Abstract
The lack of methods for the stereoselective transfer of functionalized carbenoids is one of the most significant deficiencies of Simmons–Smith cyclopropanation reactions. Outlined herein are one-pot methods for the catalytic asymmetric synthesis of halocyclopropyl alcohols with up to four stereogenic centers from achiral starting materials. The first method involves asymmetric alkyl addition to a conjugated enal to generate an allylic alkoxide followed by tandem diastereoselective iodo-, bromo- or chlorocyclopropanation to furnish halocyclopropyl alcohols. Enantioselectivities in these processes range from 89–99% and dr > 20:1 were achieved with all substrates optimized. The second method consists of an asymmetric vinylation of a saturated or aromatic aldehyde followed by a diastereoselective iodocyclopropanation to produce iodocyclopropyl alcohols with enantioselectivities between 86 and 99% and dr > 20:1. These complementary methods enable the efficient synthesis of a variety of halocyclopropyl alcohols in one-pot procedures. Preliminary efforts to functionalize iodocyclopropanes involve reaction with an excess of LiCu(n-Bu)2 to generate the cyclopropyl cuprate. This intermediate can be quenched with allyl bromides to generate the allylated cyclopropyl alcohols without loss of enantio- or diastereoselectivity.
1. Introduction
Cyclopropyl groups are found widely throughout natural product and medicinal chemistry, and cyclopropyl-containing compounds have been shown to exhibit important biological activity.1–4 Furthermore, these strained cycloalkanes are versatile intermediates in synthesis, allowing access to either functionalized cyclopropanes or ring-opened products.5 The importance of enantioenriched cyclopropanes has inspired considerable effort toward their synthesis.6–9 Remarkable progress has been made in the area of enantioselective rhodium-catalyzed decomposition of diazoesters and related precursors to afford enantio- and diastereoenriched cyclopropanes (although certain disubstituted alkenes remain challenging substrates). Complementary to these methods is halomethylmetal-mediated cyclopropanation reactions (Simmons–Smith cyclopropanations).8,10,11 In contrast to the successful rhodium catalyzed cyclopropanations, catalysts for asymmetric Simmons–Smith cyclopropanations of olefins12,13 and allylic alcohols6,14–20 have been plagued by moderate enantioselectivities, high catalyst loadings (typically 10–25 mol%)6,21 and mediocre substrate generality.22 These problems underscore the difficulty in asymmetric Simmons–Smith cyclopropanations.21
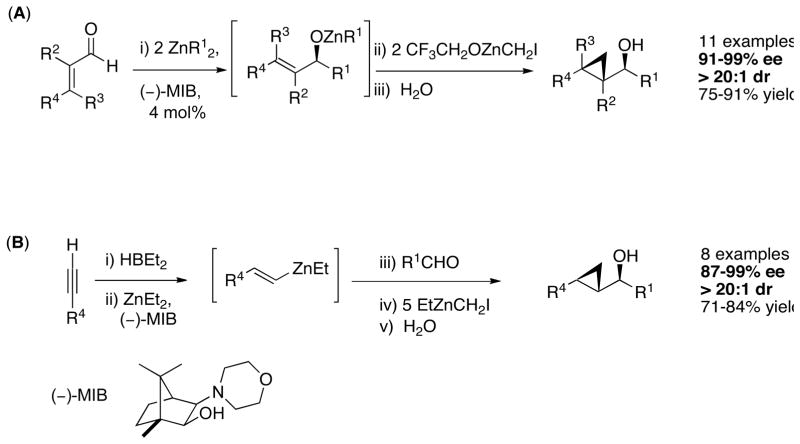
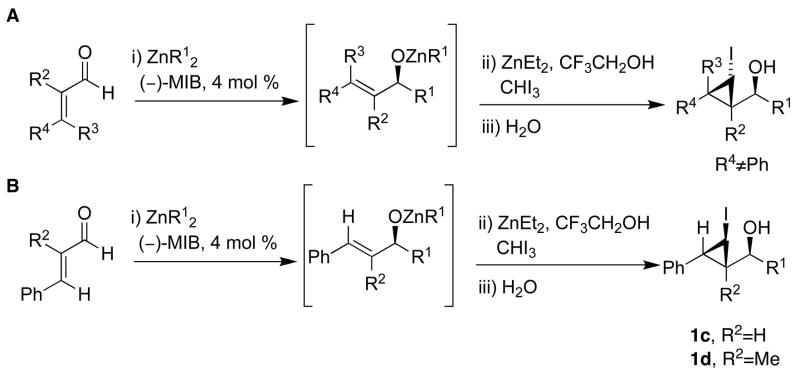
Given the formidable challenge of developing enantioselective Simmons–Smith catalysts, we considered alternative strategies for the stereoselective synthesis of cyclopropanes. We envisioned a catalytic enantioselective carbonyl addition to generate an allylic zinc alkoxide intermediate followed by a diastereoselective cyclopropanation (Scheme 1).23 Performing these reactions in tandem enables formation of three C–C bonds and up to three stereocenters in a one-pot procedure with excellent stereoselectivity.23 The two approaches we introduced consist of (A) an asymmetric addition of an alkylzinc reagent to an enal followed by diastereoselective cyclopropanation and (B) enantioselective vinyl addition to a saturated aldehyde followed by cyclopropanation. Both approaches use 4 mol % of Nugent’s amino alcohol (−)-MIB24,25 and exhibit enantioselectivities over 90% (with one exception).23 The dr’s were >20:1 in all cases. Although diastereoselective cyclopropanations of chiral allylic alcohols had been studied,26–28 these were the first examples of assembly of enantio- and diastereoenriched cyclopropyl alcohols from achiral precursors in a one-pot procedure.23
Scheme 1.
Tandem Approaches to Enantio- and Diastereoenriched Cyclopropanols
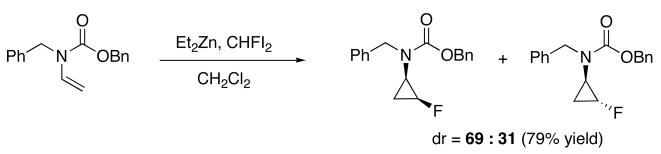
Another major limitation of the Simmons–Smith cyclopropanation is the limited number of carbenoid motifs that can be stereoselectively transferred. When carbenoid carbons bearing alkyl (CHR),29–32 halo (CHX),33–36 or alkoxy (CHOR)37 groups were employed, mixtures of diastereomers were obtained with few exceptions.38 These problems are exemplified by the fluorocyclopropanation in Scheme 2.35
Scheme 2.
Diastereoselective Fluorocyclopropanation
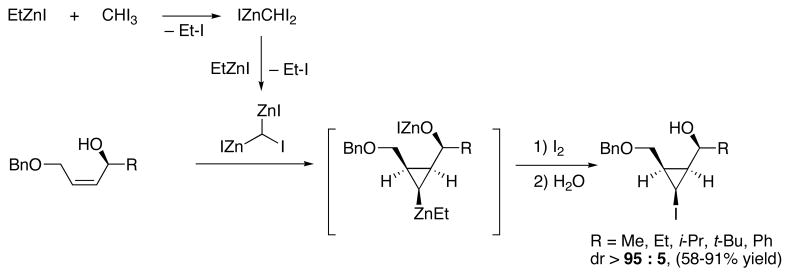
An impressive exception to the above-mentioned deficiency is Charette’s highly diastereoselective transfer of a zinc-bearing carbenoid39–41 to (Z)-allylic alcohols (Scheme 3).41 Beginning with iodoform, reaction with EtZnI generates an intermediate, IZnCHI2, that undergoes a second exchange to form the dizinc carbenoid, (IZn)2CHI. After cyclopropanation, quenching with iodine or bromine provides the cyclopropyl halide with high dr. The reaction is only moderately diastereoselective with the (E)-analogues, unless the substrate contains a very bulky t-Bu or SiMe3 group. Currently, this transformation only proceeds with substrates that bear an allylic OBn group.39–41 Moreover, prior generation and purification of the chiral alcohol is required. Metallated cyclopropanes can also be prepared by addition of organometallic reagents to cyclopropenes.9,42–44
Scheme 3.
