Abstract
Neuroimaging studies in bipolar disorder report gray matter volume (GMV) abnormalities in neural regions implicated in emotion regulation, including ventral/orbital medial prefrontal cortex (OMPFC) GMV decreases and, more inconsistently, amygdala GMV increases. We aimed to examine OMPFC and amygdala GMV in bipolar disorder, type 1 patients (BPI) versus healthy control participants (HC), and examine potential confounding effects of gender, clinical and illness history variables and psychotropic medication upon any group differences that were demonstrated in OMPFC and amygdala GMV. Images were acquired from 27 BPI (17 euthymic, 10 depressed) and 28 age- and gender-matched HC in a 3T Siemens scanner. Data were analyzed with SPM5 using voxel-based morphometry to first examine main effects of diagnostic group and gender upon whole brain (WB) GMV. Post hoc analyses were subsequently performed to examine the extent to which clinical and illness history variables and psychotropic medication contributed to GMV abnormalities in BPI in a priori and non-a priori regions demonstrated by the above VBM analyses. Here, SPPSS was used to examine the effects of these variables on magnitude of GMV in these a priori and non-a priori regions in BPI versus HC. BPI showed reduced GMV in two regions established a priori: bilateral posteromedial rectal gyrus (PMRG), but no amygdala GMV abnormalities. BPI also showed reduced GMV in two non-a priori regions: left parahippocampal gyrus and left putamen. For left PMRG GMV, there was a significant group by gender by trait anxiety interaction. GMV was significantly reduced in male low trait anxiety BPI versus male low trait anxiety HC, and in high versus low trait anxiety male BPI. Our findings show in BPI significant effects of male gender and high trait anxiety on GMV reduction in left PMRG, part of the OMPFC medial prefrontal network implicated in visceromotor and emotion regulation.
Keywords: bipolar 1 disorder, voxel-based morphometry, gender, neuroimage
1. Introduction
Bipolar disorder (BP) is a psychiatric disorder associated with severe mood dysregulation and significant morbidity (Drevets, 2000; Strakowski et al., 2000; Phillips et al., 2003b; Farrow et al., 2005). Two key neural regions are implicated in mood regulation: the medial network of the orbital medial prefrontal cortex (OMPFC) and the amygdala (Phillips et al., 2003b). The former comprises Brodmann areas (BA) 24, 25, 32, the rectal gyrus (BA14, medial regions of BA11 and 13) and a small part of the agranular anterior insula (Ongur et al., 2003), and regulates the amygdala response to emotionally-salient stimuli (Ghashghaei and Barbas, 2002; Phillips et al., 2003b; Swanson, 2003). These regions have therefore been a focus of study in mood-disordered populations, including BP and unipolar depression.
In unipolar depression, structural abnormalities in OMPFC and amygdala have been reported (Sheline, 2000; Bremner et al., 2002). Specifically, one study found volume reduction in the rectal gyrus of the OMPFC in individuals with major depression but not in other frontal cortical regions (Bremner et al., 2002). Reduction in amygdala core nuclei (consisting of the lateral, basal, and accessory basal nuclei) volume has also been reported in individuals with recurrent major depression (Sheline, 2000); whereas amygdala gray matter volume (GMV) reduction was observed in drug naïve females with major depression (Tang et al., 2007); and total amygdala volume reduction was observed in females but not males with major depression (Hastings et al., 2004). Other studies have shown no difference in either core, noncore (remaining nuclei, which include the central, medial, and periamygdaloid nuclei; Munn et al., 2007) or total (Macmaster et al., 2007) amygdala volume between individuals with major depression or a group at risk for depression. In BP, while OMPFC gray matter volume (GMV) decreases have been reported (Lopez-Larson et al., 2002; Lyoo et al., 2004; Blumberg et al., 2006; Nugent et al., 2006), the location of these GMV decreases within OMPFC is unclear. GMV reductions have been reported within the lateral OMPFC in adult (Lopez-Larson et al., 2002; Lyoo et al., 2004; Nugent et al., 2006) and adolescent (Blumberg et al., 2006), but not in first episode BP (Farrow et al., 2005). BP postmortem studies have, however, indicated glial cell number reductions in medial OMPFC (Ongur et al., 1998). Studies have found increased amygdala GMV in adult BP (Altshuler et al., 1998; Brambilla et al., 2003), although others have found reduction in amygdala volume or neuron number in patients with BPI (Berretta et al., 2007) including first episode patients (Rosso et al., 2007). Decreased amygdala structural volumes have been demonstrated in adolescent BP (DelBello et al., 2004; Chang et al., 2005) relative to age-matched healthy controls (HC). The undetermined location of the OMPFC GMV decreases and discrepant findings regarding amygdala GMV in BP are possibly due to different methodologies and populations recruited in different studies. Further examination of GMV in these neural regions using a standardized methodology, including an appropriate test of its specificity to detect GMV abnormalities in BP, is therefore required to clarify the nature of GMV abnormalities in these regions in BP.
Findings also indicate a significant effect of gender upon GMV in several neural regions. Cortical gray matter development, follows an “inverted U” course in frontal cortices (peaking at 11.0 years in girls and 12.1 years in boys; Lenroot and Giedd, 2006), in temporal cortices (peaking at 16.7 years in girls and 16.2 years in boys), and in parietal cortices (peaking at 10.2 years in girls and 11.8 years in boys; Giedd et al., 2006). These findings indicate gender differences in gray matter development in OMPFC and amygdala, but the extent to which there are differential effects of gender upon GMV in these regions in adults with BP relative to healthy adult remains unclear. No studies to date have reported gender differences in GMV in either OMPFC or amygdala in bipolar disorder. Further examination of the extent to which gender differences in GMV may impact abnormalities in GMV in BP is, therefore, required.
Potential factors, other than diagnosis of BP per se, that may contribute to abnormalities in OMPFC and amygdala GMV in BP include clinical and illness history variables in BP (for example, age of illness onset, illness duration, mood state at the time of examination, comorbid anxiety) and psychotropic medication. Although one study reported an inverse relationship between illness duration and left prefrontal GMV in BP (Lopez-Larson et al., 2002), another showed no effect of illness history variables on prefrontal cortical GMV (Hirayasu et al., 1999). An association between psychotropic medication with increases (Blumberg et al., 2006) and decreases (Nugent et al., 2006) in ventral prefrontal cortical GMV has been reported, as has decreased right inferior prefrontal cortical GMV in BP adults taking antidepressants (Lopez-Larson et al., 2002). Amygdala volumes have been negatively correlated with illness duration, but positively associated with lifetime antidepressant exposure (DelBello et al., 2004). Larger amygdala GMV has been associated with previous use of lithium or sodium valproate (Chang et al., 2005). Other studies show no effect of medication (Brambilla et al., 2003) on regional GMV in BP, or have not specifically examined this effect (Altshuler et al., 1998; Blumberg et al., 2005). No studies to date have examined potential effects of mood state at time of scan or trait anxiety upon OMPFC or amygdala GMV in BP.
