Abstract
Inhibition of the α4 subunit of both the α4β1 and α4β7 integrins has shown promise in decreasing airway inflammation and airway hyperresponsiveness in various animal models. We hypothesized that a novel, high-affinity α4β1 antagonist (LLP2A) would decrease the migration of eosinophils to the lung and ameliorate the airway hyperresponsiveness in a mouse model of ovalbumin-induced airway inflammation. To test this hypothesis, we administered LLP2A, or scrambled LLP2A (a negative control), prior to exposure of sensitized BALB/c mice to ovalbumin aerosol. We can partially prevent, or reverse, the airway inflammatory response, but not airways hyperresponsiveness, by treatment of mice with LLP2A, a synthetic peptidomimetic α4β1 antagonist LLP2A. Specifically engineered, PEGylated (PEG) formulations of this antagonist further reduce the airway inflammatory response to ovalbumin lbumin, presumably by improving the circulating half-life of the drug.
Index Words: α4β1 integrin, PEGylated antagonist, Asthma, Eosinophil, Airway hyperresponsiveness
1. Introduction
Human asthma is a spectrum of diseases characterized by persistence of airway inflammation that contributes to structural airway changes and alterations in airway mechanics, including increased airway resistance and airway hyperreactivity. The key effector cells that dictate these changes in the airways include eosinophils, mast cells, and Th-2 CD4 lymphocytes. While we believe that the primary integrins, chemokines, and cytokines that govern the migration of these cells into the lung have been identified, it is not clear which targets in which pathways will lead to future asthma therapies.
Integrins are a family of cell surface receptors involved in numerous intercellular and cell-matrix trafficking functions. Integrins exist as αβ heterodimers; the α4 subunit has proven an attractive target for blocking organ-specific inflammation. Inhibition of the α4 subunit of both the α4β7 and the α4β1 (very late antigen 4, VLA-4) integrins has shown promise in decreasing airway inflammation and airway hyperresponsiveness. Whether such an inhibitor could improve the care of patients with asthma or other inflammatory airway disorders is now being studied (Ravensberg et al., 2006).
We have previously published a series of studies describing the synthesis and activity of LLP2A (Fig. 1), a novel, high-affinity α4β1 integrin peptidomimetic antagonist (Liu et al., 2006; Peng et al., 2008; Peng et al., 2006). For example, we have shown that LLP2A binds specifically to α4β1-expressing lymphomas (Peng et al., 2008; Peng et al., 2006). The purpose of the present study was to elucidate the role of the α4β1 integrin in the recruitment of eosinophils and lymphocytes to the lung in a common mouse model of allergic airway inflammation and hyperresponsiveness. We hypothesized that there is a direct linkage between the expression and activation of the α4β1 integrin on eosinophils and lymphocytes and increased airway inflammation and hyperresponsiveness caused by exposure to ovalbumin lbumin. To test this hypothesis, we administered LLP2A, or scrambled LLP2A (S-LLP2A, Figure 1), prior to the ovalbumin exposures. We determined the response of this antagonist on the development of airway inflammation, airway hyperresponsiveness, and goblet cell hyperplasia. We further hypothesized that the bioavailability and efficacy of the synthetic α4β1 antagonist could be improved by constructing different PEGylated (PEG) formulations of the α4β1 antagonist, i.e., LLP2A conjugated to a linear 30kDa polyethylene glycol (L-PEG-LLP2A), a branch 40 kDa PEG (B-PEG-LLP2A) and a tetravalent 4-arm 10kDa PEG (T-PEG-LLP2A) (Figure 1). LLP2A, S-LLP2A and the three PEGylated antagonists were tested in subsequent in vivo experiments.
Fig. 1.
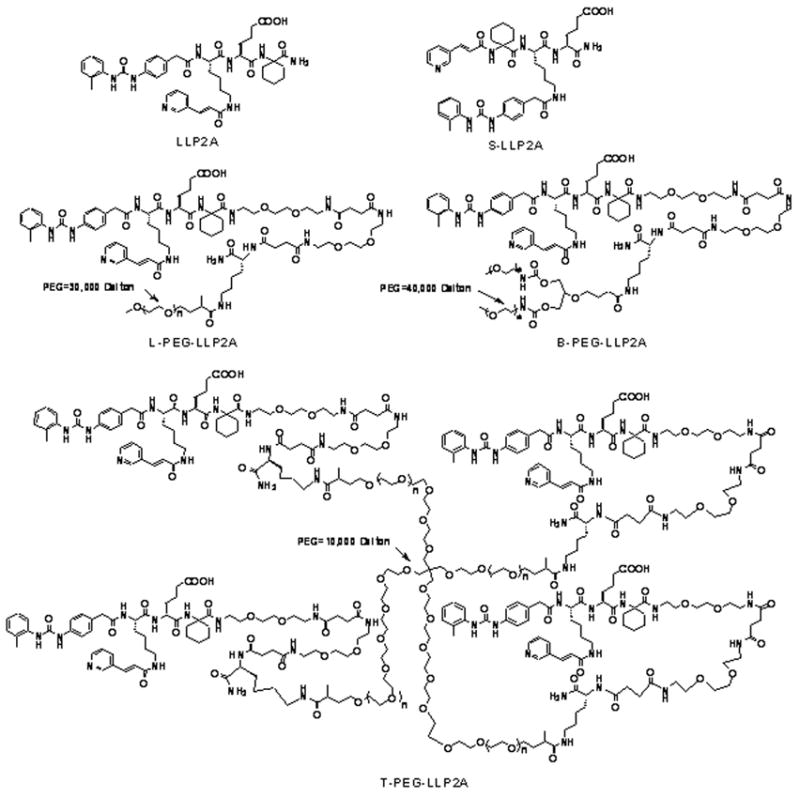
Chemical structure of LLP2A, S-LLP2A, L-PEG-LLP2A, B-PEG-LLP2A, and T-PEG-LLP2A.
2. Materials and Methods
2.1 Synthesis of LLP2A, S-LLP2A and PEGylated LLP2A conjugates
LLP2A and S-LLP2A were synthesized as previously reported (Peng et al., 2006). PEGylated LLP2A conjugates were designed to have PEG attached to the side chain of lysine (i.e., ε-amino group) on LLP2A-K (structure is shown in Scheme 1), and two hydrophilic linkers between LLP2A and K(PEG). The synthesis of LLP2A-K was performed on rink amide MBHA resin by a standard solid-phase peptide synthesis approach using Fmoc-tBu chemistry and HOBt/DIC coupling. The synthetic scheme is shown in Scheme 1. Fmoc-Lys(Boc)-OH was first coupled to the resin, followed by coupling of two linkers. Then, LLP2A was constructed as previously reported on the N-terminus of linker (Peng et al., 2006). The beads were thoroughly washed and then dried under vacuum for 1 h before adding a cleavage mixture of 95% trifluoroacetic acid (TFA): 2.5% water: 2.5% triisopropylsilane (TIS). Cleavage of compounds from the resin and removal of protecting group were achieved simultaneously over 2h at room temperature. The white crude products were precipitated with cold ether and purified by semi-preparative reversed-phase high performance liquid chromatography (RP-HPLC) to give LLP2A-K. PEGylations on the ε-amino group of LLP2A-K were achieved with linear 30 kDa PEG-NHS ester, branch 40kDa PEG-NHS ester and 4-arm 10 kDa PEG-NHS ester (Nektar Therapeutics AL Corporation, Huntsville, AL) to give L-PEG-LLP2A, B-PEG-LLP2A and TPEG-LLP2A, respectively. The following PEGylation protocol was used: 0.01 mmol of PEGNHS ester was added to LLP2A-K (2 eq. to each NHS) in 0.5 ml of dimethylsulfoxide, 0.1 ml of saturated NaHCO3 and 1 ml of water and rotated overnight at room temperature. The resulting solution was added to 50 ml diethyl ether. The precipitate was collected, washed three times with diethyl ether and dried. The white solid was dissolved in water and purified by gel filtration on a Superose 12 column (GE Healthcare, U.K.). The sizing column was run in 30% acetonitrile in water with 0.1% TFA at a flow rate of 1 ml/min, and detected at peak of 254 nm. The active fraction was collected and lyophilized to give a white powder. The identity of the compounds was confirmed by Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) on a Bruker BIFLEX III mass spectrometer (Billerica, MA).
