Abstract
Spatial and temporal control of Notch and Wingless (Wg) pathways during development is regulated at multiple levels. Here, we present an analysis of Phyllopod as a coordinated regulator of these two critical signal transduction pathways. Phyl specifically helps traffic Notch and Wg pathway components within early endocytic vesicles, thereby controlling the amount of processed signal available for causing a transcriptional response within the nucleus. In Drosophila, the EGFR pathway transcriptionally activates phyl whose product then blocks Notch and Wg signalling pathways. This provides a mechanistic basis for an antagonistic relationship between receptor tyrosine kinase and Notch/Wg pathways during development. Furthermore, this study identifies a Phyl-regulated class of endosomal vesicles that specifically include components of Notch and Wg signalling.
Keywords: Drosophila, endocytosis, Notch, Phyllopod, Wingless
Introduction
Endocytosis has emerged as a key regulatory mechanism that helps to modulate regulation of multiple signalling pathways. Activated receptors are internalized in endocytic vesicles to limit their availability and to control the magnitude of the signal. Recent studies show that endocytosis not only functions in signal attenuation, but can also have an important function in promoting signals from multiple pathways (Artavanis-Tsakonas et al, 1999; Bray, 2006; Fischer et al, 2006).
During Notch signalling, endocytosis is required in both the ligand and the receptor-expressing cell. In the signalling cell, E3 ubiquitin ligases, such as Neuralized and Mindbomb, regulate the monoubiquitnation of the ligand Delta (Dl), an essential step in its endocytosis and in its ability to signal adjacent cells (Lai et al, 2005; Wang and Struhl, 2005; Le Borgne et al, 2005b). The Clathrin-dependent trans-endocytosis of the ligand is dependent on the adaptor protein Epsin and Auxillin (Overstreet et al, 2004; Wang and Struhl, 2004; Hagedorn et al, 2006) and facilitates the co-transfer of Nextra to the signalling cell removing a block in subsequent processing of the Notch protein in the receiving cell (Parks et al, 2000). In the signal-receiving cell, the function of endocytosis in the activation of the Notch receptor is context dependent and several E3 ubiqutin ligases actively promote Notch signalling (Wilkin et al, 2004; Le Borgne et al, 2005a).
Endocytosis is also essential for active Wingless (Wg) signalling (Seto and Bellen, 2006). In Drosophila S2 cells, blocking Clathrin-dependent endocytosis causes accumulation of β-catenin and consequent loss in the transcriptional activation of a Wg target reporter (Blitzer and Nusse, 2006). Likewise, in the developing wing imaginal disc, blocking HRS-mediated transport of Wg to the late endosomes also results in an upregulation of Wg target gene expression (Seto and Bellen, 2006). Increased Wg signalling seen in these genetic backgrounds correlates with an increase in the extent of co-localization of Wg, its receptors, Arrow and Frizzled (Frz), and the adaptor protein Dishevelled (Dsh) (Blitzer and Nusse, 2006; Seto and Bellen, 2006).
The processed and activated forms of Notch and Wg pathway components in early endocytic vesicles are extremely transient and are either recycled back to the membrane or targeted to lysosomes for degradation. Earlier studies have uncovered the importance of this novel subcellular compartment using mutations that cause a general block in all endosomal trafficking (Moberg et al, 2005; Thompson et al, 2005; Vaccari and Bilder, 2005). Consequently, the components identified in such studies are globally required for endocytic regulation of many protein components and are not specific to these signalling pathways.
Mutations in phyllopod (phyl) were identified as dominant suppressors of the gain of function of sev-RasV12 in the Drosophila eye. Loss of phyl function causes a loss of R1, R6 and R7 cell types and overrepresentation of non-neuronal cone cells (Chang et al, 1995; Dickson et al, 1995). Phyl expression in the developing eye discs is dynamic and is seen at high levels at the furrow and in the R1, R6 and R7 cell types. This expression of phyl in the developing eye disc is dependent on receptor tyrosine kinase (RTK) signalling (Chang et al, 1995; Dickson et al, 1995). Phyl has been proposed to function as an adaptor protein by binding the E3 ubiquitin ligase, Sina and in promoting the degradation of the transcriptional repressor, Tramtrack (Kauffmann et al, 1996; Li et al, 1997; Tang et al, 1997). phyl is also required for the proper specification of cell types within the PNS and is required at two stages in the external sense organ (es) development. At the early stage, phyl is required for the specification of the sensory organ precursor (SOP). Loss of phyl at this stage results in loss of bristles in the adult. At a later stage, during es organ development, loss of phyl function causes the first cell division of the SOP lineage to generate two identical daughter cells resulting in transformation of cell fate (Pi et al, 2001).
In this article, we describe the specific function of Phyl in the regulation of Notch and Wg signalling in the endocytic vesicles. Loss of Phyl increases the amount of Notch, Delta (Dl) and Wg that are localized to endocytic vesicles and increases Notch and Wg signalling levels. Furthermore, as Phyl is a transcriptional target of the EGFR signal transduction cascade (Chang et al, 1995; Dickson et al, 1995), our results suggest a mechanism for the coordinated downregulation of Notch and Wg pathways by RTK signalling in the wild-type fly through the transcriptional control of Phyl.
