Abstract
The total synthesis and evaluation of iso-duocarmycin SA (5) and iso-yatakemycin (6) are detailed representing key analogues of the corresponding natural products incorporating an isomeric alkylation subunit. This pyrrole isomer of the natural alkylation subunit displayed an enhanced reaction regioselectivity and a 2-fold diminished stability. Although still exceptionally potent, the iso-DSA derivatives and natural product analogues exhibited a corresponding approximate 3- to 5-fold reduction in cytotoxic activity (L1210 IC50 for (+)-iso-duocarmycin SA = 50 pM and (+)-iso-yatakemycin = 15 pM) consistent with their placement on a parabolic relationship correlating activity with reactivity. The DNA alkylation selectivity of the resulting key natural product analogues was unaltered by the structure modification in spite of the minor groove presentation of a potential H-bond donor. Additionally, a unique o-spirocyclization with such derivatives was explored with the preparation, characterization, and evaluation of 34 that is incapable of the more conventional p-spirocyclization. Although 34 proved sufficiently stable for isolation and characterization, it displayed little stability in protic solvents (t1/2 = 0.19 h at pH 3, t1/2 = 0.20 h at pH 7), a pH independent (H+ independent) solvolysis rate profile at pH 3/4–7, and a much reduced cytotoxic potency, but a DNA alkylation selectivity and efficiency comparable to duocarmycin SA and iso-duocarmycin SA. The implications of these observations on the source of the DNA alkylation selectivity and catalysis for this class of natural products are discussed.
Introduction
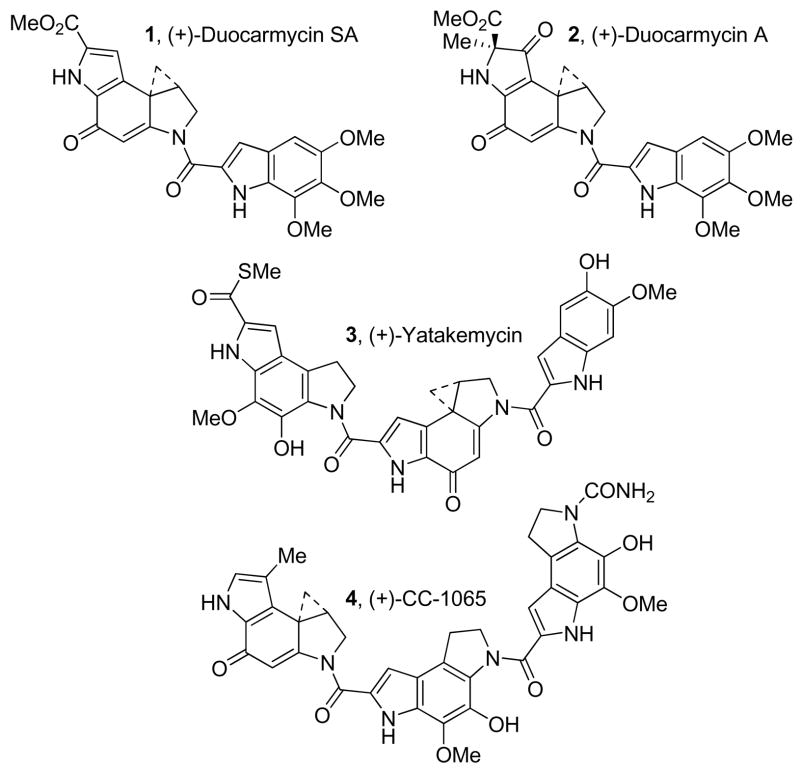
Duocarmycin SA (1, DSA)1 and yatakemycin (3)2 are the most potent (L1210 IC50 = 10 pM and 3–6 pM, respectively) members of a class of antitumor agents that also includes CC-1065 (4)3 and duocarmycin A (2, Figure 1).4 Each derives its properties from a characteristic sequence-selective alkylation of duplex DNA,5–9 in which a stereoelectronically-controlled adenine N3 addition to the least substituted carbon of the activated cyclopropane occurs within selected minor groove AT-rich sites.
Figure 1.
Natural products.
Extensive efforts using systematic alterations and simplifications in the alkylation subunits have defined key and subtle structural features that contribute to their properties. Most notable of these structural features are the stereoelectronic alignment of the cyclopropane that controls its reaction regioselectivity,10 and the cross-conjugated vinylogous amide that stabilizes the reactive cyclopropane.11 Not only have these studies provided unique insights into the relationships between structure, reactivity, and biological properties9 and the DNA alkylation selectivity,12 but they have proven key to revealing the source of catalysis9d,13–15 for the DNA alkylation reaction and have defined a fundamental parabolic relationship between reactivity and biological potency.16
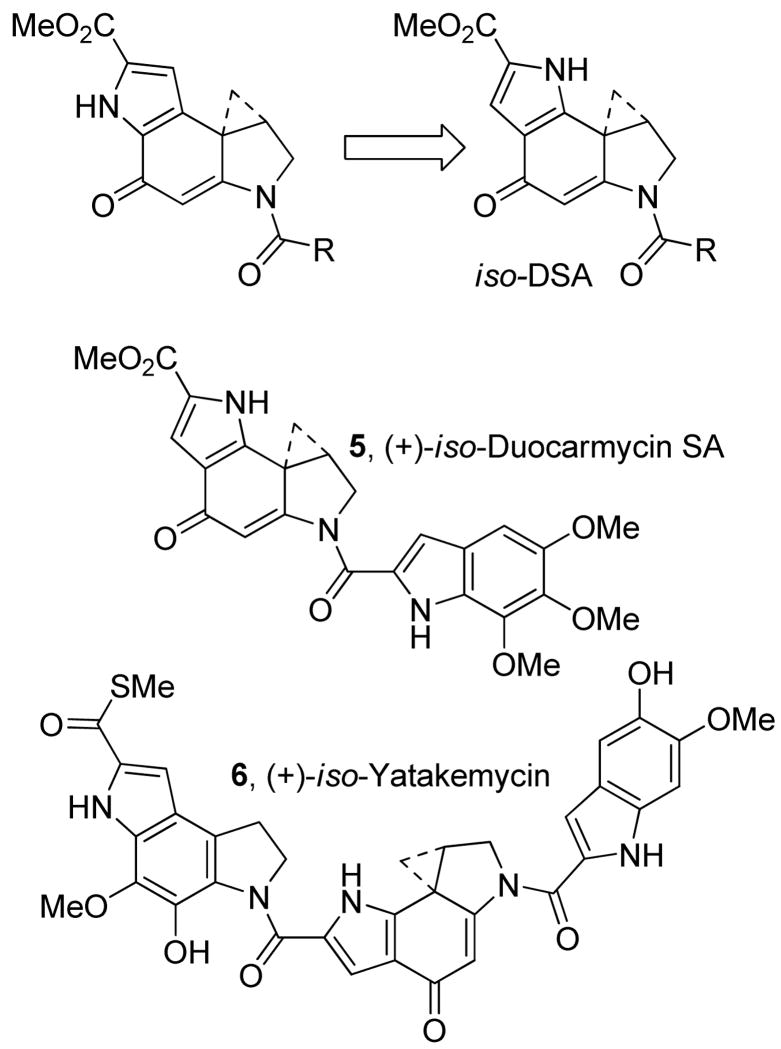
Herein, we report the synthesis, characterization, and evaluation of 6-methoxycarbonyl-1,2,8,8a-tetrahydrocyclopropa[c]pyrrolo[2,3-g]indol-4-one (iso-DSA), an isomer of the duocarmycin SA and yatakemycin alkylation subunit in which the fused pyrrole is inverted, and its incorporation into iso-duocarmycin SA (5) and iso-yatakemycin (6), key analogues of the natural products (Figure 2).
Figure 2.
iso-Duocarmycin SA (5) and iso-yatakemycin (6).