Scheme 1.
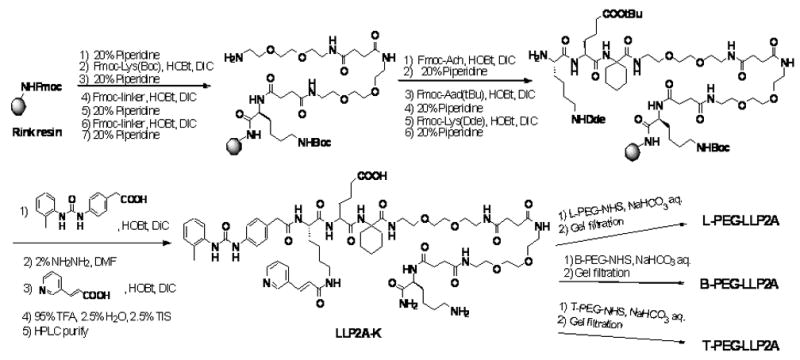
Synthetic approach to L-PEG-LLP2A, B-PEG-LLP2A, and T-PEG-LLP2A
2.2 Animals
Mice (Balb/c, 25–30 g, 8–12 wk old males) were purchased from Charles River (Wilmington, MA). Mice were certified as chronic respiratory disease free, i.e. there were no paramyxoviral, or other viral pathogens, present in these animals that might themselves provoke chronic airway inflammation (Schwarze et al., 1997; Walter et al., 2002). Animals were housed in a HEPA-filtered laminar flow cage rack with a 12-hour light/dark cycle and cared for by the staff of Animal Resource Services at UCD in AAALAC- accredited facilities. Food (Purina Rodent Chow) and water were provided ad libitum. Mice were euthanized at the end of an experiment with an overdose of pentobarbital given i.p. All protocols were reviewed and approved by our Institutional Animal Care and Use Committee.
2.3 Exposure of mice to ovalbumin aerosol
Mice were sensitized with two i.p. injections of 2 × 10 μg/0.1 ml chicken egg albumin two weeks apart (ovalbumin, grade V, ≥98% pure, Sigma, St. Louis, MO), with alum as an adjuvant (Temelkovski et al., 1998). Exposure to ovalbumin aerosols, performed using chambers and generators we have described elsewhere (Kenyon et al., 2003a), were to 10 ml of a 10 mg/ml (1%) solution, begun on day 28 after initiation of sensitization. Mice were exposed for up to 30 min, three times per week, for the duration of a given experiment. Age-matched control animals were injected i.p. with ovalbumin (sensitized), but were not exposed to ovalbumin aerosol, or were not sensitized to ovalbumin (i.p. saline injections) and were then exposed to either ovalbumin aerosol, or to filtered air. The MMAD of our nebulized particles is 1.9 ± 0.21 μm with σg 2.59 ± 0.19. Aerosol mass concentration (protein) was about 90 mg/M3, which corresponds to an efficiency of about 34% transfer of protein into the aerosol in the chambers based upon the amount of liquid actually removed from the nebulizer (Kenyon et al., 2003b).
2.4 Experimental protocol
In our initial experiments, we tested the α4β1 antagonist LLP2A in mice sensitized to ovalbumin and then exposed to either aerosolized ovalbumin or filtered air over 10 days (4 exposures) or 14 days (6 exposures). Mice were given three i.p. injections of either low dose (2 mg/kg) or high dose (20 mg/kg) of the antagonist, or a scrambled, inactive compound (2mg/kg), one hour before each of their last three ovalbumin exposures. In experiment 1, three groups of mice were exposed to filtered air and three groups were exposed to ovalbumin over 14 days (6 air + inactive compound (n=2), 6 air + low dose LLP2A (n=2), 6 air + high dose LLP2A (n=2), 6 ovalbumin + S-LLP2A (n=4), 6 ovalbumin + low dose LLP2A (n=2), 6 ovalbumin + high dose LLP2A (n=4)). In experiment 2, two groups of mice were exposed to air (4 air + S-LLP2A (n=2), 4 air + low dose LLP2A (n=2)) or ovalbumin (4 ovalbumin + S-LLP2A (n=2), 4 ovalbumin + low dose LLP2A (n=2)) for 10 days. After the third ovalbumin exposure, mice were anesthetized and placed on a mechanical ventilator to determine lung mechanics.
In our subsequent experiments, we used the 2mg/kg dose of the antagonist because it did not appear that the higher dose had increased efficacy. The antagonist LLP2A and three PEGylated formulations of the antagonist (i.e., L-PEG-LLP2A, B-PEG-LLP2A and T-PEG-LLP2A) were administered intravenously by tail vein injection in mice exposed to ovalbumin for either 7 days (3 ovalbumin exposures) or 14 days. The decision to use the intravenous route of administration with the PEGylated formulation was made after a consideration of the pharmacology of the newly synthesized antagonists, to address concerns that absorption of the larger PEGylated antagonists from a bolus dose i.p. might be impaired.
