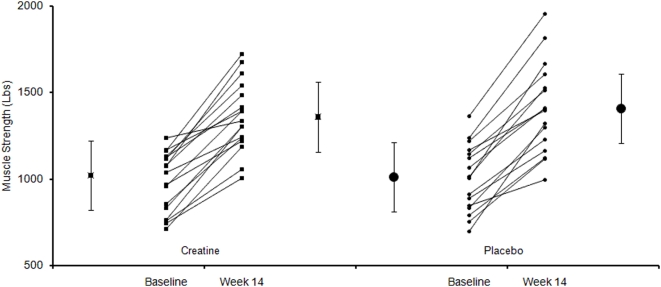
At baseline, all measures of strength were similar in the two study groups (Table 2). At week 14, average 1RM strength increased in all muscle groups (Table 2, Fig. 2). Contrary to our hypothesis, the magnitude of the increase in strength was not greater with creatine (average increase 44% for creatine and 42% for placebo, difference 2%, 95% CI −9.5% to 13.9%; P = 0.58). In addition, the rate of strength gain did not differ between groups. Dorsiflexor muscle isometric strength (MVC) did not change significantly in either group (Table 2).
Table 2. Muscle Strength and Energetics at Baseline and Changes on Study.
| Baseline | Changes wk 0 to wk 2 | Changes wk 0 to wk 14 | Differences between groups Mean changes (95% CI) | |||||
| Creatine | Placebo | Creatine | Placebo | Creatine | Placebo | Wk 0 to Wk 2 | Wk 0 to Wk 14 | |
| Strength in individual muscle groups: | ||||||||
| N | 20 | 20 | 19 | 19 | 17 | 16 | N/A | N/A |
| Ankle Dorsiflexion (lbs) | 90 (21) | 101 (21) | 5 (8) | 6 (12) | 60 (26)* | 59 (27)* | −1 (8 to 5) | 1 (−18 to 20) |
| Ankle Plantar Flexion (lbs) | 176 (44) | 174 (37) | 15 (21) | 6 (17) | 80 (39)* | 68 (54)* | 9 (−3 to 22) | 12 (−22 to 45) |
| Ankle Plantar Flexion Bent Knee (lbs) | 207 (71) | 176 (56) | 10 (19) | 23 (29) | 64 (46)* | 106 (67)* | −13 (−29 to 3) | −42 (−83 to 1) |
| Leg Presses (lbs) | 248 (55) | 245 (54) | 2 (17) | 14 (29) | 66 (56)* | 75 (49)* | −12 (−28 to 4) | −9 (−47 to 27) |
| Leg Curls (lbs) | 106 (26) | 113 (22) | 6 (10) | 4 (14) | 43 (22)* | 38 (27)* | 3 (−5 to 11) | 5 (−13 to 22) |
| Pec Deck (lbs) | 88 (23) | 94 (26) | 3 (9) | 3 (6) | 35 (15)* | 32 (14)* | 0 (−5 to 4) | 2 (−8 to 12) |
| Biceps Pushdown (lbs) | 51 (14) | 51 (12) | 1 (4) | 1 (6) | 14 (10)* | 12 (9)* | 0 (−4 to 4) | 2 (−5 to 9) |
| Triceps Curls (lbs) | 53 (10) | 54 (11) | 2 (3) | 1 (6) | 16 (8)* | 14 (8)* | 1 (−2 to 4) | 2 (−4 to 7) |
| Abdominal Crunches (rep) | 19 (5) | 17 (5) | 1 (2) | 1 (3) | 8 (4)* | 9 (5)* | 0 (−1 to 2) | −1 (−4 to 2) |
| MVC (N) | 241 (53) | 240 (61) | −1 (67) | 27 (51) | 9 (38) | 39 (50) | −28 (−76 to 9) | −30 (−65 to 5) |
| Lower Leg Flexion | (N = 16) | (N = 18) | (N = 12) | (N = 14) | (N = 13) | (N = 14) | ||
| Muscle function (31P MRS): 15-second maximal voluntary contraction (MVC) protocol: | ||||||||
| N | 17 | 17 | 13 | 12 | 14 | 13 | N/A | N/A |
| At rest (before MVC): | ||||||||
| PCr (mM) | 37 (2) | 36 (3) | −1 (3) | 0 (3) | 0 (3) | 0 (2) | −1 (−3 to 1) | 0 (−2 to 2) |
| Pi (mM) | 5.8 (2.3) | 6.1 (2.6) | 0.7 (2.9) | −0.4 (2.6) | 0.2 (2.9) | 0.2 (2.4) | 1.1 (−1.2 to 3.5) | 0.1 (−2.0 to 2.2) |