To help resolve these inconsistent findings, we first aimed to examine GMV in our a priori regions of interest, namely, OMPFC and amygdala, in adults with the conventional BP type 1 (BPI). We used voxel-based morphometry (VBM) to examine the main effect of diagnosis (BPI versus HC) upon whole brain (WB) GMV in BPI versus healthy control participants (HC), as in previous studies (Lyoo et al., 2004). VBM allows the examination of neural regions voxel-by voxel in an automated fashion, avoiding potential biases that may occur in region-of-interest (ROI) based methods. We also examined the main effect of gender upon WB GMV in this VBM analysis, as previous findings highlighted above have demonstrated a significant effect of gender upon GMV in many brain regions, including some of our a priori regions in the present study. We also included a test of the specificity of VBM to detect GMV abnormalities in BPI (see Section 2).
We secondly aimed to examine the potential effects of gender, trait anxiety, clinical and illness history variables and psychotropic medication that may have contributed to any group differences observed in GMV in our a priori (and non-a priori) regions of interest by examining relationships between these factors and GMV in a priori and non-a priori regions showing GMV abnormalities in BPI in the above VBM WB analyses.
2. Methods
2.1. Participants
Twenty-seven patients (17 euthymic; 10 in depressed episode) fulfilling DSM-IV criteria for bipolar disorder, type 1 (BPI) based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 1995) were recruited from the Western Psychiatric Institute and Clinic, Mood Disorders Treatment and Research Program, University of Pittsburgh. Exclusion criteria were: borderline personality disorder (using SCID-II criteria for borderline personality disorder); history of head injury or neurological disease; current alcohol and illicit substance abuse; non-right handedness; and failure to meet screening criteria for magnetic resonance imaging (MRI), including the presence of metal clips, heart pacemakers, claustrophobia and possibility of pregnancy for women of child-bearing age. Twelve BPI had current or lifetime comorbid anxiety disorder. Ten BPI had a previous history of substance abuse. For demographic and clinical information on BPI and HC refer to Table 1. Twenty-eight HC without personal history of psychiatric illness, using DSM-IV criteria as assessed by the SCID-I, and without history of medical illness or family history of psychiatric illness, were selected using the same exclusion criteria.
Table 1.
Demographic information and BPI characteristics, including illness duration; age of illness onset, and medication information.
| BPI (n=27) | HC (n=28) | ||
|---|---|---|---|
| GenderA | Male | 10 | 13 |
| Female | 17 | 15 | |
| Mean ageB | 31.89 (SD:7.3) | 30.82 (SD:10.56) | |
| Mood state | BPe | 17 | n/a |
| BPd | 10 | ||
| Mean age of illness onset | 20.33 (SD:6.1) | n/a | |
| Mean illness duration | 11.1 (SD:7.04) | n/a | |
| Spielberger trait anxietyC | 46.6 (SD:15.5) | 31.5 (SD:11.9)D | |
| Medication: 24 BPI were taking medication and 3 were drug free | ||
|---|---|---|
| Mood stabilizers (n=14) |
Lithium (n=12) Lamotrigine (n=1) Valproate Sodium (n=1) |
n/a |
| Antidepressants (n=10) |
Bupropion (n=2) | n/a |
| Sertraline (n=2) | ||
| Nortriptyline (n=1) | ||
| Venlafaxine (n=1) | ||
| Mirtazapine (n=1) | ||
| Citalopram (n=1) | ||
| Fluoxetine (n=1) | ||
| Duloxetine (n=1) | ||
|
| ||
| Antipsychotics (n=16) |
Aripiprazole (n=7) | n/a |
| Risperidone (n=4) | ||
| Quetiapine (n=4) | ||
| Chlorpromazine (n=1) | ||
|
| ||
| Anxiolytics/ benzodiazepines (n=7)E |
Lorazepam (n=6) | n/a |
| Alprazolam (n=1) | ||
Numbers are means; standard deviations in parentheses; BPI: bipolar I disorder patient, HC: healthy controls;
BPI and HC did not differ significantly in gender ratio (χ2=0.5, P=0.48);
BPI and HC did not differ significantly in age (z=−0.95, p=0.34);
BPI had significantly greater trait anxiety scores than HC (t (51)=3.97, P<0.001);
For two HC, data were missing;
BPI had significantly greater trait anxiety scores than HC, both BPI taking, and those not taking, anxiolytics (t (31)=3.5 and t (44)=3.4, P<0.002, respectively).
We used the Spielberger Trait Anxiety Inventory (Spielberger, 1988) to obtain trait anxiety scores as a quantitative measure of comorbid anxiety on all BPI and most HC. Written informed consent was obtained from all participants after explanations of procedures according to University of Pittsburgh Institutional Review Board guidelines.
2.2. MRI Acquisition and Image Processing
MRI structural brain images were acquired using a 3.0 Tesla Siemens Allegra MRI scanner at the University of Pittsburgh/CMU Brain Imaging Research Center (BIRC). Three dimensional sagittal high-resolution MPRAGE scans were acquired (TE: 3.04 ms, TR:1570 ms, slice thickness: 1 mm, 192 slices flip angle: 8°, FOV:200 mm, inversion time (IT): 800 ms and image matrix 256 by 256 that covered the entire brain. In fifteen participants (10 HC and 5 BPI) there were small differences in the acquisition parameters: TE: 2.48, TR: 1630 ms, slice thickness: 0.8 mm, 224 slices that had been implemented by the MR physicist. Image contrast is mostly determined by TI, which was the same in both acquisitions. We nevertheless used this difference in scan acquisition parameters as a factor in additional analyses (see below).
2.3. Statistical analysis
Processing and VBM were performed with SPM5 (Statistical Parametric Mapping, Version 5; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm), executed in Matlab 7.2 (Mathworks, Sherborn, Massachusetts). DICOM files were converted to NIFT-1 (http://nifti.nimh.nih.gov) format. Converted files were then segmented into gray and white matter and normalized to standard international consortium for brain mapping template using a unified model. In older versions of SPM, image registration and tissue classification had a circularity problem because the tissue classification required an initial registration with tissue probability maps and the registration required an initial tissue classification. A unified segmentation model (Ashburner and Friston, 2005), combining both parameters in a single generative model is included in SPM5, leading to a better probability image than earlier SPM versions. Standard SPM values were selected to bias regularization (0.0001) and FWHM cutoff (60 mm). Voxel values were modulated by the Jacobian determinants derived from the spatial normalization: brain structures that had volumes reduced after spatial normalization thereby had total counts decreased by an amount proportional to the degree of volume discounted. The final voxel resolution after normalization was 2×2×2mm. Images were smoothed with a 12-mm Gaussian kernel.
To examine the main aims of our study, and thereby determine the specificity of the diagnostic group effect upon GMV in a priori and non-a priori regions of interest, our analytic framework comprised the following four stages.