Earlier studies had proposed that all Phyllopod function can be explained by its activity as a negative regulator of a transcriptional repressor, Tramtrack (Li et al, 1997; Tang et al, 1997). The detailed phenotypic analysis presented here does not support this mechanism.
Results and discussion
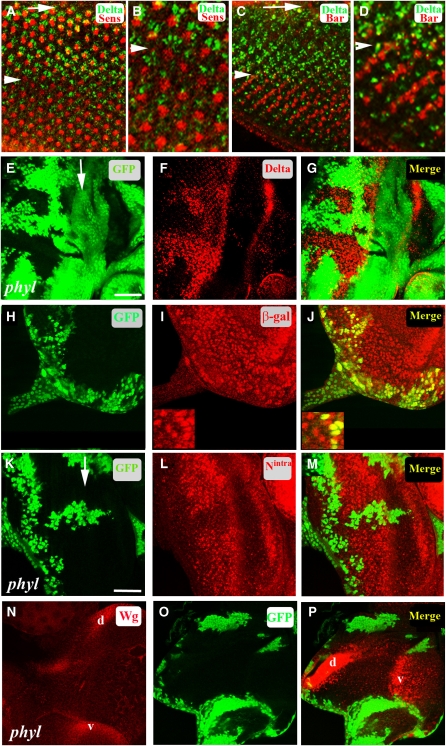
In the Drosophila third instar eye imaginal disc, an indentation called the morphogenetic furrow (MF) develops at the posterior end and sweeps across the disc in an anteriorly direction. Cell fate specification begins as the cells emerge out of the MF with the photoreceptor (R) cells differentiating first followed by the non-neuronal cone and pigment cells (Wolff and Ready, 1991). R cells express the Notch ligand Dl as they exit out of the MF. As the MF moves anteriorly and the clusters mature, Dl expression is downregulated by column 8 posterior to the furrow (Figure 1A–D). As each successive column is 2 h apart in the developmental timing, the dynamic range of this Dl expression lasts only about 16 h. Clones of cells mutated for phyl express elevated amounts of Dl in comparison to wild type (Figure 1E–G), and Dl protein in phyl mutant clones is not downregulated eight columns behind the furrow as in wild type, but continues to be expressed until the posterior end of the eye disc (Figure 1F and G). Unlike the Dl protein, the expression of the Dl-lacZ enhancer trap, in which lacZ expression is a read-out for Delta transcription is not altered in the phyl mutant tissue (Figure 1H–J), suggesting that the normal function of Phyl is in the post-transcriptional downregulation of Dl. The function of Phyl is not restricted to the signalling cell, as the Notch receptor is also elevated in cells behind the furrow in phyl mutant tissue (Figure 1K–M). This upregulation phenotype can also be seen in wing imaginal discs and in mid-pupal eye discs (Supplementary Figure S1).
Figure 1.
Phyl-mediated downregulation of Dl, Notch and Wg in the developing Drosophila eye disc. Arrows mark the morphogenetic furrow (MF). Arrowheads mark eight columns posterior to the furrow. (A–D) Temporal regulation of Dl expression in R cells. (A) Dl expression in the third instar eye disc. Dl (green) in R cells, Senseless (red) marking the R8 cell in each cluster. Dl expression is downregulated at about column 8 (arrowhead) posterior (down) to the MF. (B) A high magnification view of (A) near column 8 (arrowhead) showing downregulation of Dl (green) posterior (down) to column 8. (C) The expression of BarH1 (red) in R1 and R6 initiates at around column 8 (arrowhead) at the time when the expression of Dl (green) is downregulated in R cells. (D) A high magnification view of (C) near column 8 (arrowhead) showing downregulation of Dl posterior to column 8. (E–G) In a disc containing a phyl clone (non-green, E), Dl is overexpressed in the mutant tissue (F, red). The merged panel is shown in (G). (H–J) In a disc containing a phyl clone (non-green, H) in the background of Dl-lacZ, the level of β-galactosidase expression (I, red nuclei) remains unaltered in mutant cells. A merged panel (J) shows no difference in Dl-lacZ expression in cells mutant for phyl when compared with adjacent wild-type cells; shown in higher-magnification insets (bottom left corner of I and J). (K–M) In a disc containing a phyl clone (non-green, K), increased amount of Notch detected by an antibody against the intracellular domain of Notch (red, L) is seen in the phyl mutant cells when compared with adjacent wild-type cells. A merged image is shown in (M). (N–P) In a wild-type third instar eye disc, Wg is expressed on the dorsal (d) and ventral (v) edges of the eye disc (N). In a disc containing large phyl mutant clones (non-green in O), increased amount of Wg is seen in the mutant tissue along the dorsal/ventral edges (red in P).