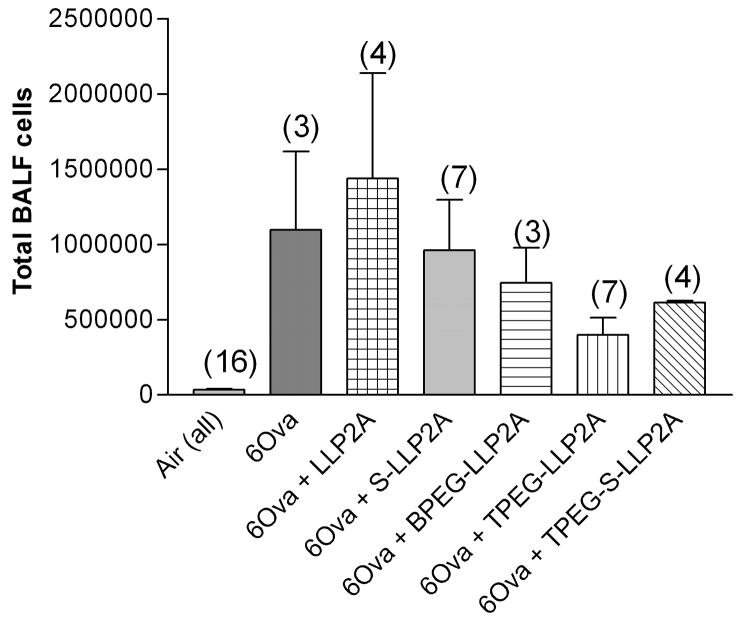
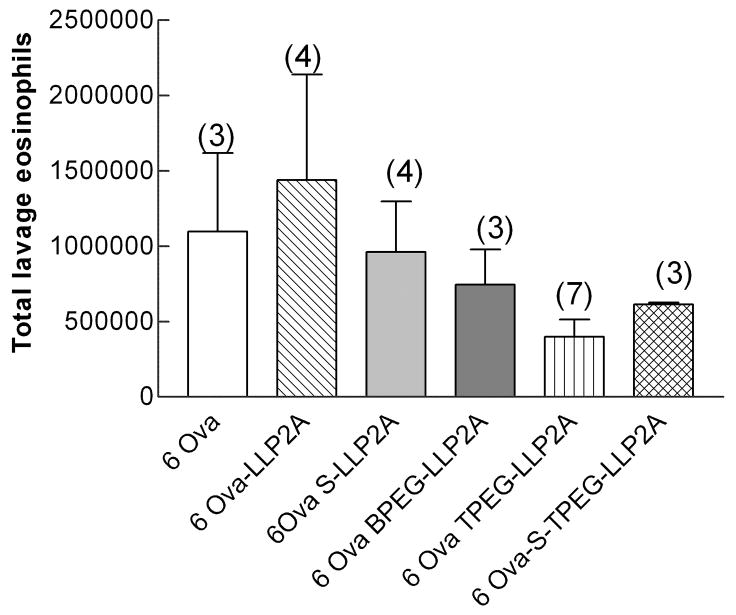
In experiment 3, four groups of mice (n=4 each) were exposed to ovalbumin for 7 days (3 exposures)—3ovalbumin + LLP2A, 3 ovalbumin + LLP2A-BPEG, 3 ovalbumin + LLP2A-LPEG, 3 ovalbumin + LLP2A-inactive. In experiment 4, six groups of mice (n=3 each) were exposed to ovalbumin for 7 days (3 exposures) or 14 days (6 exposures)—3 ovalbumin + LLP2A-TPEG, 3 ovalbumin + BPEG, 3 ovalbumin +LLP2A-inactive, 6 ovalbumin +TPEG, 6ovalbumin + BPEG, 6ovalbumin +LLP2A-inactive. Lung tissue and lavage samples were measured in all mice; however, lung physiology measurements were not done on the mice in experiment 4. To address the question of whether the LLP2A affected lung cell counts in either it’s active or scrambled form, we exposed animals in experiment 5 to either filtered air or ovalbumin six times over two weeks. Both the air-exposed and ovalbumin-exposed animals were treated with and without LLP2A, LLP2A-inactive, LLP2A-TPEG, LLP2A-inactive-TPEG (a new scrambled compound). The groups of mice for this final experiment (n=3–4 each), therefore, were air, air + LLP2A, air + S-LLP2A, air + LLP2A-TPEG, air + S-LLP2A-TPEG, 6ovalbumin, 6ovalbumin +LLP2A, 6ovalbumin + S-LLP2A, 6ovalbumin + LLP2A-TPEG, and 6ovalbumin + S-LLP2A-TPEG.
2.5 Airway inflammation, goblet cell hypertrophy, and airway fibrosis
We performed lung lavage on the animals two to five h after the last ovalbumin exposure. Mice were lavaged with two 1-ml aliquots of 1x phosphate buffered saline (PBS). Each aliquot was passed into and out of the lung twice. The total lavaged cell number was counted on a hemacytometer and the cell differentials were examined in cyto-centrifuge preparations. Lungs used for histology were cannulated via the trachea, and the mediastinal contents were removed with the lungs as a single block. Lungs were fixed for one hour at 30 cm of pressure through the tracheal cannula with 1% paraformaldehyde in PBS. The trachea was tied off to maintain the fixative in the lungs, and the lungs were removed en bloc and kept at 4°C for up to 24 h, then transferred to 70% ethanol for drying, embedding, and preparation of paraffin blocks by standard techniques. Specimens were sectioned at 5 um on a Sorvall JB4 microtome. Picro-Sirius red stain F3BA (Pfaltz and Bauer, CT) in 0.5% picric acid was performed for 30 min to stain extracellular matrix proteins in the subepithelium. Light microscopy was used for analysis of the slides. A grading system of 0–4 was established and the airways were scored by a blinded observer under 200X power (Kenyon et al., 2003b). The grading scale used was: (0-no peribronchial Sirius red stain; 1-evident Sirius red stain, but not consistently increased in all airways; 2-slight, consistent increase in Sirius red depth of stain; 3-increased, uniform stain depth throughout the airways; 4-dramatically increased depth of stain in all airways). We quantified the relative number of goblet cells in the epithelium of the conducting airways of Balb/c mice exposed to ovalbumin as cells positive for staining with the PAS-Schiff reagent. The percentage of goblet cells in the airway epithelium was quantified by counting the number of goblet cells in one representative daughter airway with a length of at least 100 basal epithelial cells (visualized at 400X magnification) divided by the total cell number counted (goblet cells + basal epithelial cells).
2.6 Plasma –SH group content
We determined the total protein sulfhydryl (R–SH) content of blood plasma by a colorimetric reaction using Ellman’s reagent (Sedlak and Lindsay, 1968) and modified the assay to use a 96-well plate format. Blood was collected by cardiac puncture, using EDTA as an anticoagulant, and plasma was separated by centrifugation for 10 min at 2000rpm. We routinely analyzed 10 μl aliquots of plasma.
2.7 Exhaled nitric oxide and nitrate/nitrite measurements
Exhaled gas was collected with a collection bag connected to the exhalation port during the first 5 min the mouse was attached to the ventilator. Placement of the bag did not affect pressure measurements. This 5-minute sample was adequate for the measurement of NO concentration in the expired air, using a Sievers Nitric Oxide analyzer (Sievers, Boulder, CO) (Silkoff et al., 1999). Lung lavage supernatants were collected and nitrate and nitrite were measured as an indicator of NO production after reduction with acidified vanadium III with the Sievers Analyzer.
