Abstract
Nutrient secretagogues activate mitochondria of the pancreatic β-cell through the provision of substrate, hyperpolarisation of the inner mitochondrial membrane and mitochondrial calcium rises. We report that mitochondrial matrix pH, a parameter not previously studied in the β-cell, also exerts an important control function in mitochondrial metabolism. During nutrient stimulation matrix pH alkalinises, monitored by the mitochondrial targeted fluorescent pH-sensitive protein mtAlpHi or 31P-NMR inorganic phosphate chemical shifts following saturation transfer. Compared with other cell types, the resting mitochondrial pH was surprisingly low, rising from pH 7.25 to 7.7 during nutrient stimulation of rat β-cells. As cytosolic alkalinisation to the nutrient was of much smaller amplitude, the matrix alkalinisation was accompanied by a pronounced increase of the ΔpH across the inner mitochondrial membrane. Furthermore, matrix alkalinisation closely correlates with the cytosolic ATP net increase, which is also associated with elevated ATP synthesis rates in mitochondria. Preventing ΔpH increases in permeabilised cells abrogated substrate-driven ATP synthesis. We propose that the mitochondrial pH and ΔpH are key determinants of mitochondrial energy metabolism and metabolite transport important for cell activation.
Keywords: ATP synthesis rates, β-cell, insulin secretion, metabolism, mtAlpHi
Introduction
The pancreatic β-cell senses a large number of signals, including peptide hormones, neurotransmitters and nutrients, thereby adjusting insulin granule exocytosis to the requirements of the organism to regulate glucose homeostasis. Oxidative metabolism of nutrients causes a plethora of intracellular changes resulting in β-cell activation (Hellman et al, 1974; Detimary et al, 1996; Maechler and Wollheim, 1999; Wollheim and Maechler, 2002; Cline et al, 2004; Ronnebaum et al, 2006). Mitochondria sense increased substrate availability and nutrient-derived signals, such as the increase in mitochondrial calcium, required to augment the rate of CO2 production during nutrient stimulation (Wollheim and Maechler, 2002; Wiederkehr and Wollheim, 2006). The resulting synthesis of ATP and intermediary metabolites act as signals linking nutrient metabolism to insulin granule exocytosis (Detimary et al, 1996; Kennedy et al, 1999; Ishihara et al, 2003). ATP causes closure of the KATP channels. This in turn results in the depolarisation of the plasma membrane potential, and calcium enters through voltage-gated channels triggering insulin granule exocytosis (Wollheim and Maechler, 2002; Wiederkehr and Wollheim, 2008). Glucose further stimulates insulin secretion by the amplifying pathway (Gembal et al, 1992), possibly depending on signaling through metabolic intermediates (Maechler and Wollheim, 1999; Ivarsson et al, 2005; Joseph et al, 2006; Wiederkehr and Wollheim, 2006). Consistent with this proposal, mitochondrial metabolite transport is required for efficient glucose-dependent insulin secretion (Joseph et al, 2006; Ronnebaum et al, 2006).
Transport of metabolites across the inner mitochondrial membrane is an active process linked directly or indirectly to the proton electrochemical gradient. According to the Nernst equation, this potential is composed of an electrical and a chemical component responsible for the transport of different sets of metabolites (Simpson and Hager, 1984; Palmieri, 2004). As the electrical and chemical potentials influence the export of ATP, metabolites and ions differentially, they may have different impacts on the export of these from the mitochondria and on their ability to act as signals in metabolism–secretion coupling.
The electrical potential across the inner mitochondrial membrane of the β-cells is augmented during nutrient stimulation (Duchen et al, 1993; Maechler and Wollheim, 1999). This hyperpolarisation increases the driving force on the ATP synthase and accelerates electrogenic transport steps such as the export of ATP in exchange for ADP (Das, 2003; Palmieri, 2004).
The proton chemical gradient is proportional to the pH difference between the mitochondrial matrix and the cytosol (ΔpH) and serves as the driving force for the import and export of a large number of metabolites across the inner mitochondrial membrane, also contributing to the driving force on the ATP synthase (Simpson and Hager, 1984; Das, 2003; Palmieri, 2004). Through its effect on metabolite transport, the ΔpH may be important for the amplifying pathway of insulin secretion.
The cytosolic pH (pHcyto) is close to neutral (pH 7.0–7.2) in various mammalian cell types. In the β-cell, pHcyto undergoes a slight alkalinisation from pH 7.0 to 7.1 in response to glucose (Shepherd and Henquin, 1995; Stiernet et al, 2007). In contrast to the pHcyto, the mitochondrial pH (pHmito) has been found to be quite alkaline, ranging from 7.6 to 8.3 in a number of different cells (Llopis et al, 1998; Matsuyama et al, 2000; Abad et al, 2004; Balut et al, 2008). The dynamics of pHmito changes with regard to their role in cell activation have not been studied to date.
Results
Metabolically active inorganic phosphate pools in INS-1 cells
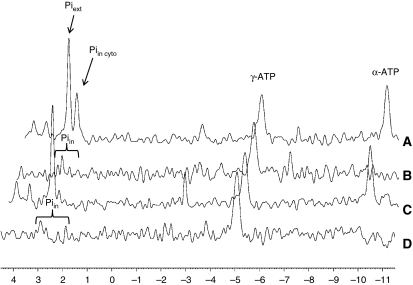
31P-NMR provides a non-invasive means of measuring intracellular pH. In particular, the chemical shift of inorganic phosphorus has been shown to be pH dependent (Gadian et al, 1979; Petroff et al, 1985). Intracellular pH was measured in INS-1 cells based on the chemical shift in the 31P-NMR spectra of intracellular inorganic phosphate (Pi). Pi–pH titration curves were obtained from the Pi and the α-phosphate of ATP chemical shift differences (see Materials and methods). The chemical shifts of α-phosphate of ATP (10.2 p.p.m.) remain constant over the range of pH changes studied here (Gadian et al, 1979). 31P-NMR measurements of the intracellular pH in INS-1 cells were obtained at resting glucose concentrations in the perifusion buffer maintained at pH 7.3. Under these conditions, the extracellular Pi peak appears at 2.72 p.p.m., and an intracellular Pi pool appears at 2.39 p.p.m. (Figure 1A). In the standard NMR spectrum, any intracellular Pi pool with a pH between 7.2 and 7.4 co-resonates with the extracellular Pi. The only intracellular Pi pool that is readily observable is the one that appears at 2.39 p.p.m., corresponding to a pH of 6.97, a value in agreement with the expected pHcyto. By performing saturation transfer experiments, we were able to detect and identify 31P-NMR resonances that correspond to Pi pools that are metabolically active as reactants for the synthesis of ATP from ADP and Pi (Cohen et al, 1978; Cline et al, 2001). We observed two metabolically active Pi pools, one at 2.37 p.p.m. and the other co-resonating with the extracellular Pi (Figure 1B). On increasing the glucose concentration to 15 mM, we observed an ∼10-fold drop in the intracellular Pi signal at 2.37 p.p.m., and the appearance of a downfield shoulder on the extracellular Pi peak (Figure 1C). Interestingly, the saturation transfer experiment revealed a shift of the downfield metabolically active Pi peak by another 47 Hz (Figure 1D). The downfield shift in the resonance suggests that this metabolically active Pi pool senses alkalinisation of a specific intracellular compartment. On increasing the glucose concentration from 2 to 15 mM, we observed an average alkalinisation from pH 7.45 to 7.73 associated with this Pi pool, whereas the Pi peak at 2.39 p.p.m. was only little affected when elevating the glucose concentration (Table I). The different Pi pools could correspond to different intracellular compartments, raising the question which compartment was undergoing alkalinisation during glucose stimulation. We speculated that it could be the mitochondrial matrix space because (1) it is a site of active ATP synthesis and (2) the mitochondrial matrix pH is more alkaline than the pHcyto.