To test if Phyl functions in the downregulation of other signalling pathways, we monitored the expression of downstream effectors of Hedgehog (Ci), Dpp (pMAD) and EGFR (pMAPK) in phyl mutant tissue and found that they remain normal (Supplementary Figure S2A–I). In addition, the localization of receptors, such as EGFR and PDGF/VEGF (PVR), which undergo endocytosis upon activation and are targeted to degradation (Jekely and Rorth, 2003), also remain unaltered in phyl mutant tissue (Supplementary Figure S2J–O). In contrast to the above-mentioned signalling pathways, we found that Wg signalling is upregulated in phyl mutant tissue. phyl mutant clones in the third instar eye discs show an increased level of Wg protein in the mutant cells that normally express Wg (Figure 1N–P). Therefore, among the many pathways tested, Phyl is specific for Notch and Wg signalling. phyl mutant clones in the third instar wing and antennal disc also show an increase in the level of Wg in mutant clones (Supplementary Figure S3).
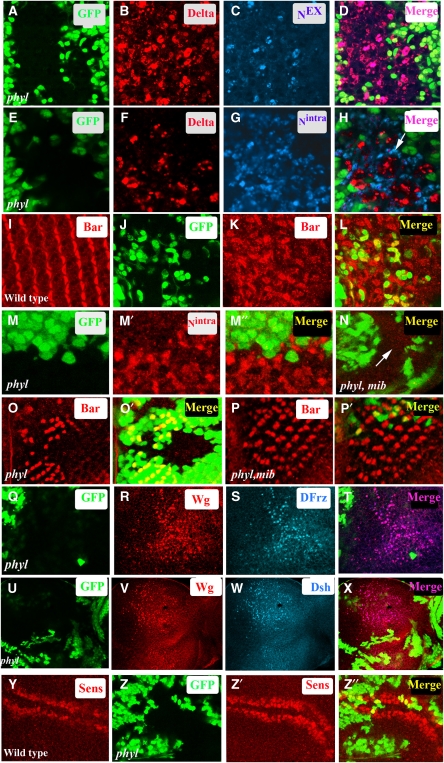
Activation of Notch is associated with the transendocytosis of the extracellular domain of the Notch receptor (Nex) in the signalling cell (Parks et al, 2000). This is followed by a series of proteolytic steps in the receiving cell generating Nintra, which migrates to the nucleus and promotes a transcriptional response (Bray, 2006). In principle, the higher level of Notch seen in phyl mutant tissue could result either from a block in the turnover of Notch before signalling or from a block in the degradation of the processed version of Notch following active signalling. To distinguish between these alternatives, we monitored the localization status of Dl and the processed versions of Notch in phyl mutant tissue. In the pupal eye disc, Dl is expressed in cone cells and is required for the differentiation of the adjacent pigment cells (Parks et al, 2000; Nagaraj and Banerjee, 2007) and this provides a nice experimental system in which we can distinguish the signalling cell from the one receiving the signal. In phyl mutant clones in the pupal eye disc, Dl co-localizes with Nex in the cone cells (Figure 2A–D). In contrast, increased level of Nintra in phyl mutant cells is seen in the adjacent signal-receiving pigment cells (Figure 2E–H) and promotes over-specification of primary pigment cell fate (Figure 2I–L). These results suggest that the accumulation of Notch in phyl mutant tissue occurs only after the activation of the Notch pathway. This was further confirmed genetically by clonal analysis, which showed that the accumulation of Nintra in phyl mutant tissue in the receiving cell is suppressed in a phyl mib double-mutant background (Figure 2M–N) in which Dl is incapable of signalling due to loss of mib function (Lai et al, 2005). The double-mutant combination of phyl, mib also shows significant rescue in the specification of Bar-expressing R1 and R6 cell types (Figure 2O–P′). Thus, the accumulation of Nintra in phyl mutant cells follows a round of signalling by an active ligand.
Figure 2.
Processed forms of Notch and activated version of Wg signalling complex accumulate in phyl mutant cells. (A–H) phyl mutant clones in pupal eye discs. (A–D) In phyl mutant cells (non-green in A and D), Dl (red, B) co-localizes with extracellular domain of Notch, Nex (blue, C). The merged panel is shown in (D). (E–H) In a pupal eye disc containing phyl mutant clones (non-green in E and H) increased level of Dl is seen in mutant tissue (red, F). The same disc co-stained with an antibody directed against the intracellular domain of Notch shows an accumulation of Nintra in adjacent pigment cells (blue, G). The merged panel shows this lack of co-localization (arrow, H). (I–L) In wild-type mid-pupal eye disc, Bar (red, I) is expressed in primary pigment cells. Mid-pupal eye disc containing phyl mutant clones (non green in J) shows an increase in the number of Bar-expressing cells (red, K). The merged panel is shown in (L). (M, N) Increased Nintra level in phyl mutant cells requires Dl signalling clones of phyl mutant tissue in third instar eye disc (non green in M) stained for Nintra (red, M′). The merged panel (M″) shows that only non-green mutant cells accumulate Notch. In phyl mib1 double-mutant clones, Nintra does not accumulate in the mutant tissue (arrow, compare M′ with N). (O–P′) Loss of Notch signalling rescues phyl mutant phenotype. phyl mutant clones (non-green, O′) show a loss of Bar-expressing R1 and R6 cell types (red, O). In phyl, mib1 double-mutant clones (non-green in P′), the phyl mutant phenotype is rescued and Bar-expressing R1 and R6 cell types are seen in the mutant tissue (red, P). (Q–T) Third instar eye disc containing phyl mutant (non-green in Q) clones shows increased levels of Wg protein (red, R). The same disc was also stained with DFrz (blue, S), showing co-localization of Wg with Dfrz (pink, T). (U–X) Third instar eye disc containing phyl mutant clones (non-green in U) shows increased levels of Wg protein (red, V). The same disc was also stained with Dsh (blue, W), showing co-localization of Wg with Dsh (pink, X). (Y–Z″) In wild-type third instar wing disc, Senseless is expressed in cells straddling the D/V boundary (red, Y). phyl mutant clones in the wing disc generated using ubx-flp (non green in Z) show an expansion of Senseless expression domain (red, Z′). The merged panel is shown in (Z″).
