Abstract
Background
Heterophyllous aquatic plants show marked phenotypic plasticity. They adapt to environmental changes by producing different leaf types: submerged, floating and terrestrial leaves. By contrast, homophyllous plants produce only submerged leaves and grow entirely underwater. Heterophylly and submerged homophylly evolved under selective pressure modifying the species-specific optima for photosynthesis, but little is known about the evolutionary outcome of habit. Recent evolutionary analyses suggested that rbcL, a chloroplast gene that encodes a catalytic subunit of RuBisCO, evolves under positive selection in most land plant lineages. To examine the adaptive evolutionary process linked to heterophylly or homophylly, we analyzed positive selection in the rbcL sequences of ecologically diverse aquatic plants, Japanese Potamogeton.
Principal Findings
Phylogenetic and maximum likelihood analyses of codon substitution models indicated that Potamogeton rbcL has evolved under positive Darwinian selection. The positive selection has operated specifically in heterophyllous lineages but not in homophyllous ones in the branch-site models. This suggests that the selective pressure on this chloroplast gene was higher for heterophyllous lineages than for homophyllous lineages. The replacement of 12 amino acids occurred at structurally important sites in the quaternary structure of RbcL, two of which (residue 225 and 281) were identified as potentially under positive selection.
Conclusions/Significance
Our analysis did not show an exact relationship between the amino acid replacements and heterophylly or homophylly but revealed that lineage-specific positive selection acted on the Potamogeton rbcL. The contrasting ecological conditions between heterophyllous and homophyllous plants have imposed different selective pressures on the photosynthetic system. The increased amino acid replacement in RbcL may reflect the continuous fine-tuning of RuBisCO under varying ecological conditions.
Introduction
Many aquatic plants exhibit marked developmental plasticity, known as heterophylly. Heterophylly occurs widely across distantly related taxa and is thought to have arisen via convergent evolution [1]. Heterophyllous species have broad, thick leaves with stomata on the water surface (floating leaves), as well as elongated, thin leaves without stomata within the water column growing from submerged shoots (submerged leaves). In addition, they sometimes produce terrestrial leaves from land shoots during droughts. In contrast, homophyllous aquatic plants produce only submerged leaves and grow entirely underwater. Each leaf type appears to be adapted to its environment morphologically and anatomically [2]–[4]. Moreover, the submerged and floating leaves have different photosynthetic properties [5]. The degree of heterophylly (the shapes of the submerged, floating, and terrestrial leaves) varies from population to population, depending on the water level fluctuation in each habitat [6], [7]. Therefore, heterophylly and submerged homophylly evolved under selective pressure modifying the species-specific optima for photosynthesis.
Potamogeton L. (Potamogetonaceae), one of the largest genera of aquatic angiosperms, is ecologically diverse and distributed in various freshwater bodies (lakes, marshes, rivers, artificial ponds, etc.), as well as in brackish water [4], [8]. The genus includes both heterophyllous and homophyllous (strictly submerged) species. Recent molecular phylogenetic analyses of Potamogeton revealed that homophylly is the ancestral state and heterophylly has evolved several times in different lineages, possibly due to parallel evolution [9], [10]. Furthermore, we have reported that the degree of heterophylly in natural hybrids depends on the maternal type in reciprocal crosses [11]. The parental taxa of the hybrid are closely related but differ ecologically; P. malaianus Miq. can survive droughts by producing terrestrial shoots and is frequently distributed in shallow and inshore areas; P. perfoliatus L. generally lacks such phenotypic plasticity, grows in deeper water, and is more shade tolerant. Despite the high similarity of the trnT-trnL intergenic region [9], all three substitutions between rbcL of the parental species are nonsynonymous [11]. Even single amino acid replacements in RbcL could account for differences in the CO2 and O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) [12]. The maternal effect on survival under drought stress and the depth distribution of reciprocal hybrids [11] has led to speculation that the amino acid difference in RbcL plays an important role in ecological adaptation.
RbcL provides all the catalytically essential residues of RuBisCO, a critical enzyme for both the reductive and oxidative photosynthetic carbon cycles. The activity of RuBisCO is thought to be limited by environmental stresses, such as oxidative stress, heat, osmotic stress, and drought [13]–[15]. The holoenzyme consists of eight large catalytic subunits (RbcLs) and eight small subunits (RbcSs). The sequence of rbcL has great phylogenetic utility because of its conserved nature [16], although substitutions occur in sites of known functional importance [17], [18]. The amino acid replacements in RbcL at the interface of subunits are correlated with the loss and gain of pyrenoids in the unicellular green alga lineage [19]. In addition, positive Darwinian selection of rbcL has been detected in cyanobacteria [20] and various taxonomic groups of land plants [21]–[23].
Potamogeton is a representative example of the adaptive evolution of heterophylly. Japanese Potamogeton taxa are highly diversified in growth forms and leaf types and represent 11 of the 14 species groups in the genus Potamogeton [9], [24]. In this study, to gain insight into whether an adaptive evolutionary process is linked to heterophylly or homophylly in Japanese Potamogeton, we tested whether there was positive selection in rbcL using phylogenetic and maximum likelihood analyses of codon substitution models [25]–[27]. We demonstrated that the evolution of ecologically divergent Japanese Potamogeton has involved the molecular adaptation of rbcL. In this article, abstract is also available in Japanese (Text S1).