Figure 1.
31P-NMR spectra revealing different phosphate pools in INS-1 cells. Representative 31P-NMR spectra of alginate-entrapped INS-1 cells. The cells were perifused in oxygenated KRB buffer containing 2.5 mM glucose (A, B) or 15 mM glucose (C, D). The control spectra of the saturation transfer experiment, with the saturation pulse downfield of Pi at 2.5 mM glucose (A) and 15 mM glucose (C), show resonances corresponding to the extracellular Pi at 2.73 p.p.m. and intracellular Pi at 2.39 p.p.m. The difference between the two spectra of the saturation transfer experiment (saturation pulse at β-ATP or downfield of Pi) reveals metabolically active intracellular Pi pools, with their chemical shifts dependent on the pH of their respective intracellular location. For ease of comparison, difference spectra (B, D) are shown inverted. (In the difference spectra, the β-ATP peaks and Pi peaks have normally negative excursion. The α-ATP peak is not affected by the saturation of the β-ATP resonance and is subtracted out in the difference.)
Table 1.
31P-NMR-measured cytosolic and mitochondrial pH in INS-1 cells
| Glucose concentration | Cytosolic | Mitochondrial |
|---|---|---|
| Glc 2.5 mM (n=5) | 6.95±0.03 | 7.45±0.13 |
| Glc 15 mM (n=5) | 7.01±0.04 | 7.73±0.13* |
| pH was determined from the chemical shift frequency of Pi referenced to the α-phosphate of ATP. | ||
| Values are reported as mean±s.e.m. | ||
| *P<0.05 compared with basal measurement. | ||
Mitochondrial matrix alkalinisation during glucose stimulation of the β-cell
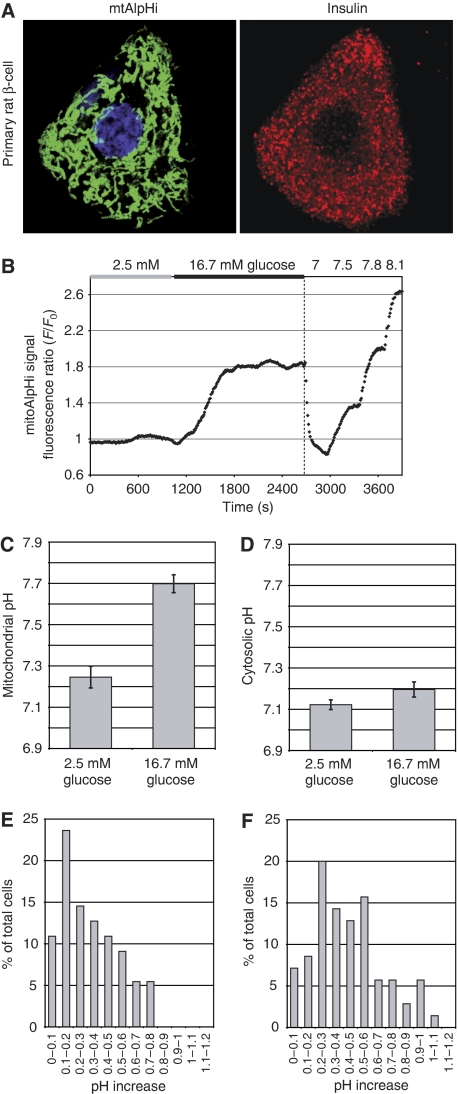
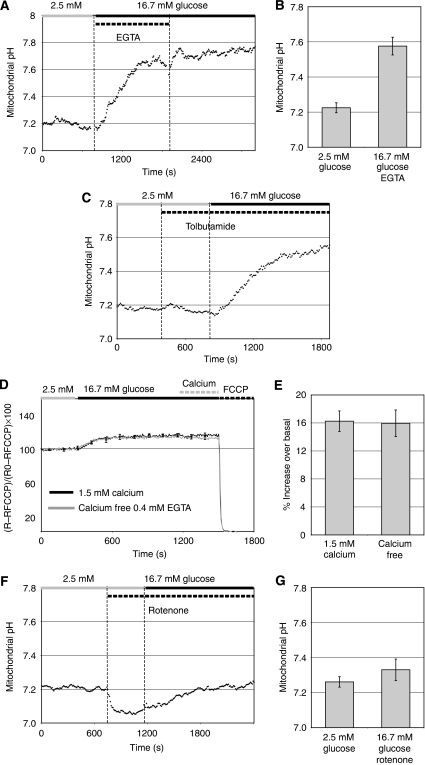
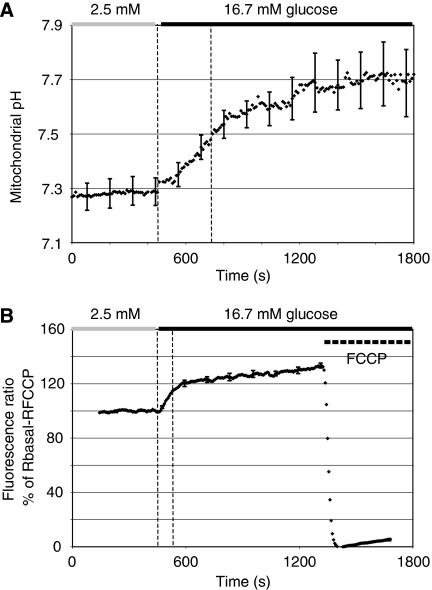
To test this possibility, we set out to measure pHmito using the pHmito probe mtAlpHi. This yellow fluorescent protein-based probe displays a close to linear fluorescence emission increase over a pH range from 7.0 to 8.5 (Abad et al, 2004). To express the mtAlpHi not only in cell lines but also in primary β-cells, we re-cloned the transgene into an adenovirus vector (Ad-tetON-mtAlpHi). After infection and induction of the tetON-mtAlpHi virus (see Materials and methods) in primary islet cells and INS-1E cells, mtAlpHi displayed a mitochondrial pattern of localisation (Figure 2A and data not shown) similar to its published distribution in HeLa cells (Abad et al, 2004). Insulin immunofluorescence was performed to confirm that mtAlpHi localisation was indeed analysed in β-cells (Figure 2A). To confirm the pH sensitivity of mitoAlpHi after adenovirus-mediated overexpression, we used ammonium chloride and FCCP. Ammonium chloride caused a large transient increase of the mtAlpHi fluorescence, consistent with its ability to alkalinise intracellular compartments (Supplementary Figure 1A). Uncoupling by FCCP also dissipates the ΔpH across the inner mitochondrial membrane, causing acidification and therefore a rapid reduction of mtAlpHi fluorescence (Supplementary Figure 1B). The mtAlpHi fluorescence is responsive to pH shifts, in the direction of both acidification and alkalinisation. Remarkably, stimulation of rat islets with 16.7 mM glucose caused an increase of the mtAlpHi fluorescence, suggesting alkalinisation of the matrix space by the nutrient (Figure 2B). Qualitatively similar results were observed after glucose stimulation of INS-1E cells (Supplementary Figure 1C). The onset of the mitochondrial alkalinisation response usually occurred rapidly after a step increase in the glucose concentration. In rat islets, the pH increase appeared more pronounced than in INS-1E cells and took 10–15 min before reaching a new steady state. In some measurements, mtAlpHi fluorescence reached a first maximum to then return to a steady state between basal and maximal fluorescence (see Figure 3A). To estimate the extent of glucose-mediated alkalinisation, we titrated the mtAlpHi fluorescence as shown in Figure 2B. Using such titration protocols, we find matrix pH to be surprisingly low both in INS-1E (pH 7.30±0.04; N=13; Figure 3B) and in islet cells (pH 7.25±0.05; N=6; Figure 2C). After glucose stimulation, the pHmito increased in INS-1E (Figure 3B; pH 7.65±0.06; N=13) and islet cells (Figure 2C; pH 7.70±0.04; N=6). In the control hepatoma cell line HepG2, matrix pH was alkaline (close to 7.8) independent of the glucose concentration (Figure 3D and E). Addition of the complex I inhibitor rotenone caused a rapid acidification of the matrix pH of HepG2 cells, demonstrating that the matrix pH can be altered in this cell type (Figure 3D). For INS-1E and rat islet, we also analysed the alkalinisation responses of the individual cells (Figure 2E and F). Alkalinisation was observed in the majority of islet cells (Figure 2F; 93%) and INS-1E cells (Figure 2E; 89%). In the rat islets, 63% of the mtAlpHi-positive cells showed alkalinisation responses between 0.2 and 0.6 pH units, with a fraction of the cells (21%) displaying even larger mitochondrial alkalinisation (Figure 2F).
