Abstract
The virulence of Pseudomonas aeruginosa and other pseudomonads was examined in a burned mouse model. P. aeruginosa M-2 was highly virulent causing 100% mortality by 38 h with an injection of 10(2) CFU by either a subcutaneous or intraperitoneal route. Subcutaneous injection of 10(2) CFU revealed rapid multiplication of the bacteria at the burn wound with 10(8) CFU/g detectable in the burned skin by 28 h postinjection, 10(5) CFU/g of liver, and 10(3) CFU/ml of blood. Non-P. aeruginosa clinical isolates were markedly less virulent; an injection of greater than or equal to 10(7) CFU caused less than or equal to 60% lethality. P. cepacia SMH colonized the burned skin of thermally injured mice, persisting at levels of 10(7) to 10(8) CFU/g of burned skin after an initial injection of 10(5) CFU. P. cepacia persisted in the burn wound for at least 3 weeks. No organ invasion was detectable throughout this period. Studies with an additional clinical isolate of P. cepacia yielded similar results. An injection of a 10(2) CFU dose revealed that the level of persistence is dose dependent. Results suggest that the tenacious persistence of P. cepacia in the burn wound may provide a model for the study of persistent colonization and infection in a compromised host.
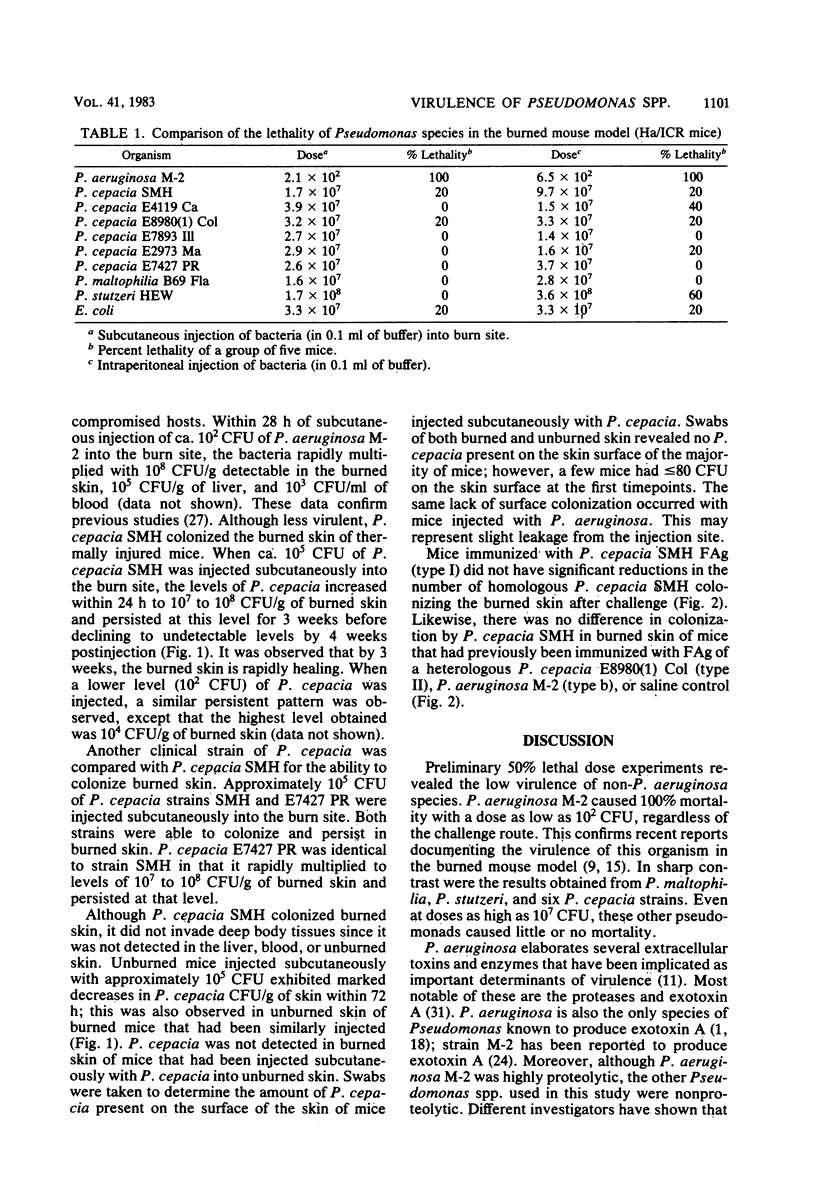
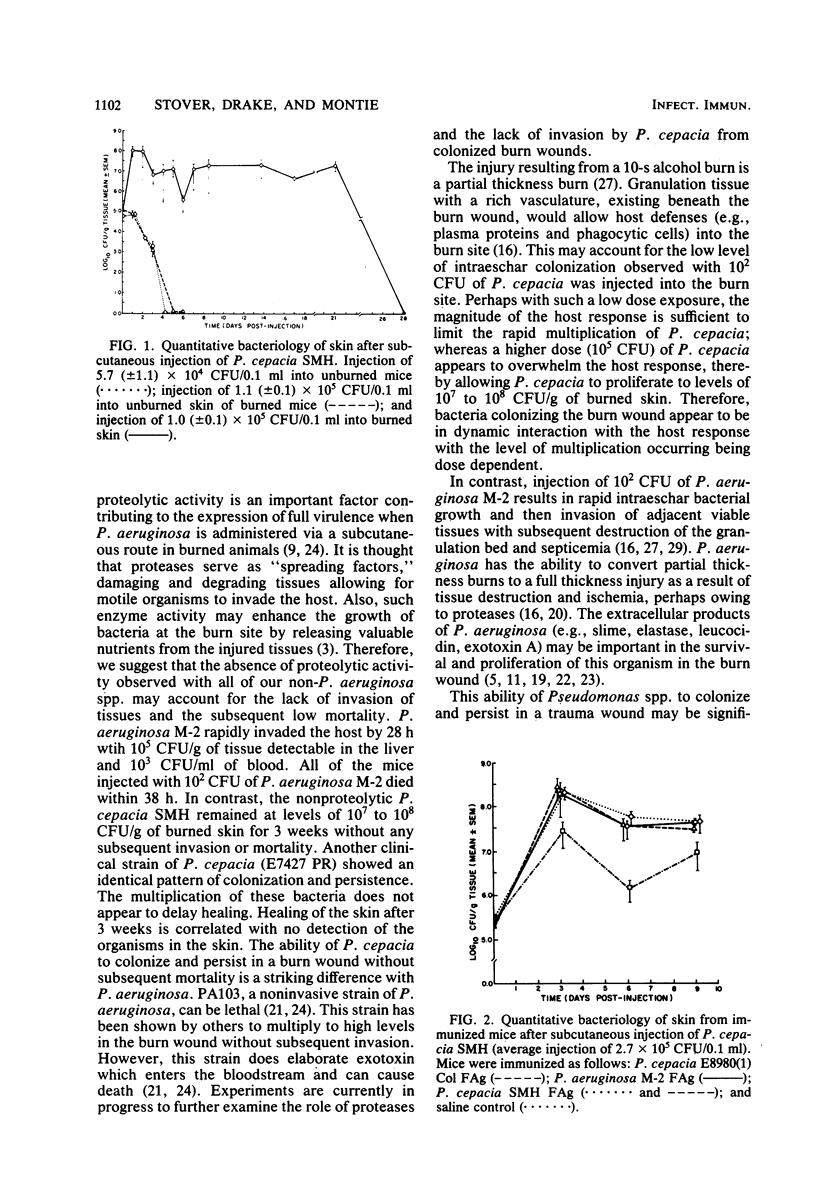
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorn M. J., Vasil M. L., Sadoff J. C., Iglewski B. H. Incidence of exotoxin production by Pseudomonas species. Infect Immun. 1977 Apr;16(1):362–366. doi: 10.1128/iai.16.1.362-366.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Favero M. S., Bond W. W., Petersen N. J. Morphological, biochemical, and growth characteristics of pseudomonas cepacia from distilled water. Appl Microbiol. 1973 Mar;25(3):476–483. doi: 10.1128/am.25.3.476-483.