Results
Evidence for positive selection in Potamogeton rbcL
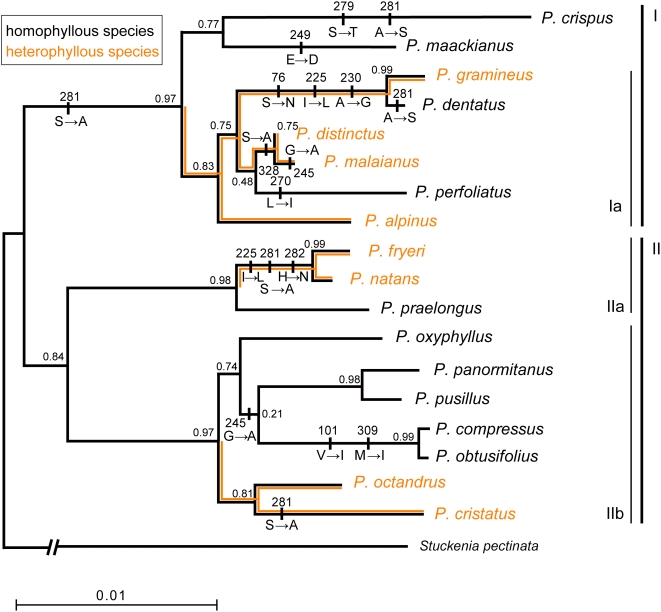
Three genes (rbcL, atpB, petA) were sequenced from 18 Japanese Potamogeton. All the nucleotide sequences determined in this study were deposited in GenBank (Table S1). The phylogenetic trees based on individual chloroplast genes were consistent with each other and with a previous phylogenetic study [9]. A robust gene tree of chloroplast DNA is essential to conduct a maximum likelihood analysis in the molecular adaptation test. We combined all four datasets (1349 rbcL, 1467 atpB, 918 petA, and 660 trnT-trnL intergenic spacer sites) to obtain a well-resolved gene tree of the Potamogeton chloroplast DNA (cpDNA tree; Fig. 1). The basal branches of the cpDNA tree were congruent with those found in a previous study of the trnT-trnL intergenic spacer [9]. The Japanese Potamogeton species were divided into group I, with broad submerged leaves, and group II, with linear submerged leaves and subepidermal bundles. The latter was further divided into subgroups IIa and IIb (Fig. 1). In addition, newly resolved terminal branches identified several species groups (Fig. 1), each of which was characterized by morphological features [9], [28], [29]. For example, subgroup Ia (Fig. 1) was characterized by the presence of overwintering subterranean turions.
Figure 1. Maximum likelihood Potamogeton cpDNA tree based on a combined 4394-bp chloroplast DNA dataset.
Approximate likelihood-ratio test measures (aLRT) are indicated as support for branches [52]. Amino acid replacements of RbcL reconstructed under the codon substitution model (M8) are also indicated. Branches with orange lines indicate three heterophyllous lineages, whereas the others were homophyllous lineages.
To test the hypothesis of positive selection in rbcL, we examined the fit of the Potamogeton rbcL sequences to codon substitution models based on the cpDNA tree (Fig. 1). Table 1 lists the parameter estimates and log-likelihood values for the eight codon substitution models of molecular evolution. In order to evaluate positive selection, we performed four likelihood ratio tests comparing M1A (nearly neutral) with M2A (positive selection), M7 (beta) with M8 (beta and ωs≥1), M8A (beta and ωs = 1) with M8 (beta and ωs≥1), and branch-site model A (ω2 = 1 fixed) with branch-site model A (ω2 estimated) (Table 2).
Table 1. Parameter estimates and log-likelihood values for Potamogeton rbcL under eight codon substitution models included in PAML.
| Model | Log-likelihood | Parameters a |
| Site-specific models | ||
| M0 : one ω | −2182.1 | ω = 0.129 |
| M1A : nearly neutral | −2167.3 | p0 a = 0.912, ω0 = 0.000 |
| p1 = 0.088, ω1 = 1.000 | ||
| M2A : positive selection | −2163.1 | p0 = 0.914, ω0 = 0.000 |
| p1 = 0.082, ω1 = 1.000 | ||
| p2 = 0.004, ω2 = 11.494 | ||
| M7 : beta | −2167.4 | p = 0.005, q = 0.0482 |
| M8 : beta & ωs≥1 | −2163.4 | p0 = 0.963 |
| p = 0.010, q = 0.314 | ||
| p1 = 0.037, ω1 = 3.825 | ||
| M8A : beta & ωs = 1 | −2167.3 | p0 = 0.912 |
| p = 0.005, q = 99.000 | ||
| p1 = 0.088, ω1 = 1.000 | ||
| Branch-site models | ||
| Foreground: Heterophylly | ||
| Model A (ω2 = 1 fixed) | −2166.7 | p0 = 0.871, ω0 = 0.000 |
| p1 = 0.064, ω1 = 1.000 | ||
| p2+p3 = 0.065 , ω2 = 1.000 | ||
| Model A (ω2 estimated) | −2162.1 | p0 = 0.920, ω0 = 0.000 |
| p1 = 0.066, ω1 = 1.000 | ||
| p2+p3 = 0.013 , ω2 = 26.150 | ||
| Foreground: Homophylly | ||
| Model A (ω2 = 1 fixed) | −2166.0 | p0 = 0.850, ω0 = 0.000 |
| p1 = 0.053, ω1 = 1.000 | ||
| p2+p3 = 0.097 , ω2 = 1.000 | ||
| Model A (ω2 estimated) | −2165.8 | p0 = 0.899, ω0 = 0.000 |
| p1 = 0.055, ω1 = 1.000 | ||
| p2+p3 = 0.046 , ω2 = 2.575 | ||
the proportion (pi) of codon sites with ωi. In models M7, M8 and M8A, ω was drawn from a beta distribution B(p, q) for a proportion (p0) of sites.