Figure 2.
Mitochondrial matrix alkalinisation in response to glucose in rat islets. Dissociated rat islet β-cells (A) or intact rat islets (B, C) were infected with an adenovirus carrying mtAlpHi under the control of the tetON promoter (Ad-tetON-mtAlpHi). Insulin immunofluorescence (A; right panel) was performed to identify β-cells expressing mtAlpHi (A; left panel). (B, C) Infected rat islets were incubated in 2.5 mM glucose and shifted to 16.7 mM glucose as indicated. For titrations of mtAlpHi fluorescence (B; see also Materials and methods), the intra-mitochondrial pH was clamped to pH values as indicated on top of the trace. (C) Average mitochondrial pH responses to glucose in rat islets (N=7). (D) Average pHcyto glucose responses in rat islets (N=9). The results are expressed as ±s.e.m. Cytosolic pH (D) was measured using the ratiometric fluorescent probe BCECF. (E, F) Histograms of single-cell analysis of matrix alkalinisation in INS-1E (E) and individual cells in intact rat islets (F). The increase of the pHmito was determined in single INS-1E (55 cells) and rat islets (70 cells from 10 islets). The cells were grouped according to the extent of alkalinisation in 0.1 pH unit increments and expressed as the percentage of total cells analysed.
Figure 3.
Mitochondrial pH in INS-1E and HepG2 cells. INS-1E cells (A–C) or HepG2 cells (D, E) expressing mtAlpHi were incubated in 2.5 mM glucose and shifted to 16.7 mM glucose as indicated. Average pHmito changes to glucose±s.e.m. in INS-1E cells (B; N=13) and HepG2 cells (E; N=4) are shown. (C) Average pHcyto changes to glucose±s.e.m. in INS-1E cells. Rotenone was used at a concentration of 2.5 μM.
Mitochondrial and cytosolic ATP synthesis rates
The results obtained after expression of mtAlpHi in INS-1 cells are consistent with the Pi shift demonstrating alkalinisation of a specific phosphate pool during glucose stimulation (Figure 1 and Table I). These results would argue that the metabolically active Pi peaks observed at ∼2.7 p.p.m. and above correspond to the mitochondrial phosphate pool. On the basis of the assignment of cytosolic and mitochondrial Pi pools, we used saturation transfer experiments to determine ATP synthesis rates in the two compartments. Although these experiments provide an absolute value for the unidirectional rate constant for ATP synthesis (kATP), the actual rates of cytosolic and mitochondrial ATP synthesis cannot be calculated without knowing the absolute pool sizes. However, as the changes in Pi concentration were calculated from the known concentration of Pi in the extracellular medium, the concentration of Pi in each intracellular compartment can be expressed in terms of the total volume of the bioreactor. This provides a total rate of ATP synthesis from each compartment and allows us to directly compare the total rates of mitochondrial and cytosolic ATP synthesis. For the cytosolic Pi pool at 2.37 p.p.m., we calculated a 5.7-fold increase in the unidirectional rate constant of ATP synthesis from Pi, but because of the significant drop in the NMR-observable intracellular Pi concentration, this translated to a 1.7-fold increase in the ATP synthesis rate (Table II). For the mitochondrial Pi pool at 2.67 p.p.m., we calculated a 2.5-fold increase in both the kATP and ATP synthesis rates (Table II).
Table 2.
31P-NMR-measured ATP synthesis rate parameters in INS-1 cells
| Cellular compartment | kATP (min−1) | ATP synthesis rate (mM/min in bioreactor volume) | ||
|---|---|---|---|---|
| Glc 2.5 mM | Glc 15 mM | Glc 2.5 mM | Glc 15 mM | |
| Cytosolic (n=4) | 2.4±1.1 | 13.7±3.1* | 0.58±0.45 | 1.0±0.37 |
| Mitochondrial (n=4) | 1.9±1.2 | 4.6±1.4 | 0.93±0.61 | 2.29±0.76 |
| kATP is the unidirectional rate constant for synthesis of ATP from ADP+Pi. ATP synthesis rate is expressed in terms of mmoles ATP per litre bioreactor volume per minute. | ||||
| Values are reported as mean±s.e.m. | ||||
| *P<0.05 compared with basal measurement. | ||||
ΔpH increases during glucose stimulation
The observed pH increases in the mitochondrial matrix of the β-cell are quite pronounced, several-fold larger than the published glucose-dependent elevation of the pHcyto (Shepherd and Henquin, 1995; Stiernet et al, 2007). To assess pHcyto changes under our experimental conditions, we used 31P-NMR in INS-1 cells (Table I) or the pH-sensitive fluorescent probe BCECF in INS-1E cells and rat islets (Figures 2D and 3C). Consistent with the published data, we find only a small increase of the pHcyto in both rat islets and INS-1E cells after glucose addition (Table I; Figures 2D and 3C). Direct comparison of pHcyto and pHmito reveals that the ΔpH across the inner mitochondrial membrane is very low at resting glucose concentrations. In rat islet cells, the ΔpH was estimated to be 0.13 pH units. At the stimulatory glucose concentration, a pronounced increase of the ΔpH to 0.51 pH units was measured.