Similar conclusion regarding Phyl function could be drawn for the Wg pathway. Upon binding of Wg to its receptor Frz, the receptor ligand complex is endocytosed and recruits Dsh to its cytoplasmic side. According to the ‘signalling endosome hypothesis' (Blitzer and Nusse, 2006; Seto and Bellen, 2006), endocytosis followed by the recruitment of cytoplasmic components of the pathway on to the endosome complex is critical for high levels of Wg signalling. As shown in Figure 2Q–X, we observe a significant cell autonomous co-localization of Wg with both Frz and Dsh in phyl mutant tissue, suggesting a block in the degradation of active signalling complex during Wg signalling. To test if this results in increased levels of Wg signalling, we generated phyl mutant clones in the wing and monitored the expression of Senseless, a target of Wg signalling (Nolo et al, 2000; Seto and Bellen, 2006). In third instar wild-type wing disc, Sens is expressed in cells that straddle the D/V boundary (Figure 2Y). In large phyl mutant clones, we observe an expansion of Sens expression domain compared with adjacent wild-type tissue (Figure 2Z–Z″).
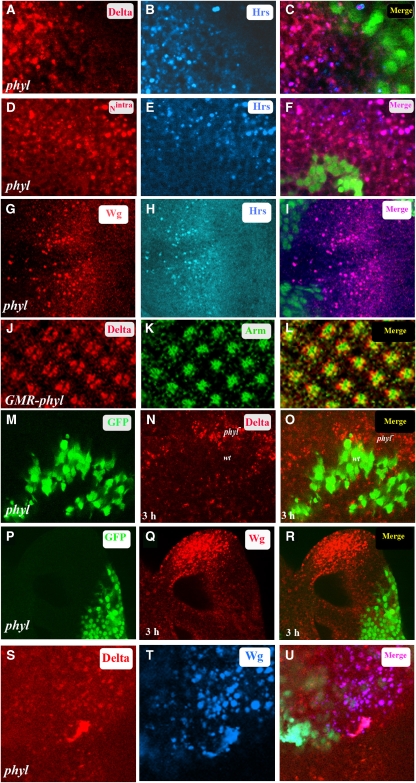
Although wild-type Dl, Notch and Wg are membrane bound (Parks et al, 1995), the accumulation of Dl (Figure 3A), Notch (Figure 3D) and Wg (Figure 3G) in phyl mutant clones is in punctate vesicles. Co-localization studies show that these vesicles are positive for the early endosomal marker, HRS (Figure 3A–I). The higher number of HRS-positive Dl, Nintra and Wg vesicles seen in phyl mutant cells as compared with adjacent wild-type cells indicates a block in the routing of Dl, Nintra and Wg from the HRS-positive endosomal compartment (Lloyd et al, 2002) to the later trafficking steps. Double staining of Nintra in endocytic vesicles in phyl mutant clones with markers for different stages of endosome maturation further shows that Nintra co-localizes with Avalanche (Avl) and Deep orange (Sevrioukov et al, 1999; Vaccari and Bilder, 2005), which mark the early endosomes, but not with Hook (Kramer and Phistry, 1996), which marks the late endosomes, suggesting that loss of phyl function results in a block in the trafficking of Nintra at the early endosome stages (Supplementary Figure S4A–I). This endosomal Nintra is extensively ubiquitinated and stains for Lysotracker Red, suggesting acidic conditions within the endosomes (Supplementary Figure S4J–O). The mere overexpression of Phyl in the eye disc does not cause membrane-associated Dl to get degraded (Figure 3J–L). Thus phyl function is required to remove signalling components from the HRS-positive endocytic vesicles after a round of signalling but not directly from the plasma membrane before signalling. To further test this hypothesis, we performed a trafficking assay (Le Borgne et al, 2005b) in which we labelled surface Dl or Wg in unfixed tissue. After allowing for the uptake of the antibody-bound Dl or Wg into endocytic vesicles, the discs were fixed and stained with secondary antibody at different time points. Following a 3 h chase, wild-type cells lose all Dl and Wg staining intracellularly due to lysosome-mediated degradation (Le Borgne et al, 2005b); however, accumulation of Dl or Wg in endocytic vesicles persists in adjacent phyl-mutant cells (Figure 3M–R). This establishes that cells mutant for phyl are competent for internalizing Dl and Wg but are restricted in subsequent trafficking steps causing an accumulation of Dl and Wg in the HRS-positive endosomes. To determine if Dl trapped in endocytic vesicles is recycled back to the membrane for additional rounds of signalling, we co-stained Dl with Rab11, which marks the recycling endosomes (Satoh et al, 2005). We found no co-localization of endocytic Dl with Rab11 (Supplementary Figure S4P–R). Finally to test if Dl and Wg localize to the same endocytic vesicles or belong to distinct non-overlapping groups of endocytic vesicles, phyl mutant clones in the eye were co-stained with Dl and Wg antibodies. Significant co-localization of Wg- and Dl-positive vesicles suggested a mechnism in a Phyl-dependent endocytic pathway that co-regulates Notch and Wg signalling levels (Supplementary Figure 3S–U).