Table 2. LRT statistics for testing the hypothesis of positive selection in Potamogeton rbcL.
| Test No. | Test model | Null model | df | 2ΔLb |
| Test 1 | M2A | M1A | 2 | 8.4* |
| Test 2 | M8 | M7 | 2 | 8.0* |
| Test 3 | M8 | M8A | -a | 7.8** |
| Test 4 | Branch-site model A (ω2 estimated) | Branch-site model A (ω2 = 1 fixed) | ||
| Foreground: Heterophylly | 1 | 9.2** | ||
| Foreground: Homophylly | 1 | 0.2 |
The test statistic for the M8A-M8 comparison is compared with 50∶50 mixture of df = 0 and 1 according to Swansson et al. (2003).
*: p<0.05, **: p<0.01.
The average ω ratio of Potamogeton rbcL was 0.129 over all 449 codon sites and lineages (Table 1, M0). Assuming that the ω ratio of all sites was either ω = 0 or ω = 1 and for the average of all the lineages in the tree, a majority of the sites (91.2%) were under purifying selection with ω = 0 in model M1A (Table 1). The remaining sites (8.8%) were under neutral evolution with ω = 1, indicating the dominant role of purifying selection in the evolution of rbcL. In model M2A, the majority of the sites (91.4%) were under purifying selection, 8.2% of the sites were under neutral evolution, and 0.4% of the sites experienced positive selection (ω = 11.494)(Table 1). The likelihood ratio test revealed that model M2A fit the data significantly better than the null model M1A (Table 2).
Model M7 assumed several codon site classes with values of ω ranging from 0 to 1 (Table 1). By contrast, 3.7% of the codons had ω = 3.825 and experienced positive selection in model M8 (Table 1). Once again, the Potamogeton rbcL sequences fit model M8, which allowed positive selection better than the null model M7 (Table 2).
In Model M8A, an additional category of sites with ω = 1 was added to the null model M7 (Table 1). The hypothesis of positive selection on Potamogeton rbcL was also supported by a test comparing M8 against the null model M8A (Table 2), which may be more robust than M1A vs. M2A or M7 vs. M8 [30].
The branch-site models allow the ω ratio to vary both among codon sites and among lineages. Heterophyllous lineages in the Potamogeton cpDNA tree were specified a priori as foreground branches that might be expected to be under positive selection (Fig. 1). In model A (ω2 estimated), a majority of the sites were under purifying selection (92.0%), whereas 6.6% of the codons were evolving neutrally throughout the tree. The remaining sites (1.34%) were either purifying (1.25%) or neutral (0.9%) on the background branches, but came under positive selection on the foreground branches with ω = 26.150 (Table 1). Test model A (ω2 estimated) fit Potamogeton rbcL significantly better than null model A (ω2 = 1 fixed; Table 1)(Table 2). Conversely, when homophyllous lineages within the genus Potamogeton were specified as foreground branches, both the test and null models had similar log-likelihood values (Tables 1 and 2).
The other chloroplast encoded genes, atpB and petA, have reported evolutionary rates similar to rbcL [31]. The average ω ratios of Potamogeton atpB and petA were 0.098 and 0.174, respectively (Tables S3 and S4). In both genes, positive selection was detected in the site-specific models analyses with a high posterior probability of evolving under positive selection for two atpB (sites 16 and 488) and one petA (site 239) residues but not in the branch-site models (Tables S3 to S5).
Features of amino acid replacement sites
Table 3 shows the distribution of 12 variable amino acid sites in RbcLs from Potamogeton and the outgroup species. Amino acid replacements at residues 225 and 281 were identified as potentially under positive selection. Most of the replacements were implied to have occurred at terminal branches of the phylogenic tree, rather than at basal ones (Fig. 1). The replacement of residue 225 (Ile225→Leu225, codon exchange ATT→CTT) was thought to have occurred twice: once in subgroup Ia and again in subgroup IIa. Residue 281 was replaced five times: three parallel changes of Ser281→Ala281 (TCT→GCT) and two of Ala281→Ser281 (GCT→TCT).