2.8 Lung compliance and resistance measurements
We measured dynamic compliance and resistance of the respiratory system with a plethysmograph for restrained animals. (Buxco Inc., Troy, NY). A subgroup of mice from experiments 1–3 underwent lung compliance and resistance measurements and lung lavage was done immediately after the forty-five minute physiology protocol. For the protocol, mice were deeply anesthetized and sedated with medetomidine, 0.5 mg/kg (Domitor, Orion Pharma, Finland), and tiletamine/zolpidem, 50 mg/kg (Telazol, Fort Dodge Laboratories, Fort Dodge, IA). Mice were ventilated at 7–8 cc/kg with a mouse ventilator (MiniVent, Harvard Apparatus, Cambridge, MA) for the duration of the procedure. Compliance and resistance measurements were made at baseline and immediately after serial 3-minute nebulizations of saline and methacholine (0.1–2.0 mg/ml). Total lung resistance and compliance parameters were computed for each breath from the flow and airway pressure signals throughout the respiratory cycle. The breath to breath calculations were then averaged over three minute intervals and recorded. The software calculated the measurements based on the equation P=QR +V/C (Q=flow, R= resistance, V=volume, C=compliance). All measurements were made in the same manner.
2.9 Statistical analysis of data
Results are presented as mean values ±S.E.M. Means were compared by t-test or by ANOVA, with Tukey’s correction for multiple comparisons applied where appropriate. A P value of 0.05 or less was taken to indicate significance. Initial analysis of apparently correlated data was by linear regression analysis, using the Prism (San Diego, CA) software package. Analysis of interactive effects was by ANCOVA. R, a common open source statistical computing package (URL: http://www.R-project.org), was used to perform this analysis.
3. Results
Balb/c mice were exposed to inhaled ovalbumin or to filtered air for seven to fourteen days and treated with LLP2A (experiments 1 and 2) or PEGylated (experiments 3 and 4) and LLP2A conjugates or scrambled LLP2A. Two mice treated with the L-PEG-LLP2A developed evidence of alveolar hemorrhage by gross observation and lung lavage, but this was not evident in subsequent experiments. The two animals were not specifically tested for evidence of acute bacterial or viral infection, but there were no neutrophils in the lung. All other animals tolerated the exposure without incident and the lungs appeared normal.
3.1 Effects of ovalbumin exposure on lung inflammatory response
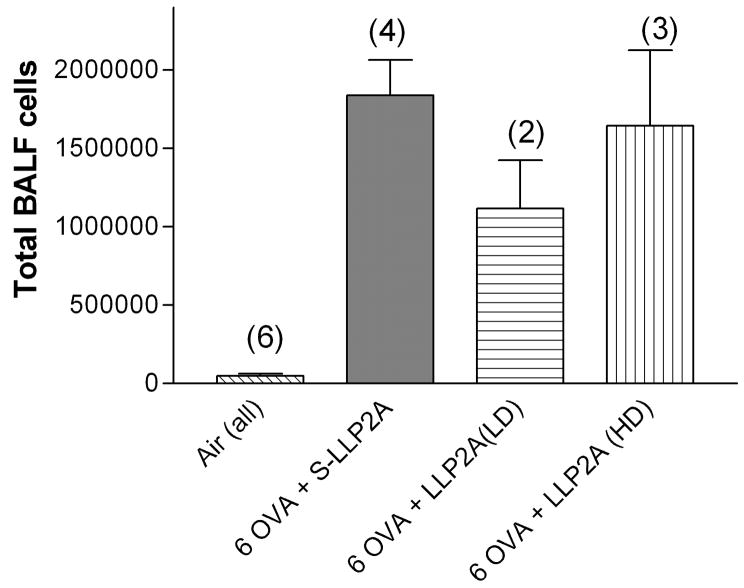
In our first experiment, we tested both low (2 mg/kg) and high dose (20 mg/kg) of LLP2A versus S-LLP2A, an inactive, scrambled compound. In all treatment groups, we observed a significant increase in the numbers of inflammatory cells recovered by lung lavage from sensitized mice exposed to ovalbumin compared to mice exposed to filtered air alone (Fig. 2). The number of lung lavage cells from the groups of mice exposed to filtered air from all time points evaluated was 6.81 ± 1.7 ×105 cells (pooled data from all groups, n= 6). The normal lung lavage from a healthy mouse contains more than 95% alveolar macrophages (Hamada et al., 2000), and our observations were consistent with this finding. There was a significant increase in the number of total lung lavage cells and eosinophils (data not shown) in each of the three ovalbumin exposed groups compared to the air exposed animals, but there was no significant differences among the ovalbumin groups given the small number of animals in each group: inactive compound (1.8 × 106 cells, n=4), low dose antagonist (1.1×106 cells, n=2), high dose antagonist (1.65×106 cells, n=3). We interpreted the results from this experiment to suggest that the high dose (20mg/kg) would not be more efficacious, if at all, than the low dose (2 mg/kg) antagonist and would have the potential to cause greater toxicity. The mean percentage of lymphocytes and neutrophils was less than 1% in all groups of mice exposed to ovalbumin in this model. The very low percentage of lymphocytes was surprising to us.
Fig. 2.
Total lung lavage cells from groups of mice exposed to ovalbumin six times over two weeks (Experiment 1). There was no significant difference among the ovalbumin exposed groups treated with either low- or high-dose LLP2A or the inactive compound S-LLP2A. The number in parentheses is the number of animals in each group. Means are expressed ± S.E.M.
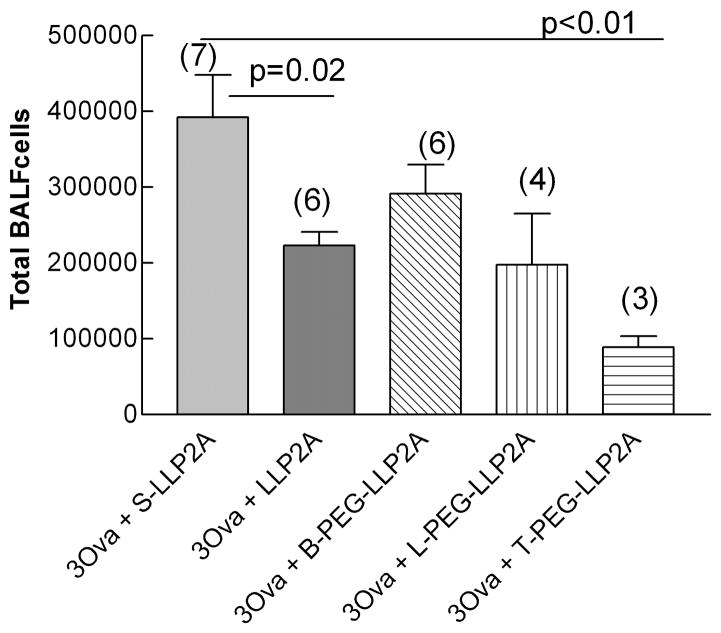
In our later experiments, we hypothesized that the addition of a PEGylated moiety to LLP2A would improve the efficacy of this compound because it would increase the time to clearance from the intravascular space. In these experiments, mice were exposed to ovalbumin for either one or two weeks and treated with either an inactive antagonist (S-LLP2A), the LLP2A antagonist (2mg/kg), or a uniquely synthesized L-, T-, or B-PEG-LLP2A compound. One week exposed mice treated with LLP2A antagonist had significantly fewer total cells in the lung lavage than mice treated with the inactive antagonist, S-LLP2A (2.23 ± 0.17 ×105 (n=6) vs. 3.91± 0.56 ×105 (n=7) respectively, P=0.02, Fig. 3A and B). Mice treated with the T-PEG-LLP2A had significantly less lung lavage cells compared to the mice treated with the inactive compound (0.89 ± 0.15 ×105 (n=3) vs. 3.92 ± 0.56 ×105 (n=7) respectively, P<0.001) and significantly less cells compared to the non-PEGylated antagonist LLP2A (2.23 ± 0.17 × 105 (n=6) vs. 0.89± 0.15 × 105 (n=3) respectively, P=0.0016). The number of cells in the lung lavage of the B-PEG-LLP2A group was higher than that of the inactive compound group, suggesting that this compound does not appear to be effective beyond the 1 week time point.