Leucine but not glutamine causes mitochondrial alkalinisation in rat β-cells
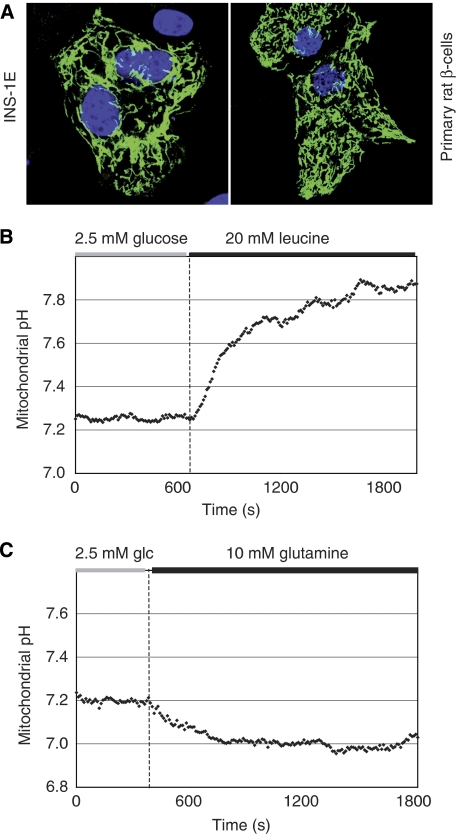
To assure that the above-described mitochondrial matrix alkalinisation occurs specifically in the islet β-cells, mtAlpHi was also placed behind the rat insulin promoter (Ad-RIP-mtAlpHi). Although expression was less strong than using the tetON system, proper targeting to mitochondria in INS-1E cells and rat islet cells (Figure 4A) and pH sensitivity of the probe was preserved. Glucose responses in rat islets were indistinguishable from those obtained with Ad-tetON-mtAlpHi (data not shown). Leucine, another insulin secretagogue, induced alkalinisation similar to glucose using Ad-RIP-mtAlpHi (Figure 4B) or Ad-tetON-mtAlpHi (data not shown). Leucine metabolism provides Acetyl-CoA for mitochondrial oxidation, bypassing many reactions required for glucose metabolism. Therefore, the alkalinisation of the matrix is not specifically linked to glucose metabolism but rather to the activation of mitochondrial oxidative metabolism. On the other hand, glutamine, which does not serve as an efficient secretagogue in the β-cell (Sener et al, 1981) and does not cause any significant increase in ATP generation (data not shown), did not alkalinise the mitochondrial matrix pH (Figure 4C). The results substantiate the conclusion that the mitochondrial matrix of pancreatic β-cells alkalinises after stimulation with nutrient secretagogues.
Figure 4.
β-Cell-specific expression of mtAlpHi for the measurement of mitochondrial pH responses to leucine and glutamine. INS-1E cells, dispersed islet cells (A) or intact rat islets (B, C) were infected with an adenovirus expressing mtAlpHi under the control of the rat insulin promoter (Ad-RIP-mtAlpHi). (A) Mitochondrial localisation of mtAlpHi was confirmed in INS-1E and primary rat β-cells. Mitochondrial matrix alkalinisation was induced by leucine (20 mM; B) but not glutamine (10 mM; C).
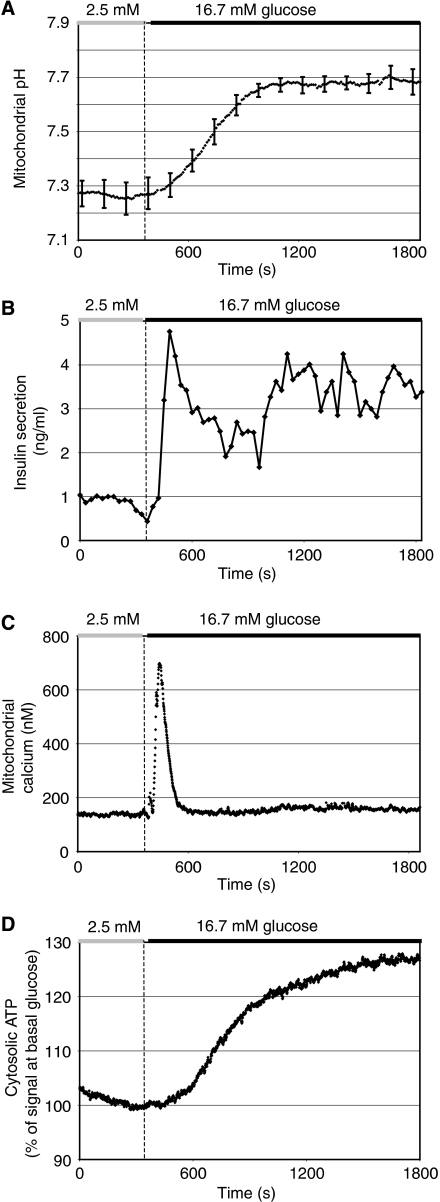
Kinetics of matrix pH alkalinisation during metabolism–secretion coupling
To place the observed matrix pH alkalinisation into the context of metabolism–secretion coupling, we compared the average pH response to glucose in rat islets (Figure 5A) with the kinetics of a number of other responses such as insulin secretion (Figure 5B), the mitochondrial calcium rise (Figure 5C) and the augmentation of cytosolic ATP levels (Figure 5D) after glucose stimulation. This comparison reveals that the new steady state of pHmito is reached relatively slowly, lagging for instance behind first-phase insulin secretion (Figure 5A and B). Furthermore, the prominent rise of mitochondrial calcium preceded the alkalinisation response (Figure 5A and C). Given the early mitochondrial calcium rise, we argued that mitochondrial calcium could be an upstream signal required to initiate matrix alkalinisation. To test this possibility, we stimulated rat islets (Figure 6A and B) or INS-1E cells (data not shown) with glucose in the absence of extracellular calcium. Under these conditions, glucose does not raise mitochondrial calcium (data not shown). Despite the absence of the calcium signal, the mitochondrial matrix pH increased rapidly from a pH of 7.22±0.03 (N=3) to an average pH of 7.58±0.05 (N=3) at high glucose concentration (Figure 6B), a rise which is about 20% smaller than under control conditions. Re-addition of calcium caused a further small alkalinisation of the pHmito (Figure 6A). The non-nutrient secretagogue tolbutamide, which closes the KATP channel, thereby raising calcium in the cytosol and mitochondria, did not cause alkalinisation (Figure 6C). Therefore, calcium is neither necessary nor sufficient to cause mitochondrial alkalinisation. We also observed that the glucose-dependent hyperpolarisation of the mitochondrial membrane potential was unchanged in calcium-free medium (Figure 6D). In the dispersed rat islet cells, the membrane potential, monitored ratiometrically with the lipophilic cation JC-1 (Park et al, 2008), was elevated by 16% in response to glucose both in the presence and in the absence of extracellular calcium (Figure 6D and E). Re-addition of calcium did not cause depolarisation of the mitochondrial membrane potential. Together with our results on pHmito changes, this finding shows that hyperpolarisation of the proton electrochemical gradient is only little affected by glucose-dependent calcium signals.
Figure 5.
Comparison of glucose-evoked mitochondrial matrix alkalinisation, insulin secretion, mitochondrial calcium and cytosolic ATP rises in rat islets. Individual rat islets were analysed as described in Figure 2. (A) Average mitochondrial pH response. For clarity, the error bars (N=7) are displayed only every 120 s. Insulin secretion (B), mitochondrial calcium (C) and cytosolic ATP (D) were measured from groups of 100–150 islets. For mitochondrial calcium or ATP measurements, islets were infected with Ad-RIP-mtAequorin or Ad-RIP-Luciferase, respectively. Insulin samples were collected every 30 s from the efflux of mitochondrial calcium measurements.