We first examined main effects, and interactions between, diagnostic group (BPI versus HC) and gender (males versus females) upon WB GMV, covarying for the total amount of GMV. Both diagnostic group and gender have been previously demonstrated to have significant effects upon WB GMV, and our study was sufficiently powered to detect significant effects of these factors on GMV in a priori regions of interest (see Section 4). This analysis allowed us, therefore, to determine in which a priori and which non-a priori regions there was a significant effect of group, but not gender, upon GMV.
We secondly examined the specificity of VBM as a method to detect a priori region GMV abnormalities in BPI. Here we re-assigned each participant (from all BPI and HC) to membership of one of two groups in which there were equal numbers of age-matched BPI and HC.
We next examined the extent to which factors common to both BPI and HC (i.e., gender, acquisition parameters, trait anxiety) may have further contributed to, and interacted with, abnormalities in GMV in BPI in a priori and non-a priori regions in GMV in BPI (relative to HC) demonstrated in the first stage of our analyses above. Here, we performed an ANOVA, including diagnostic group, gender, acquisition parameters and high vs. low trait anxiety as fixed factors, covarying for age and total amount of GMV, upon GMV extracted from the a priori and non-a priori regions showing abnormalities in GMV in BPI (relative to HC) demonstrated in the WB VBM analyses above.
We finally examined the extent to which factors specific to BPI (i.e., depressed versus euthymic mood state, age of illness onset, illness duration and medication load) may have contributed to the abnormalities in GMV in a priori and non-a priori regions in BPI demonstrated by the first stage of our analyses above. Here, we performed post hoc, t-tests and correlational analyses as appropriate to examine relationships between these BPI-specific variables and GMV values extracted from these a priori and non-a priori regions.
For the first stage of our analytic framework, we used a general linear model to examine main effects of diagnostic group and gender, the group by gender interaction, and subsequent pairwise WB between-group and between-gender comparisons, with a correction for multiple comparisons based on random field theory. Only voxels with values above an absolute gray matter (GM) threshold of 0.05 entered the analyses (Bassitt et al., 2006), resulting in a search volume of approximately 256,000 voxels. A measure of the total whole brain (WB) GMV, the total number of. voxels within each participant’s GM compartment, was entered as a covariate in an analysis of covariance (Good et al., 2001; Bassitt et al., 2006), to control for inter-individual differences in total GMV that may confound between-group analyses. VBM analyses were performed with and without age as a covariate because of the potential confounding effect of age on GMV (Good et al., 2001). Resulting statistics at each voxel were transformed to Z scores and displayed on a glass brain in standard Montreal Neurological Institute space (threshold of Z=3.31; corresponding to a statistical (uncorrected) voxel-wise threshold of p<0.001 for WB analyses). Small Volume Correction (SVC using Family-wise error, FWE; p<0.05; cluster extent threshold=5voxels) was performed on a priori regions emerging from these WB analyses, using a standardized anatomical template as defined in the WFU-Pickatlas (Maldjian et al., 2003) for regions such as BA47 and BA11. Anatomical templates have been used in previous studies to define SVC regions (Dragnaski at al., 2006). To determine the magnitude of our between-group findings, we calculated the effect size with the following formula: Cohen’s d = 2t/√df); where t is the t-test value and df is the degree of freedom.
In the second stage of our analyses, as tests of the specificity of VBM analyses to detect GMV differences between BPI and HC in our a priori regions, we created and compared two groups, each comprising both an BPI and an age-matched HC (group A with 14 HC and 14 BPI, and group B with 14 HC and with 13 BPI). We used WB VBM analyses to examine between-group differences in GMV.
In the third and fourth stages of our analyses, to examine potential effects of, gender, trait anxiety, change in acquisition parameters, their interaction with diagnostic group (stage 3), and illness variables and medication (stage 4) upon GMV in a priori and non-a priori regions in which GMV abnormalities had been demonstrated in BPI from the first-stage VBM WB analyses above, we performed the following procedures. Mean GMV values were first extracted from a priori and non-a priori regions in which GMV abnormalities were observed in BPI from the VBM WB analyses. Mean GMV values were then entered into the Statistical Package for the Social Sciences ed.13 (SPSS) to perform the following analyses. Here, we first employed an analysis of variance (ANOVA), with diagnostic group, gender, acquisition parameters and high versus low trait anxiety as fixed factors, covarying for age and total amount of GMV, testing these main effects, and interactions between these factors, upon GMV extracted from a priori and non-a priori regions in which GMV abnormalities were observed in BPI from the above VBM WB analyses. Post hoc tests examining any significant main effects and interactions were performed with Bonferroni corrections for multiple comparisons. In all BPI, relationships between GMV in a priori and non-a priori regions in which GMV abnormalities were observed in BPI from the above VBM WB analyses and mood state were then examined by comparing euthymic versus depressed BPI on GMV in these neural regions. Relationships in all BPI between a priori and non-a priori regions in which GMV abnormalities were observed in BPI from the above VBM WB analyses and age of illness onset, illness duration and medication load were examined using correlational analyses, using a corrected statistical threshold of P=0.01 to control for multiple tests.
The calculation of medication load is a novel approach for the study of medication effects in neuroimaging studies of bipolar populations (see Phillips et al., 2008 see Phillips et al., in press). It has the advantage over the study of medicated versus unmedicated individuals, as bipolar individuals (particularly BPI) who tolerate being medication free are unlikely to be matched for illness severity with those who require medication. It also has the advantage over the examination individuals taking, versus those not taking, each class of psychotropic medication, as this latter approach involves multiple comparisons of smaller numbers of individuals in each subgroup. The computation of a composite measure of total medication load, reflecting both the dose and variety of different medications taken, is, therefore, an approach that is preferable to existing strategies, and allows inclusion into neuroimaging studies BPI who are representative of the bipolar population in general, who have been medicated in real-world contexts, and typically treated with a number of different medications and medication combinations.
To compute an index of medication load for each bipolar participant, we first coded the dose of each antidepressant, mood-stabilizer, antipsychotic and anxiolytic medication as absent (0), low (1) or high (2). For antidepressants and mood-stabilizers we converted each medication into low- or high-dose groupings using a previously employed approach (Sackeim 2001). Individuals on levels 1 and 2 of these criteria were coded as low-dose, those with levels 3 and 4 as high-dose. We added a no-dose subtype for those not taking these medications. We converted antipsychotic doses into chlorpromazine dose equivalents, and coded as 0, 1 or 2, for no medication, chlorpromazine equivalents dose equal or below, or above, the mean effective daily dose (ED50) of chlorpromazine as defined by Davis and Chen (Davis and Chen, 2004). Lorazepam dose was similarly coded as, 0, 1 or 2, with reference to the midpoint of the Physician’s Desk Reference-recommended daily dose range. We generated a composite measure of total medication load, reflecting dose and variety of different medications taken, by summing all individual medication codes for each medication category for each individual bipolar participant. We used Pearson correlational analyses to examine associations between total medication load and GMV. As lithium in particular has been associated with enlarged regional GMV, particularly in ventral prefrontal cortex (Drevets et al., 1997; Moore et al., 2000), we also examined separately associations between lithium and GMV in a priori and non-a priori regions in which GMV abnormalities were observed in BPI from the above VBM WB analyses by comparing GMV in these regions in BPI taking versus those not taking lithium.