Fig. 3.
A and B. Total lung lavage cells from groups of mice exposed to ovalbumin three (one week, Figure 3a) and six times (two weeks, Figure 3b). There was a significant decrease in total cells in all groups treated with the LLP2A antagonist at one week (P=0.02). Mice treated with the T-PEG-LLP2A had significantly less lung lavage cells compared to the mice treated with the inactive compound (P<0.001) and significantly less cells compared to the non-PEGylated antagonist LLP2A (P=0.0016). The number in parentheses is the number of animals in each group. Means are expressed ± S.E.M.
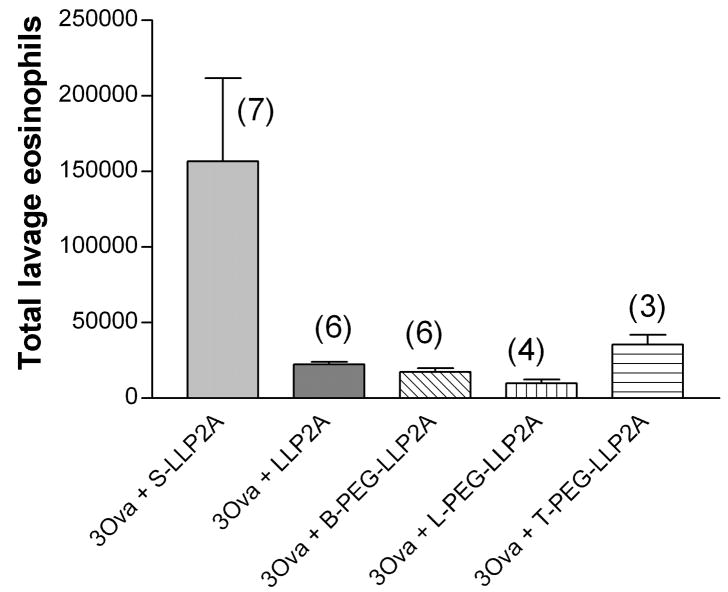
The number of eosinophils in the lung lavage of mice exposed for 1 week and treated with the active antagonist LLP2A or any of the PEGylated formulations was significantly less than in the mice treated with the inactive compound (Fig. 4A). There were 1.57 ± 0.65 ×105 (n=7) eosinophils in the mice treated with the inactive compound and between 0.98±0.05 × 105 and 2.23±0.42 × 105 eosinophils in the active-treated groups. There were no significant differences in eosinophil counts among the four active treatment groups. After 6 ovalbumin exposures (Fig. 4B), there was a trend to decreased numbers of eosinophils in the T-PEG group, but this was not significant. Again, the percentage of neutrophils in all groups was less than 1 percent, which was quite surprising. The percentage of lymphocytes in all groups exposed 6 times to ovalbumin was 8 to 39 %, but the total numbers of lymphocytes in lavage fluid was not different among the groups. All other lavageable cells were macrophages.
Fig. 4.
A and B. Total lung lavage eosinophils from groups of mice exposed to ovalbumin three (one week, Figure 4a) and six times (two weeks, Figure 4b). There was a significant decrease in total cells in all groups treated with the LLP2A antagonist at one week (P=0.05). The number in parentheses is the number of animals in each group. Means are expressed ± S.E.M.
3.2 Effects of ovalbumin exposure on lung histology and goblet cell enumeraton
Exposure of sensitized mice to ovalbumin aerosol gave rise to changes in the lungs that were consistent with those previously observed by us and others (Kenyon et al., 2003a; Kenyon and Last, 2005). We saw evidence of eosinophilic airway inflammation and mucous cell hyperplasia as the lesions evolved over two weeks time. Tissue sections from mice treated with an active antagonist demonstrated significantly less airway inflammation than mice treated with the inactive antagonist (Fig. 5A–D). As measures of airway remodeling, we quantified the number of goblet cells in mice exposed to ovalbumin for two weeks, and semi-qualitatively evaluated the degree in subepithelial collagen formation by Sirius Red staining (Kenyon et al., 2003b). There were no significant differences in the percentage of PAS-Schiff stained goblet cells in airways or the subepithelial collagen deposition score of mice treated with the active or inactive compounds. Collectively, these results suggest to us that either there is no difference between the groups, or that we are not able to determine a difference in the degree of airway remodeling at the relatively early two-week time point in mice treated with the α4β1 integrin antagonist.
Fig. 5.
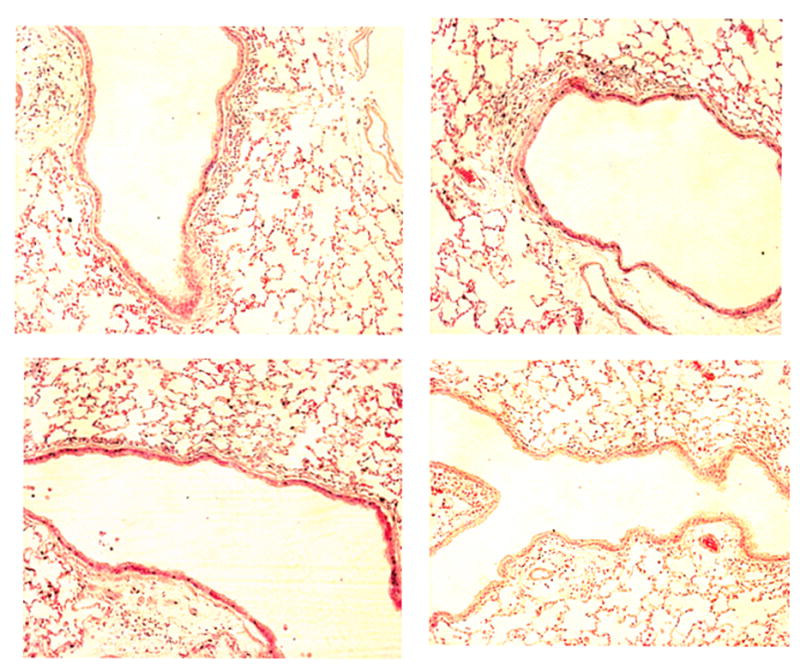
A–D. Representative lung sections from mice exposed to ovalbumin and treated with either the inactive compound S-LLP2A (a, upper left), LLP2A (b, upper right), B-PEG-LLP2A-LLP2A (c, lower left), or T-PEG-LLP2A-LLP2A (d, lower right) over one week in Experiment 2. There were fewer subepithelial inflammatory cells in both groups treated with the PEG-formulations (c, d) compared to the mice treated with the inactive compound (a). The photographed airways are of the first to second branching generation from the primary left lobar bronchus, H and E sections, 40x.