Table 3. Amino acid replacement sites in 18 Potamogeton species and Stuckenia RbcL.
| Amino acid site | 76 | 101 | 225 | 230 | 245 | 249 | 270 | 279 | 281 | 282 | 309 | 328 |
| Species | ||||||||||||
| P. crispus (−a) | S | V | I | A | G | E | L | T | S | H | M | S |
| P. maackianus (−a) | S | V | I | A | G | D | L | S | A | H | M | S |
| P. gramineus (++) | N | V | L | G | G | E | L | S | A | H | M | S |
| P. dentatus (−) | N | V | L | G | G | E | L | S | S | H | M | S |
| P. distinctus (++) | S | V | I | A | G | E | L | S | A | H | M | A |
| P. malaianus (++) | S | V | I | A | A | E | L | S | A | H | M | A |
| P. perfoliatus (−a) | S | V | I | A | G | E | I | S | A | H | M | S |
| P. alpinus (+), P. cristatus (+) | S | V | I | A | G | E | L | S | A | H | M | S |
| P. fryeri (++), P. natans (++) | S | V | L | A | G | E | L | S | A | N | M | S |
| P. praelongus (−), P. oxyphyllus (−), P. octandrus (+) | S | V | I | A | G | E | L | S | S | H | M | S |
| P. compressus (−), P. obtusifolius (−) | S | I | I | A | A | E | L | S | S | H | I | S |
| P. panormitanus (−a), P. pusillus (−) | S | V | I | A | A | E | L | S | S | H | M | S |
| Stuckenia pectinata (−a) | S | V | I | A | G | E | L | S | S | Y | M | S |
| Structural featureb | i | c | c | i | D | D | *c | D | c | D | D | *c |
| Posterior probabilities of ω≥1c | ||||||||||||
| Site-specific model (M8) | .51 | .38 | .86 | .48 | .85 | .32 | .49 | .48 | 1.0 | .86 | .43 | .38 |
| Branch-site model A (estimated ω2, Foreground: Heterophylly) | .73 | .02 | .98 | .74 | .29 | .02 | .72 | .03 | .98 | .29 | .02 | .68 |
Number corresponds to the amino acid position of spinach RbcL. (−): homophyllous plant with submerged leaves, (+): heterophyllous plant with submerged and floating leaves, (++): heterophyllous plant with submerged, floating and terrestrial leaves.
the species is also distributed in brackish water.
*: residues that are close to the active sites, c: residues that are buried in the interior of RbcL, D: residues at the intradimer interface of RbcL, i: residues at the interface between RbcL and RbcS.
probabilities above 0.95 are indicated in bold.
In order to infer the possible effects of amino acid replacement sites, the structural motif of Spinacia oleracea RbcL [32] was used (1UPP and 1RCX were obtained from the RCSB Protein Data Bank) (Table 3). Many amino acid replacements in Potamogeton RbcLs have occurred at the interface between subunits and near the active sites. Seven amino acids (76, 230, 245, 249, 279, 282, and 309) were located at the surface of the RbcL molecule. Two of them (76 and 230) were at the interface between RbcL and RbcS, whereas five (245, 249, 279, 282, and 309) were at the interface of the RbcL dimer. The remaining four (101, 225, 281, and 328) were buried within the molecule. Residue 270 was also located within the molecule, although it was not completely buried. Residues 270 and 328 were close to some of the active sites (Arg295, His298, and His327) in substrate-binding regions [32].
Discussion
In Potamogeton, heterophyllous species are distributed in freshwater and sometimes on the shore, whereas homophyllous species live entirely underwater in fresh or brackish water. The contrasting ecological conditions between heterophyllous and homophyllous plants may have imposed different selective pressures on the photosynthetic system. Our molecular evolutionary analyses indicated that Potamogeton rbcL has evolved under positive selection (Tables 1 and 2). Of 12 amino acid sites, two (sites 225 and 281) were potentially driven by positive selection (Table 3). The replacement at site 281 appeared to have originated independently in different phylogenetic lineages of both heterophyllous and homophyllous species (Fig. 1).
Contrary to our observations, positive selection was absent in the rbcL of two exclusively aquatic lineages: monocots-4 (Potamogetonaceae plus Zosteraceae) and monocots-9 (Hydrocharitaceae) [22]. Sample size may account for the differences. Compared to the enormous numbers and variety of land plant rbcL, nonsynonymous substitutions in each aquatic lineage were limited, so weak signals of positive selection could not be detected. Indeed, the presence of positive selection was shown in a combined rbcL dataset for both lineages [22]. The ω ( = dN/dS) ratio in Potamogeton rbcL (0.129) was approximately twice that found in seagrass Zostera (0.050). Ecological differences between aquatic and terrestrial habitats may account for this increase in nonsynonymous substitution in Potamogeton rbcL.
Most aquatic plants are thought to have originally thrived on land and adapted secondarily to an aquatic habitat. Therefore, the heterophyllous condition arose as the land became submerged. On the other hand, the phylogenetic tree of Alismatales [33] (Angiosperm Phylogeny Website, Version 9, June 2008 by Stevens PF [http://www.mobot.org/MOBOT/research/APweb/]) suggests that the Potamogetonaceae and its sister family Zosteraceae originated entirely from submerged seagrasses. Considering such secondary adaptation to the terrestrial environment, the heterophyllous taxa of Potamogeton are surmised to have been derived from homophyllous taxa.