3. Results
3.1.1. Examination of main effects of diagnostic group and gender upon WB GMV
WB ANOVA with diagnostic group (BPI versus HC) and gender (males versus females) as main effects, covarying for total GMV and age, revealed a significant main effect of group in our a priori regions: right OMPFC (P=0.04, corrected); and in one non-a priori region: left parahippocampal gyrus (P=0.02, corrected; Table 2).
Table 2.
Main effects of group and gender
| Region | CoordinatesA | k | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Main effect of group | Side | x | y | z | F | z | Unc. | Cor. | PB |
| Parahippocampal gyrus | L | −27 | −46 | −18 | 15.39 | 3.46 | 17 | 8 | 0.021 |
| PMRG | R | 13 | 14 | −21 | 14.11 | 3.31 | 12 | 8 | 0.044 |
|
Main effect of gender | |||||||||
| Precuneus (BA7) | R | 29 | −52 | 50 | 18.13 | 3.74 | 238 | 78 | 0.018 |
| Inferior frontal gyrus (BA47) | L | −25 | 16 | −10 | 18.09 | 3.73 | 7 | 8 | 0.011 |
| Cuneus (BA18) | L | −1 | −102 | 12 | 17.63 | 3.69 | 271 | 19 | 0.023 |
| Middle temporal gyrus (BA21) | L | −57 | 4 | −18 | 15.03 | 3.42 | 49 | 7 | 0.034 |
Talaraich and Tornoux (1998) coordinates;
P values corrected with small volume correction; BPI: Bipolar Disorder, type I; k: number of voxels in the cluster for both uncorrected and corrected values; PMRG: posteromedial rectal gyrus
There was a significant main effect of gender in several regions that did not overlap with any of the regions in which there was a significant main effect of group. Regions showing a main effect of gender included: the right pre-cuneus (BA7; P=0.018); left ventrolateral prefrontal cortex (BA47; P=0.011); left cuneus (BA18; P=0.023); and left middle temporal gyrus (BA21; P=0.034 Table 2). There was no significant group by gender interaction.
3.1.2. Effect of diagnostic group on WB GMV
Post hoc pair wise WB VBM analyses were performed to further examine the main effect of diagnostic group upon WB GM, covarying for total GMV. These analyses again revealed significantly reduced GMV in BPI relative to HC in two of our a priori regions: bilateral OMPFC, specifically, bilateral posteromedial rectal gyrus (PMRG), centered on BA14 and medial BA13 (Table 3a; Cohen’s d effect size: L=0.97 and R=0.93; P=0.01 and P=0.015, respectively; Fig. 1). WB VBM analyses additionally covarying for age revealed the same between-group differences as before with a larger cluster size (left: 32 voxels; right: 21 voxels) and effect size (Cohen’s d: L=1.04, P=0.023; R=1.0, P=0.034).
Table 3.
| Table 3a. Decreased regional gray matter volumes in BPI relative to HC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | CoordinatesA | k | |||||||
| Decreased GMV in BPI | Side | x | y | z | T | z | Unc. | Cor. | PB |
| A priori regions | |||||||||
| PMRG | R | 12 | 14 | −21 | 3.54 | 3.34 | 19 | 10 | 0.038 |
| L | −13 | 12 | −21 | 3.54 | 3.33 | 19 | 6 | 0.037 | |
| Non-a priori regions | |||||||||
| Parahippocampal gyrus (BA37) | L | −27 | −46 | −18 | 4.24 | 3.91 | 66 | 25 | 0.008 |
| Putamen | L | −25 | −15 | 6 | 3.6 | 3.38 | 33 | 30 | 0.017 |
| Table 3b. Differences in regional gray matter volumes between males and females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | CoordinatesA | K | ||||||||
| Male greater female | Side | x | y | z | T | z | Unc. | Cor. | PB | Cohen’s d |
| Inferior frontal gyrus (BA47) | L | −25 | 16 | −8 | 4.30 | 3.95 | 30 | 10 | 0.005 | 1.20 |
| Cuneus (BA18) | L | −1 | −102 | 12 | 4.23 | 3.89 | 392 | 30 | 0.01 | 1.18 |
| Cuneus (BA18) | R | 24 | −75 | 17 | 3.89 | 3.63 | 11 | 41 | 0.027 | 1.09 |
| Inferior frontal gyrus (BA45) | L | −36 | 24 | 3 | 3.84 | 3.58 | 144 | 9 | 0.007 | 1.08 |
| Inferior frontal gyrus (BA47) | R | 29 | 20 | −3 | 3.66 | 3.42 | 105 | 9 | 0.029 | 1.03 |
|
Female greater Male | ||||||||||
| Precuneus (BA7) | L | −3 | −48 | 54 | 4.03 | 3.74 | 336 | 57 | 0.016 | 1.13 |
| Superior parietal lobule (BA7) | L | −29 | −63 | 44 | 3.99 | 3.70 | 84 | 32 | 0.017 | 1.12 |
| Inferior frontal gyrus (BA11) | L | −25 | 35 | −21 | 3.82 | 3.57 | 203 | 6 | 0.019 | 1.07 |
| Middle temporal gyrus (BA21) | L | −57 | 4 | −18 | 3.66 | 3.43 | 64 | 8 | 0.029 | 1.03 |
Talaraich and Tornoux (1998) coordinates;
P values corrected with small volume correction; GMV: gray matter volume; BPI: Bipolar Disorder, type I; PMRG: posteromedial rectal gyrus; k: number of voxels in the cluster for both uncorrected and corrected values
Talaraich and Tornoux (1998) coordinates;
P values corrected with small volume correction; k: number of voxels in the cluster for both uncorrected and corrected values
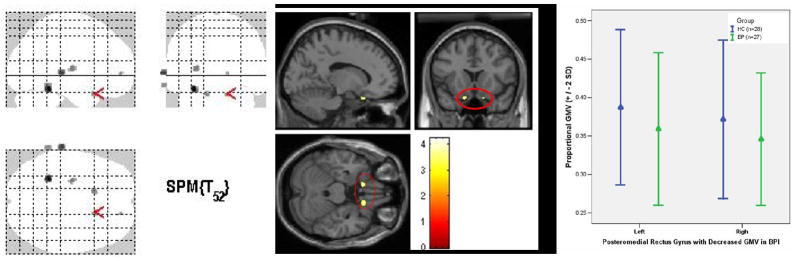
Fig. 1. Bilateral decrease in GMV in PMRG in BPI vs. HC.