Charette’s Diastereoselective Transfer of Geminal Dizinc Carbenoids to Benzyl (Z)-Allyl Ethers
Development of methods for the stereocontrolled transfer of functionalized carbenoids via the Simmons–Smith reaction would greatly expand the variety of cyclopropanes that are accessible with high enantio- and diastereoselectivity. Herein, we disclose a highly enantio- and diastereoselective one-pot synthesis of halocyclopropyl alcohols. The key steps in our approach are the generation of an enantioenriched allylic alkoxide (Scheme 1) and a novel diastereoselective halocyclopropanation reaction. A portion of this work has been communicated.23
2. Experimental Section
Representative procedures and characterization of the products are described herein. Full experimental details and characterization of all compounds are provided in the Supporting Information.
General Methods
All reactions were carried out under a nitrogen atmosphere with oven-dried glassware. The progress of reactions was monitored by thin-layer chromatography on Whatman precoated gel 60 F-254 plates and visualized by ultraviolet light or by staining with ceric ammonium molybdate stain. All manipulations involving dialkylzinc reagents were carried out under an inert atmosphere in a Vacuum Atmosphere drybox with an attached MO-40 Dritrain or by using standard Schlenk or vacuum line techniques. Unless otherwise specified, all chemicals were obtained from Aldrich, Acros, or GFS chemicals, and solvents were purchased from Fischer Scientific. Dialkylzinc compounds, except dimethyl- and diethylzinc, which are commercially available, were prepared by literature methods.45,46 Dichloromethane and hexanes were dried through alumina columns. All aldehydes were distilled prior to use and stored under N2. The 1H and 13C{1H} NMR spectra were obtained on Bruker 500 or 300 MHz Fourier transform spectrometers at the University of Pennsylvania NMR facility. Chemical shifts are reported in units of parts per million downfield from tetramethylsilane, and all coupling constants are reported in hertz. The infrared spectra were obtained using a Perkin-Elmer 1600 series spectrometer. Silica gel (230–400 mesh, Silicycle) was used for air-flashed chromatography. Deactivated silica gel was made by combining silica gel with 2.5 wt % NEt3.
Cautionary Note: Dialkylzinc reagents are pyrophoric. Caution should be used in their handling.
General Procedure A. Asymmetric Additions to Enals/Diastereoselective Iodocyclopropanations
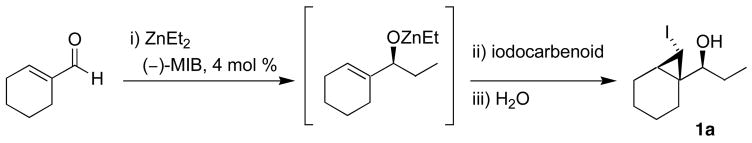
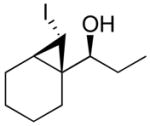
1-(7-Iodobicyclo[4.1.0]heptan-1-yl)propan-1-ol (1a)
A 10 mL Schlenk flask was charged with (−)-MIB (4.7 mg, 0.02 mmol, 4 mol %) and 1.0 mL hexanes and cooled to 0 °C. A solution of Et2Zn (1.0 mL, 1.0 M in hexanes, 1.0 mmol) was added, followed by dropwise addition of 1-cyclohexene carboxaldehyde (57 μL, 0.5 mmol). The solution was stirred at 0 °C until alkyl addition was complete by TLC (8 h). A solution of Et2Zn (1.0 mL, 1.0 M in hexanes, 1.0 mmol) and neat CF3CH2OH (72 μL, 1.0 mmol) were added slowly at 0 °C. After stirring at 0 °C for 10 min, iodoform (394 mg, 1.0 mmol) dissolved in 3.0 mL dichloromethane and 4 Å molecular sieves were added. The Schlenk flask was wrapped in aluminum foil to exclude light, and stirring was continued at room temperature for 24 h, after which the reaction mixture was quenched with a saturated solution of NH4Cl (15 mL). The organic and aqueous layers were separated, and the aqueous layer was xtracted with 3 × 20 mL dichloromethane. The combined organic layers were then washed with saturated aqueous Na2S2O3 followed by saturated aqueous NaHCO3 and then dried over MgSO4. The filtrate was concentrated in vacuo, and the residue was chromatographed on deactivated silica (10% ethyl acetate in hexanes) to afford the title compound as a light yellow oil in 66% yield. [α]D20 = +15.85 (c = 0.65, CHCl3); 1H NMR (C6D6, 500 MHz): δ 0.87 (m, 1H), 1.00 (t, 3H, J = 7.5 Hz), 1.02 (m, 2H), 1.06 (m, 1H), 1.39 (m, 5H), 1.73 (m, 1H), 1.87 (br s, 1H), 2.05 (m, 1H), 2.22 (d, 1H, J = 4.9 Hz), 3.29 (m, 1H); 13C{1H} NMR (C6D6, 75 MHz): δ 4.52, 11.19, 20.79, 21.79, 22.30, 23.06, 26.82, 27.55, 30.37, 82.96; IR (neat): 3396 (OH), 2927, 2855, 1454, 1102, 1020, 970, 790, 630 cm−1; HRMS-CI m/z 263.0285 [(M–OH)+; calcd for C10H16I: 263.0297].
General Procedure B. Asymmetric Vinylation of Aldehydes/Diastereoselective Iodocyclopropanation
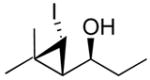
1-(2-Butyl-3-iodocyclopropyl)-2-methylpropan-1-ol (1i)
1-Hexyne (58 μL, 0.50 mmol) and diethylborane (0.50 mL, 0.50 mmol, 1.0 M in toluene) were added to a dry flask under nitrogen and stirred at room temperature for 30 min. The reaction flask was then cooled to −78 °C, (−)-MIB (4.7 mg, 0.02 mmol, 4 mol %) was added followed by Et2Zn (0.3 mL, 2.0 M in dichloromethane). The reaction mixture was then warmed to −10 °C and isobutyraldehyde (46 μL, 0.5 mmol) was added dropwise. The solution was stirred for 8 h at −10 °C until vinyl addition was complete by TLC. The volatile materials, including the Et3B byproduct, were removed in vacuo at 0 °C. Hexanes (2.0 mL) was added and the volatile materials were again removed under reduced pressure. This step was repeated two more times to ensure removal of Et3B. Et2Zn (1.5 mL, 1.5 mmol, 1.0 M in dichloromethane), iodoform (3.0 mmol, 6 equiv in 5.0 mL dichloromethane), and activated 4 Å molecular sieves were added at 0 °C. The Schlenk flask was covered with aluminum foil to exclude light and the reaction mixture was stirred at room temperature for 24 h. The reaction was then quenched by addition of a saturated solution of NH4Cl (15 mL). The organic and aqueous layers were separated then the aqueous layer was extracted with 3 × 20 mL dichloromethane. The combined organic layers were then washed with saturated aqueous Na2S2O3, and saturated aqueous NaHCO3 and dried over MgSO4. The filtrate was concentrated in vacuo, and the residue was chromatographed on deactivated silica (3% ethyl acetate in hexanes) to afford the title compound as a colorless oil in 64% yield. [α]D20 = +16.66 (c = 0.50, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 2.93 (dd, 1H, J = 4.4 Hz, 7.2 Hz), 2.57 (dd, 1H, J = 4.4, 7.8 Hz), 1.73 (m, 1H), 1.52 (br s, 1H), 1.43 (m, 2H), 1.31 (m, 4H), 0.91 (d, 6H, J = 6.67 Hz), 0.86 (t, 3H, J = 7.1), 0.83 (m, 1H), 0.42 (m, 1H); 13C{1H} NMR (CDCl3, 125 MHz): δ 79.02, 34.52, 34.26, 33.85, 30.83, 22.74, 21.08, 18.85, 18.00, 14.30, −3.53; IR (neat): 3421 (OH), 2958, 2857, 1465, 1379, 1224, 998, 801 cm−1; HRMS-CI m/z 279.0609 [(M-OH)+; calcd for C11H20I: 279.0609].