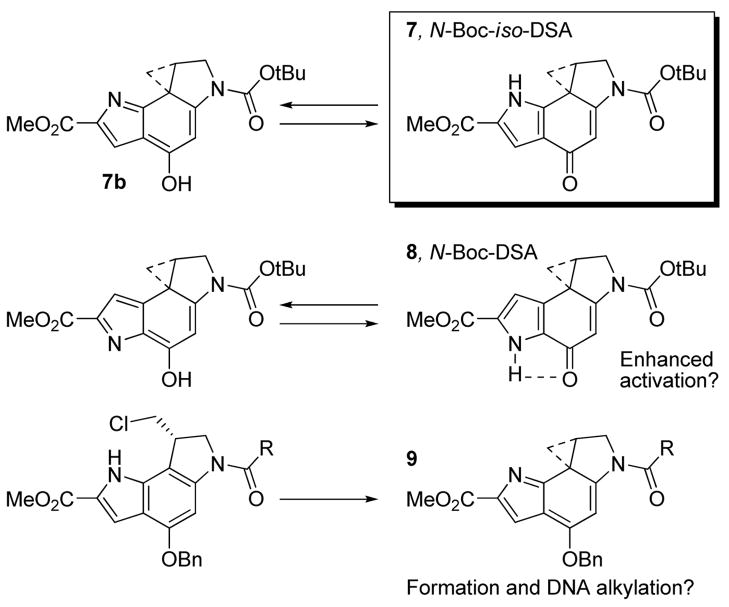
The examination of the iso-DSA alkylation subunit was expected to address three important questions. First, would the introduction of a fixed H-bond donor on the minor groove interacting face of the molecule affect the DNA alkylation selectivity or efficiency? It was our expectation that this would have a minimal impact on the natural enantiomers, but may have a much more significant impact on the unnatural enantiomers where the distance to the adjacent 5′-base on the alkylated strand is closest to this center.17 Second, it was not clear what impact this seemingly simple change would have on the reactivity of the molecule. That is, does it now possess a significant competing vinylogous amide structure that will serve to destabilize the alkylation subunit activating it for nucleophilic attack or will it further stabilize the structure by removing (moving) an internal H-bond that may serve to activate DSA for nucleophilic attack? Although contrary to our own expectations, the latter has been suggested by Skibo18 to be a significant contributing factor for duocarmycin SA. Thus, the examination of 7 was viewed as key to understanding how structure impacts reactivity. Finally, we were interested in establishing what impact this structural change would have on the regioselectivity of the cyclopropane nucleophilic addition now that the acidic indole NH is proximal to the reacting center and even whether the cyclohexadienone structure would be required for spirocyclization and DNA alkylation. It is possible that an o-spirocyclization involving the indole NH may provide comparable, albeit more reactive, intermediates (e.g., 9) capable of alkylating DNA.
Results and Discussion
Synthesis of N-Boc-iso-Duocarmycin SA
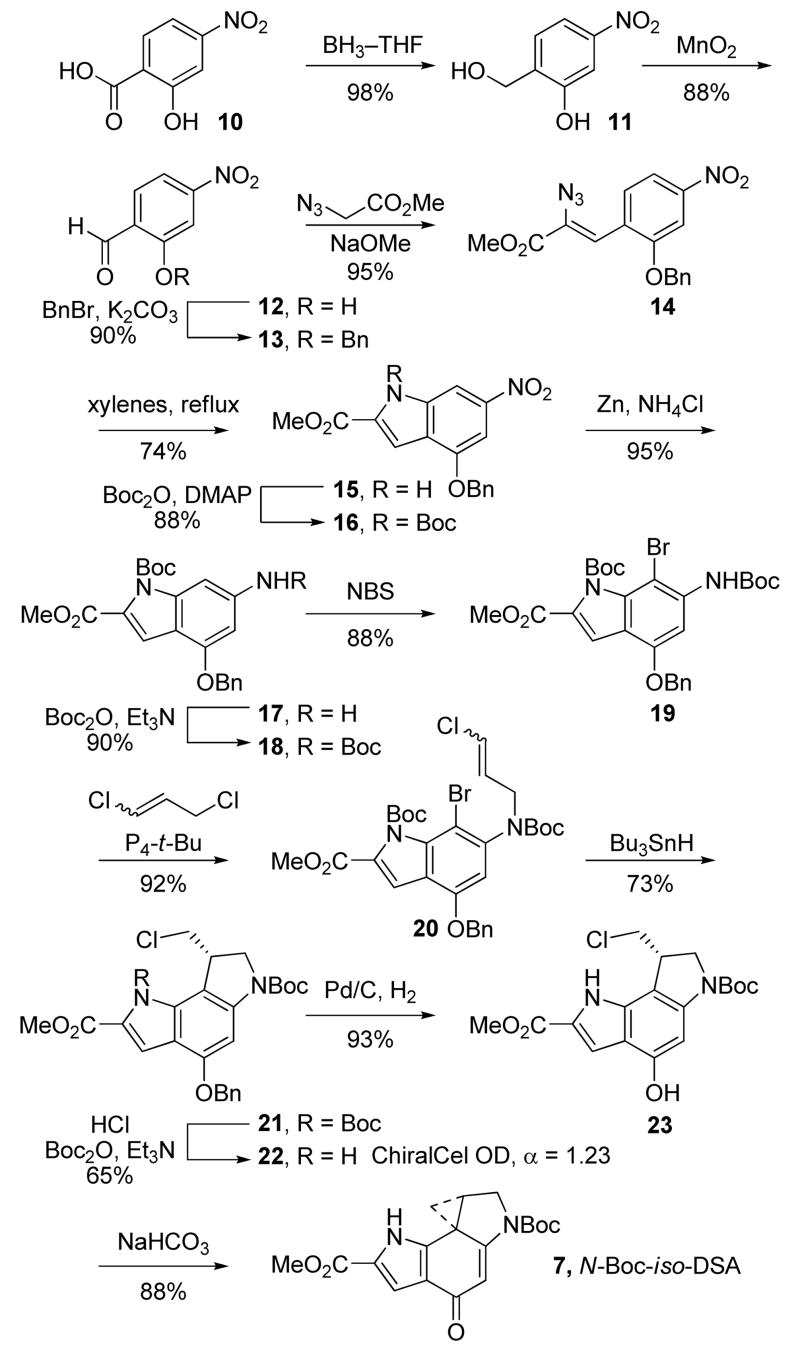
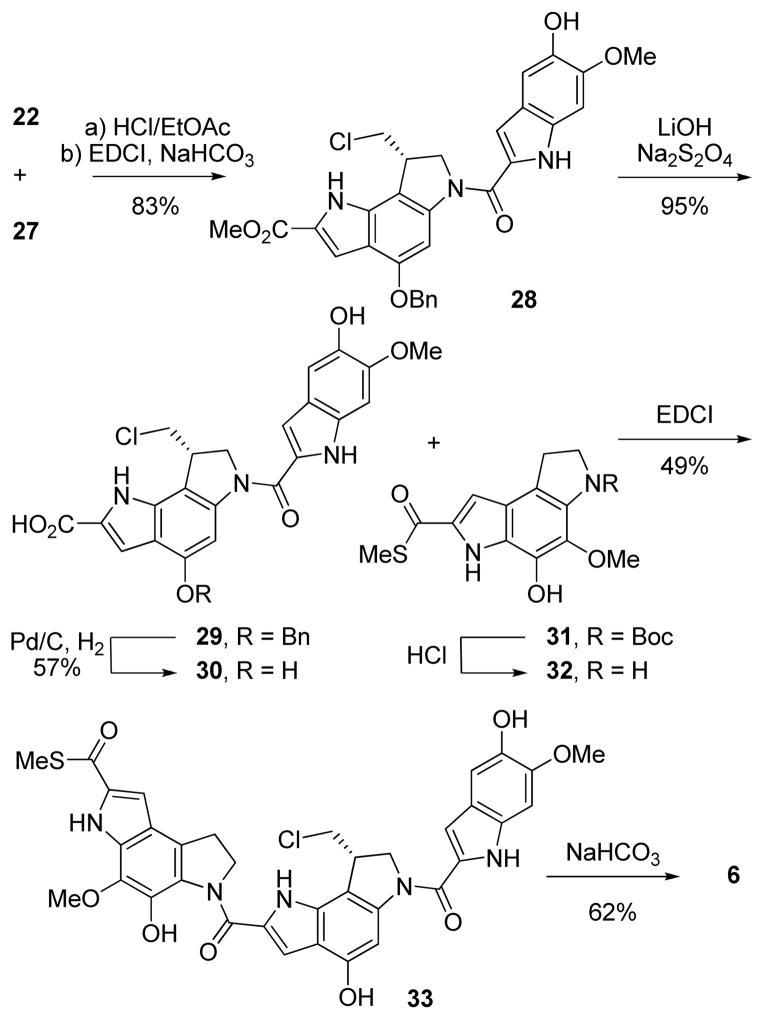
The synthesis19 of the modified alkylation subunit began with reduction of 2-hydroxy-4-nitrobenzoic acid with BH3–THF to afford the corresponding benzyl alcohol 11 (THF, 0–23 °C, 18 h, 98%) that was subsequently oxidized to aldehyde 12 (7 equiv of MnO2, EtOAc, 80 °C, 2.5 h, 88%), Scheme 1. Protection of the free phenol as a benzyl ether afforded 13 (2 equiv of BnBr, 1.5 equiv of K2CO3, DMF, 0–23 °C, 18 h, 90%) that was converted to the styryl azide 14 by condensation with methyl azidoacetate (4 equiv, 4 equiv of Na, MeOH, −15 to 0 °C, 24 h, 95%). Formation of indole 15 was achieved using a thermally-induced Hemetsberger cyclization (xylenes, 145 °C, 3 h, 74%) to provide 15 that was protected as its N-Boc derivative 16 (1.5 equiv of Boc2O, 0.1 equiv of DMAP, THF, 23 °C, 2 h, 89%). Reduction of the aryl nitro group of 16 to the corresponding aniline 17 was carried out smoothly with zinc nanopowder and ammonium chloride20 (10 equiv of Zn, 15 equiv of NH4Cl, 5:1 acetone/H2O, 23 °C, 5 min, 95%) in excellent yields. Alternative attempts to reduce 16 using tin(II) chloride provided lower yields and generated by-products that proved difficult to separate from the desired aniline. The zinc nanopowder reduction, which proceeds at remarkable rates and at nearly neutral pH, leaves the labile indole N-Boc intact in a reaction for which the workup simply entails filtration removal of the zinc nanopowder. Following N-Boc protection of the aniline 17 (1.5 equiv of Boc2O, 1.2 equiv of Et3N, THF, 23 °C, 24 h, 90%), 18 underwent a regioselective bromination (1.2 equiv of NBS, THF, 23 °C, 4 h, 88%) to afford 19. N-Alkylation of the carbamate with 1,3-dichloropropene enlisting phosphazene base P4-t-Bu (1-tert-butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)-phosphoranylidenamino]-2Λ5,4Λ5-catenadi(phosphazene), 1.5 equiv of 1,3-dichloropropene, 1.2 equiv of P4-t-Bu, benzene, 23 °C, 3 h, 92%) afforded the free radical cyclization precursor 20 as a mixture of geometrical isomers in good yield. The use of the exceptionally strong, non-nucleophilic P4-t-Bu base as its commercially available solution in hexanes provided exceptional yields of the desired alkylation product without the competitive hydroxide-promoted ester hydrolysis often observed with the more common use of NaH. The final ring of the alkylation subunit was constructed using a Bu3SnH-mediated 5-exo-trig aryl radical–alkene cyclization21 (1.2 equiv of Bu3SnH, 0.3 equiv of AIBN, benzene, 80 °C, 3.5 h, 73%). A thorough removal of all residual tin by repeated chromatography and trituration with hexanes improved the conversions in the subsequent Boc-deprotection. Additionally at