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitracopoulos G., Sensakovic J. W., Bartell P. F. Slime of Pseudomonas aeruginosa: in vivo production. Infect Immun. 1974 Jul;10(1):152–156. doi: 10.1128/iai.10.1.152-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Pseudomonas in the hospital. Hosp Pract. 1976 Feb;11(2):63–70. doi: 10.1080/21548331.1976.11706501. [DOI] [PubMed] [Google Scholar]
- Gelbart S. M., Reinhardt G. F., Greenlee H. B. Pseudomonas cepacia strains isolated from water reservoirs of unheated nebulizers. J Clin Microbiol. 1976 Jan;3(1):62–66. doi: 10.1128/jcm.3.1.62-66.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari T., Reyes M. P., Brooks N. Pseudomonas cepacia septic arthritis due to intra-articular injections of methylprednisolone. Can Med Assoc J. 1977 Jun 4;116(11):1230–1235. [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Craven R. C., Holder I. A. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect Immun. 1982 Jan;35(1):281–288. doi: 10.1128/iai.35.1.281-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Doyle-Huntzinger D., Craven R. C., Holder I. A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect Immun. 1982 Dec;38(3):1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan P., Holder I. A., MacMillan B. G. Burn wounds: microbiology, local host defenses, and current therapy. CRC Crit Rev Clin Lab Sci. 1973 Jul;4(1):61–100. doi: 10.3109/10408367309151684. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Iglewski B. H., Pollack M. Mechanism of action of Pseudomonas aeruginosa exotoxin A in experimental mouse infections: adenosine diphosphate ribosylation of elongation factor 2. Infect Immun. 1978 Jan;19(1):29–33. doi: 10.1128/iai.19.1.29-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Anderson S. E., Jr Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect Immun. 1978 Mar;19(3):1092–1096. doi: 10.1128/iai.19.3.1092-1096.