General Procedure C. Asymmetric Addition to an Enal/Diastereoselective Bromocyclopropanation
3-Bromo-2, 2-dimethylcyclopropyl)propan-1-ol (2b)
A 10 mL Schlenk flask was charged with (−)-MIB (4.7 mg, 0.02 mmol, 4 mol %) and cooled to 0 °C. A solution of Et2Zn (0.50 mL, 2.0 M in dichloromethane) was added, followed by dropwise addition of 3-methyl-2-butenal (48 μL, 0.5 mmol). The reaction was stirred at 0 °C for 8 h until the addition was complete by TLC. A solution of Et2Zn (1.25 mL, 2.5 mmol, 2.0 M in dichloromethane) and neat CF3CH2OH (179 μL, 2.5 mmol) were added slowly at 0 °C. After stirring at 0 °C for 5 min, bromoform (218 μL, 2.5 mmol) was injected into the reaction mixture. The flask was covered with aluminum foil and the solution was stirred at room temperature for 24 h. The reaction was quenched with saturated NH4Cl (10 mL), and the organic and aqueous layers were separated. The aqueous layer was extracted with 3 × 15 mL dichloromethane. The combined organic layers were then washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the residue was chromatographed on deactivated silica (3% ethyl acetate in hexanes) to afford the title compound as a colorless oil in 75% yield. [α]D20 = + 33.75 (c = 0.5, CHCl3); 1H NMR (CDCl3, 300 MHz): δ 3.48 (m, 1H), 2.97 (d, 1H, J = 7.46 Hz), 2.02 (br s, 1H), 1.58 (m, 2H), 1.12 (s, 3H), 1.11 (s, 3H), 0.97 (t, 3H, J = 7.65 Hz), 0.89 (dd, 1H, J = 7.46, 9.68 Hz); 13C{1H} NMR (CDCl3, 75 MHz): δ 72.29, 35.56, 33.86, 29.81, 27.48, 21.15, 18.38, 10.25; IR (neat): 3457 (OH), 2930, 1450, 1254, 1169, 1112, 1010, 966, 698 cm−1; HRMS-CI m/z 190.1058 [(M–OH)+; calcd for C8H14Br: 190.1053].
General Procedure D. Asymmetric Addition to an Enal/Diastereoselective Chlorocyclopropanation
1-(3-Chloro-2,2-dimethylcyclopropyl)ethanol (3b)
A 10 mL Schlenk flask was charged with (−)-MIB (4.7 mg, 0.02 mmol, 4 mol %) and cooled to 0 °C. A solution of Et2Zn (0.50 mL, 1.0 mmol, 2.0 M in dichloromethane) was added followed by dropwise addition of 3-methyl-2-butenal (48 μL, 0.5 mmol). The reaction was stirred at 0 °C for 8 h until alkyl addition was complete by TLC. A solution of Et2Zn (1.25 mL, 2.5 mmol, 2.0 M in dichloromethane) and neat CF3CH2OH (179 μL, 2.5 mmol) were added slowly at 0 °C. After stirring at 0 °C for 5 min, dibromochloromethane (212 μL, 2.5 mmol) was added. The Schlenk flask was wrapped with aluminum foil and the reaction mixture stirred at room temperature for 24 h. The reaction was then quenched with saturated NH4Cl (15 mL), the organic and aqueous layers were separated, and the aqueous layer was extracted with 3 × 20 mL dichloromethane. The combined organic layers were then washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the residue was chromatographed on deactivated silica (3% ethyl acetate in hexanes) to afford the title compound as a colorless oil in 70% yield. [α]D20 = + 11.20 (c = 0.5, CHCl3); 1H NMR (CDCl3, 300 MHz): δ 3.55 (m, 1H), 3.01 (d, 1H, J = 7.47 Hz), 1.88 (br s, 1H), 1.59 (m, 2H), 1.14 (s, 3H), 1.08 (s, 3H), 0.98 (t, 3H, J = 7.52 Hz), 0.89 (dd, 1H, J = 7.47, 9.77 Hz); 13C{1H} NMR (CDCl3, 75 MHz): δ 70.79, 43.66, 34.23, 29.93, 27.34, 21.53, 16.23, 10.22; IR (neat): 3407 (OH), 2859, 1450, 1259, 1085, 801, 749 cm−1; HRMS-CI m/z 145.0779 [(MOH)+; calcd for C8H14Cl: 145.0784].
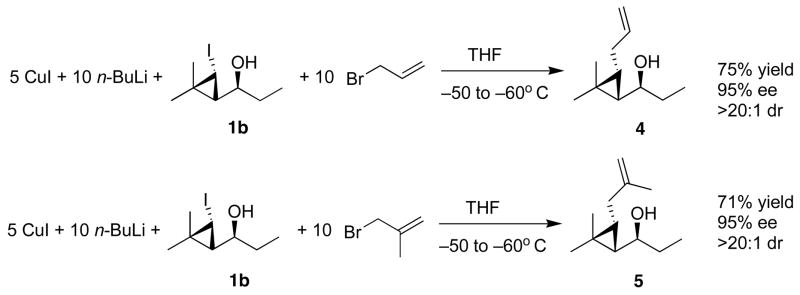
General Procedure E. Allylation of Iodocyclopropyl Alcohols
1-(3-Allyl-2,2-dimethylcyclopropyl)propan-1-ol (4)
An oven-dried 25.0 mL round-bottom flask that had been thoroughly purged with N2 was charged with CuI (1.4 mmol, 266 mg, 5 equiv) and dry THF (3.0 mL) and was cooled to −50 °C. A solution of n-BuLi (1.12 mL, 2.5 M in decane, 2.8 mmol, 10 equiv) was slowly added and allowed to stir for 10 min at this temperature. The iodocyclopropyl alcohol, 1-(3-iodo-2,2-dimethylcyclopropyl)propan-1-ol (0.28 mmol) in dry THF (2 mL), was added to the resulting black solution of LiCu(n-Bu)2 and the reaction mixture was stirred for 30 min at −50 °C. Allyl bromide (121 μL, 1.4 mmol) was added, and the solution was stirred for 30 min and quenched with saturated NH4Cl (10 mL). The organic and aqueous layers were separated, and the aqueous layer was extracted with 3 × 10 mL diethyl ether. The combined organic layers were then washed with brine and dried over MgSO4. The filtrate was concentrated in vacuo, and the residue was chromatographed on deactivated silica (5% ethyl acetate in hexanes) to afford the title compound as a colorless oil in 75% yield. [α]D20 = + 9.40 (c = 0.5, CHCl3); 1H NMR (CDCl3, 300 MHz): δ 6.02 (m, 1H), 5.22 (m, 1H), 5.09 (m, 1H), 3.30 (m, 1H), 2.11 (m, 2H), 1.66 (m, 2H), 1.10 (t, 3H, J = 7.37 Hz), 1.02 (s, 3H), 0.91 (s, 3H), 0.75 (dt, 1H, J = 7.1, 8.3 Hz), 0.67 (dd, 1H, J = 8.3, 9.8 Hz); 13C{1H} NMR (CDCl3, 75 MHz): δ 139.78, 114.55, 70.54, 33.85, 31.56, 29.45, 29.28, 26.03, 22.15, 15.75, 10.44; IR (neat): 3459 (OH), 2929, 1619, 1376, 1114, 958, 801, 726, 660, 597 cm−1; HRMS-CI m/z 151.1478 [(M-OH)+; calcd for C11H19:151.1491].
3. Results and Discussion
3.1. Asymmetric Alkylation of Enals/Diastereoselective Iodocyclopropanation
As outlined in the Introduction, the stereoselective transfer of substituted carbenoids in Simmons–Smith reactions remains a significant challenge. Development of such methods would enable synthesis of cyclopropanes not accessible by state-of-the-art protocols. When we initiated our studies, no precedent existed regarding stereoselectivity in alkoxide-directed halocyclopropanations. Subsequent to our initial communication in 2005,23 a study involving iodocyclopropanation of unfunctionalized olefins mediated by CrCl2 appeared. High diastereoselectivities were obtained in some cases, although the reactions were not enantioselective.47 Furthermore, only terminal alkenes were observed to undergo iodocyclopropanation under the reported conditions.