Figure 6.
Mitochondrial calcium signals play little or no role in mitochondrial matrix alkalinisation or hyperpolarisation of the electrical potential in β-cell mitochondria. (A) Intact rat islets expressing mtAlpHi were stimulated with 16.7 mM glucose in the absence of extracellular calcium (KRBH containing 16.7 mM glucose, 0 mM calcium chloride, 0.4 mM EGTA). As indicated, EGTA was then removed and 1.5 mM calcium chloride was added. (B) Glucose responses of the pHmito in the absence of extracellular calcium. Values are presented as mean±s.e.m. (N=4). (C) Rat islets expressing mtAlpHi were stimulated with tolbutamide (500 μM) followed by glucose (16.7 mM) in the continued presence of tolbutamide. (D) Ratiometric measurements of JC-1 fluorescence changes during glucose stimulation (Materials and methods). Dispersed rat islet cells were loaded with JC-1. Changes in fluorescence ratios from basal levels were normalised to ratio differences between resting and maximal depolarisation induced by 10 μM FCCP and expressed as percent changes of the mitochondrial membrane potential. At the end of the trace, EGTA was removed and 1.5 mM calcium was added to the cells lacking extracellular calcium. Average glucose responses in the presence (black trace) or absence of extracellular calcium (grey trace; 0.4 mM EGTA) are shown. (E) Quantification of the ratio changes. (F) Rat islets expressing mtAlpHi were treated with 1 μM rotenone and then stimulated with 16.7 mM glucose. (G) Average pHmito responses of rat islets to glucose in the presence of 1 μM rotenone.
Effect of respiratory chain activity and hyperpolarisation of the mitochondrial membrane on matrix alkalinisation
Next, we tested how mitochondrial respiratory function may relate to matrix alkalinisation. After inhibition of the respiratory chain complexes I by rotenone, a rapid acidification was noticed both in rat islets (Figure 6F) and in INS-1E cells (data not shown). Therefore, respiratory chain-dependent proton pumping is required to maintain the ΔpH. Addition of rotenone at the time of glucose stimulation almost completely suppressed the alkalinisation response to the nutrient in rat islets (Figure 6G). The results show that respiratory chain function is a key driving force for matrix alkalinisation.
Interestingly, the electrical potential and matrix pH changes did not follow the same kinetics during glucose stimulation (Figure 7). While mtAlpHi fluorescence increased quite steadily over a time period of 10 min in INS-1E cells (Figure 7A), the electrical potential as measured with JC-1 initially rose rapidly, followed by a slow second phase (Figure 7B). Half-maximal hyperpolarisation was reached after 88 s. In the case of the pH measurement, the half-maximal response was only reached 270 s after glucose addition. This result argues that net proton extrusion by the respiratory chain from the mitochondrial matrix can be buffered at least during initiation of the response.
Figure 7.
Kinetic comparison of matrix alkalinisation and the increase of the mitochondrial electrical potential. INS-1E cells expressing mtAlpHi (A; N=6) or loaded with JC-1 (B; N=7) were stimulated with 16.7 mM glucose. For clarity, standard errors are included only every 120 s. FCCP (10 μM) was used to depolarise the mitochondria.
Role for matrix pH in mitochondrial ATP generation
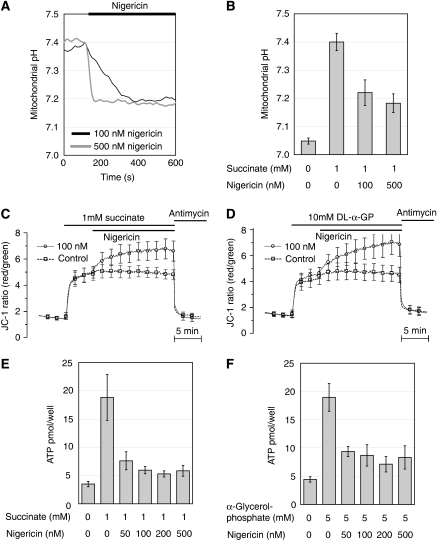
We noted a close correlation between alkalinisation and net ATP changes after the glucose stimulus (Figure 5A and D). Our results suggest that mitochondrial alkalinisation could contribute to enhanced energy metabolism. To substantiate this possibility, we performed experiments in permeabilised INS-1E cells, a preparation which allows the manipulation of the pHmito. INS-1E cells were permeabilised with Staphylococus aureus α-hemolysin toxin, which preserves mitochondrial function and retains cytosolic proteins, while rendering the plasma membrane permeable to nucleotides and other small molecular weight compounds (Maechler et al, 1997). The K+/H+ ionophore nigericin was utilised to lower matrix pH under conditions of clamped pHcyto (Nicholls, 2006). The effective concentration of nigercin was determined by monitoring mtAlpHi fluorescence in the permeabilised cells. The addition of the mitochondrial substrate succinate (1 mM) increased matrix pH from 7.04 to 7.4, yielding a ΔpH of 0.36 units, similar to the effect of glucose in intact cells. Nigercin at both 100 and 500 nM significantly reduced the matrix pH in the continued presence of 1 mM succinate (Figure 8A and B). The same concentrations of nigericin further hyperpolarised the mitochondrial membrane potential as recorded with JC-1 when added after succinate stimulation (Figure 8C). DL-α-glycerolphosphate (effective concentration 5 mM), which directly provides reducing equivalents at complex II of the respiratory chain, caused a similar hyperpolarisation as 1 mM succinate (Figure 8D). Again, nigericin polarised the mitochondrial membrane potential further. This hyperpolarisation reflects a compensatory effect to the loss of ΔpH striving to maintain a constant electrochemical gradient across the inner mitochondrial membrane (Nicholls, 2006). Therefore, nigericin should only marginally affect the electrochemical potential and consequently the driving force on the ATP synthase. As a read-out of mitochondrial energy metabolism, we measured total ATP synthesised from ADP in the permeabilised cells. Succinate or α-glycerolphosphate increased ATP generation approximately five-fold (Figure 8E and F) when compared with control in the absence of exogenous nutrient. Nigericin strongly reduced substrate-induced ATP synthesis. Succinate-dependent ATP production, for instance, was reduced by more than 80% by 100 nM nigericin. Therefore, the pH gradient is a key determinant of mitochondrial substrate-mediated ATP synthesis.
Figure 8.
Lowering of the pHmito in permeabilised cells strongly reduces mitochondrial ATP synthesis. INS-1E cells were permeabilised with α-hemolysin toxin and analysed for mitochondrial matrix pH changes (A, B), mitochondrial membrane potential polarisation (C, D) and ATP generation (E, F). Mitochondria of the permeabilised cells were stimulated with either 1 mM succinate (A, B, C, E) or 10 mM DL-α-glycerolphosphate (D, F). (A) Nigericin caused acidification of the mitochondrial matrix at both 100 and 500 nM. (B) Quantification of the mitochondrial pH measurements (N=4–7). The same concentrations (C, D and data not shown) resulted in hyperpolarisation of the inner mitochondrial membrane (N=6–8). Nigericin strongly reduced ATP generation from both 1 mM succinate (E) and 10 mM DL-α-glycerolphosphate (F) oxidation (N=3–8). Values are presented as mean±s.e.m.