3.3 Marker of oxidant stress
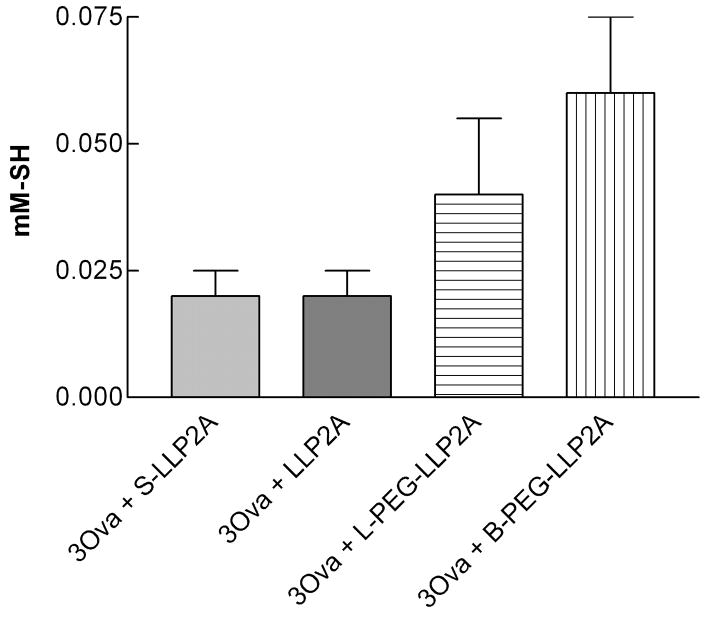
To test the hypothesis that exposure to antigen could cause local and systemic oxidative stress, we analyzed the total R-SH content of blood plasma collected from mice exposed to ovalbumin lbumin, and from their matched controls that breathed only filtered air. As shown in Fig. 6, we found increased total R-SH, suggesting less oxidative stress, in blood plasma in mice treated with B-PEG-LLP2A compared to mice treated with the inactive compound (P=0.045). Interestingly, there was no difference in the R-SH content in plasma between the inactive and active, non-PEGylated antagonist. Any apparent reduction in total plasma R-SH could have been due to oxidation of the R–SH groups on plasma proteins, but a decrease in plasma protein, predominantly albumin, could also have been a factor.
Fig. 6.
Total R-SH (sulfhydryl) plasma concentration from one week (3 ovalbumin) exposed mice treated with inactive compound S-LLP2A or LLP2A or B-PEG-LLP2A- or L-PEG-LLP2A compounds. We found increased total R-SH, suggesting less oxidative stress, in blood plasma in mice treated with B-PEG-LLP2A compared to mice treated with the inactive compound (P=0.045).
3.4 Exhaled NO concentration and nitrite/nitrate from lung lavage
Exhaled nitric oxide (FeNO) concentration was measured in some, but not all, groups of mice. FeNO did not differ significantly among the groups and the individual readings showed a high degree of variability. We measured nitrate/nitrite (NOX) in lung lavage fluid to more accurately determine reactive nitrosative stress. NOX concentrations in lung lavage fluid (5–9uM) were not significantly different between any groups of mice. We concluded that differences in lung inflammation seen with the integrin antagonist were independent of any effects on nitrosative stress.
3.5 Lung compliance and resistance
Dynamic compliance (Cdyn) and resistance (Rrs) of the respiratory system were measured in anesthetized, tracheotomized mice 1 to 3 h after their last ovalbumin exposure. As expected, in our first experiment we found a significant decrease in the respiratory system compliance and a significant increase in the resistance in the lungs of mice exposed to ovalbumin compared to the lungs of mice exposed to filtered air. There were no differences among the three ovalbumin exposed groups (Fig. 7A and B). Similarly, in the subsequent experiments testing the physiologic effects of the PEGylated compounds, we found no statistically significant differences in Cdyn (Fig. 8A and B) and Rrs among any of the groups exposed to ovalbumin lbumin. The α4β1 antagonist did not appear to ameliorate physiological responses to inhaled methacholine.
Fig. 7.

A and B. Change in total lung compliance (a) and lung resistance (b) after inhalation of increasing doses of nebulized methacholine (0–2 mg/ml) compared to baseline in Experiment 1. Filled squares indicates all mice from combined air groups (n=7), filled circles indicates 3 ovalbumin + low dose LLP2A (n=2), open square indicates 6 ovalbumin + low dose LLP2A (n=2), open triangle indicates 6 ovalbumin + high dose LLP2A (n=3), open circle indicates 6 ovalbumin + S-LLP2A (n=4). Means are expressed ± S.E.M.
Fig. 8.
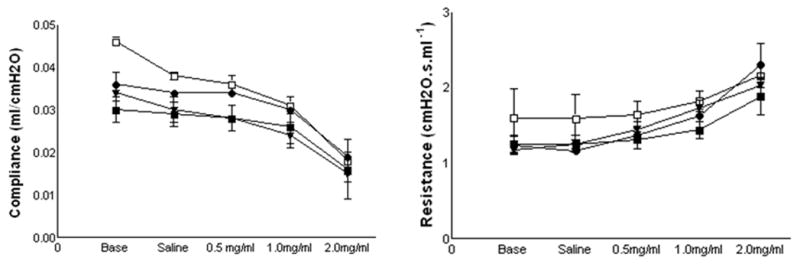
Change in total lung compliance (a) and lung resistance (b) after inhalation of increasing doses of nebulized methacholine (0–2 mg/ml) compared to baseline in Experiment 4. Filled squares indicates 3 ovalbumin + S-LLP2A (n=4), filled circles indicates 3 ovalbumin + LLP2A (n=4), filled triangles indicates 3 ovalbumin + BPEG- LLP2A (n=4), open square indicates 3 ovalbumin + LPEG-LLP2A (n=4). Means are expressed ± S.E.M.
4. Discussion
We have previously demonstrated a sequence of inflammatory and structural airway changes in the lungs of BALB/c mice sensitized and exposed to ovalbumin aerosols for one to eight weeks (Kenyon et al., 2003a; Kenyon and Last, 2005). For this study, we hypothesized that the administration of the novel, small molecule α4β1 antagonist LLP2A would inhibit the migration of lymphocytes and eosinophils into the lung in mice exposed to ovalbumin for one to two weeks by affecting α4β1 integrin function.
We can summarize our results as follows. Upon exposure to ovalbumin aerosol for one week, sensitized BALB/c mice develop eosinophilic airway inflammation and airways hyperresponsiveness. The i.p. administration of LLP2A, our α4β1 antagonist, can partially prevent, or reverse, the inflammatory response, but not the airways hyperresponsiveness, caused by ovalbumin exposure at the one week time point. The in vivo efficacy of this LLP2A antagonist can be improved by the addition of a specific tetravalent PEGylated formulation (T-PEG-LLP2A), presumably by increasing drug bioavailability.