Intrigued by the potential synthetic utility of enantioenriched halocyclopropane derivatives, we investigated the catalytic asymmetric generation of enantio- and diastereoenriched iodocyclopropanes via diastereoselective transfer of iodocarbenoids. Previously, Hashimoto demonstrated that the preparation of iodocyclopropane derivatives could be performed using a combination of CHI3 and ZnEt2. Unfortunately, they observed low yields and stereoselectivities.33
In our approach to iodocyclopropanes, we initially conducted the asymmetric addition/iodocyclopropanation as in Scheme 1, but used CHI3 and ZnEt2 in a 1:1 ratio to generate Hashimoto’s33 carbenoid, EtZnCHI2. In the presence of the allylic alkoxide addition product, we were pleased to find that EtZnCHI2 mediated the cyclopropanation to form iodocyclopropane 1a with excellent diastereoselectivity (Scheme 4, Table 1). Similar levels of diastereoselectivity were observed with dichloromethane, hexanes, and toluene. We opted for dichloromethane due to the greater solubility of iodoform in this solvent. The conversion and yield were low, however, possibly due to the moderate reactivity of this carbenoid (Table 1, entry 1). The more reactive halo zinc carbenoid, IZnCHI2, did not improve the conversion to iodocyclopropane 1a (entry 2). Next we turned to Shi-type zinc carbenoids, which contain electron-withdrawing substituents such as CF3CO2− bound to the zinc.48 The cyclopropanation reactions employing CF3CO2ZnCHI2 exhibited enhanced reactivity, with complete consumption of the allylic alkoxide in under 24 h at 20 °C. Unfortunately, the diastereoselectivity was compromised (3:1, entry 3). Lowering the reaction temperature improved the diastereoselectivity (10:1) as well as isolated yield of the product to 25% (entry 4). We were unsuccessful at further optimizing this process with CF3CO2ZnCHI2, and began to investigate the impact of different electron-withdrawing ligands on the zinc, such as CF3CH2O− (entry 5–7). Gratifyingly, combination of CF3CH2OH, ZnEt2, CHI3, and activated 4 Å molecular sieves resulted in the formation of the desired iodocyclopropane 1a in 66% yield with excellent diastereoselectivity (entry 7).
Scheme 4.
Asymmetric Addition/Diastereoselective Iodocyclopropanation Optimization (see Table 1).
Table 1.
Optimization of the Iodocyclopropanation to Form 1a in Scheme 4 by Screening Zinc Carbenoids and Reaction Conditions.
| entry | carbenoid | equiv | T (°C) | dr | yield (%)a | comment |
|---|---|---|---|---|---|---|
| 1 | EtZnCHI2 | 5 | 20 | >20:1 | nd | low conversionb |
| 2 | IZnCHI2 | 5 | 20 | >20:1 | nd | low conversionb |
| 3 | CF3CO2ZnCHI2 | 2 | 20 | ~3:1 | 18 | many products |
| 4 | CF3CO2ZnCHI2 | 2 | 0 | ~10:1 | 25 | |
| 5 | CF3CH2OZnCHI2 | 2 | 20 | >20:1 | 30 | |
| 6 | CF3CH2OZnCHI2 | 5 | 0 | >20:1 | nd | low conversionb |
| 7 | CF3CH2OZnCHI2 | 2 | 20 | >20:1 | 66 | 4 Å MS added |
After 24 h.
Some allylic alcohol was recovered in these runs.
The optimized conditions for the iodocyclopropanation reaction in Table 1 with our new reagent, CF3CH2OZnCHI2, were then applied to a series of substrates (Scheme 5). Di- and trisubstituted alkenes underwent iodocyclopropanation with good yields (Table 2). Importantly, after optimization only a single diastereomer was observed by 1H NMR spectroscopy in each case.23 This is particularly impressive, because the allylic alkoxide intermediates in Scheme 5 can be grouped into three different classes: those exhibiting A1,2 strain, A1,3 strain, or neither A1,2 nor A1,3 strain in one of the diastereomeric transition states for the iodocyclopropanation. To facilitate discussion of diastereoselectivity in the cyclopropanations, the structures in Scheme 5 are drawn such that interaction between R1 and R2 (R2 ≠ H, R3 = H) leads to A1,2 strain, whereas interaction between R1 and R3 (R3 ≠ H, R2 = H) results in A1,3 strain. Neither significant A1,2 nor A1,3 strain is present when R2 = R3 = H. In Table 2, entries 1, 4, and 7 exhibited A1,2 in the less favorable diastereomeric transition states while entries 2, 5, 6, and 8 exhibited A1,3 strain. Entry 3, with R2 = R3 = H did not exhibit significant A1,2 or A1,3 strain, yet still underwent iodocyclopropanation with excellent diastereoselectivity to yield 1c. In contrast to these cyclopropanations, highly diastereoselective epoxidations of allylic alcohols or allylic alkoxides proceeded only when A1,2 or A1,3 strain was present in one of the diastereomeric transitions states.49–52 It is noteworthy that when the addition/iodocyclopropanation reaction was carried out sequentially with isolation and purification of the allylic alcohols followed by iodocyclopropanation, 1a and 1b were again obtained with high dr (>20:1), in analogy with the one-pot procedures.
Scheme 5.
Asymmetric Alkylation of Enals/Diastereoselective Iodocyclopropanation (see Table 2).
Table 2.
One-Pot Asymmetric Alkyl Addition/Diastereoselective Iodocyclopropanation (Scheme 5).
| entry | product | % ee (% y) | dra | entry | product | % ee (% y) | dra |
|---|---|---|---|---|---|---|---|
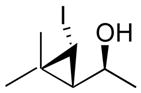
| 1 |
 1a
1a
|
99 (66) | > 20:1 | 5 |
 1e
1e
|
98 (74) | > 20:1 |
| 2 |
 1b
1b
|
95 (62) | > 20:1 | 6 |
 1fb
1fb
|
99 (78) | > 20:1 |
| 3 |
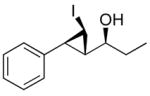 1c
1c
|
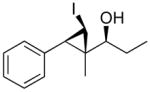
89 (79) | > 20:1 | 7 |
 1g
1g
|
98 (70) | > 20:1 |
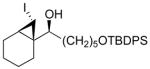
| 4 |
 1d
1d
|
96 (56) | > 20:1 | 8 |
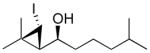 1h
1h
|
96 (60) | > 20:1 |
Determined by 1H NMR analysis of the crude reaction mixture.
10 mol% MIB was used.
To our knowledge, these results constitute the first examples of transfer of halogenated carbenoids in a Simmons–Smith cyclopropanation with excellent control of stereochemistry. Noteworthy is that up to four stereogenic centers can be established from achiral precursors in this one-pot procedure.
The stereochemistry of the iodocyclopropanes was established by X-ray crystal structure determinations of 1a and 1b. Both these structures have syn cyclopropyl alcohols (see Supporting Information). Surprisingly, the iodide is cis to the carbinol in 1a and 1b, which is likely the sterically more demanding position (Scheme 5A). It has been demonstrated that the stereochemistry of related iodocyclopropanes can be successfully assigned using proton coupling constants.33,43,53,54 The stereochemistry of the remaining entries in Table 2 were assigned on the basis of H–H coupling constants between CHI and the remaining hydrogens of the cyclopropane ring. The coupling constants for hydrogens with a cis disposition on the cyclopropane ring are approximately 8 Hz while those with a trans relationship exhibit coupling constants of 3–5 Hz. For example, the two trans hydrogens attached to the cyclopropane in 1a exhibit J = 4.9 Hz and the cis cyclopropane hydrogens in 1b exhibit J = 7.8 Hz. Likewise, in entries 5–8 the carbinol and iodide were cis by 1H NMR spectroscopy (for a complete listing of the coupling constants used to assign stereochemistry, see the Supporting Information). Surprisingly, the coupling constants between the two cyclopropane hydrogens in 1d exhibit J = 8.4 Hz, indicating that the iodide is trans to the carbinol. Likewise, the coupling constant between the benzylic hydrogen and the CHI in 1c is 8.2 Hz, indicating the iodide and carbinol are also trans.