| PCr/Pi | 7.6 (3.7) | 7.3 (3.6) | −0.3 (6.1) | 0.4 (4.8) | 0.5 (6.8) | −1.1 (4.0) | −0.7 (−5.3 to 3.8) | 1.6 (−2.9 to 6.1) |
| pH | 7.15(0.06) | 7.12(0.05) | −0.04(0.07) | −0.02(0.06) | −0.03(0.06) | −0.02(0.05) | −0.02(−0.08 to 0.03) | −0.01(−0.06 to 0.03) |
| Immediately following a 15-sec MVC: | ||||||||
| PCr (mM) | 18 (4) | 16 (5) | −2 (6) | −1 (5) | −2 (5) | −1 (8) | −1 (−5 to 4) | −1 (−6 to 5) |
| % of resting PCr | 48 (11) | 44 (13) | −4 (15) | −4 (10) | −4 (12) | −3 (21) | 0 (−11 to 10) | −1 (−14 to 13) |
| Pi (mM) | 23 (6) | 25 (6) | 2 (8) | 2 (4) | −2 (6) | 0 (8) | 0 (−5 to 6) | −2 (−8 to 4) |
| PCr/Pi | 0.9 (0.5) | 0.7 (0.3) | 0.1 (0.5) | −0.1 (0.3) | 0.0 (0.5) | 0.0 (0.5) | 0.0 (−0.4 to 0.3) | 0.0 (−0.4 to 0.4) |
| pH | 7.09 (0.23) | 7.06 (0.13) | −0.11 (0.26) | 0.03 (0.20) | −0.02 (0.38) | 0.01 (0.13) | −0.14(−0.34 to 0.05) | −0.03(−0.26 to 0.20) |
| PCr recovery (t1/2, sec) | 30 (9) | 29 (12) | −2 (12) | −1 (15) | −4 (9) | −4 (16) | −1 (−12 to 11) | 0 (−10 to 11) |
| Muscle function (31P MRS): 6 minute exercise protocol: | ||||||||
| N | 15 | 15 | 10 | 10 | 12 | 10 | N/A | N/A |
| At rest : | ||||||||
| PCr (mM) | 37 (3) | 37 (2) | −1 (3) | −1 (3) | 0 (2) | 0 (3) | 0 (−3 to 3) | 0 (−2 to 3) |
| Pi (mM) | 5.4 (2.6) | 5.1 (2.4) | 0.9 (3.5) | 0.7 (2.9) | −0.4 (1.9) | −0.1 (3.1) | 0.1 (−2.8 to 3.1) | −0.3 (−2.6 to 2.0) |
| PCr/Pi | 10.0 (8.0) | 9.6 (5.9) | −2.7 (7.6) | −2.1 (4.3) | −0.9 (7.6) | 0.9 (6.5) | −0.6 (−6.4 to 5.2) | −1.8 (−8.2 to 4.6) |
| pH | 7.13(0.08) | 7.09(0.06) | −0.03(0.08) | 0.02(0.10) | 0.02(0.09) | 0.03(0.09) | −0.05(−0.13 to 0.04) | −0.01(−0.09 to 0.07) |
| Immediately following the 6-minute exercise protocol: | ||||||||
| PCr (mM) | 8 (7) | 7 (4) | 2 (6) | −1 (4) | −1 (6) | −1 (5) | 3 (−2 to 7) | 0 (−5 to 5) |
| % of resting PCr | 23 (20) | 19 (11) | 5 (16) | −3 (10) | −2 (15) | −2 (14) | 8 (−5 to 20) | 0 (−13 to 13) |
| Pi (mM) | 33 (8) | 35 (5) | 0 (8) | 2 (4) | 1 (8) | 1 (7) | −2 (−8 to 4) | 0 (−7 to 7) |
| PCr/Pi | 0.4 (0.5) | 0.2 (0.2) | 0.1 (0.3) | −0.1(0.1) | 0.0 (0.4) | 0.0 (0.2) | 0.2 (0.0 to 0.4) | 0.0 (−0.3 to 0.4) |
| pH | 6.67(0.16) | 6.67(0.25) | −0.06 (0.18) | 0.04(0.12) | −0.03 (0.21) | 0.07 (0.13) | −0.02(−0.16 to 0.13) | −0.10(−0.26 to 0.06) |
| PCr recovery (t1/2, sec) | 36 (16) | 46 (18) | −1 (28) | −2 (15) | −1 (17) | −10 (20) | 1 (−21 to 22) | 9 (−7 to 25) |
Data are mean (SD). The mean differences and the 95% confidence intervals between treatment groups of the changes from baseline at weeks 2 and 14 were determined by unpaired t-tests. Asterisks (*) are used to indicate within-group changes from baseline that are statistically significant by paired t-test (P<0.05).