A. Glass brain with arrow in the right PMRG
B. Anatomical localization of bilateral GMV reduction in PMRG in BPI. Right: cluster size 19 voxels, z=3.2, P=0.015 (x=12, y=14, z=−21). Left: cluster size 19 voxels, z=3.31, P=0.01 (x=−13, y=12, z=−21).
C. Mean and standard deviation of PMRG GMV in BPI and HC.
BPI: patients with bipolar disorder type I, HC: healthy controls. SE: standard error of the mean. Statistical threshold=P<0.05, using small volume correction
GMV was also reduced in BPI in two non-a priori regions: the left parahippocampal gyrus (Cohen’s d: 1.18, P=0.008) and left putamen (Cohen’s d: 1.0, P=0.017; see Table 3a). WB VBM analyses additionally covarying for age revealed the same between-group differences as before with a larger cluster size and effect size (Cohen’s d: L=1.28, P=0.002 and Cohen’s d: L=1.06, P=0.01, respectively; Table 3a). There were no significant increases in WB GMV in BPI relative to HC.
3.1.3. Effect of gender on WB GMV
Post hoc pairwise WB VBM analyses were performed to further examine the main effect of gender upon WB GM, covarying for age and total GMV. These analyses revealed GMV differences between all males and all females in regions other than those showing differences in GMV between BPI and HC. Males had increased GMV in bilateral cuneus (BA18; R: Cohen’s d=1.09, P=0.03; L: Cohen’s d=1.18, P=0.01), bilateral ventrolateral prefrontal cortex (BA47; R: Cohen’s d=1.03, P=0.03; L: Cohen’s d=1.2, P=0.01) and left inferior frontal gyrus (BA45; Cohen’s d=1.08, P=0.01). Females had increased GMV in the left ventromedial prefrontal cortex, but not in the PMRG (BA11; Cohen’s d=1.07, P=0.02), left middle temporal gyrus (BA21; Cohen’s d=1.03, P=0.03), left precuneus (BA7; Cohen’s d=1.13, P=0.02) and left superior parietal lobule (BA7; Cohen’s d=1.12, P=0.02; Table 3b).
3.2. Specificity of VBM to detect a priori region GMV abnormalities in BPI
There were no significant GMV differences (P<0.001, uncorrected) between groups comprising an approximately equal number of BPI patients and HC in any a priori regions. Between-group differences were observed in non-a priori regions, but did not survive WB FWE correction for multiple comparisons.
3.3. Examining potential effects of gender, change in acquisition parameters and trait anxiety upon GMV in a priori and non-a priori regions showing between-group differences in GMV
To further examine the extent to which factors common to both BPI and HC (i.e., gender, acquisition parameters, trait anxiety) may have contributed to, and interacted with, the reduction in GMV in BPI in a priori regions, i.e., bilateral PMRG, we performed an ANOVA, including diagnostic group, gender, acquisition parameters and high vs. low trait anxiety as fixed factors, covarying for age and total amount of GMV, on extracted GMV values in bilateral PMRG in all BPI and HC (Tables 4a and b).
Table 4.
| Table 4a: Relationship between PMRG, Putamen and parahippocampal gyrus GMV and diagnostic group, gender, scan (acquisition parameters) and trait anxiety | ||||||||
|---|---|---|---|---|---|---|---|---|
| PMRG |
Left |
|||||||
| Left | Right | Putamen | Parahippocampal Gyrus | |||||
| F | P | F | P | F | P | F | P | |
| Main effect of group | (1,36)2.14 | 0.15 | (1,36)2.00 | 0.17 | (1,36)18.13 | <0.001 | (1,36)7.5 | 0.009 |
| Main effect of gender | (1,36)0.08 | 0.77 | (1,36)0.18 | 0.67 | (1,36)0.001 | 0.97 | (1,36)0.01 | 0.91 |
| Main effect of scan | (1,36)0.00 | 0.97 | (1,36)0.01 | 0.92 | (1,36)0.03 | 0.85 | (1,36)<0.001 | 0.99 |
| Main effect of trait anxiety | (1,36)3.79 | 0.06 | (1,36)2.57 | 0.12 | (1,36)0.91 | 0.34 | (1,36)0.64 | 0.43 |
| Interaction group by gender | (1,36)0.52 | 0.47 | (1,36)0.40 | 0.53 | (1,36)0.52 | 0.48 | (1,36)0.003 | 0.95 |
| Interaction group by scan | (1,36)0.54 | 0.47 | (1,36)0.52 | 0.47 | (1,36)1.62 | 0.21 | (1,36)0.04 | 0.84 |
| Interaction group by trait anxiety | (1,36)2.00 | 0.17 | (1,36)3.08 | 0.09 | (1,36)0.85 | 0.36 | (1,36)0.04 | 0.85 |
| Interaction group by gender by scan | (2,36)2.56 | 0.09 | (2,36)1.24 | 0.30 | (2,36)0.17 | 0.84 | (2,36)0.62 | 0.54 |
| Interaction group by gender by trait anxiety | (2,36)3.97 | 0.03 | (2,36)1.73 | 0.19 | (2,36)2.58 | 0.09 | (2,36)1.1 | 0.34 |
| Interaction group by scan by trait anxiety | (2,36)0.78 | 0.46 | (2,36)0.19 | 0.83 | (2,36)0.99 | 0.38 | (2,36)0.21 | 0.81 |
| interaction group by gender by scan by trait anxiety | (1,36)0.00 | 0.97 | (1,36)0.02 | 0.88 | (1,36)0.58 | 0.45 | (1,36)0.66 | 0.42 |
| Table 4b. Post hoc tests to examine significant findings regarding the relationship between left PMRG GMV and diagnostic group, gender, and trait anxiety | ||||||
|---|---|---|---|---|---|---|
| Post hoc tests to examine the significant interaction group by gender by anxiety in left PMRG (P<0.01 to control for multiple tests) | ||||||
| F | P | F | P | F | P | |
| Min effect of group | Min effect of anxiety | Iteraction group by anxiety | ||||
| Gender male | (1,16)0.63 | 0.44 | (1,16)5.87 | 0.03 | (1,16)7.59 | 0.01 |
| Gender female | (1,25)5.18 | 0.03 | (1,25)0.03 | 0.87 | (1,25)0.61 | 0.44 |
| Main effect of group | Main effect of gender | Interaction group by gender | ||||
| Anxiety low | (1,22)15.39 | <0.001a | (1,22)0.38 | 0.54 | (1,22)0.79 | 0.38 |
| Anxiety high | (1,19)0.48 | 0.50 | (1,19)1.97 | 0.18 | (1,19)1.60 | 0.22 |
| Main effect of gender | Main effect of anxiety | Interaction gender by anxiety | ||||
| Group BPI | (1,21)1.54 | 0.23 | (1,21)2.44 | 0.13 | (1,21)4.12 | 0.06 |
| Group HC | (1,20)0.00 | 1.00 | (1,20)0.06 | 0.81 | (1,20)0.16 | 0.69 |
| Post hoc tests to examine the significant group x anxiety interaction in males in left PMRG (P<0.005 to control for multiple tests) | ||||||
| F | P | |||||
|
| ||||||
| Main effect of group | ||||||
| Anxiety low | (1,10)43.63 | <0.001b | ||||
| Anxiety high | (1,4)0.34 | 0.59 | ||||
| Main effect of anxiety | ||||||
| Group BPI | (1,6)37.20 | <0.001c | ||||
| Group HC | (1,8)0.04 | 0.84 | ||||
PMRG: posteromedial rectal gyrus. The statistical threshold used was: P<0.05. The trend for main effect of trait anxiety resulted form all high trait anxiety individuals having slower left PMRG GMV than all high trait anxiety individuals
BPI: bipolar disorder, type I; HC: healthy controls
: All low trait anxiety BPI had significantly smaller left PMRG GMV than all low trait anxiety HC
: All male low trait anxiety BPI had significantly smaller left PMRG GMV than all male low trait anxiety HC
: All male high trait anxiety BPI had significantly smaller left PMRG GMV than all male low trait anxiety BPI
In the right PMRG there were no significant main effects or interactions (all F<2.6, P>0.1). In the left PMRG a trend toward significance was found with a main effect of trait anxiety (F[1,36]=3.79, P=0.06). Here, larger GMV was associated with lower trait anxiety in all BPI and HC (low trait anxiety: mean left PMRG GMV=0.3355; high trait anxiety: mean left PMRG GMV=0.3305). A significant interaction of group by gender by trait anxiety (F [2,36]=3.97, P=0.03) was found. No other significant main effects or interactions were found (all F<3, P>0.05; Table 4a).