Terrestrial plants are commonly exposed to temperatures of 40 to 50°C for several hours a day [34]. Heterophyllous taxa usually produce floating or terrestrial leaves in the summer [3], [35]. The temperature of the floating leaves can exceed 30°C [36]. To improve their intrinsic thermal stability, proteins have more hydrophobic residues with branched side chains and more charged residues, at the expense of uncharged polar residues [37]. Molecular evolutionary analysis of cyanobacteria RbcL suggests that adaptive replacement, an increase in hydrophobic residues and a decrease in uncharged polar residues, occurred in a clade of hot spring strains [20]. In Potamogeton RbcL, the uncharged polar residue Ser was replaced with the hydrophobic residue Ala at site 281 (Table 3). This amino acid replacement might increase the thermal stability of RbcL in the heterophyllous taxa. However, recent evolutionary analyses of RuBisCO suggested that amino acid site 281 underwent parallel genetic changes; the Ala in almost all C3 photosynthesis species was replaced by Ser in the C4 group [23]. The replacement of Ala281 by Ser281 occurred in two homophyllous species (P. crispus and P. dentatus), and the reverse from Ser281 to Ala281 occurred in two independent heterophyllous lineages (Fig. 1). A significant correlation was found between the presence or absence of heterophylly and the amino acid at residue 281 (p<0.05 by Fisher's exact test). The detection of positive selection in heterophyllous lineages suggested that selective pressure on this chloroplast gene was higher for heterophyllous lineages than for homophyllous lineages. In the evolutionary analyses of rbcL sequences from over 3000 species representing green plants and other phototroph lineages, the neighboring residues 279 and 282 in helix 4 were identified as evolving under positive selection [22]. This suggests that a replacement at these amino acid sites could be involved in the adaptation of specificity or catalytic efficiency to widespread environmental variation in temperature and dryness in both terrestrial land plants (C4 and C3 plants) and Potamogeton.
Many aquatic plants have acquired carbon-concentrating mechanisms to overcome the potentially low, fluctuating supply of CO2 for underwater photosynthesis [38]. A strategy of C4-like carbon fixation operates in several freshwater homophyllous species [38]–[40] and seagrasses [41]. The evolutionary analysis of the RuBisCO gene revealed that five amino acid sites in rbcL evolved under positive selection in terrestrial C4 monocots [23]. At three amino acid sites, residues of the C4-like seagrass Zostera were fixed and shared with those of terrestrial C4 monocots (Ile101, Ile270, and Ser281)[42]. In Potamogeton, some homophyllous plants share residues with C4 monocots (Ile101, Ile270, Ser281, and Ile309; Table 3). The homophyllous species P. perfoliatus, which is located terminally in the cpDNA tree, had Ile270 (Fig. 1, Table 3). This plant tends to be shade tolerant and grows in deep fresh water and brackish water [11]. Its sister species P. malaianus is heterophyllous, shade intolerant, and distributed mainly in shallow, inshore freshwater [11]. Our ongoing studies indicate that underwater heat stress induces the transcription of key enzymes in C4 photosynthesis (phosphoenolpyruvate carboxylase and pyruvate, orthophosphate dikinase) in submerged leaves of P. perfoliatus but not in those of P. malaianus. This suggests that amino acid replacement in P. perfoliatus RbcL could be involved in acquiring C4-like photosynthesis. However, the selective pressure involved appeared to be mild, as no positive selection specific to homophyllous lineages was detected.
Conclusion
Our analysis did not show an exact relationship between the amino acid replacements and heterophylly or homophylly but revealed that positive selection has affected the Potamogeton rbcL. Polyploidy and aneuploidy, major forces in diversification, are common within the genus [43]. We are now conducting phylogenetic analyses of four nuclear genes to infer the polyploid history of Japanese Potamogeton. Our preliminary data suggest that heterophylly has evolved through allopolyploid formation. The increased amino acid replacement in rbcL may reflect the adaptive fine-tuning of RuBisCO in the alternation of growth forms (heterophyllous or homophyllous) and nuclear genome setsz.
Materials and Methods
Japanese Potamogeton species and the allied species Stuckenia pectinata (L.) Börner were used in the analyses (Table S1). Total DNA was prepared previously for a phylogenetic analysis of Japanese Potamogeton based on the trnT-trnL region [9]. We categorized the Potamogeton species into three groups based on growth form and leaf type: homophyllous species (submerged leaves), floating-leaved heterophyllous species (submerged and floating leaves), and terrestrial-leaved heterophyllous species (submerged, floating, and terrestrial leaves), according to the descriptions of Kadono (1984) [44]. The production of terrestrial leaves has been observed in some homophyllous species (P. dentatus and P. perfoliatus) under cultivation or on a few anomalous occasions in the field [11], [44]–[46]. Nevertheless these species were treated as homophyllous, as their terrestrial leaves did not seem to contribute to persistent survival.
Amplification and sequencing of chloroplast-encoded genes
The amplification and sequence analysis of chloroplast-encoded genes (rbcL, atpB, and petA) were performed as described previously [9]. Twenty-five PCR cycles at 94°C for 30 s, 59°C for 40 s, and 72°C for 60 s were performed. The forward and reverse primers used for amplification are listed in Table S2.