Strength was measured as the 1 repetition maximum (1RM) for each muscle group (in pounds) except for the rectus abdominus, in which strength was assessed as the number of sit-ups completed in 30 seconds; and maximum voluntary contraction (MVC, in Newtons) of the tibialis anterior muscle, which was assessed during dorsiflexion of the right foot. Muscle function was evaluated using two exercise protocols that were performed inside a 1.9T magnet. The first protocol consisted of a 15-second maximum voluntary contraction (MVC) that was used to assess the rate of PCr recovery (an index of oxidative capacity). The second protocol consisted of 36 consecutive MVCs, one every 10 seconds, with cycles of 6 seconds of contraction and 4 seconds of relaxation. This latter protocol was used to assess muscle fatigue.
Abbreviations: Rep, Repetitions; PCr, phosphocreatine; Pi, inorganic phosphate; PCr recovery (t ½), time needed to recover half of the baseline PCr value.
Figure 2. Muscle strength before and after 14 weeks of study.
Large rectangles represent the average values, while smaller rectangles individual data before and after creatine supplementation. Similarly, large circles represent the average values, while smaller circles individual data before and after placebo supplementation. Data are the sum of strength (1 RM) in 8 muscle groups in subjects randomized to receive creatine monohydrate or placebo. Strength increased significantly within each treatment group (P<0.01), but there was no significant difference between groups in the magnitude of the increase (average increase 44 and 42% (difference 2%, 95% CI −9.5% to 13.9%) in creatine and placebo, respectively, P = 0.58).
Effects on muscle energetics and fatigue
All measures of muscle energetics, including muscle oxidative capacity, were similar across groups at all time points, and were not significantly affected by creatine supplementation (Table 2). Likewise, the fatigue profile (the rate of force decline during the 6-minute exercise protocol) did not differ between or within the two groups at any time point (data not shown). Among subjects randomized to creatine, there was a weak association between changes in plasma creatine levels and changes in resting PCr (r = 0.53, P = 0.09) and no relationship with post-exercise PCr. In the two treatment groups combined, PCr recovery following 15-sec MVC improved significantly after PRT (T1/2 = −3.89 [−0.04 to −7.74] seconds, P = 0.047).
Effects on body composition
Body weight and composition were similar at baseline (Table 3). By week 14, LBM had increased in both groups, with a greater increase in the creatine group (P = 0.01). Body weight increased only in the creatine group. No differences between groups were noted in total, trunk or limb fat content at any time point.
Table 3. Body Composition at Baseline and Changes on Study.
| Baseline | Changes wk 0 to wk 2 | Changes wk 0 to wk 14 | Differences between groups Mean changes (95% CI) | |||||
| Creatine | Placebo | Creatine | Placebo | Creatine | Placebo | Wk 0 to Wk 2 | Wk 0 to Wk 14 | |
| N | 20 | 20 | 19 | 19 | 17 | 16 | N/A | N/A |
| DEXA Results (kg) | ||||||||
| Lean body mass | 56.0 (7.3) | 57.4 (6.6) | 0.9 (1.9) | 0.4 (1.2) | 2.3 (1.4)* | 0.9 (1.4)* | 0.5 (−0.5 to 1.5) | 1.4 (0.3 to 2.4) |
| Limb (arm+leg) LBM | 25.5 (3.8) | 26.2 (3.4) | 0.6 (0.9)* | −0.0 (0.7) | 1.1 (0.9)* | 0.7 (1.1)* | 0.7 (0.1 to 1.3) | 0.5 (−0.3 to 1.2) |
| Total body fat | 13.7 (6.4) | 11.8 (4.8) | 0.2 (0.8) | 0.2 (0.7) | 0.3 (1.8) | 0.4 (2.0) | −0.0 (−0.5 to 0.4) | −0.0 (−1.4 to 1.3) |
| Trunk fat | 8.2 (4.0) | 6.7 (3.1) | 0.0 (0.5) | 0.1 (0.5) | 0.0 (1.2) | 0.3 (1.5) | −0.2 (−0.5 to 0.1) | −0.3 (−1.2 to 0.7) |
| Limb (arm+leg) fat | 4.8 (2.2) | 4.5 (1.8) | 0.2 (0.4) | 0.0 (0.3) | 0.2 (0.6) | 0.0 (0.6) | 0.2 (−0.1 to 0.4) | 0.2 (−0.2 to 0.6) |
| MRI Results | ||||||||
| Thigh muscle CSA (cm2) | 136.7 (24.0) | 142.9 (18.3) | 4.3 (4.8) | 0.7 (4.8) | 12.2 (7.8)* | 9.3 (8.1)* | 3.6 (−0.0 to 7.3) | 2.9 (−3.3 to 9.1) |
| Thigh % EMCL | 4.2 (1.3) | 4.9 (2.1) | −0.1 (1.4) | −0.6 (1.6) | −0.1 (1.5) | −1.0 (2.3) | 0.6 (−0.6 to 1.7) | 0.9 (−0.6 to 2.4) |
| Thigh SAT area (cm2) | 36.2 (18.7) | 38.6 (20.3) | −0.8 (2.9) | 2.4 (13.5) | −0.5 (4.1) | 4.7 (14.6) | −3.2 (−10.3 to 3.9) | −5.2 (−13.1 to 2.7) |
| Calf muscle CSA (cm2) | 74.7 (12.7) | 73.9 (13.3) | 0.2 (2.6) | −0.1 (2.8) | 1.5 (3.8) | 0.1 (3.5) | 0.3 (−1.7 to 2.2) | 1.4 (−1.4 to 4.1) |
| Calf % EMCL | 4.7 (1.6) | 4.6 (1.9) | 0.8 (1.7) | 0.5 (1.9) | 1.1 (1.9) | 0.9 (2.0) | 0.3 (−1.0 to 1.6) | 0.2 (−1.2 to 1.7) |
| Calf SAT area (cm2) | 8.2 (5.6) | 7.6 (5.4) | −0.3 (1.6) | 0.6 (2.1) | 0.2 (1.3) | 0.4 (0.7) | −0.9 (−2.2 to 0.4) | −0.2 (−1.0 to 0.6) |
Data are mean (SD). Limb fat is the sum of fat in the arms and legs. The mean differences and 95% confidence intervals between treatment groups of the changes from baseline at weeks 2 and 14 were determined by unpaired t-tests. Differences between groups in changes from baseline that are statistically significant (P<0.05) are highlighted in bold. Asterisks (*) are used to indicate within-group changes from baseline that are statistically significant by paired t-test (P<0.05).