Figure 3.
Defects in endocytic trafficking of Dl, Nintra and Wg in phyl mutant cells. (A–I) phyl mutant clones in third instar eye disc. GFP marks wild-type cells and non-GFP cells are mutant for phyl. (A–C) In phyl mutant cells (non-green in C), Dl (red, A) co-localizes with HRS, which marks endocytic vesicles (blue, B). The merged panel is shown in (C). (D–F) In phyl mutant cells (non-green in F), Nintra (red, D) co-localizes with HRS (blue, E). The merged panel (F) shows this co-localization (pink, F). (G–I) In phyl mutant cells (non-green in I), Wg (red, G) co-localizes with HRS (blue, H). The merged panel shows this co-localization (pink, I). (J–L) GMR-phyl third instar eye disc stained for Dl (red, J) and Arm marking the apical tips of the R cells (green, K). The merged panel (L) shows co-localization of Dl and Arm in the plasma membrane (yellow, L). (M–R) Block in endocytic trafficking of Dl and Wg in phyl mutant cells. Live labelling followed by a 3 h chase in third instar eye disc containing a phyl mutant clone (non-green in M and P) stained for Dl (red in N) or Wg (red in Q). Following the chase, Dl and Wg proteins were detected only in phyl mutant cells and not in adjacent wild-type cells where they have been degraded (O and R). (S–U) Dl and Wg co-localize to the same endocytic vesicles. Third instar eye disc containing clones of phyl (non-green tissue in U) stained with Dl (red, S) and Wg (blue, T). The merged panel (U) shows co-localization of Dl and Wg in the endocytic vesicles.
Previous studies have suggested a function of Phyl in binding Sina and a function of this complex in the regulation of the transcriptional repressor, Tramtrack (Kauffmann et al, 1996; Li et al, 1997; Tang et al, 1997). Several lines of evidence suggest that the results on Phyl presented here do not directly involve this mechanism. First, in contrast to the function of Tramtrack in transcriptional regulation, the increased level of Dl and Nintra in phyl mutant cells occurs by a post-transcriptional mechanism (Figure 1H–J). Second, forced expression of Tramtrack does not cause localization defects of Dl and Nintra (Supplementary Figure S5A–I). Third, we observe the phyl phenotypes in cell types, such as the pre-cluster R cells and in the cells of the antennal disc and the wing pouch where Tramtrack is not expressed. Fourth, sinaA16 mutant clones do not show accumulation of Dl or Nintra seen in phyl (Supplementary Figure S5J–O). Most definitively, in double-mutant clones of phyl and ttk, the accumulation of Nintra is similar to that seen in adjacent phyl mutant tissue (Supplementary Figure S5P–S). Finally, Wg expression remains unaltered in GMR-phyl third instar eye disc (not shown) and in sina mutant clones (Supplementary Figure S5T–V). We conclude that the function of phyl in the regulation of Notch and Wg signalling is brought about by a mechanism that is independent of Sina and Tramtrack.
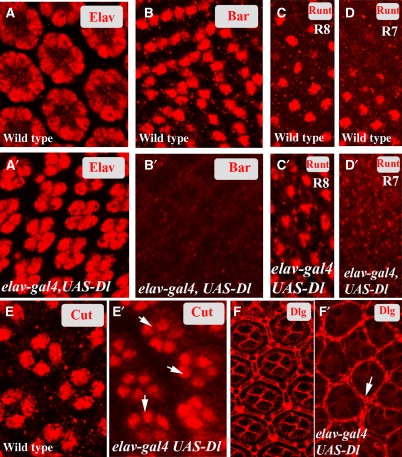
The phenotypic consequences of the loss of Phyl function in the developing eye is the loss of R1, R6 and R7, over-specification of the non-neuronal cone and pigment cells and a loss in the specification of bristle complex (Chang et al, 1995; Dickson et al, 1995), and as elaborated earlier (Figure 1E–G), loss of phyl function results in an increase in the level of Notch signalling. To determine if the cellular phenotypes of phyl mutants can be phenocopied upon overexpression of Dl in the same cells that normally express it, we used elav-Gal4, UAS-Dl combination in which Dl is expressed in the same cells as in wild type but does not undergo downregulation at column 8 (Supplementary Figure S6). elav-Gal4, UAS-Dl third instar eye discs show a loss in the specification of R1, R6 and R7 and an over-specification of non-neuronal cone and pigment cell types (Figure 4A–F′) identical to that seen in phyl mutants (Chang et al, 1995; Dickson et al, 1995). We conclude that the phenotypes of phyl loss of function are due to increased accumulation of Delta and Notch and the consequent increase in Notch signalling.
Figure 4a.