Post-hoc analyses to examine the above significant three-way interaction revealed a significant effect of group in all low trait anxiety participants (F[1,22]=15.39, P<0.001), and a significant group by trait anxiety two-way interaction in all male participants (F[1,16]= 7.59, P=0.01), but not in females (Table 4b).
The significant effect of group in all low trait anxiety participants resulted from all low trait anxiety BPI having smaller left PMRG GMV than all low trait anxiety HC (low trait anxiety BPI: mean left PMRG GMV=0.33; low trait anxiety HC: mean left PMRG GMV=0.34). Post-hoc tests to explore the above two-way interaction of group x trait anxiety in all male participants first revealed a significant effect of group in low- but not high-trait anxiety males (F[1,10]=43.63, P<0.001), with low trait anxiety male BPI showing smaller left PMRG GMV than low trait anxiety male HC (low trait anxiety male BPI: mean left PMRG GMV=0.34; low trait anxiety male HC: mean left PMRG GMV=0.36). There was also a main effect of trait anxiety in all male BPI (F[1,6]=37.2, P<0.001): high trait anxiety male BPI had smaller left PMRG GMV than low trait anxiety male BPI (high trait anxiety male BPI: mean left PMRG GMV=0.344; low trait anxiety male BPI: mean left PMRG GMV=0.345). No other significant main effects or interactions were found from these post-hoc analyses (F<6; P>0.03; Table 4b).
To further examine the extent to which factors common to both BPI and HC may have contributed to, and interacted with, the reduction in GMV in BPI in non-a priori regions, i.e., left parahippocampal gyrus and left putamen, we performed an ANOVA, including diagnostic group, gender, acquisition parameters and high vs. low trait anxiety as fixed factors, covarying for age and total amount of GMV, on extracted GMV values in these regions in all BPI and HC. No significant main effects or interaction on GMV were found in either region. There was only a weak trend for a three-way group by gender by trait anxiety interaction (P=0.09) in the left putamen (Table 4b).
3.4. Examination of illness variables and medication load in BPI a priori and non-a priori regions
We performed additional post hoc, t-tests and correlational analyses as appropriate upon GMV values extracted from bilateral PMRG to examine the extent to which illness variables specific to BPI (i.e,. depressed vs. euthymic mood state, age of illness onset, illness duration and medication load) may have contributed to the reduction in GMV in these a priori regions in BPI. There was no significant difference in either left or right PMRG GMV volume between euthymic and depressed BPI (Table 5a). There were no significant relationships between either left or right PMRG GMV and age of illness onset, disease duration, or medication load in all BPI, although there were trends for negative correlations between medication load and bilateral PMRG GMV in all BPI (r=−0.4, P<0.05; Table 5a). There was no significant difference between either left or right PMRG GMV between BPI taking versus those not taking lithium (Table 5a).
Table 5.
| Table 5a: Effects of mood state, illness variables and medication upon PMRG GMV in BPI | |||
|---|---|---|---|
| PMRG | GMV | ||
| Euthymic versus depressed BPI | R | t (25)=0.29 | P=0.77 |
| L | t (25)=1.13 | P=0.27 | |
| Age of illness onset | R | r=0.15 | P=0.46 |
| L | r=0.023 | P=0.91 | |
| Illness duration | R | r=0.015 | P=0.94 |
| L | r=0.14 | P=0.49 | |
| Lithium | R | t (25)=0.45 | P=0.66 |
| L | t (25)=1.57 | P=0.13 | |
| Medication load | R | r=−0.41 | P=0.034* |
| L | r=−0.41 | P=0.032* | |
| Table 5b. Effects of mood state, illness variables and medication upon left putamen and left parahippocampal gyrus GMV in BPI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-a priori region | Euthymic vs. depressed BPI | Age of illness onset | Illness duration | Lithium (taking versus not taking) | Medication load | |||||
| Left putamen | t (25)=0.36 | P=0.2 | r=0.24 | p=0.23 | r=0.031 | p=0.88 | t (25)=1.4 | P=0.17 | r=−0.45 | P=0.02* |
| Left parahippocampal gyrus | t (25)=1.6 | p=0.12 | r=0.2 | p=0.31 | r=0.031 | p=0.88 | t (25)=0.74 | p=0.47 | r=−0.45 | p=0.02* |
Trend findings: We used a statistical threshold of P=0.01 to control for multiple tests.
We performed similar analyses to examine the extent to which illness variables specific to BPI may have contributed to the reductions in GMV in non-a priori regions in BPI, i.e., left parahippocampal gyrus and left putamen. There were trends for negative correlations between medication load and left putamen (r=−0.45, P=0.02) and left parahippocampal gyrus (r=−0.451, P=0.02; Table 5b).