α4 integrins are highly expressed on lymphocytes and have been used in the treatment of lymphoma and multiple sclerosis. α4β1 (very late antigen 4, VLA-4) is expressed on all circulating leukocytes, except neutrophils, and binds to vascular cell adhesion molecule-1 (VCAM-1) (Cortijo et al., 2006). α4 integrins and their binding sites, therefore, are attractive therapeutic targets in inflammatory airways diseases, such as asthma and chronic bronchitis. In asthma, there is a direct link between the α4 integrins and the allergic inflammatory cytokine, interleukin-5 (IL-5). IL-5 is a cytokine that promotes the development of eosinophilic airway inflammation in asthma and encourages the development of podosome formation of eosinophils and the adherence of α4β1 to VCAM-1 in the lung (Johansson et al., 2004). At least two preliminary clinical trials of a α4β7 antagonist has been performed in asthma (Ravensberg et al., 2006; Vanderslice et al., 2004).
Various animal models of allergic airways inflammation have been used to investigate the effect of α4 integrin inhibitors in the lung (Borchers et al., 2001; Henderson et al., 1997; Kanehiro et al., 2000; Kraneveld et al., 1997; Okigami et al., 2007). A dual α1 integrin small molecule antagonist decreases airways hyperresponsiveness and the recruitment of leukocytes into the airway of Brown Norway rats exposed to aerosolized antigen (Cortijo et al., 2006). The administration of a small molecule α4β1 antagonist reduced airways hyperresponsiveness to inhaled carbachol and airway inflammatory cell infiltration in sheep exposed to Ascaris antigen (Abraham et al., 2000). In rabbits exposed to Alternaria tenuis antigen, the administration of a monoclonal antibody to α4β1 reduced airways hyperresponsiveness during the early asthmatic reaction phase. It was hypothesized that VLA-4 integrin was a trigger for mast cell degranulation (Gascoigne et al., 2003). Our results differ from these studies performed in other animal models in that we found a significant anti-inflammatory effect of the α4β1 antagonist, but no effect on airways hyperresponsiveness. This perhaps can be explained by our use of BALB/c mice. This strain demonstrates significant airways hyperresponsiveness at baseline (as seen by our data) and is considered a ‘high-responder’ to inhaled allergen. Any beneficial effect of the integrin inhibitor on inflammation may not carry over to an effect on lung function. On the other hand, different types of drugs were used in all of these studies, and the observed lung function responses may reflect the differences in experimental design.
It is well known that small molecule inhibitors vary in potency. Recently, important improvements in the design and potency of small molecule integrin inhibitors have followed elucidation of the crystalline structure of the integrin (αvβ3) in 2001 (Xiong et al., 2001). The α4 integrins, in particular, have become attractive targets in the pharmaceutical industry for the development of small molecule inhibitors (Pepinsky et al., 2005; Singh et al., 2004; Wattanasin et al., 2001). In general, integrins have been difficult targets for the design of small molecule inhibitors because of their high molecular weight, a lack of detailed structural information, and multiple antagonist specificities.
We have previously developed α4β1 antagonists using a unique ‘one-bead, one-compound, combinatorial library method (Liu et al., 2006; Peng et al., 2006). To address the issue of bioavailability of our small molecule antagonists, we hypothesized that the PEG compound would remain in the blood circulation longer than the non-PEGylated formulations and would be more efficacious. We covalently attached the antagonist to PEG, based upon the work of Pepinsky and colleagues, who administered a PEGylated α4β1 small molecule inhibitor (1 mg/kg) to female Lewis mice injected with myelin basic protein, an injury model of encephalitis (Pepinsky et al., 2005). Compared to a non-PEGylated inhibitor (BIO5192, 30mg/kg), the PEGylated compound reduced the number of days of the rats had an increased paralysis score, thereby suggesting that PEGylation improved the bioavailability and potency of the compound. We found a similar effect on inflammation in the lung with the PEGylated compounds; in addition, we found that the different homologues of the PEGylated molecule had significantly different properties.
The number of inflammatory cells in the lung lavage of ovalbumin -exposed mice was significantly less in the animals injected with the T-PEG-LLP2A, compared to those treated with the non-PEGylated compound at the one week time point. Certainly, the tetravalent T-PEG structure had more binding sites for α4β1 integrin than did the other compounds, but we would suggest that the shape of the T-PEGylated modification to the antagonist also decreased the serum binding of the inhibitor to a greater degree than either the L-PEG-LLP2A or B-PEG-LLP2A compounds. Other groups have found less serum protein binding in the PEG-modified compounds; this may also vary among the PEGylated structures (Pepinsky et al., 2005), but we did not specifically test this hypothesis.
Several limitations to our study are apparent. First, we did not measure the pharmacokinetics of the PEGylated compounds in the mouse to determine optimal dosing. However, we believe that the magnitude of the anti-inflammatory effect of the compounds seen at the dosing interval chosen demonstrates efficacy. Further studies that test different dosing regimens may be warranted in the future. Second, we did not confirm the deposition of the synthesized compounds in the lung by in vivo imaging; however, we have shown previously that dye-labeled LLP2A can be imaged in subcutaneous xenografts of Molt-4 (α4β1-positive) tumors (Peng et al., 2006). We attempted to address this question by performing preliminary experiments to image fluorescently labeled inhibitors with a whole animal CAT scanner. Unfortunately, we were unable to identify these compounds in the lung and suspect that the level of detection of the instrument was insufficient to detect the labeling of the lung parenchyma. Third, the number of animals that were tested with each compound was small. We invested significant effort in assuring the purity of each drug and were constrained by the limited amount of these antagonists in each experiment. In this regard, these experiments should be considered proof-of-concept. Fourth, though the antagonist had a potent anti-inflammatory effect, we did not find an effect on airways hyperresponsiveness. This discrepancy is not unique and has been reported in a host of other small animal studies. This finding reinforces the point that, as in asthma, eosinophilic airway inflammation and airway hyperresponsiveness may be independent processes, each with its own cascades of mediators that may or may not overlap. Our findings should not discourage, and we believe strongly encourage, further testing of these inhibitors in pre-clinical asthma models.
Consistent with the findings of others, we found that a synthetic, small molecule antagonist for α4β1 can inhibit the infiltration of eosinophils and lymphocytes into the lungs of mice exposed to ovalbumin lbumin. In addition, we demonstrated that specifically engineered PEGylated antagonists can improve the efficacy of these potential therapeutic agents. Still, these results provide evidence that encourages the further development of α4β1 inhibitors in inflammatory airways diseases and suggests that several drug designs should be tested in pre-clinical studies to optimize efficacy.
Acknowledgments
We thank Lisa Franzi for technical assistance in performing these experiments and Dr. Jerold Last for his review of the manuscript. This work was supported by grants from the NIH (NHLBI (NJK) and NCI (KSL)).