Discussion
In the pancreatic β-cell, nutrients play a dual role, providing substrates as well as generating signals for the activation of mitochondria essential for insulin secretion. Here, we report that the pHmito alkalinises during nutrient stimulation and suggest that variations of this parameter act as a regulator of mitochondrial metabolism, specifically ATP generation. Our results using either 31P-NMR or the pH-sensitive protein probe mtAlpHi show that pHmito is not constant but is modulated by the metabolic state of the cell.
We suggest that nutrient stimulation of the β-cell causes an imbalance in proton handling of mitochondria, which results in the net export of protons from the matrix space. Proton export could result from influx of positive charge, for instance, in the form of ions, under conditions where the mitochondrial membrane potential would be maintained through the extrusion of protons by the respiratory chain. Continued influx of cations such as calcium may result in sufficient compensatory proton export to overcome the buffering capacity of the matrix space. Here we find that alkalinisation still occurs under conditions that prevent mitochondrial calcium influx. Nevertheless, removal of extracellular calcium decreased the mitochondrial alkalinisation response by 20%, arguing that net calcium accumulation in the mitochondria contributes marginally to the observed increase of pHmito. A second possible mechanism is the net export of negatively charged metabolites. For instance, citrate is exported from the mitochondria together with protons (Joseph et al, 2006), which could contribute to matrix alkalinisation. Whether export of metabolites such as citrate contributes to matrix alkalinisation has not yet been tested. Subsequently, rates of proton-coupled transport are reduced, resulting in a new steady state. Alternatively, a new steady state may be reached as the sum of the electrical and chemical potentials becomes limited by the proton motive force that β-cell mitochondria are able to generate.
Mitochondrial alkalinisation to glucose was observed neither in HeLa cells (Llopis et al, 1998) nor in HepG2 cells (this study). Nutrient-dependent mitochondrial alkalinisation, therefore, seems to be specific for β-cells. This may be due to the fact that the β-cell is designed to sense nutrients, and secondly, because glycolysis-derived pyruvate is effectively funneled into the β-cell mitochondria for oxidative metabolism in this highly respiratory cell type (Sekine et al, 1994; Schuit et al, 1997). Other cell types that fulfill these criteria may exhibit similar nutrient-dependent pHmito changes, a possibility that remains to be tested.
It is noteworthy that the oxidative substrate leucine mimicked the effect of glucose on β-cell pHmito. On the other hand, the non-secretagogue substrate glutamine (Sener et al, 1981) did not alkalinise. The pronounced alkalanisation to nutrient secretagogues only, suggests that mitochondrial matrix alkalinisation could be an important metabolic event determining the β-cell secretory response.
A surprising finding of this study is the very low matrix pH value under resting conditions in INS-1 cells and rat islet β-cells. In a number of other cell types, including cultured primary cells and cell lines, mitochondrial matrix pH is alkaline, varying from pH 7.6 to 8.3 (Llopis et al, 1998; Matsuyama et al, 2000; Abad et al, 2004). As a consequence of the low pHmito of the resting β-cell, the ΔpH is also small (0.13 pH units in rat islet cells). During glucose stimulation, the ΔpH of primary β-cell mitochondria increases markedly, reaching 0.51 pH units. Compared with the small pHcyto changes in rat islets (0.08 pH units; see also Shepherd and Henquin, 1995; Stiernet et al, 2007), the pHmito increase is pronounced (0.45 pH units), yielding an increase of the ΔpH across the inner mitochondrial membrane of 0.37 pH units. Thereby, the proton chemical gradient adds −22 mV to the proton electrochemical potential, a significant contribution to the hyperpolarisation of the inner mitochondrial membrane by glucose.
The mitochondrial matrix alkalinisation to glucose is relatively slow. Comparison with the kinetics of first- and second-phase insulin secretion would argue that alkalinisation is principally important during the second phase. No correlation was observed between the rapid initial rise of the mitochondrial calcium signal and matrix alkalinisation. Mitochondrial calcium signals were not necessary for the observed alkalinisation during glucose stimulation, nor was calcium alone sufficient to raise the mitochondrial matrix pH. Calcium removal slows glucose metabolism (Hellman et al, 1974), which could explain the small attenuation of matrix alkalinisation.
The halftime of the mitochondrial alkalinisation is also slower than the increase of the electrical potential. Extrusion of few protons from the matrix space is sufficient to build up a membrane potential, while resulting in little alkalinisation due to the high proton-buffering capacity of the mitochondrial matrix. Alkalinisation ensues as the net proton export overcomes mitochondrial buffering. However, the pH increase may be counteracted by the formation of protons from oxidative metabolism. Considering the many aspects of mitochondrial metabolism that affect pHmito without impacting on the electrical potential, these kinetic differences are not unexpected.
Alkalinisation correlates quite closely with the net increase of cytosolic ATP. Similarly, oxygen consumption was found to increase for about 15 min after an initial rapid rise in respiration (Hutton and Malaisse, 1980). The respiratory chain is the key driving force for the maintenance of the ΔpH across the inner mitochondrial membrane of the β-cell, as inhibition of complex I resulted in a rapid acidification of the matrix space. These results also confirm the close correlation between mitochondrial ATP synthesis and alkalinisation.
The 31P-NMR saturation transfer experiments substantiate the link between mitochondrial alkalinisation and ATP synthesis during glucose stimulation. The results obtained for pHmito are in good agreement with mtAlpHi recordings. Remarkably, the acceleration of ATP synthesis as measured by 31P-NMR was higher in the mitochondrial compared with the cytosolic compartment. Consequently, the relative contribution of mitochondria to ATP synthesis increases at stimulatory glucose concentrations. To our knowledge, this is the first study reporting pHmito measurements with 31P-NMR as well as mitochondrial unidirectional rate constants for ATP synthesis and ATP synthesis rates in situ.
The association between matrix alkalinisation and mitochondrial ATP synthesis was further corroborated in α-toxin permeabilised INS-1E cells by direct manipulation of the pHmito. In the presence of the K+/H+ ionophore nigericin, substrate-mediated alkalinisation was strongly attenuated, accompanied by the expected hyperpolarisation of the electrical potential maintaining the driving force on the ATP synthase. This manoeuvre caused a dramatic reduction of ATP synthesis both for succinate, which must be taken up by mitochondria, and for α-glycerolphosphate, which directly transfers electrons to the respiratory chain. These findings underline the importance of the pHmito and/or the ΔpH in mitochondrial ATP generation. Low pHmito has also been implicated in the inhibition of ATP synthesis in isolated mitochondria from cardiomyocytes (Rouslin, 1987).
To date, only mitochondrial calcium has been suggested to serve as a signal linking nutrient stimulation to the metabolic activation of mitochondria (for a review, see Wiederkehr and Wollheim, 2008). Our work points to the importance of mitochondrial matrix alkalinisation in this process. We propose that the increased ΔpH ensures the sustained uptake of pyruvate and Pi for oxidative metabolism and ATP synthesis during sustained glucose-dependent insulin secretion. This study puts mitochondrial matrix pH on the map as a signal associated with metabolic activation.
Materials and methods
Most chemicals and reagents used for the experiments were from Sigma and Fluka Chemie (Switzerland). Coelenterazine was purchased from Calbiochem. Beetle luciferin was obtained from Promega.