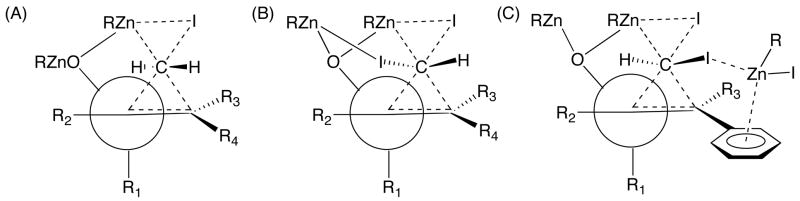
It is generally accepted that Simmons–Smith cyclopropanations proceed via a “butterfly-type” transition state as shown in Figure 1A.6,21 It is possible, however, that removal of the departing iodide could be aided by a second zinc center. On the basis of this model, and the stereochemistry of the products in entries 1, 2, and 5–8, we propose (Figure 1B) that the iodide transferred with carbenoid carbon weakly coordinates to one of the zinc centers, lowering the energy of this transition state and leading to excellent control over the stereoselectivity (Figure 1B). Although we are unaware of direct precedent for such interactions in zinc chemistry, it is known that alkyl halides, such as dichloromethane and methyl iodide, can coordinate to transition metal complexes with formation of LnM–XCH2Y adducts55–58 and that ortho-diiodobenzene can chelate to transition metals through the iodides in the solid state and solution.59,60
Figure 1.
(A) Butterfly-type transition state for cyclopropanation and proposed transition states for iodocyclopropanation leading to the cis (B) and trans (C) products. It is possible that an additional zinc center is involved in removing the iodide leaving group from the carbenoid.
The intimate details of the transition state in these iodocyclopropanation reactions are currently unknown, as are the factors responsible for the dramatic reversal in stereochemistry observed in entries 3 and 4 of Table 2. Two scenarios can be imagined, however. The interaction of Zn(II) with unsaturated π-systems has been indicated in the control of stereoselective reactions.61,62 Furthermore, we have recently observed π-interactions between Zn(II) and a C–C double bond in the solid state,63 Bochmann and coworkers have characterized η2-binding of toluene to Zn(C6F5)2 in the solid state,64,65 and Vigalok and coworkers presented solid state and solution evidence for Zn(II) interactions with alkyne π-systems.66 Although the number of zinc centers and the nature of their interaction with the phenyl substituent in the cyclopropanation transition state remain to be elucidated, we speculate that Zn-π interactions may be responsible for the observed reversal in diastereoselectivity in substrates bearing phenyl groups at the R4 position (Scheme 5). The second scenario is based on the well-known interaction of iodine with aromatic π-systems.67–69 We are unaware of precedent for related interactions with C-I bonds. Furthermore, the orientation and distance between the C-I σ* and phenyl π-system do not seem appropriate for such an interaction.
Preliminary studies to probe the impact of the electronic character of the π-system on the diastereoselectivity in the iodocyclopropanation were undertaken. Under the conditions employed in Table 2, cinnamaldehyde and 4-trifluromethyl cinnamaldehyde were subjected to ethyl addition/iodocyclopropanation. As shown in Table 2, cinnamaldehyde underwent the addition/iodocyclopropanation with >20:1 dr. In contrast, the trifluoromethyl analogue resulted in 5:1 diastereoselectivity under identical reaction conditions, although the reaction only went to 75% completion. The major diastereomer was the same in both cases. These results indicate that the electron density of the aryl π-system impacts the diastereoselectivity, which would be expected if the transition state in Figure 1C is operating. It is likely that the diastereoselectivity and conversion with the trifluoromethyl substrate could be optimized.
3.2. Asymmetric Vinylation of Aldehydes/Diastereoselective Iodocyclopropanation
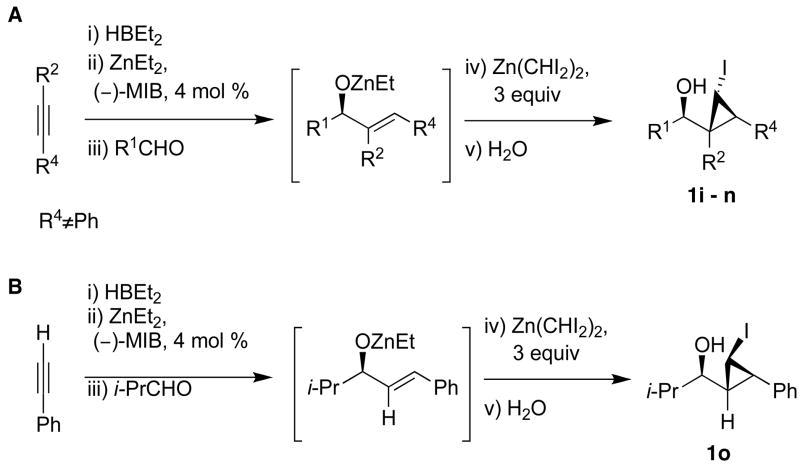
We have found that the asymmetric vinylation of aldehydes, developed by Oppolzer,70,71 is a versatile method to prepare allylic alcohols with high enantioselectivity.49,51,72,73 In our earlier study23 we employed the addition of vinyl groups to saturated aldehydes en route to cyclopropyl alcohols (Scheme 1B). Following Oppolzer’s hydroboration/transmetallation method, we generated vinylzinc reagents in situ. In the presence of (−)-MIB, clean vinylation of aldehydes proceeded to furnish the allylic alkoxide intermediates. After removal of the volatile materials (including the triethylborane) under reduced pressure, addition of diethylzinc and CH2I2 (5.0 equiv each) was performed at 0 °C. This procedure allowed preparation of cyclopropyl alcohols with high enantio- and diastereoselectivity and in high yields (Scheme 1B).
Our procedure for vinylation/cyclopropanation was then applied to the vinylation of aldehydes/iodocyclopropanation (Scheme 6). As in Table 2, the substituents are numbered to facilitate identification of A1,2 strain in the diastereomeric iodocyclopropanation transition states. Unfortunately, the reaction was thwarted by low conversion and poor diastereoselectivity in the iodocyclopropanation. We reasoned that CF3CH2OZnCHI2 might interact with the excess of vinylzinc reagent and interfere with the iodocyclopropanation. To circumvent this problem we generated 1 equiv of vinylzinc species, making it the limiting reagent, performed the asymmetric addition to the aldehyde, and then screened different iodocyclopropanating reagents. The carbenoids IZnCHI2, EtZnCHI2 and CF3CO2ZnCHI2 led to formation of multiple products and/or low conversion in the iodocyclopropanation. We found, however, that 3.0 equiv of zinc bis(carbenoid), Zn(CHI2)2 were optimal for this reaction. Use of Zn(CHI2)2 enabled the synthesis of iodocyclopropyl alcohol building blocks 1i – 1m with excellent selectivities (Table 3, entries 1–5). Unexpectedly, substrates containing an aromatic ring gave diminished yields (entries 6 and 7). Although the reasons for this behavior are puzzling, we found that increasing the concentration of the zinc carbenoid from 0.24 to 0.42 M resulted in an increase in the yields of 1n and 1o to around 50%. The diastereoselectivities remained very high under the more concentrated conditions. It is interesting to note that the products in entries 1–6 (Table 3) have a cis arrangement of the carbinol and iodide, while 1o in entry 7 exhibits a trans disposition of these groups (Scheme 6B). This result is consistent with those in Scheme 5B and Figure 1.
Scheme 6.
Hydroboration, Transmetallation to Zinc, and Asymmetric Vinyl Addition/Diastereoselective Iodocyclopropanation (see Table 3).
Table 3.
One-Pot Asymmetric Vinyl Addition/Diastereoselective Iodocyclopropanation from Scheme 6.
| entry | product | % ee (% y) | dra | entry | product | % ee (% y) | dra |
|---|---|---|---|---|---|---|---|
| 1 |
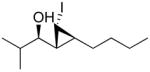 1i
1i
|
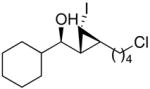
92 (64) | > 20:1 | 5 |
 1m
1m
|
93 (80) | > 20:1 |
| 2 |
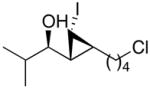
 1j
1j
|
94 (78) | > 20:1 | 6 |
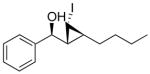 1n
1n
|
94 (52) | > 20:1 |
| 3 |
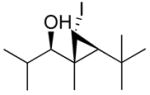 1k
1k
|
86 (67) | > 20:1 | ||||
| 4 |
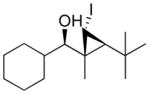 1l
1l
|
87 (70) | > 20:1 | 7 |
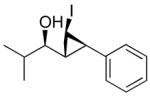 1o
1o
|
99 (50) | > 20:1 |
Determined by 1H NMR analysis of the crude reaction mixture.