Abbreviations: DEXA, dual energy X-ray absorptiometry; MRI, magnetic resonance imaging; CSA, cross-sectional area; SAT, subcutaneous adipose tissue; EMCL, extramyocellular fat (fat infiltration).
Thigh muscle CSA increased in both groups at week 14, but the magnitude of the increase did not differ significantly between groups. There were no changes in calf CSA in either group. Likewise, no changes in muscle or subcutaneous fat were noted at any time point in either treatment group.
Biochemical and safety measures
There were no significant differences between groups in biochemical parameters at baseline (Table 4). Average plasma creatine levels increased significantly in the creatine group, with no change in those on placebo (P≤0.001 vs. placebo). Serum creatinine concentrations increased more with creatine than placebo (P = 0.001 and 0.002 at weeks 2 and 14, respectively). Triglycerides increased with creatine at week 14 (P = 0.04 vs. placebo). Total cholesterol decreased significantly in the placebo group at week 14, but the difference between groups was not statistically significant. At week 2, both insulin and HOMA-IR decreased transiently in the creatine group (P = 0.04 and 0.05 vs. placebo, respectively). There were no differences in lactate, glucose or CK levels between or within groups at any time point.
Table 4. Safety and Biochemical Measurements at Baseline and Changes on Study.
| Baseline | Changes wk 0 to wk 2 | Changes wk 0 to wk 14 | Differences between groups Mean changes (95% CI) | |||||
| Creatine | Placebo | Creatine | Placebo | Creatine | Placebo | Wk 0 to Wk 2 | Wk 0 to Wk 14 | |
| N | 20 | 20 | 19 | 19 | 17 | 16 | N/A | N/A |
| Plasma creatine (µg/mL) | 4.6 (1.4) | 4.6 (2.2) | 26.0 (25.5)* | −0.4 (1.4) | 22.1 (18.0)* | −1.4 (1.4)* | 26.4 (12.9 to 39.8) | 23.5 (13.6 to 33.4) |
| Serum creatinine (mg/dL) | 1.0 (0.2) | 1.0 (0.1) | 0.2 (0.2) | 0.0 (0.1) | 0.2 (0.2) | 0.1 (0.1) | 0.2 (0.1 to 0.3) | 0.2 (0.1 to 0.3) |
| Creatine kinase (U/L) | 111 (51) | 147 (75) | 20 (57) | −15 (84) | 20 (61) | 8 (64) | 35 (−13 to 84) | 12 (−33 to 57) |
| Triglycerides (mg/dL) | 169 (89) | 222 (199) | 91 (362) | 65 (199) | 78 (165) | −18 (75) | 26 (−166 to 218) | 96 (4 to 188) |
| Cholesterol (mg/dL) | 183 (47) | 185 (46) | 1 (19) | −4 (23) | −6 (22) | −10 (18)* | 4 (−10 to 18) | 4 (−1 to 19) |
| Lactate (mmol/L) | 1.4 (0.7) | 1.1 (0.4) | 0.0 (0.9) | 0.0 (0.5) | 0.0 (1.1) | −0.1 (0.7) | −0.1 (−0.5 to 0.4) | −0.1 (−0.8 to 0.6) |
| Glucose (mg/dL) | 94 (12) | 90 (9) | −2 (12) | −1 (9) | −6 (14) | 0 (8) | −1 (−8 to 6) | −6 (−14 to 3) |
| Insulin (µIU/mL) | 15.9 (5.9) | 14.3 (5.2) | −1.6 (6.1) | 3.0 (6.6) | 1.2 (10.5) | 2.7 (7.3) | −4.6 (−9.1 to −0.2) | −1.5 (−8.1 to 5.1) |
| HOMA-IR | 3.6 (1.2) | 3.3 (1.5) | −0.5 (1.5) | 0.6 (1.5) | 0.2 (2.5) | 0.6 (1.7) | −1.0 (−2.0 to 0.0) | −0.4 (−2.0 to 1.2) |
Data are the mean (SD). All measurements were performed under fasting conditions. The significance of differences between treatment groups in the changes from baseline at weeks 2 and 14 was determined by unpaired t-tests, and differences that are statistically significant (P<0.05) are highlighted in bold. Asterisks (*) are used to indicate within-group changes from baseline that are statistically significant by paired t-test (P<0.05).