(A–F′) Phenotypic consequences of the lack of Dl downregulation in fate specification of neuronal and non-neuronal cell types. (A) Each wild-type ommatidium shows eight R cells positive for ELAV. (A′) elav-gal4 UAS-Dl ommatidia contain only five R cells per cluster positive for ELAV. (B) In the wild-type larval eye disc, BarH1 expression highlights R1 and R6. (B′) BarH1 expression is missing in elav-gal4 UAS-Dl larval eye discs. (C, D) In wild-type larval eye discs, Runt marks R8 (C) and, at a lower plane, R7 (D) cells. (C′, D′) In elav-gal4 UAS-Dl eye discs, Runt is expressed normally in R8 (C′) but no R7 expression is detected (D′). (E) Wild-type pupal eye disc, stained for Cut, shows expression in four cone cells per cluster. (E′) In elav-gal4 UAS-Dl pupal eye discs, five cone cells are usually detected in each cluster (arrows). (F) Wild-type mid-pupal eye disc, stained for Disc large (Dlg) marking membranes shows a single row of pigment cells surrounding each ommatidial cluster. (F′) In elav-gal4 UAS-Dl, inappropriate specification of pigment cells is seen (arrow).
Previous studies have shown that the transcriptional activation of phyl in the eye disc is directly controlled by the EGFR pathway (Chang et al, 1995; Dickson et al, 1995). phyl expression in R cells initiates at the furrow and peaks by column 8, closely following the profile of Dl expression. Combining this earlier work with the results presented in this article, we propose a model in which activation of the EGFR pathway causes transcription of phyl, which then promotes the trafficking of Dl, Nintra and Wg into a degradation pathway.
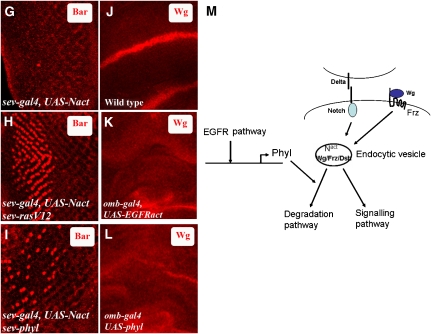
To seek genetic evidence for this suppression of Notch and Wg signalling by EGFR, we expressed the activated but membrane-tethered version of Notch (UAS-Nact) (Doherty et al, 1996), in cells behind the furrow under the control of sev-gal4. As reported previously, this combination activates Notch signalling in a ligand-independent manner and causes a loss of R1 and R6 (Tomlinson and Struhl, 2001) (Figure 4G).We combined sev-gal4 UAS-Nact with sev-RasV12 in which the RTK signal is kept artificially high in sev-expressing cells. This results in a rescue in the specification of R1 and R6 cell fates (Figure 4H). To test if the phenotypes seen with EGFR can be phenocopied by Phyl, we combined sev-gal4, UAS-Nact with sev-phyl in which Phyl and Nact are coexpressed. This too results in a significant rescue in the specification of R1 and R6 cell types (Figure 4I). Likewise, in the wing imaginal disc, ectopic activation of EGFR pathway in the pouch cells using omb-gal4, UAS-EGFRact causes a loss of Wg expression at the D/V boundary (Figure 4K). Once again, this phenotype can be phenocopied by overexpression of Phyl instead of EGFRact in the pouch cells (Figure 4L). Finally, EGFRts mutant clones in the eye disc show increased level of Wg, which is localized in endocytic vesicles (Supplementary Figure S7), a phenotype identical to that seen in phyl mutant clones. These results suggest that increased EGFR signalling through Phyl is required for promoting the degradation of Nintra and Wg in developing imaginal discs.
Figure 4b.
(G–L) EGFR and Phyl downregulate Notch and Wg signalling. (G) In sev-gal4, UAS-Nact third instar eye disc, Bar expression is virtually eliminated. (H) In sev-gal4 UAS-Nact sev-RasV12, Bar expression is partially restored in R1 and R6. (I) A combination of sev-gal4 UAS-Nact with sev-phyl also partially restores Bar expression to R1 and R6. (J) Wild-type expression of Wg in the third instar wing imaginal disc; Wg is expressed at the D/V boundary of the wing disc. (K) In omb-Gal4, UAS-EGFRact, Wg expression is lost at the D/V boundary. (L) In omb-Gal4, UAS-phyl combination as well, Wg expression is lost at the D/V boundary. (M) A model for Phyllopod in the negative regulation of Notch and Wg signalling. Phyl expression is dependent on the EGFR/Ras/MAPK kinase pathway. Activation of Phyl expression promotes degradation of Dl, Nintra and Wg. In the absence of Phyl, Dl/Nintra and Wg are trapped in endocytic vesicles. Excess endosomal Nintra and Wg/DFrz/Dsh signalling complex trapped in the endocytic vesicles causes an increase in signalling levels. During normal development, EGFR activation, through the transcriptional upregulation of Phyl, will downregulate Notch and Wg signals once their developmental function has been accomplished.
In this article, we report a specific negative regulatory mechanism for the Notch and Wg pathways that functions at the level of endocytic vesicles. The adaptor protein, Phyllopod, allows a balanced level of activated components of Notch and Wg pathways to be made available after a round of signalling. As phyl is a transcriptional target of EGFR signalling, these observations suggest a negative cross talk between RTK and Notch /Wg pathways (Figure 4M).