3.3. Asymmetric Alkylation of Enals/Diastereoselective Bromo- and Chlorocyclopropanation
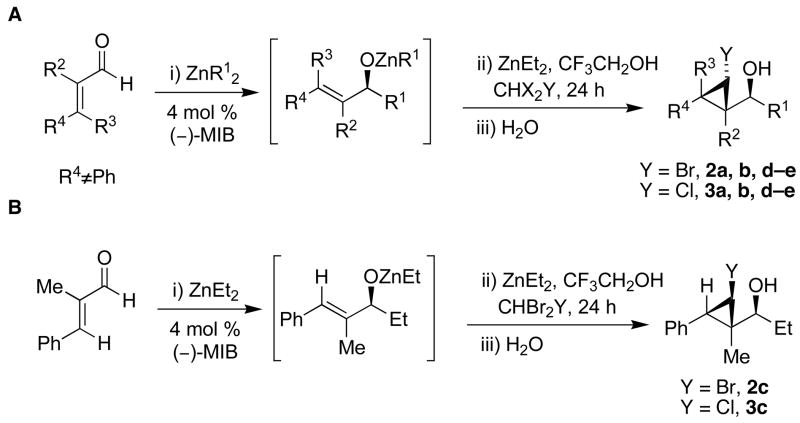
Encouraged by the successful iodocyclopropanations above, we turned our attention to the synthesis of other halocyclopropyl alcohols. To the best of our knowledge no highly enantio- or diastereoselective methods for bromo- or chlorocyclopropanation were known at the outset of our work, although Charette has reported the quenching of a zinc metallocyclopropane with bromine.39 We hoped that bromo- and chlorocyclopropyl alcohols could be generated using an approach similar to the iodocyclopropanation. It was unclear how changing the halide would impact the diastereoselectivity, especially if there is a zinc–halide interaction such as that proposed in Figure 1B.
The first step in formation of the carbenoid is a zinc–halogen exchange reaction. Knochel has shown that alkyl exchange between dialkylzinc reagents and alkyl iodides proceeds rapidly.74,75 Thus, we desired to initiate the bromocyclopropanation with CHBrI2; however, this reagent is not readily available. The need to prepare polyhalomethane precursors for the halocyclopropanation would make the methodology less attractive and useful. We therefore focused on commercially available polyhalomethane precursors such as bromoform (Scheme 7). To overcome the lower reactivity of bromoform relative to iodoform in the exchange reaction with diethylzinc in the bromocyclopropanation, we generated an excess of the cyclopropanating reagents under more concentrated conditions. Thus, use of 1.11 M CF3CH2OZnCHBr2 (5 equiv) led to formation of the bromocyclopropane with high diastereoselectivity, but with low conversion (after 24 h at rt). Slightly better yields (around 60%) were obtained after 50 h with 1.11 M carbenoid. A further increase in carbenoid concentration to 2.50 M and changing the solvent to dichloromethane resulted in higher yields in Scheme 7 (70 – 80% yield, Table 4, entries 1–5). Under these conditions, substrates possessing trisubstituted double bonds underwent full conversion to the bromocyclopropane products within 24 h at ambient temperature, yielding a single diastereomer with > 95% ee. Allylic alkoxides possessing disubstituted double bonds, such as those derived from ethyl addition to trans-cinnamaldehyde and (Z)-4-(benzyloxy)-but-2-enal did not undergo bromocyclopropanation under these conditions. The stereochemistry of the bromocyclopropyl alcohols 2b was determined by X-ray analysis (Table 4, entry 2, see Supporting Information). The relationship between bromide and carbinol in entries 1, 2, 4, and 5 was determined to be cis by measurement of the coupling constants between the cyclopropane hydrogens by 1H NMR. The stereochemistry of 2c (entry 3), however, was assigned as trans on the basis of the coupling constant of the cyclopropyl C–H’s (J = 8.18 Hz) and X-ray analysis. The trans stereochemistry of 2c is consistent with the result of iodocyclopropanation reaction of related substrates (1c, 1d, and 1o).
Scheme 7.
Asymmetric Alkylation of Enals/Diastereoselective Bromo- and Chlorocyclopropanations (Table 4). 10 mol% (−)-MIB was used with dimethylzinc
Table 4.
One-Pot Asymmetric Alkyl Addition/Diastereoselective Bromo- and Chlorocyclopropanation from Scheme 7.
| entry | product | % ee (% y) | dra | entry | product | % ee (% y) | dra |
|---|---|---|---|---|---|---|---|
| 1 |
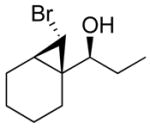 2a
2a
|
99 (70) | > 20:1 | 6 |
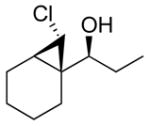 3a
3a
|
99 (65) | > 20:1 |
| 2b |
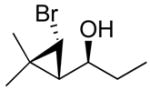 2b
2b
|
95 (75) | > 20:1 | 7b |
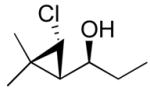 3b
3b
|
95 (70) | > 20:1 |
| 3b |
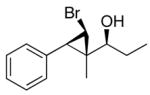 2c
2c
|
96 (80) | > 20:1 | 8 |
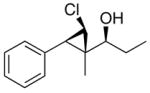 3c
3c
|
96 (70) | > 20:1 |
| 4c |
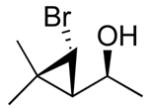 2d
2d
|
99 (70) | > 20:1 | 9c |
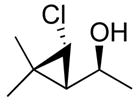 3d
3d
|
99 (59) | > 20:1 |
| 5 |
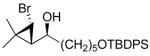 2e
2e
|
97 (77) | > 20:1 | 10 |
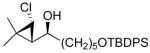 3e
3e
|
97 (70) | > 20:1 |
Determined by 1H NMR analysis of the crude reaction mixture.
Stereochemistry assigned by X-ray analysis, see SI.
10 mol % MIB was used with ZnMe2.
The synthesis of chlorocyclopropyl alcohols was then attempted using bromodichloromethane as the carbenoid precursor (Scheme 7). Initially, 5 equiv each diethylzinc, bromodichloromethane, and trifluoroethanol were mixed to generate a 1.11 M solution of the carbenoid CF3CH2OZnCHCl2 at room temperature. Under these conditions, reactions reached a maximum of 60% conversion after 60 h. Increasing the concentration of the carbenoid to 2.50 M resulted in consumption of the allylic alkoxide over 50 h. Despite significant efforts to optimize the chlorocyclopropanation with this carbenoid, the results were not satisfactory. Fortunately, we found that the tandem asymmetric addition/diastereoselective chlorocyclopropanation could be achieved by combining 5 equiv each of diethylzinc, dibromochloromethane, and trifluoroethanol to generate a 2.5 M carbenoid, CF3CH2OZnCHClBr. At this concentration, the consumption of the allylic alkoxide was complete in 24 h at room temperature. After optimization, yields ranged from 59–70% with dr >20:1 (Table 4, entries 6–10).
The stereochemistry of chlorocyclopropyl alcohols was assigned by X-ray structure determination of 3b (entry 7) and measurement of the H–H coupling constants of the cyclopropyl moiety by NMR spectroscopy, as outlined for the bromo- and iodocyclopropyl alcohols above (also see the Supporting Information). The stereochemical outcome of these tandem reactions was analogous to those observed in the iodo- and bromocyclopropanation reactions. In particular, the trans relationship between the chloride and the carbinol in 3c was confirmed by independent synthesis, prepared from the hydroxy-directed dichlorocyclopropanation followed by the hydride reduction using Bu3SnH/AIBN.76,77
The synthesis of fluorocyclopropyl alcohols still remains elusive due to the lack of available fluorohalomethane precursors. We are currently investigating the stereoselective preparation of the corresponding fluorohalomethanes.