this stage, chiral phase chromatography was not successful at separating the enantiomers of 21. To circumvent this, both Boc groups were removed (4 N HCl/EtOAc, 23 °C, 6 h), and the indoline nitrogen was selectively reprotected (1.5 equiv of Boc2O, 2 equiv of Et3N, THF, 25 °C, 18 h, 65%). Separation of the enantiomers of 22 was now possible and conducted effectively using a Chiralcel OD semipreparative HPLC column (2 × 25 cm, 2% i-PrOH/hexane, 7 mL/min, α = 1.23).17 O-Debenzylation of 22 (0.2 wt. equiv of 10% Pd/C, 1 atm H2, THF–MeOH, 23 °C, 1 h, 93%) followed by treatment of the resulting phenol 23 with mild base afforded N-Boc-iso-DSA (7) in superb conversion (sat. aqueous NaHCO3, DMF, 23 °C, 1 h, 88%), Scheme 1 (natural enantiomer shown).
Scheme 1.
Synthesis of iso-Duocarmycin SA (iso-DSA)
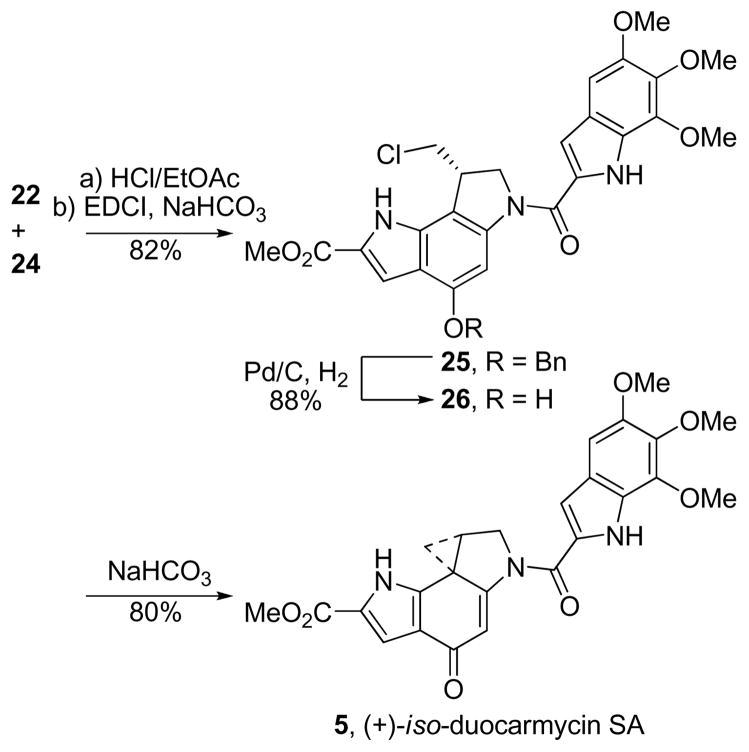
Duocarmycin SA (1) represents the most stable and most potent member of the natural products that contain the two subunit structure. Consequently, the isomeric alkylation subunit was incorporated into the key analogue iso-duocarmycin SA (5) as shown in Scheme 2. Thus, Boc deprotection of 22 (4 N HCl/EtOAc, 23 °C, 45 min) followed by direct coupling of the resulting indoline hydrochloride salt with 5,6,7-trimethoxyindole-2-carboxylic acid22 (24, 1.1 equiv, 1.0 equiv of NaHCO3, 4 equiv of EDCI, DMF, 23 °C, 30 min, 82%) afforded 25 (natural enantiomer shown). The benzyl group was removed (0.2 wt. equiv of 10% Pd/C, 1 atm H2, THF–MeOH, 23 °C, 3 h, 88%) affording 26 that was smoothly spirocyclized under mild conditions to provide iso-duocarmycin SA (5, sat. aqueous NaHCO3, DMF, 23 °C, 2 h, 80%).
Scheme 2.
Synthesis of iso-Yatakemycin
A second key natural product analogue that we elected to examine was iso-yatakemycin (6) since yatakemycin itself represents the most exciting of the natural products isolated to date. Central to its unique sandwiched three-subunit structure, yatakemycin contains the same alkylation subunit found in duocarmycin SA that we now replaced with the isomeric alkylation subunit. The simplified right-hand subunit of the natural product as well as the left-hand PDE thiomethyl ester found in yatakemycin were incorporated unchanged into iso-yatakemycin (6). Notably, this left-hand subunit also constitutes the central and right-hand subunits found in CC-1065, but is capped with a thiomethyl ester unique to the yatakemycin structure. The most remarkable features of this “sandwiched” three subunit arrangement are the enhanced rates and efficiencies of DNA alkylation, the near identical DNA alkylation selectivities of the two enantiomers, and their enhanced cytotoxic potency including those of the unnatural enantiomers.23,24 The synthesis of iso-yatakemycin diverged from that of iso-duocarmycin SA following chromatographic separation of the enantiomers of 22. Treatment of 22 with 4 N HCl/EtOAc, followed by immediate coupling of the resulting indoline hydrochloride salt with 5-hydroxy-6-methoxyindole-2-carboxylic acid (27, 1.5 equiv, 1.1 equiv of NaHCO3, 4 equiv of EDCI, DMF, 23 °C, 2.5 h, 83%) afforded 28 (only natural enantiomer shown). Hydrolysis of the methyl ester of 28 initially posed a challenge, as standard LiOH hydrolysis conditions afforded product yields of only 20–30%. Previous studies with similar substrates25 suggested that oxidation contributes to the poor yields, and that addition of a mild reducing agent such as sodium dithionite as well as rigorous degassing of the solvents would improve the conversion. This simple modification was successful (3 equiv of Na2S2O4, 10 equiv of LiOH–H2O, 3:2:1 THF–MeOH–H2O, 23 °C, 6.5 h, 95%) and yielded carboxylic acid 29 in superb yield. Removal of the benzyl ether (0.2 wt. equiv of 10% Pd/C, 1 atm H2, THF–MeOH, 23 °C, 6 h, 57%) yielded the polar intermediate 30. Treatment of thioester 3123 with 4 N HCl/EtOAc (23 °C, 45 min) followed by coupling of the free amine with carboxylic acid 30 (4 equiv of EDCI, DMF, 23 °C, 45 min, 49%) afforded 33. Notably, purification of 33 must be conducted quickly, as slow or repeated silica gel chromatography was found to promote the spirocyclization to 6. LC/MS analysis of the reaction mixture following chromatography on silica gel at times revealed a nearly 1:1 mixture of seco:spiro products, indicating the ease with which the final ring closure proceeds. Compound 33 was carried forward into the final spirocyclization reaction (sat. aqueous NaHCO3, DMF, 23 °C, 1 h, 62%) to provide iso-yatakemycin (6), (Scheme 3).
Scheme 3.