Mutations of the endocytic machinery, such as the core ESCRT complex components vsp 25 and vsp 23, also cause accumulation of Nintra in endosomes and upregulation of Wg signalling (Moberg et al, 2005; Thompson et al, 2005; Vaccari and Bilder, 2005). However, in contrast to phyl, loss-of-function mutations in ESCRT complex components result in increased apoptosis and non-cell autonomous cell proliferation in the undifferentiated cells ahead of the MF. Phyl is not expressed in these cells nor does its inactivation in the 2nd instar eye disc show defects in the localization of Dl and Notch. The function of Phyl is restricted to specific developmental events that involve EGFR-mediated regulation of Notch and Wg pathways. ESCRT components function broadly and their loss causes multiple defects not seen in phyl mutants, such as defective membrane trafficking and loss of apicobasal polarity. Finally, activation of Notch signalling in ESCRT mutations is independent of ligand function (Thompson et al, 2005), whereas the phenotypic consequences of phyl mutation are found to be dependent on signalling from a functional ligand (Figure 2L).
Our studies suggest that Phyl regulates the residence time of the components of Notch and Wg signalling pathways in early endocytic vesicles. It is important to point out that Phyl loss of function gives rise to a gain-of-function phenotype for Notch and Wg. Thus, trapping of these receptors in endocytic vesicles increases, rather than attenuates, the signal. This post-transcriptional downregulation of Notch and Wg signalling by Phyl allows fine-tuning of the signal and creates a delicate balance between active signalling of Notch/Wg pathways and their degradation by the lysosomal pathway. This balance provides a mechanism for the modulation of the strength of signals during development. Phyl is a novel adaptor protein whose cytoplasmic partners remain to be identified in future biochemical analysis. However, this genetic loss-of-function study allows us to identify specific endosomal components that exclusively include Notch and Wg signalling components that are regulated by Phyl. As EGFR, Notch and Wg pathways function together in several developmental scenarios, this mechanism provides an effective means for the coordinated temporal and spatial regulation of development by the combined actions of these pathways.
Materials and methods
Drosophila stocks
phyl2245, mib1, UAS-Dl, sev-Gal4 were obtained from the Bloomington stock centre. Ubx-Flp (on X) was obtained from H Bellen (Jafar-Nejad et al, 2005). UAS-Nact was obtained from YN Jan (Doherty et al, 1996). UAS-RasV12 was obtained from GM Rubin (Karim and Rubin, 1998).
Immunohistochemistry
Larval or pupal eye discs were dissected in PBS and fixed in 4% formaldehyde in PBS for 40 min. The fixed tissue was permeabilized in PBST (0.4% Triton X-100) and incubated in primary antibody anti-Delta, 1/100 (Parks et al, 1995), anti-Bar 1/50 (Hayashi et al, 1998), anti-Cut 1/20 (Chang et al, 1995), anti-Dlg 1/20 (Woods et al, 1997), anti-β-Gal 1/50 mouse (Promega), 1/200 rabbit (Capel), anti-HRS 1/500 (Lloyd et al, 2002), anti-Nintra 1/20 (Vaccari and Bilder, 2005), anti-Nextra 1/20 (Vaccari and Bilder, 2005), anti-Ubi 1/100 mouse (Jekely and Rorth, 2003) (Affiniti), anti-Ubi 1/200 rabbit (Moberg et al, 2005) (Dako Cytomation), anti-Wg mouse 1/30 (Neumann and Cohen, 1997), anti-Avl chicken 1/200 (Vaccari and Bilder, 2005), anti-Hook, rabbit 1/100 (Kramer and Phistry, 1996), anti-Dor G.pig 1/100 (Sevrioukov et al, 1999), anti-Rabll rabbit 1/200 (Satoh et al, 2005), anti-Sens 1/500 G.Pig (Nolo et al, 2000), anti-DFrz Rat 1/100 (Ataman et al, 2008), anti-Dsh rabbit 1/200 (Shimada et al, 2001; Packard et al, 2002, 2003; Speese and Budnik, 2007; Ataman et al, 2008) overnight, washed in PBST three times and incubated in appropriate secondary florescent-labelled antibody. Images were captured using a Bio-Rad Confocal microscope.
Lysotracker staining
Lysotracker staining was performed on unfixed tissue. Eye discs were dissected in Schneider's medium at room temperature and stained for Lysotracker red 99 (Invitrogen) at a dilution of 1/1000 for 5 min. The discs were washed twice in PBS and mounted in Vectashield and images were captured using Bio-Rad confocal microscope.