Molecular evolutionary analysis
Maximum likelihood (ML) trees were produced online (http://www.atgc-montpellier.fr/phyml/) using the program PhyML [47]. Five starting trees (neighbor-joining and four equally parsimonious trees) were examined with the substitution model HKY85, and the phylogenetic tree with the largest likelihood was selected. Stuckenia pectinata was used as the outgroup. Two length mutations (a 9-bp deletion in P. gramineus and P. dentatus atpB and a 6-bp insertion in P. praelongus petA) were treated as missing data and excluded from the analysis.
Molecular adaptation tests on the Potamogeton rbcL codon sites and reconstruction of the ancestral rbcL sequences were performed using maximum-likelihood models and programs included in PAML ver. 4.1 [25]–[27], [48], [49]. The models used the nonsynonymous/synonymous substitution rate ratio (ω = dN/dS) as an indicator of selective pressure and allowed the ratio to vary among codon sites. We used five site-specific codon substitution models: null models for testing positive selection (M1A, M7, and M8A) and models allowing for positive selection (M2A and M8) [50], [51]. The likelihood ratio test was used to compare these alternative models. Using model 0 included in the same program, the average nonsynonymous/synonymous substitution rate ratio (ω = dN/dS) was also estimated.
In contrast to the site-specific models, which assume the same ω ratio among all of the lineages in the tree, the branch-site models let the ω ratio vary among both sites and lineages [26], [27]. To clarify whether positive selection is linked to the evolution of heterophylly or homophylly, the branch-site models implemented in PAML ver. 4.1 (the modified branch-site model A) [27], [48] used heterophylly or homophylly to define branches tested for positive selection (foreground branches). As a single origin of heterophylly is more parsimonious than multiple origins, subgroup Ia was assigned as a heterophyllous lineage for the selection test, in addition to the other monophyletic lineages of heterophyllous species (Fig. 1). Such a definition of heterophyllous lineage was considered logical, as the homophyllous species included in subgroup Ia (P. dentatus and P. perfoliatus) has been observed to produce anomalous terrestrial leaves [11], [44]–[46].
The posterior probabilities that a site was drawn from a given ω site class were calculated using the parameter estimates from model M8 or branch-site model A (ω2 estimated, foreground: heterophylly) and a procedure called the Bayes empirical Bayes method [51]. A site was considered potentially under positive selection when it had probabilities above 0.95. All the above molecular evolutionary analyses were also carried out for the other chloroplast genes, atpB and petA.
To identify the amino acid position for the structural motif, the quaternary structure of RbcL was inferred from data for Spinacia oleracea L. [32] (1UPP and 1RCX obtained from the RCSB Protein Data Bank: http://www.rcsb.org/pdb).
Supporting Information
List of Potamogeton and Stuckenia species with GenBank accession numbers of chloroplast genes examined in the present study.
(0.05 MB DOC)
List of primers used for PCR.
(0.03 MB DOC)
Parameter estimates and log-likelihood values for Potamogeton atpB under eight codon substitution models included in PAML.
(0.05 MB DOC)
Parameter estimates and log-likelihood values for Potamogeton petA under eight codon substitution models included in PAML.
(0.05 MB DOC)
LRT statistics for testing the hypothesis of positive selection in Potamogeton chloroplast genes.
(0.05 MB DOC)
Abstract in Japanese.
(0.03 MB DOC)
Acknowledgments
We thank Dr. Hisayoshi Nozaki (The University of Tokyo) for discussion of the molecular evolution of rbcL.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Cook CDK. Aquatic plant book. The Hague: SPB Academic Publishing; 1990. p. 228. [Google Scholar]
- 2.Arber A. Water plants: a study of aquatic angiosperms. Cambridge: Cambridge University Press; 1920. p. 436. [Google Scholar]
- 3.Sculthorpe CD. The biology of aquatic vascular plants. London: Edward Arnold; 1967. p. 610. [Google Scholar]
- 4.Hutchinson GE. A treatise on limnology. 3. Limnological Botany. New York: Wiley; 1975. p. 672. [Google Scholar]
- 5.Frost-Christensen H, Sand-Jensen K. Comparative kinetics of photosynthesis in floating and submerged Potamogeton leaves. Aquat Bot. 1995;51:121–134. DOI 10.1016/0304-3770(95)00455-9. [Google Scholar]
- 6.Cook SA, Johnson MP. Adaptation to heterogeneous environments. I. Variation in heterophylly in Ranunculus flammula L. Evolution. 1968;22:496–516. doi: 10.1111/j.1558-5646.1968.tb03988.x. [DOI] [PubMed] [Google Scholar]
- 7.Wooten JW. Experimental investigations of the Sagittaria graminea complex: transplant studies and genecology. J Ecol. 1970;58:233–242. [Google Scholar]
- 8.Kadono Y. Distribution and habitat of Japanese Potamogeton. Bot Mag Tokyo. 1982;95:63–76. [Google Scholar]