Synthesis of O-Benzyl-iso-duocarmycin SA
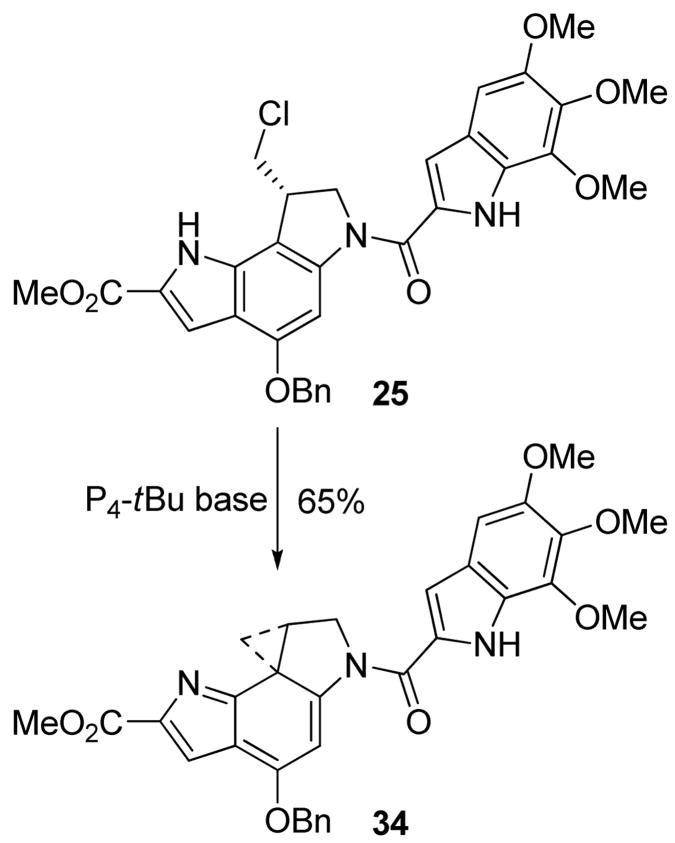
In order to further probe the activation capabilities of the isomeric alkylation subunit,26,27 deliberate efforts to promote a potential o-spirocyclization were undertaken enlisting protected substrates that preclude the conventional p-spirocyclization. Thus, benzyl ether 25 was treated with phosphazene base P4-t-Bu (2.2 equiv, DMF, 23 °C, 6.5 h, 27%) to afford the ortho-spirocyclized product 34. Upon disappearance of starting material by LC/MS, the products of the reaction were subjected to chromatography taking care to avoid the use of chlorinated and nucleophilic solvents and provided the sensitive product 34. Remarkably, this product proved surprisingly stable to chromatography and extended times in solution. Nonetheless, this material was much more reactive and much less stable than iso-duocarmycin SA and the yield of purified 34 reflects this intrinsic reactivity. The use of alternative and more conventional strong bases including NaH and DBU (DMF) to promote the o-spirocyclization provided little to no product 34 by TLC or LC/MS. Although CH2Cl2 (vs DMF) was unsuccessful in supporting the o-pirocyclization reaction of 25, the use of MeCN provided an even more effective and manageable o-spirocyclization. With an additional increase in the amount of base (3.3 equiv) and with an increasing experience with the isolation and chromatographic purification of 34, the o-spirocyclization was optimized (3.3 equiv of P4-t-Bu, CH3CN, 23 °C, 3 h) to the point of providing 34 in 65% yield in this solvent. Although not as extensively examined, analogous initial efforts to promote the o-spirocyclization of the N-Boc derivative 22 were not successful at providing the corresponding product as a stable isolatable intermediate.
The 1H NMR characterization of 34 displayed the expected three well-defined cyclopropane CH signals at δ 2.37 (1H, dd, J = 7.2, 7.8 Hz), 1.99 (1H, ddd, J = 3.6, 6.0, 8.4 Hz), and 0.79 (1H, t, J = 6.0 Hz) and the characteristic diastereotopic signals for C2-H2 exhibiting a large geminal coupling constant (δ 4.00, 1H, d, J = 12.0 Hz and δ 3.47 1H, dd, J = 3.6, 12.0 Hz) analogous to those observed with duocarmycin SA itself. The most diagnostic 1H NMR signals proved to be the disappearance of the indole NH (δ 11.08, 1H, br s in 25) and a marked shift in the C4-H from δ 7.91 (1H, br s) in the precursor 25 to δ 4.62 (1H, s) in 34. This latter remarkable C4-H shift is indicative of an aromatic CH and its conversion to a signal behaving as the β-CH of an isolated electron-rich olefin (enamide). Similarly, the UV spectrum (MeCN) of 34 (λmax = 304 nm (ε 21,800)) proved easily distinguishable from that of 1 (λmax = 355 nm (ε 27,800), 313 nm (ε 17,100)) or 5 (λmax = 342 nm (ε 17,800), 267 nm (ε 15,600)) exhibiting its longest wavelength absorption at a significantly shorter wavelength. Finally and interestingly, optically active 34 exhibited a large rotation value analogous to those characteristic of this family including 1 and 5, but it is of an opposite sign ((−)-34 is the natural enantiomer) and the series does not exhibit the characteristic sign inversion going from 25 to 34.
Solvolyis Reactivity and Regioselectivity
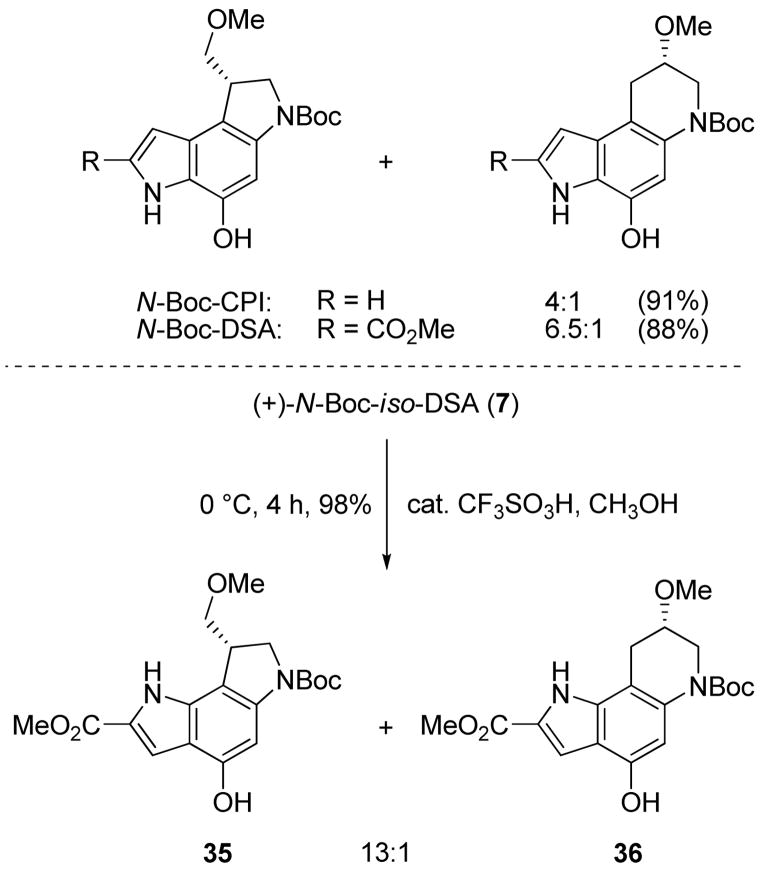
Important diagnostic features of the alkylation subunits of this class of compounds are their relative solvolytic reactivity and the site of cyclopropane cleavage. Past studies have revealed that these agents participate in a characteristic acid-catalyzed, stereoelectronically-controlled ring-opening reaction with predominant nucleophilic addition to the least substituted cyclopropane carbon. However, both the reactivity of the alkylation subunit as well as the stereoelectronic alignment of the cyclopropane have been shown to influence the intrinsic regioselectivity of such additions.10,11 Exemplifying such trends, the addition regioselectivity is much greater with N-Boc-CBI (>20:1)28 than N-Boc-CPI (4:1) because of the cyclopropane stereoelectronic alignment, whereas that of N-Boc-DSA (6.5:1)29 exceeds that of N-Boc-CPI (4:1) because of its reduced reactivity. Acid-catalyzed addition of methanol to N-Boc-iso-DSA (7) under identical conditions (cat. CF3SO3H, CH3OH, 0 °C, 4 h, 98%) provided a 93:7 (13:1) mixture of two products (Scheme 5).
Scheme 5.