4. Discussion
The goal of our study was to increase understanding of pathophysiologic neural mechanisms of BP by examining GMV in two key neural regions implicated in mood regulation. We aimed first to localize OMPFC GMV abnormalities and determine the nature of amygdala GMV in BPI. We used VBM as an objective mean of examining regional GMV, and included a test of the specificity of this methodology to detect GMV abnormalities in BPI. Our main finding of reduced GMV in OMPFC in BPI is consistent with previous findings (Blumberg et al., 2006; Lopez-Larson et al., 2002; Lyoo et al., 2004; Nugent et al., 2006), and, additionally, we have localized this to the posteromedial rectal gyrus (PMRG). This region, in the medial prefrontal network of the OMPFC, is important for visceromotor and emotion regulation (Ongur et al., 2003), and is implicated in reappraisal of emotional stimuli in both children (Levesque et al., 2004) and adults (Phan et al., 2004). Decreased PMRG GMV may, therefore, be associated with mood dysregulation in BPI. We did not demonstrate any amygdala GMV abnormality in BPI, in contrast to functional neuroimaging studies, that have demonstrated abnormally increased amygdala activity to emotional stimuli in BPI (Blumberg et al., 2005; Altshuler et al., 2005). Our findings therefore suggest that decreased amygdala GMV may not underlie the abnormal amygdala activity observed in BPI. It is, however, possible that a type II error led to our findings regarding amygdala GMV in BPI versus HC in this study.
To distinguish between potential effects of diagnosis and gender upon WB GMW we examined the effect of gender, covarying for total amount of GMV and age, on WB GMV in all BPI and HC. Our analyses revealed a significant effect of gender upon GMV in regions other than those in which a significant effect of diagnostic group was observed. Specifically, all males, regardless of diagnosis, had increased GMV relative to all females in a variety of bilateral prefrontal and occipital cortical regions. Females showed GMV increases relative to males in different prefrontal, visual and temporal cortical regions. These findings may reflect the different patterns of development of cortical regions in male and females that have been previously observed (e.g. Lenroot and Giedd, 2006; Giedd et al., 2006). Importantly, there was no overlap between findings regarding the effect of diagnostic group and effect of gender upon WB GMV. These findings therefore indicate distinct effects of diagnostic group and gender upon WB GMV in the participants in our study.
Additional VBM analyses on two subject groups, each comprising similar numbers of BPI and HC, demonstrated no significant effect of group upon GMV in a priori regions, and therefore allowed us to be confident that the PMRG GMV decrease demonstrated in BPI was not an artifact of VBM analyses.
Post-hoc analyses examining the potential effects of gender, changes in acquisition parameters and trait anxiety upon PMRG GMV in BPI relative to HC revealed a significant interaction only between diagnostic groups, gender and trait anxiety upon left PMRG GMV. Here, we found a significant GMV reduction in left PMRG in all low trait anxiety BPI versus low trait anxiety HC, and, more specifically, in low trait anxiety male BPI versus low trait anxiety male HC. We also found significant GMV reduction in left PMRG in high versus low trait anxiety male BPI, and a trend overall for reduced left PMRG GMV in all high versus low trait anxiety HC and BPI. The PMRG role in emotion regulation has been indicated previously (Ongur et al., 2003; Ongur and Price 2000; Phillips et al., 2003a). Reduced GMV in PMRG may therefore be associated with the emotion dysregulation of BPI. Our findings further suggest that GMV reduction in this region and relationship with emotion dysregulation may be specific to male BPI. The significant reduction in PMRG GMV evident for low, but not high, trait anxiety male BPI relative to their healthy male counterparts may have resulted from the fact that in all high-trait anxiety individuals there was a trend, relative to all low trait anxiety individuals, for decreased left PMRG GMV. Thus significant decreases in left PMRG GMV in male BPI may manifest more in the low trait anxiety subgroup.
We employed a novel method for examination of potential contribution of total medication load, rather than separate effects of individual psychotropic medication classes, to abnormalities in GMV in BPI. There were trends for negative correlations between medication load and GMV in bilateral PMRG, consistent with previous findings showing an association between psychotropic medication and decreases (Nugent et al., 2006) in ventral prefrontal cortical GMV, and between antidepressants and decreased right inferior prefrontal cortical GMV, in BP adults Lopez-Larson et al., 2002). Our findings are not consistent with other findings, however, showing a potential effect of either lithium or sodium valproate in protecting against glial loss in amygdala (Chang et al., 2005; Bowley et al., 2002) and ventromedial prefrontal cortex (Moore et al., 2000; Manji et al., 2001), and an association between increased inferior prefrontal cortical GMV and psychotropic medication use (Blumberg et al., 2006). We showed no significant relationship in BPI between any illness history variable and PMRG GMV, in support of previous findings (Brambilla et al., 2003). Other studies have, however, shown an inverse relationships between illness duration and left prefrontal GMV (Lopez-Larson et al., 2002), and between amygdala volume and illness duration (DelBello et al., 2004), in BPI. Possible reasons for the discrepancy between our findings and those of other studies are differences in age at scan, illness duration and lithium exposure in our adult BPI relative to the younger populations in some earlier studies. It is also possible that complex relationships between illness duration and lithium exposure may underlie the finding of normal amygdala GMV in the BPI in our study.
There were two non-a priori findings in BPI: decreased left-sided parahippocampal gyrus and putamen GMV. The parahippocampal gyrus has multiple, direct connections with the hippocampus and the amygdala (Altshuler et al., 2005). A dynamic functional relationship between amygdala and parahippocampal gyrus may be protective against potentially harmful experiences (McNaughton and Corr, 2004). The parahippocampal gyrus has, furthermore, been implicated in the response to emotional stimuli (e.g. Lang et al., 2003; Surguladze et al., 2006), and decreased parahippocampal gyral activity to emotional words has previously been reported in BP (Malhi et al., 2007); however our finding regarding the parahippocampal gyrus was left- rather than right-sided. The putamen, together with other subcortical limbic regions, is important in the response to rewarding emotional stimuli (Surguladze et al., 2005), and greater activity in the putamen has previously been demonstrated in BPI relative to HC (e.g. Lawrence et al, 2004). There are, however, inconsistent findings regarding striatal volumes in BP that may be related to inconsistencies in data acquisition and age effects (Bonelli et al., 2006). Our findings of reduced parahippocampal gyral and putamen GMV in BPI may underlie the dysfunctional regulation of emotional experiences in BPI. We also observed a trend toward negative correlations with psychotropic medication load and GMV in both left putamen and left parahippocampal gyrus in BPI. Together with findings regarding medication load on bilateral PMRG GMV, these findings suggest an effect of medication load per se on reducing GMV in BPI. Further examination of effects of clinical variables and medication upon GMV in these regions should be the focus of future studies in BPI.