- 9.Iida S, Kosuge K, Kadono Y. Molecular phylogeny of Japanese Potamogeton species in light of noncoding chloroplast sequences. Aquat Bot. 2004;80:115–127. DOI 10.1016/j.aquabot.2004.08.005. [Google Scholar]
- 10.Lindqvist C, Laet JD, Haynes RR, Aagesen L, Keener BR, et al. Molecular phylogenetics of an aquatic plant lineage, Potamogetonaceae. Cladistics. 2006;22:568–588. doi: 10.1111/j.1096-0031.2006.00124.x. DOI 10.1111/j.1096-0031.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 11.Iida S, Yamada A, Amano M, Ishii J, Kadono Y, et al. Inherited maternal effects on the drought tolerance of a natural hybrid aquatic plant, Potamogeton anguillanus. J Pl Res. 2007;120:473–481. doi: 10.1007/s10265-007-0087-y. DOI 10.1007/s10265-007-0087-y. [DOI] [PubMed] [Google Scholar]
- 12.Galmés J, Flexas J, Keys AJ, Cifre J, Mitchell RAC, et al. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ. 2005;28:571–579. DOI 10.1111/j.1365-3040.2005.01300.x. [Google Scholar]
- 13.Moreno J, Spreitzer RJ. C172S Substitution in the chloroplast-encoded large subunit affects stability and stress-induced turnover of ribulose-1,5-bisphosphate carboxylase/oxygenase. J Biol Chem. 1999;274:26789–26793. doi: 10.1074/jbc.274.38.26789. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Mao H, Ruan X, Xu Q, Gong Y, et al. Association of heat-induced conformational change with activity loss of Rubisco. Biochem Biophys Res Commun. 2002;290:1128–1132. doi: 10.1006/bbrc.2001.6322. DOI 10.1006/bbrc.2001.6322. [DOI] [PubMed] [Google Scholar]
- 15.Parry MAJ, Andralojc PJ, Khan S, Lea PJ, Keys AJ. Rubisco activity: effects of drought stress. Ann Bot. 2002;89:833–839. doi: 10.1093/aob/mcf103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert VA, Backlund A, Bremer K, Chase MW, Manhart JR, et al. Functional constraints and rbcL evidence for land plant phylogeny. Ann Mo Bot Gard. 1994;81:534–567. [Google Scholar]
- 17.Kellogg EA, Juliano ND. The structure and function of RUBISCO and their implications for systematic studies. Am J Bot. 1997;84:413–428. [PubMed] [Google Scholar]
- 18.Wolfe AD, dePamphilis CW. Alternate paths of evolution for the photosynthetic gene rbcL in four nonphotosynthetic species of Orobanche. Pl Mol Biol. 1997;33:965–977. doi: 10.1023/a:1005739223993. DOI 10.1023/A:1005739223993. [DOI] [PubMed] [Google Scholar]
- 19.Nozaki H, Onishi K, Morita E. Differences in pyrenoid morphology are correlated with differences in the rbcL genes of members of the Chloromonas lineage (Volvocales, Chlorophyceae). J Mol Evol. 2002;55:414–430. doi: 10.1007/s00239-002-2338-9. DOI 10.1007/s00239-002-2338-9. [DOI] [PubMed] [Google Scholar]
- 20.Miller SR. Evidence for the adaptive evolution of the carbon fixation gene rbcL during diversification in temperature tolerance of a clade of hot spring cyanobacteria. Mol Ecol. 2003;12:1237–1246. doi: 10.1046/j.1365-294x.2003.01831.x. DOI 10.1046/j.1365-294X.2003.01831.x. [DOI] [PubMed] [Google Scholar]
- 21.Kapralov MV, Filatov DA. Molecular adaptation during adaptive radiation in the Hawaiian endemic genus Schiedea. PLoS ONE. 2006;1:e8. doi: 10.1371/journal.pone.0000008. DOI 10.1371/journal.pone.0000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapralov MV, Filatov DA. Widespread positive selection in the photosynthetic Rubisco enzyme. BMC Evol Biol. 2007;7:73. doi: 10.1186/1471-2148-7-73. DOI 10.1186/1471-2148-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christin PA, Salamin N, Muasya AM, Roalson EH, Russier F, et al. Evolutionary switch and genetic convergence on rbcL following the evolution of C4 photosynthesis. Mol Biol Evol. 2008;25:2361–2368. doi: 10.1093/molbev/msn178. DOI 10.1093/molbev/msn178. [DOI] [PubMed] [Google Scholar]
- 24.Wiegleb G. Notes on pondweeds: outlines for a monographical treatment of the genus Potamogeton L. Feddes Repert. 1988;99:249–266. [Google Scholar]
- 25.Yang Z, Nielsen R, Goldman N, Pedersen A-MK. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155:431–449. doi: 10.1093/genetics/155.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Nielsen R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol. 2002;19:908–917. doi: 10.1093/oxfordjournals.molbev.a004148. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Nielsen R, Yang Z. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol Biol Evol. 2005;22:2472–2479. doi: 10.1093/molbev/msi237. DOI 10.1093/molbev/msi237. [DOI] [PubMed] [Google Scholar]
- 28.Wiegleb G, Kaplan Z. An account of the species of Potamogeton L. (Potamogetonaceae). Folia Geobot. 1998;33:241–316. [Google Scholar]
- 29.Kaplan Z, Štěpánek J. Genetic variation within and between populations of Potamogeton pusillus agg. Plant Syst Evol. 2003;239:95–112. DOI 10.1007/s00606-002-0252-7. [Google Scholar]