Abbreviations: HOMA-IR: Homeostasis model assessment of insulin resistance (20).
To convert to SI units: creatine µg/mL to µmol/L multiply by 7.625; creatinine mg/dL to µmol/L multiply by 88.4; triglycerides mg/dL to mmol/L multiply by 0.01129; cholesterol mg/dL to mmol/L multiply by 0.02586; glucose mg/dL to mmol/L multiply by 0.05551; insulin µIU/mL to pmol/L multiply by 7.175.
With regard to the pre-defined toxicity management guidelines, 4 subjects, all in the creatine group, experienced increases in serum creatinine levels to >1.8 mg/dL. In all four cases, creatinine levels had returned to <1.8 mg/dL at week 14. CK levels increased to >450 U/liter 18 times in 14 subjects (10 on placebo and 4 on creatine). The increases were transient and likely related to exercise, and all had resolved by week 14.
Discussion
In the present study, we found that PRT consistently increased muscle strength in HIV-infected men (Fig. 2). Although subjects receiving creatine supplementation had a greater increase in LBM than those on placebo, our results provided no evidence that creatine augmented the increase in strength derived from PRT. Thus, the increase in LBM in the creatine group was of no measurable functional benefit. Moreover, using 31P-MRS, we found no difference between groups in skeletal muscle energy metabolism at rest, during or following muscle contractions, or in the fatigue profile. Overall, these data indicate that PRT is a highly effective means of increasing strength in individuals with HIV infection, but supplementation with creatine, though safe, confers no additional increase in strength in this population.
Other studies of creatine supplementation have provided mixed results. In studies of healthy volunteers, short-term creatine supplementation (5–30 days) increased muscle size [30] and indices of short-term high intensity exercise performance, including muscle strength and power output [31], [32] as well as intramuscular PCr levels assessed by both 31P-MRS [33] and biopsy [34]. Chronic use of creatine (up to 12 months) has been associated with increases in total body weight [35], [36]. In contrast, other studies have failed to demonstrate a positive effect of creatine supplementation on strength, body composition or muscle energy metabolism [18]–[21]. The responses to creatine supplementation in patients with chronic diseases have been similarly mixed, with some studies showing positive effects [37]–[42] and others showing no benefit [43]–[46].
The pre-treatment PCr concentrations observed in the current study are consistent with those in the same muscle in healthy young and older adults [24], suggesting that no deficiency of PCr existed in this group of HIV-positive subjects. Although other studies have shown that creatine supplementation increased PCr levels in healthy adults with normal baseline levels [47] we saw no such increase in our subjects with HIV infection. We followed a protocol for creatine loading and maintenance that had been successfully applied in previous studies [13]. In view of the potential role of carbohydrate availability and insulin in stimulating the transport of creatine into muscle cells [22], we provided subjects with beverages containing 20 g of carbohydrate for consumption with each dose of study medication. Importantly, we raised plasma creatine to levels seen in previous studies [48]. Nonetheless, we saw no change in intramuscular PCr concentration at rest or following exercise. It is possible that factors specific to HIV infection or its therapies may account for this failure. For example, some HIV protease inhibitors inhibit glucose uptake by skeletal muscle [49], [50] and might have also interfered with creatine uptake into muscle. In addition use of another antiretroviral agent, zidovudine, has been associated with an accelerated rate of depletion of PCr during exercise in HIV-infected subjects [51] and may have had a similar effect in our population. An assessment of the effects of specific antiretroviral medications on outcome measures was not possible in view of the relatively small size of our study.
In our study, administration of creatine for two weeks prior to the exercise intervention was associated with a modest increase in thigh CSA that approached a level of statistical significance (p = 0.06 vs. placebo). Likewise, subjects in the creatine group had a significantly greater increase in LBM following exercise training (p = 0.01 vs. placebo). Since these changes were not accompanied by increases in muscle strength or improvement in indices of muscle fatigue or mitochondrial energy metabolism, it is conceivable that the increases in CSA and LBM could be explained by increases in muscle water content, rather than functional muscle mass. Indeed, increases in muscle water content during creatine treatment have been reported in prior studies [52]. An increase in water content in muscle may precede increases in muscle protein synthesis, which may confer functional (fitness) and physiological (e.g. glucose disposal) benefits with a longer period of follow-up. Moreover, increasing muscle size may be considered to be desirable in persons using creatine and exercise for aesthetic purposes.