3.4. Functionalization of Iodocyclopropyl Alcohols
Despite extensive studies directed toward the synthesis of functionalized cyclopropane derivatives, the stereoselective preparation of cyclopropanes bearing alkyl substituents on each carbon remains challenging.38,78,79 A potentially useful approach to these multisubstituted cyclopropanes involves the generation and elaboration of metallated cyclopropanes. We envisioned that the halocyclopropyl alcohols introduced in the previous sections could be valuable precursors to metallated cyclopropanes if the stereochemistry of the metallation could be controlled. To explore this possibility, we prepared organocuprate reagents from iodocyclopropyl alcohol 1b as illustrated in Scheme 8. Previous work with organocopper(I) reagents has shown that formation of LiCuR2 complexes stabilizes the resulting organocuprates toward thermal decomposition and increases the nucleophilicity of these reagents.80,81 Thus, we generated the cuprate by combining n-butyllithium and copper(I) iodide (2:1) in dry THF at −50 °C. This reagent was then added into a cold solution of the iodocyclopropyl alcohol 1b at the same temperature. After 30 min, the reaction mixture was treated with excess allyl or methallyl bromide for an additional 30 min at −50 °C. The reactions were worked up and the desired 1,2,3-substituted cyclopropane derivatives 4 and 5 were isolated in good yields as single diastereomers, as judged by 1H NMR spectroscopy of the crude reaction mixture. The stereochemistry of the allylated cyclopropanes 4 and 5 was assigned on the basis of the cyclopropyl hydrogens H–H coupling constants of 8.3 and 8.1 Hz, respectively (see Supporting Information).
Scheme 8.
Allylation of Iodocyclopropyl Alcohol 1b using Organocuprate Chemistry.
The control of stereochemistry in these metallation reactions likely arises from the stereochemical stability of the resulting cyclopropyl cuprates.82 These preliminary experiments suggest that our halocyclopropyl alcohols will be useful in the stereoselective generation of multisubstituted cyclopropane derivatives.
4. Conclusions and Outlook
The most significant limitation of the Simmons–Smith cyclopropanation reaction is the lack of efficient methods to transfer functionalized carbenoids with high stereoselectivity. To address this problem, we have introduced the first examples of highly diastereoselective halocyclopropanation reactions. Two methods for the synthesis of iodocyclopropane derivatives have been developed. The first is initiated with a catalytic asymmetric addition of dialkylzinc reagents to a conjugated enal. The resulting allylic alkoxide is then treated with our newly developed carbenoid, (CF3CH2O)ZnCHI2, to perform the iodocyclopropanation. Complementary to this method is the generation of the allylic alkoxide intermediate by enantioselective vinylation of saturated aldehydes. Thus, hydroboration of an alkyne, transmetallation of the boron-bound alkenyl group to zinc, and catalytic asymmetric vinylation of an aldehyde leads to an allylic alkoxide that undergoes iodocyclopropanation with Zn(CHI2)2. Using these methods, iodocyclopropyl alcohols with up to four stereogenic centers can be prepared in a one-pot procedure with ee’s between 87 and 99% and dr >20:1. Beginning with enals and using a similar strategy, we have introduced the first highly stereoselective examples of bromo- and chlorocyclopropanation reactions. The resulting bromo- and chlorocyclopropyl alcohols also have high ee’s and dr’s. In most cases, the alkoxide directed halocyclopropanation resulted in product with the halide and carbinol cis (dr >20:1 in all cases). However, an unexpected reversal of the diastereoselectivity was found when the allylic alkoxide contained a styryl group, resulting in a trans relationship between the halide and carbinol (dr >20:1).
Our initial studies indicate that the iodocyclopropanols can be metallated with LiCu(n-Bu)2 and quenched with allylbromides, producing allylated cyclopropyl alcohols with high diastereoselectivity. These multisubstituted cyclopropanes would be difficult to prepare by other methods.
Development of general methods for the enantioselective cyclopropanation of alkenes and allylic alcohols remains a challenging goal in organic synthesis. Our approach, involving enantioselective carbonyl additions followed by tandem diastereoselective cyclopropanations circumvents difficulties associated with enantioselective cyclopropanations and enables the efficient one-pot synthesis of functionalized cyclopropanes. Given the rapid increase in molecular complexity with defined stereochemical outcome, we anticipate that these methods will be useful in enantioselective synthesis.
Supplementary Material
Procedures, full characterization, and stereochemical assignments of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the NSF (CHE-065210) and NIH (National Institute of General Medical Sciences GM58101) for partial support of this work. We are also grateful to Akzo Nobel Polymer Chemicals for a gift of dialkylzinc reagents.
References
- 1.Pietruszka J. Chem Rev. 2003;103:1051–1070. doi: 10.1021/cr010027g. [DOI] [PubMed] [Google Scholar]
- 2.Wessjohann LA, Brandt W, Thiemann T. Chem Rev. 2003;103:1625–1648. doi: 10.1021/cr0100188. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson WA. Tetrahedron. 2001;57:8589–8627. [Google Scholar]
- 4.Salaun J. Top Curr Chem. 2000;207:1–67. [Google Scholar]
- 5.de Meijere A, editor. Small Ring Compounds in Organic Synthsis VI. Vol. 207 Springer; Berlin: 2000. [Google Scholar]
- 6.Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 7.Charette AB, Marcoux JF. Synlett. 1995:1197–1207. [Google Scholar]
- 8.Simmons HE, Smith RD. J Am Chem Soc. 1959;81:4256–4264. [Google Scholar]
- 9.Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- 10.Charette AB, Beauchemin A. Org React. 2001;58:1–415. [Google Scholar]
- 11.Denmark SE, Christenson BL, O’Connor SP, Noriaki M. Pure Appl Chem. 1996;68:23–27. [Google Scholar]
- 12.Long J, Yuan Y, Shi Y. J Am Chem Soc. 2003;125:13632–13633. doi: 10.1021/ja030488e. [DOI] [PubMed] [Google Scholar]
- 13.Long J, Du H, Li K, Shi Y. Tet Lett. 2005;46:2737–2740. [Google Scholar]
- 14.Charette AB, Molinaro C, Brochu C. J Am Chem Soc. 2001;123:12168–12175. doi: 10.1021/ja0108382. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Yoshioka M, Ohno M, Kobayashi S. Tet Lett. 1992;33:2575–2578. [Google Scholar]
- 16.Denmark SE, O’Connor SP. J Org Chem. 1997;62:3390–3401. doi: 10.1021/jo9702397. [DOI] [PubMed] [Google Scholar]
- 17.Imai N, Sakamoto K, Takahashi H, Kobayashi S. Tet Lett. 1994;35:7045–7048. [Google Scholar]
- 18.Takahashi H, Yoshioka M, Shibasaki M, Ohno M, Imai N, Kobayashi S. Tetrahedron. 1995;51:12013–12026. [Google Scholar]
- 19.Voituriez A, Charette AB. Adv Syn & Catal. 2006;348:2363–2370. [Google Scholar]
- 20.Balsells J, Walsh PJ. J Org Chem. 2000;65:5005–5008. doi: 10.1021/jo991704y. [DOI] [PubMed] [Google Scholar]
- 21.Charette AB. In: In The Chemistry of Organozinc Compounds. Rappoport Z, Marek I, editors. John Wiley & Sons, Ltd.; West Sussex: 2006. pp. 237–286. [Google Scholar]
- 22.For an exception see: Shitama H, Katsuki T. Angew Chem, Int Ed. 2008;47:2450–2453. doi: 10.1002/anie.200705641.