The major product is derived from attack at the least-substituted cyclopropane carbon analogous to the regioselectivity observed with the natural alkylation subunits, and the minor product was identified as the ring-expansion product generated by addition to the more-substituted carbon. Chiral HPLC analysis (ChiralCel AD column, 15% i-PrOH/hexane) of 36 derived from enantiomerically pure 7 alongside both enantiomers of the ring-expansion product derived from racemic 7 indicate a clean SN2 ring-opening of 7 with no loss of stereochemical integrity at the reacting center (Supporting Information Figure S1). Thus, N-Boc-iso-DSA (7) exhibits an enhanced intrinsic reaction regioselectivity (13:1 vs 6.5:1) relative to N-Boc-DSA (8) despite the fact that it is 2-fold more reactive (see below). Although it is possible that this results from an altered and enhanced stereoelectronic alignment of the reacting cyclopropane, it is also conceivable that the in-plane placement of the proximal electronegative indole NH of 7, which is absent with 8, disfavors nucleophilic attack at the closer, more substituted cyclopropane carbon.
The relative stability of each alkylation subunit as measured by acid-catalyzed solvolysis has been shown to correlate directly with the biological potency (cytotoxic activity) of the compounds and to be significantly impacted by subtle structural features.16 The solvolysis of N-Boc-iso-DSA at pH 3.0 (50% MeOH–buffer, buffer = 4:1:20 v/v/v 0.1 M citric acid, 0.2 M Na2HPO4, H2O) was monitored by UV (Supporting Information Figure S2) with the disappearance of the long wavelength absorption at 295 nm of the iso-DSA chromophore and with the appearance of a shorter wavelength absorption at 265 nm attributable to the solvolysis product. The solvolysis half-life of N-Boc-iso-DSA (k = 2.2 × 10−6 s−1, t1/2 = 89 h) was found to be two-fold shorter than that of N-Boc-DSA (t1/2 = 177 h).30 This would indicate that there is little to no contribution made by the second vinylogous amide embedded in the pyrrole structure towards the stability of the alkylation subunit. In fact, the opposite appears to be true. Interestingly and indirectly, this slightly enhanced reactivity of N-Boc-iso-DSA relative to N-Boc-DSA itself suggests that the putative internal H-bond of N-Boc-DSA (see Figure 3), which is absent in this isomer, is not contributing to activation or catalysis of a nucleophilic addition as suggested by Skibo.18
Figure 3.
Alternate reactive forms of iso-duocarmycin SA.
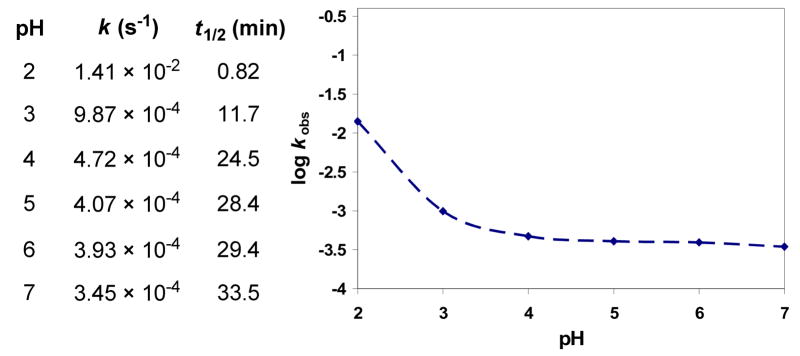
In contrast, but consistent with experimental observations made during its preparation, the o-spirocyclized isomer 34 (O-benzyl-iso-duocarmycin SA) proved to be 470-fold more reactive than 7, exhibiting a short but measurable solvolysis half-life at pH 3 (k = 1.00 × 10−3 s−1, t1/2 = 0.19 h). Additionally and unlike N-Boc-iso-DSA for which no measurable solvolysis is observed at pH 7, 34 also exhibited rapid solvolysis at pH 7 (50% MeOH–H2O, k = 9.77 × 10−4 s−1, t1/2 = 0.20 h). Since 34 exhibited little difference in intrinsic reactivity at pH 3 versus pH 7 albeit under conditions that enlist different solvent and buffer conditions, we established its full pH rate profile conducted under more comparable conditions using a universal buffer (boric acid/citric acid/Na3PO4).31 As the initial studies suggested, the rate of solvolysis of 34 proved independent of pH within the range of pH 3/4–7 indicating that it constitutes an uncatalyzed (vs acid-catalyzed) reaction above pH 3–4 (Figure 4). Only in the range of pH 2–3 did 34 exhibit a characteristic linear dependence on pH indicating its acid-catalyzed solvolysis below ca. pH 3.
Figure 4.
Rates of solvolysis (k), half-life (t1/2), and pH rate profile for solvolysis of 34 at pH 2–7. Importantly, the intrinsic reactivity of 34 indicates that while o-spirocyclization of iso-DSA represents an alternative mode of activation,26,27 it is less productive than the more conventional p-spirocyclization.
Cytotoxic Activity
The results of the in vitro cytotoxic evaluation of N-Boc-iso-DSA, iso-duocarmycin SA and iso-yatakemycin are summarized in Table 1 along with the results of the comparison duocarmycin SA derivatives. Both the natural and unnatural enantiomers of iso-duocarmycin SA were found to be 5-fold less potent than their duocarmycin SA counterparts. This closely follows the trend observed in the comparison of their relative solvolytic reactivities (2-fold less stable). As expected, the natural and unnatural enantiomers of iso-duocarmycin SA exhibit a 10-fold difference in potency, identical to the difference seen with duocarmycin SA itself.
Table 1.
In Vitro Cytotoxic Activity, L1210
| Compound | IC50 (pM) |
|---|---|
| (+)-8, (+)-N-Boc-DSA | 6000 |
| (−)-8, ent-(−)-N-Boc-DSA | 60000 |
| (+)-7, (+)-N-Boc-iso-DSA | 33000 |
| (−)-7, ent-(−)-N-Boc-iso-DSA | 330000 |
| (+)-1, (+)-duocarmycin SA | 10 |
| (−)-1, ent-(−)-duocarmycin SA | 100 |
| (+)-5, (+)-iso-duocarmycin SA | 50 |
| (−)-5, ent-(−)-iso-duocarmycin SA | 550 |
| (+)-3, (+)-yatakemycin | 6 |
| (−)-3, ent-(−)-yatakemycin | 6 |
| (+)-6, (+)-iso-yatakemycin | 15 |
| (−)-6, ent-(−)-iso-yatakemycin | 30 |
| (−)-34 (natural enantiomer) | 6000 |
| (+)-34 (unnatural enantiomer) | 7000 |
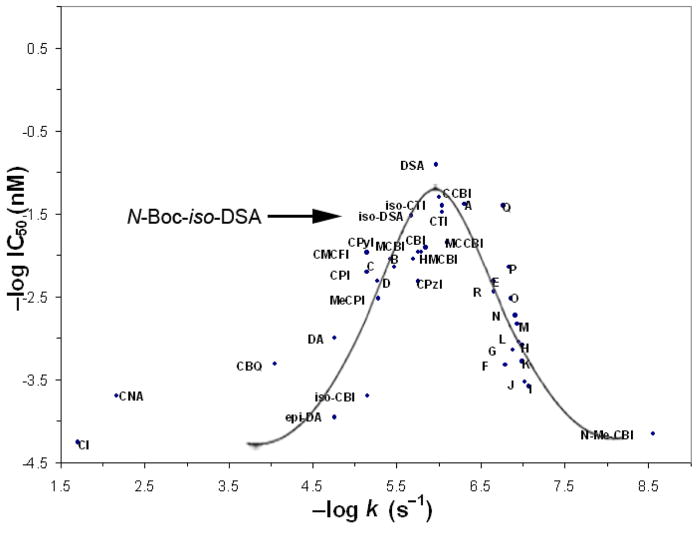
The simple alkylation subunit N-Boc-iso-DSA (7) also exhibited an analogous decreased cytotoxic potency compared to N-Boc-DSA. Thus, a 5-fold difference in potency between the two series was observed, and the natural enantiomer was 10-fold more potent than the unnatural enantiomer similar to the trend observed with iso-duocarmycin SA and N-Boc-DSA. Moreover, when N-Boc-iso-DSA is compared to the full set of preceding alkylation subunits placing it on the parabolic plot, its relative cytotoxic activity closely approaches that expected of a derivative exhibiting its relative reactivity (Figure 5).
Figure 5.
Parabolic relationship between rate of solvolysis and cytotoxic activity of the alkylation subunits.
Satisfyingly, the natural enantiomer of iso-yatakemycin (6) also exhibited a cytotoxic potency (IC50 = 15 pM) in line with its 2-fold greater reactivity than yatakemycin itself (IC50 = 6 pM). Unlike the natural product and a series of yatakemycin analogues containing the DSA alkylation subunit where the two enantiomers display indistinguishable cytotoxic activity,23,24 the unnatural enantiomer of iso-yatakemycin proved ca. two-fold less active than the natural enantiomer (30 vs 15 pM) and ca. 5-fold less active than either enantiomer of yatakemycin. It is tempting to suggest that this slightly less effective relative behavior of the unnatural enantiomer with 6 may result from the minor groove presentation of a H-bond donor for which the unnatural enantiomer is uniquely sensitive.17 However, its activity still closely follows expectations (2-fold more reactive and 5-fold less potent) and the differences are so small, that the effect, if operative, must be minor.