All subjects showed robust increases in strength in eight important muscle groups following 12 weeks of PRT. These results provide further evidence of the beneficial effects of PRT in individuals with HIV infection, as has been reported previously in smaller studies [2]–[10]. In addition, the rate of PCr recovery following a 15-second MVC improved in the group as a whole. At baseline, average PCr recovery rate (T1/2) was 29 seconds, which is approximately 24% slower than those of healthy young and old adults studied with the same apparatus and using comparable methodology [24]. Following training, PCr recovery rates approached those in healthy untrained adults (T½≈23 seconds[24]). Because PCr recovery under the conditions applied in the present study reflects the capacity for oxidative phosphorylation, these data suggest that PRT improved mitochondrial energy metabolism in patients with HIV infection. Overall, these benefits provide further evidence that persons with HIV infection can adapt to a demanding exercise-training program.
Because HIV-infected persons on ART are at risk of developing metabolic and morphologic abnormalities, including peripheral lipoatrophy [53], we examined the effects of creatine supplementation and PRT on fat distribution and fasting lipids and glucose homeostasis. The observed preservation of subcutaneous fat during PRT suggests that this type of exercise did not promote or exacerbate lipoatrophy. Overall, we saw no evidence of improvement in metabolic and morphologic outcomes with either creatine supplementation or exercise. Because renal function is impaired in some patients with HIV infection [54], clinicians should be aware of the possibility that elevations in serum creatinine may occur in patients using creatine supplementation.
In summary, in this randomized, placebo-controlled trial of the effects of progressive resistance training with and without creatine supplementation in HIV-positive subjects, significant gains in strength were achieved with 12 weeks of PRT with no further benefit from creatine. The medical use of creatine supplementation in this population may be limited to aesthetic purposes, rather than to improve functional capacity. However, the efficacy and safety of PRT demonstrates its potential therapeutic benefit in preventing or reversing muscle weakness.
Supporting Information
CONSORT Checklist
(0.06 MB DOC)
Trial Protocol
(0.10 MB DOC)
Acknowledgments
We wish to thank Sid Shastri of Jarrow Universal Herbs, Inc., who generously provided creatine and placebo capsules. We are also grateful to our colleagues for assistance with data collection and analyses: Kirsten Johansen, MD, Gustavo del Puerto, Ian Lanza, MS, Danielle Wigmore, MS, Peyton Jacob, PhD, Michael Wen, MS, and Clara Shayevich, NP; and Barbara Chang and Joy Hirai of the SFGH GCRC core lab.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was performed under a grant from the National Center for Complementary and Alternative Medicine of the National Institutes of Health (AT00491, DK45833, DK54615, RR-00083). Creatine monohydrate and placebo were donated by Jarrow Universal Herbs, Inc., but they provided neither funds for this study nor any input on data analysis.
References
- 1.Grinspoon S, Mulligan K. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003;36:S69–78. doi: 10.1086/367561. [DOI] [PubMed] [Google Scholar]
- 2.Shevitz AH, Wilson IB, McDermott AY, Spiegelman D, Skinner SC, et al. A comparison of the clinical and cost-effectiveness of 3 intervention strategies for AIDS wasting. J Acquir Immune Defic Syndr. 2005;38:399–406. doi: 10.1097/01.qai.0000152647.89008.2b. [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon S, Corcoran C, Parlman K, Costello M, Rosenthal D, et al. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting. A randomized, controlled trial. Ann Intern Med. 2000;133:348–355. doi: 10.7326/0003-4819-133-5-200009050-00010. [DOI] [PubMed] [Google Scholar]
- 4.Rigsby LW, Dishman RK, Jackson AW, Maclean GS, Raven PB. Effects of exercise training on men seropositive for the human immunodeficiency virus-1. Med Sci Sports Exerc. 1992;24:6–12. [PubMed] [Google Scholar]
- 5.Lox CL, McAuley E, Tucker RS. Aerobic and resistance exercise training effects on body composition, muscular strength, and cardiovascular fitness in an HIV-1 population. Int J Behav Med. 1996;3:55–69. doi: 10.1207/s15327558ijbm0301_5. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Storer TW. Exercise regimens for men with HIV. Jama. 2000;284:175–176. [PubMed] [Google Scholar]