- 23.Kim HY, Lurain AE, García-García P, Carroll PJ, Walsh PJ. J Am Chem Soc. 2005;127:13138–13139. doi: 10.1021/ja0539239. [DOI] [PubMed] [Google Scholar]
- 24.Nugent WA. J Chem Soc, Chem Commun. 1999:1369–1370. [Google Scholar]
- 25.Chen YK, Jeon SJ, Walsh PJ, Nugent WA. Organic Synthesis. 2005;82:87–92. [Google Scholar]
- 26.Ratier M, Castaing M, Godet JY, Pereyre MJ. J Chem Res Miniprint. 1978:2309–2318. [Google Scholar]
- 27.Charette AB, Lebel H. J Org Chem. 1995;60:2966–2967. [Google Scholar]
- 28.Charette AB, Mathieu S, Fournier JF. Synlett. 2005:1779–1782. [Google Scholar]
- 29.Friedrich EC, Biresaw G. J Org Chem. 1982;47:1615–1618. [Google Scholar]
- 30.Mariano PS, Bay E, Watson DG, Rose T, Bracken C. J Org Chem. 1980;45:1753–1762. [Google Scholar]
- 31.Groth U, Schollkopf U, Tiller T. Liebigs Ann Chem. 1991:857–60. [Google Scholar]
- 32.Sugimura T, Katagiri T, Tai A. Tetrahedron Lett. 1992;33:367–368. [Google Scholar]
- 33.Miyano S, Hashimoto H. Bull Chem Soc Jap. 1974;47:1500–1503. [Google Scholar]
- 34.Miyano S, Matsumoto Y, Hashimoto H. J Chem Soc, Chem Commun. 1975:364–365. [Google Scholar]
- 35.Tamura O, Hashimoto M, Kobayashi Y, Katoh T, Nakatani K, Kamada M, Hayakawa I, Akiba T, Terashima S. Tetrahedron. 1994;50:3889–3904. [Google Scholar]
- 36.Sugimura T, Futagawa T, Mori A, Ryu I, Sonoda N, Tai A. J Org Chem. 1996;61:6100–6103. doi: 10.1021/jo960448b. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher RJ, Motherwell WB, Popkin ME. Chem Commun. 1998:2191–2192. [Google Scholar]
- 38.Charette AB, Lemay J. Angew Chem Int Ed. 1997;36:1090–1092. [Google Scholar]
- 39.Charette AB, Gagnon A, Fournier JF. J Am Chem Soc. 2002;124:386–387. doi: 10.1021/ja017230d. [DOI] [PubMed] [Google Scholar]
- 40.Fournier JF, Charette AB. Eur J Org Chem. 2004:1401–1404. [Google Scholar]
- 41.Fournier JF, Mathieu S, Charette AB. J Am Chem Soc. 2005;127:13140–13141. doi: 10.1021/ja054328+. [DOI] [PubMed] [Google Scholar]
- 42.Fox JM, Yan N. Curr Org Chem. 2005;9:719–732. [Google Scholar]
- 43.Yan N, Liu X, Fox JM. J Org Chem. 2008;73:563–568. doi: 10.1021/jo702176x. [DOI] [PubMed] [Google Scholar]
- 44.Liao LA, Fox JM. J Am Chem Soc. 2002;124:14322–14323. doi: 10.1021/ja0278234. [DOI] [PubMed] [Google Scholar]
- 45.Rozema MJ, Sidduri A, Knochel P. J Org Chem. 1992;57:1956–1958. [Google Scholar]
- 46.Langer F, Schwink L, Devasagayaraj A, Chavant PY, Knochel P. J Org Chem. 1996;61:8229–8243. doi: 10.1021/jo961129n. [DOI] [PubMed] [Google Scholar]
- 47.Takai K, Toshikawa S, Inoue A, Kokumai R, Hirano M. J Organomet Chem. 2007;692:520–529. [Google Scholar]
- 48.Yang ZQ, Lorenz JC, Shi Y. Tet Lett. 1998;39:8621–8624. [Google Scholar]
- 49.Lurain AE, Carroll PJ, Walsh PJ. J Org Chem. 2005;70:1262–1268. doi: 10.1021/jo048345d. [DOI] [PubMed] [Google Scholar]
- 50.Rowley Kelly A, Lurain AE, Walsh PJ. J Am Chem Soc. 2005;127:14668–14674. doi: 10.1021/ja051291k. [DOI] [PubMed] [Google Scholar]
- 51.Lurain AE, Maestri A, Kelly AR, Carroll PJ, Walsh PJ. J Am Chem Soc. 2004;126:13608–13609. doi: 10.1021/ja046750g. [DOI] [PubMed] [Google Scholar]
- 52.Adam W, Wirth T. Acc Chem Res. 1999;32:703–710. [Google Scholar]
- 53.Seyferth D, Yamazaki H, Alleston DL. J Org Chem. 1963;28:703–706. [Google Scholar]
- 54.Miyano S, Hashimoto H. Bull Chem Soc Jpn. 1973;46:892–897. [Google Scholar]
- 55.Arndtsen BA, Bergman RG. Science. 1995;270:1970–1973. [Google Scholar]
- 56.Burk MJ, Segmuller B, Crabtree RH. Organometallics. 1987;6:2241–2246. [Google Scholar]
- 57.Fukuyama T, Czech PT, Gladysz JA, Fenske RF. Organometallics. 1989;8:1806–1810. [Google Scholar]
- 58.Winter CH, Arif AM, Gladysz JA. J Am Chem Soc. 1987;109:7560–7561. [Google Scholar]
- 59.Crabtree RH, Faller JW, Mellea MF, Quirk JM. Organometallics. 1982;1:1361–1366. [Google Scholar]
- 60.Albietz PJJ, Cleary Brian P, Paw W, Eisenberg R. Inorg Chem. 2002;41:2095–2108. doi: 10.1021/ic025506s. [DOI] [PubMed] [Google Scholar]
- 61.Marek I, Beruben D, Normant JF. Tet Lett. 1995;36:3695–3698. [Google Scholar]
- 62.Beruben D, Marek I, Normant JF, Platzer N. J Org Chem. 1995;60:2488–2501. [Google Scholar]
- 63.Wooten A, Carroll PJ, Maestri AG, Walsh PJ. J Am Chem Soc. 2006;128:4624–4631. doi: 10.1021/ja058700x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guerrero A, Martin E, Hughes DL, Kaltsoyannis N, Bochmann M. Organometallics. 2006;25:3311–3313. [Google Scholar]
- 65.Walker DA, Woodman TJ, Hughes DL, Bochmann M. Organometallics. 2001;20:3772–3776. [Google Scholar]
- 66.Bukhaltsev E, Goldberg I, Cohen R, Vigalok A. Organometallics. 2007;26:4015–4020. [Google Scholar]
- 67.Benesi HA, Hildebrand JH. J Am Chem Soc. 1949;71:2703–2707. [Google Scholar]
- 68.Mulliken RS. J Am Chem Soc. 1952;74:811–824. [Google Scholar]
- 69.Cheng PY, Zhong D, Zewail AH. J Chem Phys. 1996;105:6216–6248. [Google Scholar]
- 70.Oppolzer W, Radinov RN. Helvetica Chimica Acta. 1992;75:170–173. [Google Scholar]
- 71.Oppolzer W, Radinov RN. J Am Chem Soc. 1993;115:1593–1594. [Google Scholar]
- 72.Lurain AE, Walsh PJ. J Am Chem Soc. 2003;125:10677–10683. doi: 10.1021/ja035213d. [DOI] [PubMed] [Google Scholar]
- 73.Chen YK, Lurain AE, Walsh PJ. J Am Chem Soc. 2002;124:12225–12231. doi: 10.1021/ja027271p. [DOI] [PubMed] [Google Scholar]
- 74.Knochel P. Chemtracts, Organic Chemistry. 1995;8:205–221. [Google Scholar]
- 75.Knochel P, Singer RD. Chem Rev. 1993;93:2117–2188. [Google Scholar]
- 76.Mohamadi F, Still WC. Tet Lett. 1986;27:893–896. [Google Scholar]
- 77.Nishii Y. Perkin Trans. 1;1996:1243–1249. [Google Scholar]
- 78.Liu P, Jacobsen EN. J Am Chem Soc. 2001;123:10772–10773. doi: 10.1021/ja016893s. [DOI] [PubMed] [Google Scholar]
- 79.Araki S, Shiraki H, Tanaka T, Nakano H, Subburaj K, Hirashita T, Yamamura H, Kawai M. Chem Eur J. 2001;7:2784–2790. doi: 10.1002/1521-3765(20010702)7:13<2784::aid-chem2784>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 80.Whitesides GM, Fisher WFJ, Filippo JSJ, Bashe RW, House HO. J Am Chem Soc. 1969;91:4871–4882. [Google Scholar]
- 81.House HO, Fischer WFJ. J Org Chem. 1968;33:949–951. [Google Scholar]
- 82.Hiyama H, Yamamoto H, Nishio K, Kitatani K, Nozaki H. Bull Chem Soc Jpn. 1979;52:3632–3637. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedures, full characterization, and stereochemical assignments of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.