Finally, the natural enantiomer of O-benzyl-iso-duocarmycin SA (34), which represents the reactive o-spirocyclization isomer, proved to be 120-fold less active than iso-duocarmycin SA (L1210 IC50 = 6 nM vs 50 pM) and 600-fold less active than duocarmycin SA itself, consistent with its relative reactivity (470-fold more reactive than N-Boc-iso-DSA). Interestingly although not probed extensively, the unnatural enantiomer of 34 (IC50 = 7 nM) was found to approach the activity of the natural enantiomer. It is tempting to suggest that since the activation features responsible for DNA alkylation catalysis that lead to the typical characteristic distinctions are not operative for 34, it is not surprising that the two enantiomers of 34 display comparable biological properties.
DNA Alkylation Selectivity and Efficiency
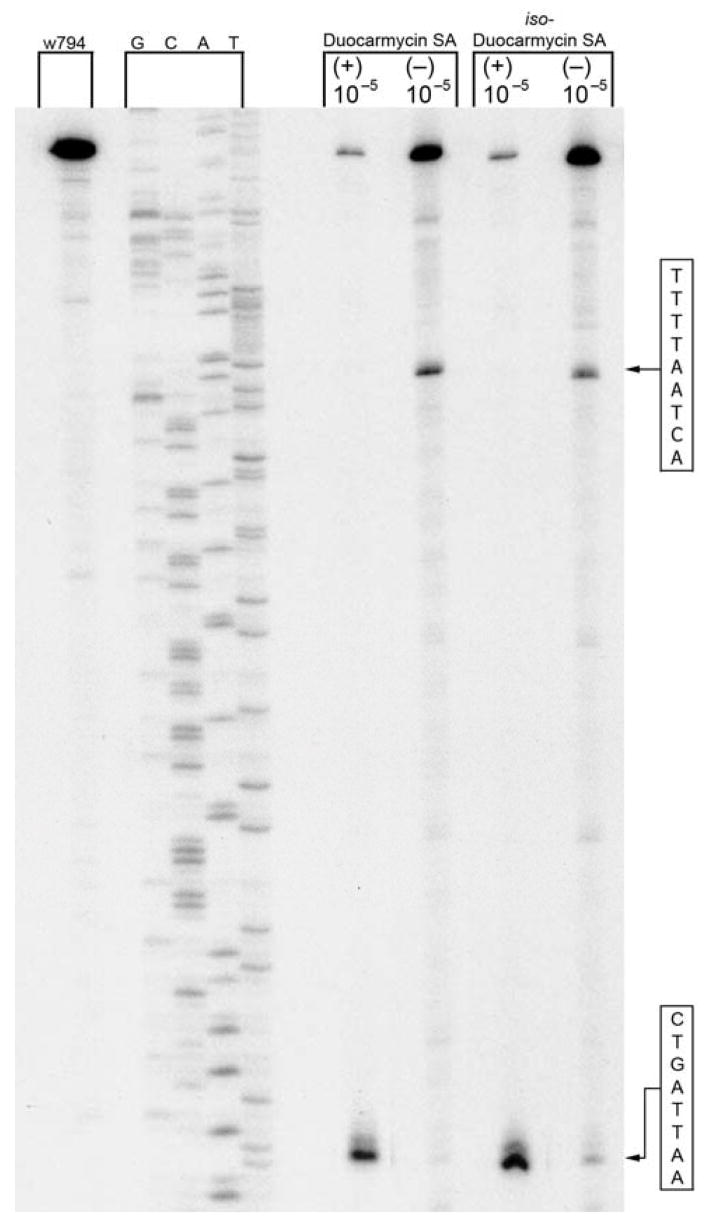
The DNA alkylation properties of the agents were examined in w794 and w836 duplex DNA and were compared to their respective natural product counterparts enlisting protocols described in detail in earlier studies.15 Figure 6 illustrates the alkylation selectivity of both natural and unnatural iso-duocarmycin SA alongside (+)- and ent-(−)-duocarmycin SA. Satisfyingly, each enantiomer of iso-duocarmycin SA alkylated the same site as its duocarmycin SA counterpart, displaying the same characteristic and enantiomerically distinguishable selectivity. Like duocarmycin SA, the unnatural enantiomer of iso-duocarmycin SA proved less efficient and slower at alkylating DNA than its natural enantiomer. Although not represented in Figure 6, the only notable distinction observed was that (+)- and ent-(−)-iso-duocarmycin SA exhibit a decreased alkylation efficiency compared to (+)- and ent-(−)-duocarmycin SA after 24 h. After 48 h (Figure 6), the differences in efficiency either disappear or are much less noticeable. Thus, the final efficiencies of DNA alkylation are not readily distinguishable if the reactions are allowed to progress to completion, but the rates of DNA alkylation are different with that of duocarmycin SA being perceptibly faster.
Figure 6.
Thermally-induced strand cleavage of w794 DNA (144 bp, nucleotide no. 5238–138) after DNA–agent incubation with duocarmycin SA and iso-duocarmycin SA (48 h, 23 °C), removal of unbound agent by EtOH precipitation and 30 min thermolysis (100 °C), followed by denaturing 8% PAGE and autoradiography. Lane 1, control DNA; lanes 2–5, Sanger G, C, A, and T sequencing standards; lanes 6 and 7, (+)-duocarmycin SA and ent-(−)-duocarmycin SA (1, 1 × 10−5 M); lanes 8 and 9, (+)-iso-duocarmycin SA and ent-(−)-iso-duocarmycin SA (5, 1 × 10−5 M).
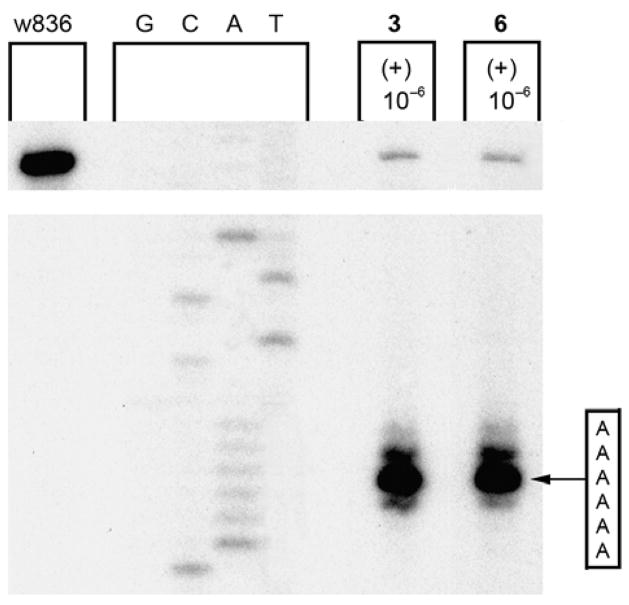
A similar trend was observed with iso-yatakemycin. After 24 h, (+)- and ent-(−)-iso-yatakemycin show approximately 2–5 fold less alkylation than (+)- and ent-(−)-yatakemycin. After 48 h (Figure 7), (+)-yatakemycin and (+)-iso-yatakemycin exhibit similar levels of alkylation. The largest distinction observed between iso-duocarmycin SA and iso-yatakemycin was the altered and identical alkylation selectivity of the two enantiomers of 6 and their comparable efficiencies of DNA alkylation. This mirrors the now characteristic observations made with yatakemycin itself and related sandwiched analogues and has been discussed in detail elsewhere.23,24
Figure 7.
Thermally-induced strand cleavage of w836 DNA (146 bp, nucleotide no. 5189–91) after DNA–agent incubation with yatakemycin and iso-yatakemycin (48 h, 23 °C), removal of unbound agent by EtOH precipitation and 30 min thermolysis (100 °C), followed by denaturing 8% PAGE and autoradiography. Lane 1, control DNA; lanes 2–5, Sanger G, C, A, and T sequencing standards; lane 6, (+)-yatakemycin (3, 1 × 10−6 M); lane 7, (+)-iso-yatakemycin (6, 1 × 10−6 M).
The key issues addressed with these studies were that the isomeric alkylation subunit did not alter the intrinsic DNA alkylation selectivity of either duocarmycin SA or yatakemycin, but the modification did slow the rate and potentially decrease the efficiency of DNA alkylation despite its enhanced intrinsic reactivity.
The DNA alkylation behavior of the simple alkylation subunit N-Boc-iso-DSA versus N-Boc-DSA was also examined (Supporting Information Figure S3). Most significant in the comparisons is the indistinguishable behavior of N-Boc-iso-DSA and N-Boc-DSA itself. Both enantiomers of both agents alkylate the same sites displaying the same DNA alkylation selectivity (5′-AA and 5′-TA). In addition to this unusual identical enantiomeric selectivity that has been interpreted in detail for N-Boc-DSA itself5 and that is much less selective than duocarmycin SA, both N-Boc-iso-DSA and N-Boc-DSA alkylate DNA much less efficiently (103–104 times) and kinetically much slower than the full length agents.