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Taylor N. S., Callahan L. T., 3rd Exotoxin production by clinical isolates of pseudomonas aeruginosa. Infect Immun. 1977 Mar;15(3):776–780. doi: 10.1128/iai.15.3.776-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger C. B., Snell K., Holder I. A. Experimental studies on the pathogenesis of infections due to Pseudomonas aeruginosa: direct evidence for toxin production during Pseudomonas infection of burned skin tissues. J Infect Dis. 1977 Oct;136(4):555–561. doi: 10.1093/infdis/136.4.555. [DOI] [PubMed] [Google Scholar]
- Scharmann W. Formation and isolation of leucocidin from Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):283–291. doi: 10.1099/00221287-93-2-283. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Hashman N., Reinherz G., Merzbach D. Nosocomial Pseudomonas cepacia infection associated with chlorhexidine contamination. Am J Med. 1982 Aug;73(2):183–186. doi: 10.1016/0002-9343(82)90176-0. [DOI] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- TEPLITZ C., DAVIS D., MASON A. D., Jr, MONCRIEF J. A. PSEUDOMONAS BURN WOUND SEPSIS. I PATHOGENESIS OF EXPERIMENTAL PSEUDOMONAS BURN WOUND SEPSIS. J Surg Res. 1964 May;4:200–216. doi: 10.1016/s0022-4804(64)80026-3. [DOI] [PubMed] [Google Scholar]
- Taplin D., Bassett D. C., Mertz P. M. Foot lesions associated with Pseudomonas cepacia. Lancet. 1971 Sep 11;2(7724):568–571. doi: 10.1016/s0140-6736(71)92150-7. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. The role of proteases and exotoxin A in the pathogenicity of Pseudomonas aeruginosa infections. Scand J Infect Dis Suppl. 1981;29:13–19. [PubMed] [Google Scholar]
- Zuravleff J. J., Yu V. L. Infections caused by Pseudomonas maltophilia with emphasis on bacteremia: case reports and a review of the literature. Rev Infect Dis. 1982 Nov-Dec;4(6):1236–1246. doi: 10.1093/clinids/4.6.1236. [DOI] [PubMed] [Google Scholar]