- 30.Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Mol Biol Evol. 2003;20:18–20. doi: 10.1093/oxfordjournals.molbev.a004233. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe KH, Li W-H, Sharp PM. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA. 1987;84:9054–9058. doi: 10.1073/pnas.84.24.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight S, Anderson I, Brändén C-I. Crystallographic analysis of ribulose 1,5-bisphosphate carboxylase from spinach at 2·4 Å resolution subunit interactions and active site. J Mol Biol. 1990;215:113–160. doi: 10.1016/S0022-2836(05)80100-7. DOI 10.1016/S0022-2836(05)80100-7. [DOI] [PubMed] [Google Scholar]
- 33.Les DH, Cleland MA, Waycott M. Phylogenetic studies in Alismatidae. II. Evolution of marine angiosperms (seagrasses) and hydrophily. Syst Bot. 1997;22:443–463. [Google Scholar]
- 34.Larcher W. Ökophysiologie der Pflanzen, 5. Auflage. Stuttgart: Eugen Ulmer GmbH & Co; 1994. p. 408. [Google Scholar]
- 35.Wiegleb G, Kadono Y. Growth and development of Potamogeton malaianus in southwestern Japan. Nord J Bot. 1989;9:167–178. [Google Scholar]
- 36.Ikusima I. Seitaigakukouza 7 Suikaishokubutsugunraku no butsushitsuseisan I – Suiseishokubutsu- (Matter production in aquatic plant community I Aquatic plants) (In Japanese) Tokyo: Kyoritsu shuppan; 1972. p. 98. [Google Scholar]
- 37.Kumar S, Nussinov R. How do thermophilic proteins deal with heat? Cell Mol Life Sci. 2001;58:1216–1233. doi: 10.1007/PL00000935. DOI 10.1007/PL00000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maberly SC, Madsen TV. Freshwater angiosperm carbon concentrating mechanisms: process and patterns. Funct Plant Biol. 2002;29:393–405. doi: 10.1071/PP01187. DOI 10.1071/PP01187. [DOI] [PubMed] [Google Scholar]
- 39.Holaday AS, Bowes G. C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiol. 1980;65:331–335. doi: 10.1104/pp.65.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnin NC, Cooley BA, Reiskind JB, Bowes G. Regulation and localization of key enzymes during the induction of kranz-less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiol. 1997;115:1681–1689. doi: 10.1104/pp.115.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touchette BW, Burkholder JM. Overview of the physiological ecology of carbon metabolism in seagrasses. J Exp Mar Bio Ecol. 2000;250:169–205. doi: 10.1016/s0022-0981(00)00196-9. DOI 10.1016/S0022-0981(00)00196-9. [DOI] [PubMed] [Google Scholar]
- 42.Kato Y, Aioi K, Omori Y, Takahata N, Satta Y. Phylogenetic analyses of Zostera species based on rbcL and matK nucleotide sequences: Implications for the origin and diversification of seagrasses in Japanese waters. Genes Genet Syst. 2003;78:329–342. doi: 10.1266/ggs.78.329. [DOI] [PubMed] [Google Scholar]
- 43.Les DH. Taxonomic implications of aneuploidy and polyploidy in Potamogeton (Potamogetonaceae). Rhodora. 1983;85:301–323. [Google Scholar]
- 44.Kadono Y. Comparative ecology of Japanese Potamogeton: an extensive survey with special reference to growth form and life cycle. Jpn J Ecol. 1984;34:161–172. [Google Scholar]
- 45.Kaplan Z. Phenotypic plasticity in Potamogeton (Potamogetonaceae). Folia Geobot. 2002;37:141–170. [Google Scholar]
- 46.Amano M, Ohno M, Suda R, Iida S, Kadono Y, et al. Origin and present status of Potamogeton×inbaensis in a natural population of Oitoike pond, Kitakyushu-city, Japan (in Japanese with English abstract). Bunrui. 2008;8:129–139. [Google Scholar]
- 47.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. DOI 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. DOI 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Bielawski JP. Statistical methods for detecting molecular adaptation. Trends Ecol Evol. 2000;15:496–503. doi: 10.1016/S0169-5347(00)01994-7. DOI 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong WSW, Nielsen R. Detecting selection in noncoding regions of nucleotide sequences. Genetics. 2004;167:949–958. doi: 10.1534/genetics.102.010959. DOI 10.1534/genetics.102.010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z, Wong WSW, Nielsen R. Bayes empirical bayes inference of amino acid sites under positive selection. Mol Biol Evol. 2005;22:1107–1118. doi: 10.1093/molbev/msi097. DOI 10.1093/molbev/msi097. [DOI] [PubMed] [Google Scholar]
- 52.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–552. doi: 10.1080/10635150600755453. DOI 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Potamogeton and Stuckenia species with GenBank accession numbers of chloroplast genes examined in the present study.
(0.05 MB DOC)
List of primers used for PCR.
(0.03 MB DOC)
Parameter estimates and log-likelihood values for Potamogeton atpB under eight codon substitution models included in PAML.
(0.05 MB DOC)
Parameter estimates and log-likelihood values for Potamogeton petA under eight codon substitution models included in PAML.
(0.05 MB DOC)
LRT statistics for testing the hypothesis of positive selection in Potamogeton chloroplast genes.
(0.05 MB DOC)
Abstract in Japanese.
(0.03 MB DOC)