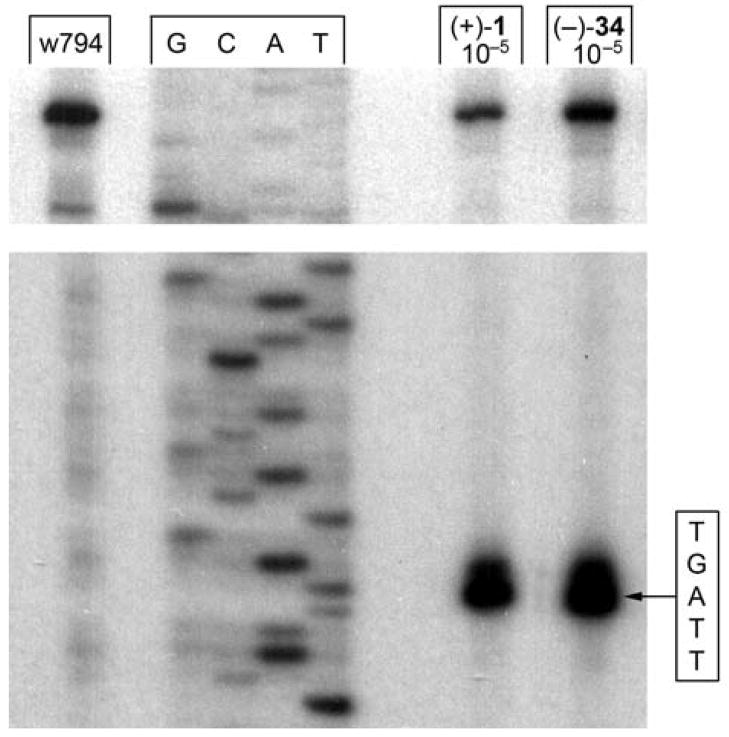
Additionally, we examined the DNA alkylation properties of 34 that contains the unusual o- versus p-spirocyclization and the much greater reactivity. Remarkably, both enantiomers of 34 alkylated DNA with selectivities analogous to each of the enantiomers of iso-duocarmycin SA (5) and duocarmycin SA (1). This is illustrated nicely in Figure 8 where the natural enantiomer of 34 alkylates the single high affinity site in w794 like the natural enantiomer of duocarmycin SA (1). Moreover, it did so with an efficiency nearly equivalent to that of 1 despite its greater reactivity and instability. Although the results of these comparisons reaffirm many features contributing to the DNA alkylation selectivity and source of catalysis for 1, the most prominent of these are that 1) the source of catalysis is not what determines the selectivity of DNA alkylation,9 2) DNA backbone phosphate protonation or Lewis acid coordination of the C4-carbonyl is not contributing to or controlling the DNA alkylation selectivity,14 and 3) the alkylation selectivity of 34 is consistent with studies that indicate it is derived from the intrinsic noncovalent binding selectivity of 1 and 34.12,15,24
Figure 8.
Thermally-induced strand cleavage of w794 DNA (144 bp, nucleotide no. 5238–138) after DNA–agent incubation with duocarmycin SA and 34 (22 h, 23 °C), removal of unbound agent by EtOH precipitation and 30 min thermolysis (100 °C), followed by denaturing 8% PAGE and autoradiography. Lane 1, control DNA; lanes 2–5, Sanger G, C, A, and T sequencing standards; lane 6, (+)-duocarmycin SA (1, 1 × 10−5 M); lane 7, (−)-34 (natural enantiomer, 1 × 10−5 M).
Conclusions
The synthesis, characterization, and evaluation of a key pyrrole isomer of the duocarmycin SA alkylation subunit were conducted and its incorporation into two key analogues of the natural products is described: iso-duocarmycin SA (5) and iso-yatakemycin (6). This isomeric variant of the naturally occurring DNA alkylation subunit displayed an enhanced intrinsic reaction regioselectivity and a 2-fold diminished stability. Its derivatives and natural product analogues exhibited a potent cytotoxic activity exhibiting only 3- to 5-fold reduction in activity relative to the corresponding natural products consistent with its placement on a parabolic relationship correlating activity and reactivity. The DNA alkylation selectivity of the resulting derivatives and the natural product analogues was unaltered by the structural modification despite the presentation of a potential H-bond donor to the minor groove floor when bound or alkylated with DNA. Additionally, a unique o-spirocyclization for activation of DNA alkylation was explored with 34 that proved sufficiently stable for customary characterization, yet 470-fold more reactive than N-Boc-iso-DSA (7, t1/2 = 89 h at pH 3; stable at pH 7). It displayed this enhanced reactivity not only at pH 3 (t1/2 = 0.19 h), but it also proved nearly equally reactive at pH 7 where both N-Boc-iso-DSA (7) and N-Boc-DSA (8) are stable and exhibit no reactivity. As such, it exhibited a much reduced cytotoxic potency but, remarkably, it was found to alkylate DNA with a selectivity and efficiency analogous to those of duocarmycin SA (1) and iso-duocarmycin SA. In addition to providing unique insights into the sources of the DNA alkylation selectivity and catalysis for this class of natural products, these studies indicate that this o-spirocyclization27,32 constitutes an additional activation pathway,26 although it is energetically less favorable and less productive than the more conventional p-spirocyclization.
Supplementary Material
Full experimental details are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Scheme 4.
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA41986) and the Skaggs Institute for Chemical Biology. We thank Dr. Mark S. Tichenor for supplying 27 and 31 enlisted to prepare iso-yatakemycin. KSM is a Skaggs Fellow.
References
- 1.Ichimura M, Ogawa T, Takahashi K, Kobayashi E, Kawamoto I, Yasuzawa T, Takahashi I, Nakano H. J Antibiot. 1990;43:1037–1038. doi: 10.7164/antibiotics.43.1037. [DOI] [PubMed] [Google Scholar]
- 2.Igarashi Y, Futamata K, Fujita T, Sekine A, Senda H, Naoki H, Furumai T. J Antibiot. 2003;56:107–113. doi: 10.7164/antibiotics.56.107.Structure revision: Tichenor MS, Kastrinsky DB, Boger DL. J Am Chem Soc. 2004;126:8396–8398. doi: 10.1021/ja0472735.
- 3.Martin DG, Biles C, Gerpheide SA, Hanka LJ, Krueger WC, McGovren JP, Mizsak SA, Neil GL, Stewart JC, Visser J. J Antibiot. 1981;34:1119–1125. doi: 10.7164/antibiotics.34.1119. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi I, Takahashi K, Ichimura M, Morimoto M, Asano K, Kawamoto I, Tomita F, Nakano H. J Antibiot. 1988;41:1915–1917. doi: 10.7164/antibiotics.41.1915. [DOI] [PubMed] [Google Scholar]
- 5.Duocarmycin SA, Boger DL, Johnson DS, Yun W. J Am Chem Soc. 1994;116:1635–1656. [Google Scholar]
- 6.Yatakemycin: Parrish JP, Kastrinsky DB, Wolkenberg SE, Igarishi Y, Boger DL. J Am Chem Soc. 2003;125:10971–10976. doi: 10.1021/ja035984h.Trzupek JD, Gottesfeld JM, Boger DL. Nature Chem Biol. 2006;2:79–82. doi: 10.1038/nchembio761.
- 7.CC-1065: Hurley LH, Lee C-S, McGovren JP, Warpehoski MA, Mitchell MA, Kelly RC, Aristoff PA. Biochemistry. 1988;27:3886–3892. doi: 10.1021/bi00410a054.Hurley LH, Warpehoski MA, Lee CS, McGovren JP, Scahill TA, Kelly RC, Mitchell MA, Wicnienski NA, Gebhard I, Johnson PD, Bradford VS. J Am Chem Soc. 1990;112:4633–4649.Boger DL, Johnson DS, Yun W, Tarby CM. Bioorg Med Chem. 1994;2:115–135. doi: 10.1016/s0968-0896(00)82007-6.Boger DL, Coleman RS, Invergo BJ, Sakya SM, Ishizaki T, Munk SA, Zarrinmayeh H, Kitos PA, Thompson SC. J Am Chem Soc. 1990;112:4623–4632.
- 8.Duocarmycin A: Boger DL, Ishizaki T, Zarrinmayeh H, Munk SA, Kitos PA, Suntornwat O. J Am Chem Soc. 1990;112:8961–8971.Boger DL, Ishizaki T, Zarrinmayeh H. J Am Chem Soc. 1991;113:6645–6649.Boger DL, Yun W, Terashima S, Fukuda Y, Nakatani K, Kitos PA, Jin Q. Bioorg Med Chem Lett. 1992;2:759–765.Boger DL, Yun W. J Am Chem Soc. 1993;115:9872–9873.