Footnotes
Conflict of Interest statement:
The authors have no conflict of interest relevant to the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WM, Gill A, Ahmed A, Sielczak MW, Lauredo IT, Botinnikova Y, Lin KC, Pepinsky B, Leone DR, Lobb RR, Adams SP. A small-molecule, tight-binding inhibitor of the integrin alpha (4) beta (1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am J Respir Crit Care Med. 2000;162:603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- Borchers MT, Crosby J, Farmer S, Sypek J, Ansay T, Lee NA, Lee JJ. Blockade of CD49d inhibits allergic airway pathologies independent of effects on leukocyte recruitment. Am J Physiol Lung Cell Mol Physiol. 2001;280:L813–821. doi: 10.1152/ajplung.2001.280.4.L813. [DOI] [PubMed] [Google Scholar]
- Cortijo J, Sanz MJ, Iranzo A, Montesinos JL, Nabah YN, Alfon J, Gomez LA, Merlos M, Morcillo EJ. A small molecule, orally active, alpha4beta1/alpha4beta7 dual antagonist reduces leukocyte infiltration and airway hyper-responsiveness in an experimental model of allergic asthma in Brown Norway rats. Br J Pharmacol. 2006;147:661–670. doi: 10.1038/sj.bjp.0706658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne MH, Holland K, Page CP, Shock A, Robinson M, Foulkes R, Gozzard N. The effect of anti-integrin monoclonal antibodies on antigen-induced pulmonary inflammation in allergic rabbits. Pulm Pharmacol Ther. 2003;16:279–285. doi: 10.1016/S1094-5539(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–3092. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol. 2004;31:413–422. doi: 10.1165/rcmb.2004-0099OC. [DOI] [PubMed] [Google Scholar]
- Kanehiro A, Takeda K, Joetham A, Tomkinson A, Ikemura T, Irvin CG, Gelfand EW. Timing of administration of anti-VLA-4 differentiates airway hyperresponsiveness in the central and peripheral airways in mice. Am J Respir Crit Care Med. 2000;162:1132–1139. doi: 10.1164/ajrccm.162.3.9910100. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Gohil K, Last JA. Susceptibility to ovalbumin lbumin-induced airway inflammation and fibrosis in inducible nitric oxide synthetase-deficient mice: mechanisms and consequences. Topical Appl Pharmacol. 2003a;191:2–11. doi: 10.1016/s0041-008x(03)00227-8. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Last JA. Reversible and irreversible airway inflammation and fibrosis in mice exposed to inhaled ovalbumin. Inflamm Res. 2005;54:57–65. doi: 10.1007/s00011-004-1325-6. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Ward RW, Last JA. Airway fibrosis in a mouse model of airway inflammation. Toxicol Appl Pharmacol. 2003b;186:90–100. doi: 10.1016/s0041-008x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- Kraneveld AD, van Ark I, Van Der Linde HJ, Fattah D, Nijkamp FP, Van Oosterhout AJ. Antibody to very late activation antigen 4 prevents interleukin-5-induced airway hyperresponsiveness and eosinophil infiltration in the airways of guinea pigs. J Allergy Clin Immunol. 1997;100:242–250. doi: 10.1016/s0091-6749(97)70231-8. [DOI] [PubMed] [Google Scholar]
- Liu R, Peng L, Han H, Lam KS. Structure-activity relationship studies of a series of peptidomimetic ligands for alpha (4) beta (1) integrin on Jurkat T-leukemia cells. Biopolymers. 2006;84:595–604. doi: 10.1002/bip.20588. [DOI] [PubMed] [Google Scholar]
- Okigami H, Takeshita K, Tajimi M, Komura H, Albers M, Lehmann TE, Rolle T, Bacon KB. Inhibition of eosinophilia in vivo by a small molecule inhibitor of very late antigen (VLA)-4. Eur J Pharmacol. 2007;559:202–209. doi: 10.1016/j.ejphar.2006.11.065. [DOI] [PubMed] [Google Scholar]
- Peng L, Liu R, Andrei M, Xiao W, Lam KS. In vivo optical imaging of human lymphoma xenograft using a library-derived peptidomimetic against {alpha} 4{beta} 1 integrin. Mol Cancer Ther. 2008;7:432–437. doi: 10.1158/1535-7163.MCT-07-0575. [DOI] [PubMed] [Google Scholar]
- Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Lee WC, Cornebise M, Gill A, Wortham K, Chen LL, Leone DR, Giza K, Dolinski BM, Perper S, Nickerson-Nutter C, Lepage D, Chakraborty A, Whalley ET, Petter RC, Adams SP, Lobb RR, Scott DM. Design, synthesis, and analysis of a polyethelene glycol-modified (PEGylated) small molecule inhibitor of integrin {alpha}4{beta}1 with improved pharmaceutical properties. J Pharmacol Exp Ther. 2005;312:742–750. doi: 10.1124/jpet.104.075648. [DOI] [PubMed] [Google Scholar]
- Pretolani M, Ruffie C, Lapa e Silva JR, Joseph D, Lobb RR, Vargaftig BB. Antibody to very late activation antigen 4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J Exp Med. 1994;180:795–805. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabb HA, Olivenstein R, Issekutz TB, Renzi PM, Martin JG. The role of the leukocyte adhesion molecules VLA-4, LFA-1, and Mac-1 in allergic airway responses in the rat. Am J Respir Crit Care Med. 1994;149:1186–1191. doi: 10.1164/ajrccm.149.5.8173758. [DOI] [PubMed] [Google Scholar]
- Ravensberg AJ, Luijk B, Westers P, Hiemstra PS, Sterk PJ, Lammers JW, Rabe KF. The effect of a single inhaled dose of a VLA-4 antagonist on allergen-induced airway responses and airway inflammation in patients with asthma. Allergy. 2006;61:1097–1103. doi: 10.1111/j.1398-9995.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- Richards IM, Kolbasa KP, Hatfield CA, Winterrowd GE, Vonderfecht SL, Fidler SF, Griffin RL, Brashler JR, Krzesicki RF, Sly LM, Ready KA, Staite ND, Chin JE. Role of very late activation antigen-4 in the antigen-induced accumulation of eosinophils and lymphocytes in the lungs and airway lumen of sensitized brown Norway rats. Am J Respir Cell Mol Biol. 1996;15:172–183. doi: 10.1165/ajrcmb.15.2.8703473. [DOI] [PubMed] [Google Scholar]
- Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Silkoff PE, Stevens A, Pak J, Bucher-Bartelson B, Martin RJ. A method for the standardized offline collection of exhaled nitric oxide. Chest. 1999;116:754–759. doi: 10.1378/chest.116.3.754. [DOI] [PubMed] [Google Scholar]
- Singh J, Adams S, Carter MB, Cuervo H, Lee WC, Lobb RR, Pepinsky RB, Petter R, Scott D. Rational design of potent and selective VLA-4 inhibitors and their utility in the treatment of asthma. Curr Top Med Chem. 2004;4:1497–1507. doi: 10.2174/1568026043387520. [DOI] [PubMed] [Google Scholar]
- Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;53:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderslice P, Biediger RJ, Woodside DG, Berens KL, Holland GW, Dixon RA. Development of cell adhesion molecule antagonists as therapeutics for asthma and COPD. Pulm Pharmacol Ther. 2004;17:1–10. doi: 10.1016/j.pupt.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–175. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanasin S, Weidmann B, Roche D, Myers S, Xing A, Guo Q, Sabio M, von Matt P, Hugo R, Maida S, Lake P, Weetall M. Design and synthesis of potent and selective inhibitors of integrin VLA-4. Bioorg Med Chem Lett. 2001;11:2955–2958. doi: 10.1016/s0960-894x(01)00586-8. [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]