Rat islet isolation and culture conditions
Islets were isolated from 200 to 300 g male Wistar rats (Janvier, France) by collagenase (Roche Diagnostics, Switzerland) digestion as described (Franklin et al, 2005). Islets were cultured in a humidified atmosphere containing 5% CO2 in RPMI-1640 medium (Invitrogen, Switzerland) containing 11 mM glucose, 10 mM Hepes, 10% heat-inactivated fetal calf serum (Brunschwig AG, Switzerland), 50 μg/ml penicillin, 100 μg/ml streptomycin and 100 μg/ml gentamicin (Essex Chemie AG, Switzerland) (islet medium).
Cell culture conditions
INS-1E cells were cultured in RPMI 1640 medium containing 11 mM glucose (Invitrogen, Switzerland) supplemented with 10 mM Hepes (pH 7.3), 10% (v/v) heat-inactivated fetal calf serum (Brunschwig AG, Switzerland), 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 50 μg/ml penicillin and 100 μg/ml streptomycin (INS medium). HepG2 cells were grown in DMEM medium containing 5.6 mM glucose, 4 mM L-glutamine and 1 mM sodium pyruvate (Invitrogen, Switzerland) and 10% (v/v) heat-inactivated fetal calf serum.
Intracellular pH measurements using 31P-NMR phosphate chemical shifts
INS-1 cell pellets were re-suspended in a 3% alginate solution in PBS (w/v). Beads of ∼1–2 mm diameter were formed by extruding the slurry from a 28-gauge needle dropping into a 1.1% BaCl2 solution. The cells were allowed to recover overnight in a spinner flask containing INS medium. Alginate-entrapped INS-1 cells were perifused in the bioreactor in the bore of the AVANCE-500 NMR spectrometer, and 31P-NMR spectra and saturation transfer experiments were acquired during step changes of metabolic substrates. 31P-NMR spectra were continuously collected in 20-min experiments (3–4 measurements for each substrate level). The samples were oxygenated, and physiological pH and temperature were maintained.
The chemical shift of Pi by 31P-NMR has been shown to be pH dependent, and the differences in the Pi frequency from the α-phosphate resonance of ATP (α-ATP) can be used to determine intracellular pH values after calibration (Gadian et al, 1979; Petroff et al, 1985). A calibration curve of Pi chemical shift frequencies with respect to pH was determined with Pi in buffered salt solutions.
ATP synthesis rate measurements
Changes in the intensity of Pi as measured by 31P-NMR saturation transfer techniques are indicative of and proportional to the rate of ATP synthesis by that cellular pool of Pi. ATP synthesis rates are calculated as k−1 × [Pi]in, where k−1 is the rate constant, k (min−1)=δM/M0 × 1/T1, and [Pi]in is the intracellular concentration of the metabolically active Pi pool. δM/M0 is the change in the intracellular Pi signal when the terminal phosphate of ATP is saturated compared with the Pi signal with a saturation pulse offset an equal distance downfield from the Pi resonance (δM/M0=(M0−Mz)/M0). T1 was measured using standard inversion-recovery experiments in separate studies. T1 for extracellular Pi was calculated to be 4.13 s and T1 for intracellular Pi was calculated to be 1.58 s at basal glucose. The intracellular Pi concentration was calculated from the relative signal intensities of the extracellular (Piext) and intracellular (Piin) Pi.
Recombinant adenoviruses
The adenoviruses expressing mitochondrially targeted Aequorin (Ad-RIP-mitoAequorin) or cytoplasmic Luciferase (Ad-RIP-Luciferase) were constructed as described (Ishihara et al, 2003; Brun et al, 2004). The mtAlpHi DNA was cloned into the pTRE-Shuttle vector and re-cloned into the Adeno-X virus plasmid backbone for doxycycline-inducible expression (CLONTECH Laboratories). The tetON virus carrying the transcriptional regulator was provided with the expression system from CLONTECH Laboratories. Adenoviruses were amplified in HEK293 cells.
Virus infection protocols
At 1 day after plating, cultured cells were infected for 90 min at 37°C. After 2–3 days, the cells were analysed. Freshly isolated rat islets were recovered for 2 h in islet medium and were then infected for 90 min. For perifusion experiments, 100–150 infected rat islets were placed onto A431 extracellular-matrix-coated Thermanox cover slips (15 mm diameter, Nalge Nunc, USA) (Ishihara et al, 2003). For cytosolic and pHmito measurements, 20 islets were placed in the centre of a matrix-coated 25 mm glass coverslip (Menzel GmbH, Germany).
Luminescence measurements
Mitochondrial calcium and cytosolic ATP measurements were performed as described (Kennedy et al, 1996; Ishihara et al, 2003). Measurements were performed in Krebs-Ringer bicarbonate Hepes buffer (KRBH): 140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 10 mM Hepes, 5 mM NaHCO3, 2.5 mM glucose pH 7.4.
Single-cell imaging
Expression of the mtAlpHi gene was induced with 1 μg/ml of doxycycline after infection with the Ad-tetON-mtAlpHi and Ad-tetON regulatory virus. mtAlpHi measurements on attached rat islets were performed 2–3 days after infection. Titration of the pHmito was performed as described (Abad et al, 2004). Image acquisition was performed on an inverted microscope (Zeiss Axiovert 200M, Carl Zeiss AG, Switzerland), with an array laser confocal spinning disk (QLC100, VisiTech, UK). Cells were imaged using a 63 × (numerical aperture 1.4) oil-immersion objective (Carl Zeiss AG) and excited using 488 nm laser light. The emission wavelength was 535 nm. Images were acquired every 10 s and were analysed using Metafluor 6.3 Software (Universal Imaging, Molecular Devices Corporation, USA).
For pHcyto measurements, attached INS-1E or rat islets were washed once in KRBH and loaded with the pH indicator BCECF (1 μM; Molecular Probes) for 8 min at 37°C. The pHcyto was measured ratiometrically exciting with laser light of 440/488 nm and measuring the emission at 535 nm in KRBH containing sulfinpyrazone (100 μM).
Membrane potential measurements
Cell suspensions from isolated rat islets were prepared as described (Franklin et al, 2005). Dispersed islet cells from about 50 rat islets were seeded in black-walled 96-well plates (Greiner bio-one GmbH, Germany). Before the measurement, the cells were transferred for 1 h to islet medium (4 mM glucose). The cells were washed with KRBH 0.1% bovine serum albumin (fraction V, AppliChem, Germany) and incubated for 30 min at 37°C in the presence of 350 nM JC-1 (Invitrogen, Switzerland). They were then washed twice with KRBH 0.1% bovine serum albumin and incubated for 20 min. JC-1 fluorescence was measured ratiometrically at 37°C. The wavelengths used were 490 nm excitation/540 nm emission (green; monomer) and 540 nm excitation/590 nm emission (red; J-aggregates) in a multi-well fluorescence reader (FlexStation, Molecular Devices Corporation, USA) as described (Park et al, 2008).