- 9.Reviews: Boger DL, Johnson DS. Angew Chem Int Ed Engl. 1996;35:1438–1474.Boger DL. Acc Chem Res. 1995;28:20–29.Boger DL, Johnson DS. Proc Natl Acad Sci U S A. 1995;92:3642–3649. doi: 10.1073/pnas.92.9.3642.Boger DL, Garbaccio RM. Acc Chem Res. 1999;32:1043–1052.Tichenor MS, Boger DL. Natural Prod Rep. 2008;25:220–226. doi: 10.1039/b705665f.
- 10.(a) Boger DL, Mesini P. J Am Chem Soc. 1995;117:11647–11655. [Google Scholar]; (b) Boger DL, Mesini P. J Am Chem Soc. 1994;116:11335–11348. [Google Scholar]; (c) Boger DL, Mesini P, Tarby CM. J Am Chem Soc. 1994;116:6461–6462. [Google Scholar]
- 11.(a) Boger DL, Turnbull P. J Org Chem. 1998;63:8004–8011. [Google Scholar]; (b) Boger DL, Turnbull P. J Org Chem. 1997;62:5849–5863. [Google Scholar]
- 12.(a) Boger DL, Munk SA, Zarrinmayeh H. J Am Chem Soc. 1991;113:3980–3983. [Google Scholar]; (b) Boger DL, Zarrinmayeh H, Munk SA, Kitos PA, Suntornwat O. Proc Natl Acad Sci U S A. 1991;88:1431–1435. doi: 10.1073/pnas.88.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Boger DL, Coleman RS, Invergo BJ, Zarrinmayeh H, Kitos PA, Thompson SC, Leong T, McLaughlin LW. Chem Biol Interact. 1990;73:29–52. doi: 10.1016/0009-2797(90)90107-x. [DOI] [PubMed] [Google Scholar]; (d) Boger DL, Wysocki RJ, Ishizaki T. J Am Chem Soc. 1990;112:5230–5240. [Google Scholar]; (e) Boger DL, Coleman RS, Invergo BJ. J Org Chem. 1987;52:1521–1530. [Google Scholar]; (f) Boger DL, Sakya SM. J Org Chem. 1992;57:1277–1284. [Google Scholar]
- 13.Boger DL, Garbaccio RM. Bioorg Med Chem. 1997;5:263–276. doi: 10.1016/s0968-0896(96)00238-6. [DOI] [PubMed] [Google Scholar]
- 14.(a) Ambroise Y, Boger DL. Bioorg Med Chem Lett. 2002;12:303–306. doi: 10.1016/s0960-894x(01)00740-5. [DOI] [PubMed] [Google Scholar]; (b) Boger DL, Garbaccio RM. J Org Chem. 1999;64:5666–5669. doi: 10.1021/jo990762g. [DOI] [PubMed] [Google Scholar]; (c) Ellis DA, Wolkenberg SE, Boger DL. J Am Chem Soc. 2001;123:9299–9306. doi: 10.1021/ja010769r. [DOI] [PubMed] [Google Scholar]
- 15.Boger DL, Munk SA, Zarrinmayeh H, Ishizaki T, Haught J, Bina M. Tetrahedron. 1991;47:2661–2682. [PubMed] [Google Scholar]
- 16.(a) Parrish JP, Hughes TV, Hwang I, Boger DL. J Am Chem Soc. 2004;126:80–81. doi: 10.1021/ja038162t. [DOI] [PubMed] [Google Scholar]; (b) Boger DL, Yun W. J Am Chem Soc. 1994;116:5523–5524. [Google Scholar]; (c) Boger DL, Munk SA, Ishizaki T. J Am Chem Soc. 1991;113:2779–2780. [Google Scholar]; (d) Boger DL, Ishizaki T, Sakya SM, Munk SA, Kitos PA, Jin Q, Besterman JM. Bioorg Med Chem Lett. 1991;1:115–120. [Google Scholar]
- 17.Boger DL, Yun W. J Am Chem Soc. 1994;116:7996–8006. [Google Scholar]
- 18.LaBarbera DV, Skibo EB. J Am Chem Soc. 2006;128:3722–3727. doi: 10.1021/ja057289a. [DOI] [PubMed] [Google Scholar]
- 19.Review: Boger DL, Boyce CW, Garbaccio RM, Goldberg J. Chem Rev. 1997;97:787–828. doi: 10.1021/cr960095g.
- 20.Jacobsen MF, Moses JE, Adlington RM, Baldwin JE. Org Lett. 2005;7:641–644. doi: 10.1021/ol047594l. [DOI] [PubMed] [Google Scholar]
- 21.(a) Boger DL, Boyce CW, Garbaccio RM, Searcey M. Tetrahedron Lett. 1998;39:2227–2230. [Google Scholar]; (b) Patel VF, Andis SL, Enkema JK, Johnson DA, Kennedy JH, Mohamadi F, Schultz RM, Soose DJ, Spees MM. J Org Chem. 1997;62:8868–8874. [Google Scholar]
- 22.Boger DL, Ishizaki T, Zarrinmayeh H, Kitos PA, Suntornwat O. J Org Chem. 1990;55:4499–4502. [Google Scholar]
- 23.(a) Tichenor MS, Trzupek JD, Kastrinsky DB, Shiga F, Hwang I, Boger DL. J Am Chem Soc. 2006;128:15683–15696. doi: 10.1021/ja064228j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tichenor MS, MacMillan KS, Trzupek JD, Rayl TJ, Hwang I, Boger DL. J Am Chem Soc. 2007;129:10858–10869. doi: 10.1021/ja072777z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Boger DL, Bollinger B, Hertzog DL, Johnson DS, Cai H, Mesini P, Garbaccio RM, Jin Q, Kitos PA. J Am Chem Soc. 1997;119:4987–4998. [Google Scholar]; (b) Boger DL, Hertzog DL, Bollinger B, Johnson DS, Cai H, Goldberg J, Turnbull P. J Am Chem Soc. 1997;114:4977–4986. [Google Scholar]
- 25.(a) Boger DL, Coleman RS. J Am Chem Soc. 1988;110:4796–4807. [Google Scholar]; (b) Boger DL, Coleman RS. J Am Chem Soc. 1988;110:1321–1323. [Google Scholar]; (c) Boger DL, Coleman RS. J Am Chem Soc. 1987;109:2717–2727. [Google Scholar]
- 26.(a) Tse W, Boger DL. Chem Biol. 2004;11:1607–1617. doi: 10.1016/j.chembiol.2003.08.012. [DOI] [PubMed] [Google Scholar]; (b) Wolkenberg SE, Boger DL. Chem Rev. 2002;102:2477–2496. doi: 10.1021/cr010046q. [DOI] [PubMed] [Google Scholar]
- 27.(a) MacMillan KS, Boger DL. J Am Chem Soc. 2008;130:0000–0000. doi: 10.1021/ja806593w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Asai A, Nagamura S, Saito H. J Am Chem Soc. 1994;116:4171–4177. [Google Scholar]; (c) Nagamura S, Kobayashi E, Gomi K, Saito H. Bioorg Med Chem. 1996;4:1379–1391. doi: 10.1016/0968-0896(96)00132-0. [DOI] [PubMed] [Google Scholar]
- 28.(a) Boger DL, Ishizaki T, Wysocki RJ, Jr, Munk SA, Kitos PA, Suntornwat O. J Am Chem Soc. 1989;111:6461–6463. [Google Scholar]; (b) Boger DL, Ishizaki T, Kitos PA, Suntornwat O. J Org Chem. 1990;55:5823–5832. [Google Scholar]; (c) Boger DL, Ishizaki T. Tetrahedron Lett. 1990;31:793–796. [Google Scholar]; (d) Boger DL, Munk SA. J Am Chem Soc. 1992;114:5487–5496. [Google Scholar]
- 29.Boger DL, Goldberg J, McKie JA. Bioorg Med Chem Lett. 1996;6:1955–1960. [Google Scholar]
- 30.(a) Boger DL, Machiya K, Hertzog DL, Kitos PA, Holmes D. J Am Chem Soc. 1993;115:9025–9036. [Google Scholar]; (b) Boger DL, Machiya K. J Am Chem Soc. 1992;114:10056–10058. [Google Scholar]
- 31.(a) Perrin DD, Dempsey B. Buffers for pH and Metal Ion Control. Chapman and Hall; London: 1979. p. 156. [Google Scholar]; (b) Boger DL, Garbaccio RM. J Org Chem. 1999;64:5666–5669. doi: 10.1021/jo990762g. [DOI] [PubMed] [Google Scholar]
- 32.(a) Boger DL, Garbaccio RM, Jin Q. J Org Chem. 1997;62:8875–8891. [Google Scholar]; (b) Boger DL, Garbaccio RM. J Org Chem. 1999;64:8350–8362. doi: 10.1021/jo991301y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental details are provided. This material is available free of charge via the Internet at http://pubs.acs.org.