Supplementary Material
Supplementary Data
Acknowledgments
We thank S Artavanis Tsakonas, H Bellen, G Fischer, I Hariharan, YN Jan, E Lai, GM Rubin, F Schweisguth, G Struhl and the Bloomington stock centre for fly stocks. We are grateful to H Bellen for providing anti-HRS and anti-Senseless antibody, M Muskavitch for guinea pig anti-Delta antibody, Helmut Kramer for anti-Dor and anti-hook antibody, David Bilder for anti-Avl antibody, Vivian Budnik for Dfrz antibody and T Uemura for anti-Dsh antibody. Monoclonal anti-Delta, anti-Cut and anti-Dlg antibodies developed by S Artavanis Tsakonas, G Rubin and C Goodman, respectively, were obtained from the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by NIH grant EY08152 to UB.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 [DOI] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V (2008) Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron 57: 705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R (2006) A critical role for endocytosis in Wnt signaling. BMC Cell Biol 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Chang HC, Solomon NM, Wassarman DA, Karim FD, Therrien M, Rubin GM, Wolff T (1995) phyllopod functions in the fate determination of a subset of photoreceptors in Drosophila. Cell 80: 463–472 [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Dominguez M, van der Straten A, Hafen E (1995) Control of Drosophila photoreceptor cell fates by phyllopod, a novel nuclear protein acting downstream of the Raf kinase. Cell 80: 453–462 [DOI] [PubMed] [Google Scholar]
- Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN (1996) Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev 10: 421–434 [DOI] [PubMed] [Google Scholar]
- Fischer JA, Eun SH, Doolan BT (2006) Endocytosis, endosome trafficking, and the regulation of Drosophila development. Annu Rev Cell Dev Biol 22: 181–206 [DOI] [PubMed] [Google Scholar]
- Hagedorn EJ, Bayraktar JL, Kandachar VR, Bai T, Englert DM, Chang HC (2006) Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J Cell Biol 173: 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kojima T, Saigo K (1998) Specification of primary pigment cell and outer photoreceptor fates by BarH1 homeobox gene in the developing Drosophila eye. Dev Biol 200: 131–145 [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ (2005) Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell 9: 351–363 [DOI] [PubMed] [Google Scholar]
- Jekely G, Rorth P (2003) Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep 4: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim FD, Rubin GM (1998) Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125: 1–9 [DOI] [PubMed] [Google Scholar]
- Kauffmann RC, Li S, Gallagher PA, Zhang J, Carthew RW (1996) Ras1 signaling and transcriptional competence in the R7 cell of Drosophila. Genes Dev 10: 2167–2178 [DOI] [PubMed] [Google Scholar]
- Kramer H, Phistry M (1996) Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J Cell Biol 133: 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM (2005) The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development 132: 2319–2332 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Bardin A, Schweisguth F (2005a) The roles of receptor and ligand endocytosis in regulating Notch signaling. Development 132: 1751–1762 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Remaud S, Hamel S, Schweisguth F (2005b) Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol 3: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li Y, Carthew RW, Lai ZC (1997) Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell 90: 469–478 [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ (2002) Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108: 261–269 [DOI] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK (2005) Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell 9: 699–710 [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U (2007) Combinatorial signaling in the specification of primary pigment cells in the Drosophila eye. Development 134: 825–831 [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM (1997) Long-range action of Wingless organizes the dorsal–ventral axis of the Drosophila wing. Development 124: 871–880 [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ (2000) Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102: 349–362 [DOI] [PubMed] [Google Scholar]
- Overstreet E, Fitch E, Fischer JA (2004) Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development 131: 5355–5366 [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V (2002) The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111: 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V (2003) Wnts and TGF beta in synaptogenesis: old friends signalling at new places. Nat Rev Neurosci 4: 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA (2000) Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127: 1373–1385 [DOI] [PubMed] [Google Scholar]
- Parks AL, Turner FR, Muskavitch MA (1995) Relationships between complex Delta expression and the specification of retinal cell fates during Drosophila eye development. Mech Dev 50: 201–216 [DOI] [PubMed] [Google Scholar]
- Pi H, Wu HJ, Chien CT (2001) A dual function of phyllopod in Drosophila external sensory organ development: cell fate specification of sensory organ precursor and its progeny. Development 128: 2699–2710 [DOI] [PubMed] [Google Scholar]
- Satoh AK, O'Tousa JE, Ozaki K, Ready DF (2005) Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132: 1487–1497 [DOI] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ (2006) Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol 173: 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H (1999) A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol Cell 4: 479–486 [DOI] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T (2001) Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol 11: 859–863 [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V (2007) Wnts: up-and-coming at the synapse. Trends Neurosci 30: 268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Neufeld TP, Kwan E, Rubin GM (1997) PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell 90: 459–467 [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM (2005) Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell 9: 711–720 [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Struhl G (2001) Delta/Notch and Boss/Sevenless signals act combinatorially to specify the Drosophila R7 photoreceptor. Mol Cell 7: 487–495 [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 9: 687–698 [DOI] [PubMed] [Google Scholar]
- Wang W, Struhl G (2004) Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131: 5367–5380 [DOI] [PubMed] [Google Scholar]
- Wang W, Struhl G (2005) Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development 132: 2883–2894 [DOI] [PubMed] [Google Scholar]
- Wilkin MB, Carbery AM, Fostier M, Aslam H, Mazaleyrat SL, Higgs J, Myat A, Evans DA, Cornell M, Baron M (2004) Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol 14: 2237–2244 [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF (1991) The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development 113: 841–850 [DOI] [PubMed] [Google Scholar]
- Woods DF, Wu JW, Bryant PJ (1997) Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet 20: 111–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data