- 7.Yarasheski KE, Tebas P, Stanerson B, Claxton S, Marin D, et al. Resistance exercise training reduces hypertriglyceridemia in HIV-infected men treated with antiviral therapy. J Appl Physiol. 2001;90:133–138. doi: 10.1152/jappl.2001.90.1.133. [DOI] [PubMed] [Google Scholar]
- 8.Roubenoff R, Wilson IB. Effect of resistance training on self-reported physical functioning in HIV infection. Med Sci Sports Exerc. 2001;33:1811–1817. doi: 10.1097/00005768-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Robinson FP, Quinn LT, Rimmer JH. Effects of high-intensity endurance and resistance exercise on HIV metabolic abnormalities: a pilot study. Biol Res Nurs. 2007;8:177–185. doi: 10.1177/1099800406295520. [DOI] [PubMed] [Google Scholar]
- 10.Dolan SE, Frontera W, Librizzi J, Ljungquist K, Juan S, et al. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med. 2006;166:1225–1231. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DA, Perry PJ. The efficacy of ergogenic agents in athletic competition. Part I: Androgenic-anabolic steroids. Ann Pharmacother. 1992;26:520–528. doi: 10.1177/106002809202600414. [DOI] [PubMed] [Google Scholar]
- 12.Smith DA, Perry PJ. The efficacy of ergogenic agents in athletic competition. Part II: Other performance-enhancing agents. Ann Pharmacother. 1992;26:653–659. doi: 10.1177/106002809202600510. [DOI] [PubMed] [Google Scholar]
- 13.Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol. 2003;94:651–659. doi: 10.1152/japplphysiol.00755.2002. [DOI] [PubMed] [Google Scholar]
- 14.Mujika I, Padilla S. Creatine supplementation as an ergogenic aid for sports performance in highly trained athletes: a critical review. Int J Sports Med. 1997;18:491–496. doi: 10.1055/s-2007-972670. [DOI] [PubMed] [Google Scholar]
- 15.Kilduff LP, Vidakovic P, Cooney G, Twycross-Lewis R, Amuna P, et al. Effects of creatine on isometric bench-press performance in resistance-trained humans. Med Sci Sports Exerc. 2002;34:1176–1183. doi: 10.1097/00005768-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Gilliam JD, Hohzorn C, Martin D, Trimble MH. Effect of oral creatine supplementation on isokinetic torque production. Med Sci Sports Exerc. 2000;32:993–996. doi: 10.1097/00005768-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Izquierdo M, Ibanez J, Gonzalez-Badillo JJ, Gorostiaga EM. Effects of creatine supplementation on muscle power, endurance, and sprint performance. Med Sci Sports Exerc. 2002;34:332–343. doi: 10.1097/00005768-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Culpepper RM. Creatine supplementation: safe as steak? South Med J. 1998;91:890–892. doi: 10.1097/00007611-199809000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Snow RJ, McKenna MJ, Selig SE, Kemp J, Stathis CG, et al. Effect of creatine supplementation on sprint exercise performance and muscle metabolism. J Appl Physiol. 1998;84:1667–1673. doi: 10.1152/jappl.1998.84.5.1667. [DOI] [PubMed] [Google Scholar]
- 20.Kinugasa R, Akima H, Ota A, Ohta A, Sugiura K, et al. Short-term creatine supplementation does not improve muscle activation or sprint performance in humans. Eur J Appl Physiol. 2004;91:230–237. doi: 10.1007/s00421-003-0970-8. [DOI] [PubMed] [Google Scholar]
- 21.Syrotuik DG, Bell GJ. Acute creatine monohydrate supplementation: a descriptive physiological profile of responders vs. nonresponders. J Strength Cond Res. 2004;18:610–617. doi: 10.1519/12392.1. [DOI] [PubMed] [Google Scholar]
- 22.Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol. 1996;271:E821–826. doi: 10.1152/ajpendo.1996.271.5.E821. [DOI] [PubMed] [Google Scholar]
- 23.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964–972. [PubMed] [Google Scholar]
- 24.Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- 25.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- 26.Karatzaferi C, Giakas G, Ball D. Fatigue profile: a numerical method to examine fatigue in cycle ergometry. Eur J Appl Physiol Occup Physiol. 1999;80:508–510. doi: 10.1007/s004210050626. [DOI] [PubMed] [Google Scholar]
- 27.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 28.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–870. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Hespel P, Op't Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, et al. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536:625–633. doi: 10.1111/j.1469-7793.2001.0625c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volek JS, Ratamess NA, Rubin MR, Gomez AL, French DN, et al. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur J Appl Physiol. 2004;91:628–637. doi: 10.1007/s00421-003-1031-z. [DOI] [PubMed] [Google Scholar]
- 32.Volek JS, Kraemer WJ, Bush JA, Boetes M, Incledon T, et al. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J Am Diet Assoc. 1997;97:765–770. doi: 10.1016/S0002-8223(97)00189-2. [DOI] [PubMed] [Google Scholar]