Interestingly, the majority of our significant findings regarding GMV reductions in a priori – and non-a priori - regions in BPI were localized to the left hemisphere, and were more prominent in males in the left PMRG. The well-documented anterior/posterior, i.e., fronto-occipital, asymmetry in humans, with larger right than left frontal cortex (Weinberger et al., 1982), occurs to a greater extent in males relative to females (Bear et al, 1986), and may be more prominent in right-handers (Narr et al., 2007). The greater right than left-sided frontal cortical GMV therefore expected in right-handed male HC and BPI in the present study may have led to any reduction in frontal cortical GMV in male BPI to be manifest more prominently in the left hemisphere. The greater right than left-sided anterior relative to posterior cortical GMV expected in all (right-handed) participants in the present study may also have led to any reduction in GMV in anterior limbic and paralimbic structures, including the parahippocampal gyrus and putamen, being more prominent in the left hemisphere in BPI. A recent VBM study, however, showed increased rather than decreased gray matter in BPI in the left parahippocampal gyrus (Chen et al., 2007)
We had a large effect size of 0.98 for between-group differences in bilateral PMRG GMV. With this effect size, and the number of participants we recruited, our study was adequately powered (using P=0.05) to detect between-group differences in a priori ROIs (Kraemer and Kupfer, 2006). The number of participants recruited was also similar to that in previous structural neuroimaging studies in BP (Blumberg et al., 2006). While a possible limitation of our study was the employment of VBM because of the potential problem with spatial normalization (Oakes et al., 2007), VBM has been employed previously in the examination of volumetric abnormalities in BP (e.g., Chen 2007). Furthermore, many potential concerns regarding registration were alleviated because of the unified segmentation model in SPM5, which leads to a better probability image than earlier SPM versions. An interesting methodological focus for future studies in bipolar – and other psychiatric – populations would be to compare findings from region-of-interest tracing and whole brain VBM-based analytic methodologies.
Other potential limitations to the study included the inclusion of BPI with previous substance abuse and different medication histories, and the inherent difficulties in distinguishing medication effects from effects of illness severity. We did, however, examine separately the main effects of illness history variables and medication load upon GMV abnormalities in the BPI in our study. Our approach for the examination of medication load is preferable to existing strategies that examine effects of different types of psychotropic medication separately, and also allows inclusion into neuroimaging studies BPI who are representative of the bipolar population in general, who have been medicated in real-world contexts, and typically treated with a number of different medications and medication combinations. It is clearly extremely difficult to recruit medication-naïve BP, particularly BPI, unmedicated BPI or BPI matched for medication history into neuroimaging studies. Recruitment of medication-naïve or unmedicated BPI may bias selection of participants to those with less severe illness, who can tolerate being medication-free, but who are not representative of the bipolar population as a whole (Phillips et al., 2008 Phillips et al., in press).
We demonstrate reduced OMPFC GMV in BPI, and are the first to localize this abnormality to the PMRG, part of the OMPFC medial cortical network implicated in emotion regulation, that appear to be specific to male BPI and to high trait anxiety individuals. We do not show abnormalities in amygdala GMV. Our findings indicate that structural abnormalities, specifically within the OMPFC medial network, may underlie the affective instability in BPI, particularly male BPI. Future studies in BPI should examine further effects of gender, trait anxiety, and psychotropic medication upon development of these regional GMV abnormalities throughout the course of the illness.
Acknowledgments
All work was carried out within the Department of Psychiatry, University of Pittsburgh, neuroimaging data was collected at the Brain Imaging Research Center, University of Pittsburgh and Carnegie Mellon University. We thank Dr. KJ Jung, S Kurdilla and D Vizslay for their help acquiring neuroimaging data. The research in this study was supported in part by a NARSAD Independent Investigator Award to MLP and 5R01 MH076971-01. MLP is the NARSAD Nellie Blumenthal Investigator. JRCA is supported by CAPES (Brazilian foundation, #190105-2)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archives General Psychiatry. 1998;55(7):663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. American Journal Psychiatry. 2005;162(6):1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bassitt DP, Neto MR, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. European Archives Psychiatry Clinical Neuroscience 2006;??:???–???. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biological Psychiatry 2007;??:???–???. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183(3):308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, Martin L, Gerard E, Charney DS, Peterson BS. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biological Psychiatry. 2006;59(7):611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Kapfhammer HP, Pillay SS, Yurgelun-Todd DA. Basal ganglia volumetric studies in affective disorder: what did we learn in the last 15 years? Journal of Neural Transmission. 2006;113(2):255–268. doi: 10.1007/s00702-005-0372-7. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002;52(5):404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. Journal of Psychiatric Ressearch. 2003;37(4):287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002;51:4–273. doi: 10.1016/s0006-3223(01)01336-1. [1-page reference?] [DOI] [PubMed] [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, Simeonova DI, Barnes P, Ketter T, Reiss AL. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biological Psychiatry. 2005;58(3):197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Chen X, Wen W, Malhi GS, Ivanovski B, Sachdev PS. Regional gray matter changes in bipolar disorder: a voxel-based morphometric study. Australian and New Zealand Journal Psychiatry. 2007;41(4):327–336. doi: 10.1080/00048670701213229. [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. Journal of Clinical Psychopharmacology. 2004;24(2):192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorder. 2004;6(1):43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48(8):813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biological Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon ML, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV (SCID-I): User’s Guide and Interview, Research Version. Biometrics Research Department, New York Psychiatric Institute; New York: 1995. [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Molecular Cellular Endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29(5):952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. Subgenual cingulate cortex volume in first-episode psychosis. American Journal Psychiatry. 1999;156(7):1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry. 2006;59(11):990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biological Psychiatry. 2004;55(6):648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Macmaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, Lynch M, Rose M, Moore GJ, Rosenberg DR. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biological Psychiatry 2007;??:???–???. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. Journal of Affective Disorders. 2007;97(1–3):109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Bipolar disorder: leads from the molecular and cellular mechanisms of action of mood stabilizers. British Journal of Psychiatry Suppl. 2001;41:s107–119. [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356(9237):1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, Ratnanather JT, Huang H, Todd RD, Miller MI, Botteron KN. Amygdala volume analysis in female twins with major depression. Biological Psychiatry. 2007;62(5):415–422. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Luders E, Thompson PM, Woods RP, Robinson D, Szeszko PR, Dimtcheva T, Gurbani M, Toga AW. Asymmetries of cortical shape: effects of handedness, sex and schizophrenia. Neuroimage. 2007;34(3):939–948. doi: 10.1016/j.neuroimage.2006.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30(2):485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ. Integrating VBM into the General Linear Model with voxelwise anatomical covariates. Neuroimage. 2007;34(2):500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States America. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003a;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003b;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication Effects in Neuroimaging Studies of Bipolar Disorder. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.07071066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Killgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biological Psychiatry. 2007;61(6):743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48(8):791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Professional Manual for the State–Trait Anger Expression Inventory. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disorder. 2000;2(3 Pt 1):148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Annals of the New York Academy of Sciences. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: a voxel-based morphometric magnetic resonance imaging study. Psychiatry Research. 2007;156(1):83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Luchins DJ, Morihisa J, Wyatt RJ. Asymmetrical volumes of the right and left frontal and occipital regions of the human brain. Annals of Neurology. 1982;11(1):97–100. doi: 10.1002/ana.410110118. [DOI] [PubMed] [Google Scholar]