INS-1E cell permeabilisation with α-hemolysin toxin
INS-1E cells were washed with Ca2+-free KRBH and then permeabilised with 1 μg/ml of Staphylococus aureus α-hemolysin toxin (Sigma) in intracellular buffer (140 mM KCl, 5 mM NaCl, 7 mM MgSO4, 1 mM KH2PO4, 20 mM HEPES, 10.2 mM EGTA, 1.65 mM CaCl2, pH 7.0 titrated with KOH) for 10 min at 37°C (Maechler et al, 1997). After the permeabilisation step, the cells were washed with intracellular buffer containing 0.5% BSA before incubation in intracellular buffer supplemented with 1 mM ATP (no BSA) (or 10 μM ADP when ATP synthesis was monitored). pHmito was recorded in intracellular buffer solution but was measured otherwise as described for intact cells. ATP production was measured after 15 min incubation of INS-1E cells in 48-well plates (2 × 105 cells/well) using a bioluminescence assay kit (HS II, Roche Diagnostics, Switzerland). Measurement of the mitochondrial membrane potential was performed in 96-well plates (5 × 104 cells/well), and cells were loaded with JC-1 and monitored in an intracellular buffer solution containing 1 μM JC-1.
Insulin assay
Insulin was measured during mitochondrial calcium recordings in the effluent of the perifusion chamber every 30 s. Insulin concentrations were determined using an enzyme immunoassay (SPI bio, France).
Supplementary Material
Supplementary Figure 1
Supplementary Figure Legend
Acknowledgments
We thank N Aebischer, A Gjinovci and D Nappey for expert technical assistance, and Dr D Poburko as well as Dr S Arnaudeau for advice with the single-cell imaging. We gratefully acknowledge the continued support by the Swiss National Foundation (310000-116750/1) and EuroDia (LSHM-CT-2006-518153), a European-Community-funded project under framework program 6. GWC was funded by NIH/NIDDK R01 DK0710771.
References
- Abad MF, Di Benedetto G, Magalhaes PJ, Filippin L, Pozzan T (2004) Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J Biol Chem 279: 11521–11529 [DOI] [PubMed] [Google Scholar]
- Balut C, vandeVen M, Despa S, Lambrichts I, Ameloot M, Steels P, Smets I (2008) Measurement of cytosolic and mitochondrial pH in living cells during reversible metabolic inhibition. Kidney Int 73: 226–232 [DOI] [PubMed] [Google Scholar]
- Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR (2004) The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol 167: 1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI (2004) 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 279: 44370–44375 [DOI] [PubMed] [Google Scholar]
- Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI (2001) In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J Biol Chem 276: 20240–20244 [DOI] [PubMed] [Google Scholar]
- Cohen SM, Ogawa S, Rottenberg H, Glynn P, Yamane T, Brown TR, Shulman RG (1978) P nuclear magnetic resonance studies of isolated rat liver cells. Nature 273: 554–556 [DOI] [PubMed] [Google Scholar]
- Das AM (2003) Regulation of the mitochondrial ATP-synthase in health and disease. Mol Genet Metab 79: 71–82 [DOI] [PubMed] [Google Scholar]
- Detimary P, Jonas JC, Henquin JC (1996) Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology 137: 4671–4676 [DOI] [PubMed] [Google Scholar]
- Duchen MR, Smith PA, Ashcroft FM (1993) Substrate-dependent changes in mitochondrial function, intracellular free calcium concentration and membrane channels in pancreatic beta-cells. Biochem J 294 (Part 1): 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB (2005) {beta}-cell secretory products activate {alpha}-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54: 1808–1815 [DOI] [PubMed] [Google Scholar]
- Gadian DG, Radda GK, Richards RE, Seeley PJ (1979) In Biological Application of Magnetic Resonance, Shulman RG (ed), pp 463–535. New York: Academic Press [Google Scholar]
- Gembal M, Gilon P, Henquin JC (1992) Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 89: 1288–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B, Idahl LA, Lernmark A, Sehlin J, Taljedal IB (1974) The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J 138: 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton JC, Malaisse WJ (1980) Dynamics of O2 consumption in rat pancreatic islets. Diabetologia 18: 395–405 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB (2003) Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5: 330–335 [DOI] [PubMed] [Google Scholar]
- Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in ‘t Veld P, Renstrom E, Schuit FC (2005) Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54: 2132–2142 [DOI] [PubMed] [Google Scholar]
- Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alarcon C, Rhodes CJ, Newgard CB (2006) The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 281: 35624–35632 [DOI] [PubMed] [Google Scholar]
- Kennedy ED, Rizzuto R, Theler JM, Pralong WF, Bastianutto C, Pozzan T, Wollheim CB (1996) Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J Clin Invest 98: 2524–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA (1999) Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J Biol Chem 274: 13281–13291 [DOI] [PubMed] [Google Scholar]
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY (1998) Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci USA 95: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler P, Kennedy ED, Pozzan T, Wollheim CB (1997) Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic beta-cells. EMBO J 16: 3833–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402: 685–689 [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC (2000) Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol 2: 318–325 [DOI] [PubMed] [Google Scholar]
- Nicholls DG (2006) Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J Biol Chem 281: 14864–14874 [DOI] [PubMed] [Google Scholar]
- Palmieri F (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch 447: 689–709 [DOI] [PubMed] [Google Scholar]
- Park KS, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou JC, Marchetti P, Demaurex N, Wollheim CB (2008) Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 283: 33347–33356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG (1985) Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology 35: 781–788 [DOI] [PubMed] [Google Scholar]
- Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV (2006) A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281: 30593–30602 [DOI] [PubMed] [Google Scholar]
- Rouslin W (1987) Factors affecting the reactivation of the oligomycin-sensitive adenosine 5′-triphosphatase and the release of ATPase inhibitor protein during the re-energization of intact mitochondria from ischemic cardiac muscle. J Biol Chem 262: 3472–3476 [PubMed] [Google Scholar]
- Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M (1997) Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272: 18572–18579 [DOI] [PubMed] [Google Scholar]
- Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, Girotti M, Marie S, MacDonald MJ, Wollheim CB, Rutter GA (1994) Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem 269: 4895–4902 [PubMed] [Google Scholar]
- Sener A, Somers G, Devis G, Malaisse WJ (1981) The stimulus-secretion coupling of amino acid-induced insulin release. Biosynthetic and secretory responses of rat pancreatic islet to L-leucine and L-glutamine. Diabetologia 21: 135–142 [DOI] [PubMed] [Google Scholar]
- Shepherd RM, Henquin JC (1995) The role of metabolism, cytoplasmic Ca2+, and pH-regulating exchangers in glucose-induced rise of cytoplasmic pH in normal mouse pancreatic islets. J Biol Chem 270: 7915–7921 [DOI] [PubMed] [Google Scholar]
- Simpson DP, Hager SR (1984) Bicarbonate-carbon dioxide buffer system: a determinant of the mitochondrial pH gradient. Am J Physiol 247: F440–F446 [DOI] [PubMed] [Google Scholar]
- Stiernet P, Nenquin M, Moulin P, Jonas JC, Henquin JC (2007) Glucose-induced cytosolic pH changes in beta-cells and insulin secretion are not causally related: studies in islets lacking the Na+/H+ exchange R NHE1. J Biol Chem 282: 24538–24546 [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB (2006) Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 147: 2643–2649 [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB (2008) Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium 44: 64–76 [DOI] [PubMed] [Google Scholar]
- Wollheim CB, Maechler P (2002) Beta-cell mitochondria and insulin secretion: messenger role of nucleotides and metabolites. Diabetes 51(Suppl 1): S37–S42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure Legend