- 33.Smith SA, Montain SJ, Matott RP, Zientara GP, Jolesz FA, et al. Effects of creatine supplementation on the energy cost of muscle contraction: a 31P-MRS study. J Appl Physiol. 1999;87:116–123. doi: 10.1152/jappl.1999.87.1.116. [DOI] [PubMed] [Google Scholar]
- 34.Greenhaff PL, Bodin K, Soderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am J Physiol. 1994;266:E725–730. doi: 10.1152/ajpendo.1994.266.5.E725. [DOI] [PubMed] [Google Scholar]
- 35.Earnest CP, Snell PG, Rodriguez R, Almada AL, Mitchell TL. The effect of creatine monohydrate ingestion on anaerobic power indices, muscular strength and body composition. Acta Physiol Scand. 1995;153:207–209. doi: 10.1111/j.1748-1716.1995.tb09854.x. [DOI] [PubMed] [Google Scholar]
- 36.Kreider RB, Ferreira M, Wilson M, Grindstaff P, Plisk S, et al. Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Komura K, Hobbiebrunken E, Wilichowski EK, Hanefeld FA. Effectiveness of creatine monohydrate in mitochondrial encephalomyopathies. Pediatr Neurol. 2003;28:53–58. doi: 10.1016/s0887-8994(02)00469-1. [DOI] [PubMed] [Google Scholar]
- 38.Tarnopolsky MA, Roy BD, MacDonald JR. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve. 1997;20:1502–1509. doi: 10.1002/(sici)1097-4598(199712)20:12<1502::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 39.Gordon A, Hultman E, Kaijser L, Kristjansson S, Rolf CJ, et al. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res. 1995;30:413–418. [PubMed] [Google Scholar]
- 40.Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62:1771–1777. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- 41.Walter MC, Lochmuller H, Reilich P, Klopstock T, Huber R, et al. Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study. Neurology. 2000;54:1848–1850. doi: 10.1212/wnl.54.9.1848. [DOI] [PubMed] [Google Scholar]
- 42.Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, et al. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS ONE. 2007;2:e991. doi: 10.1371/journal.pone.0000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarnopolsky M, Mahoney D, Thompson T, Naylor H, Doherty TJ. Creatine monohydrate supplementation does not increase muscle strength, lean body mass, or muscle phosphocreatine in patients with myotonic dystrophy type 1. Muscle Nerve. 2004;29:51–58. doi: 10.1002/mus.10527. [DOI] [PubMed] [Google Scholar]
- 44.Kornblum C, Schroder R, Muller K, Vorgerd M, Eggers J, et al. Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial DNA deletions: a placebo-controlled, double-blind 31P-MRS crossover study. Eur J Neurol. 2005;12:300–309. doi: 10.1111/j.1468-1331.2004.00970.x. [DOI] [PubMed] [Google Scholar]
- 45.Walter MC, Reilich P, Lochmuller H, Kohnen R, Schlotter B, et al. Creatine monohydrate in myotonic dystrophy: a double-blind, placebo-controlled clinical study. J Neurol. 2002;249:1717–1722. doi: 10.1007/s00415-002-0923-x. [DOI] [PubMed] [Google Scholar]
- 46.Zange J, Kornblum C, Muller K, Kurtscheid S, Heck H, et al. Creatine supplementation results in elevated phosphocreatine/adenosine triphosphate (ATP) ratios in the calf muscle of athletes but not in patients with myopathies. Ann Neurol. 2002;52:126; author reply 126–127. doi: 10.1002/ana.10197. [DOI] [PubMed] [Google Scholar]
- 47.Rawson ES, Clarkson PM, Price TB, Miles MP. Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol Scand. 2002;174:57–65. doi: 10.1046/j.1365-201x.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 48.Derave W, Marescau B, Vanden Eede E, Eijnde BO, De Deyn PP, et al. Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol. 2004;97:852–857. doi: 10.1152/japplphysiol.00206.2004. [DOI] [PubMed] [Google Scholar]
- 49.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 50.Nolte LA, Yarasheski KE, Kawanaka K, Fisher J, Le N, et al. The HIV protease inhibitor indinavir decreases insulin- and contraction-stimulated glucose transport in skeletal muscle. Diabetes. 2001;50:1397–1401. doi: 10.2337/diabetes.50.6.1397. [DOI] [PubMed] [Google Scholar]
- 51.Sinnwell TM, Sivakumar K, Soueidan S, Jay C, Frank JA, et al. Metabolic abnormalities in skeletal muscle of patients receiving zidovudine therapy observed by 31P in vivo magnetic resonance spectroscopy. J Clin Invest. 1995;96:126–131. doi: 10.1172/JCI118012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balsom PD, Soderlund K, Ekblom B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994;18:268–280. doi: 10.2165/00007256-199418040-00005. [DOI] [PubMed] [Google Scholar]
- 53.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 54.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Checklist
(0.06 MB DOC)
Trial Protocol
(0.10 MB DOC)