Abstract
Many phytochemicals in fruits and vegetables have been shown to have cancer-inhibitory effects in animal studies. These effects on cancer, however, have not been clearly demonstrated in human studies. This study investigated the association between fruit and vegetable intakes and the risk of adenomatous polyps. Participants were part of the Tennessee Colorectal Polyp Study. Eligible participants aged 40–75 y were recruited from patients undergoing colonoscopy at 2 medical centers in Nashville, Tennessee from 2003 to 2005. Cases had at least one adenoma and controls were polyp free. Dietary intake was assessed using a self-administered FFQ. Associations between dietary intakes and adenoma risk were evaluated using unconditional logistic regression with restricted cubic function spline. In multivariate analyses of 764 cases and 1517 controls, increased intakes of total fruits, berries, fruit juice, and green leafy vegetables were associated with reduced adenoma risk. The odds ratio for upper tertile intake compared with lower was 0.66 (95% CI = 0.51–0.86) for total fruits, 0.64 (95% CI = 0.47–0.87) for berries, 0.72 (95% CI = 0.56–0.92) for fruit juice, and 0.74 (95% CI = 0.58–0.96) for green vegetables. This study provides additional evidence that high total fruit intake and certain fruit and vegetable intakes may be associated with a reduced risk of colorectal adenomas.
Introduction
Fruits and vegetables contain a wide variety of potential cancer-inhibitory nutrients and other phytochemicals. Substantial evidence has indicated a biological link between dietary constituents and carcinogenesis (1,2). Nonetheless, results from previous epidemiologic studies on the associations of fruit and vegetable intakes with colorectal cancer have been inconsistent and a recent summary report suggests fruits and vegetables may have only limited protective effects on colorectal cancer (3). Many case-control studies reported inverse associations with the intake of certain fruits and vegetables (4–9), whereas most prospective cohort studies showed much weaker or even no associations with these foods (10–14). Colorectal adenomas as established precursors of colorectal cancers have drawn research interest. Intervention studies have been conducted with diets high in fruits and vegetables on adenoma recurrence (15–18), because recurrence allows a shorter trial and reduced sample size (19). However, these randomized intervention trials did not find significant benefits for higher fruit and vegetable intakes in reducing the risk of adenoma recurrence (15,17,18) or rectal mucosal cell proliferation rates (20).
Many factors may contribute to the inconsistency and contradiction (21). One possible biological explanation is that dietary components may affect an early stage of carcinogenesis and, thus, may only affect primary adenomas, not adenoma recurrence (19,22). In support of this possibility, previous epidemiologic studies have found that intakes of total fruits (22–25) and total vegetables (26–28) were associated with a reduced adenoma risk. These studies also suggested that intakes of certain fruits or vegetables, such as citrus fruits (22), legumes (22,28), dry beans (16), and green leafy vegetables (22,27) might be strongly protective against colorectal adenoma. However, no associations have been reported in some other studies (29–31).
In this article, we examine whether intakes of fruits and vegetables were related to a reduced risk of primary colorectal adenomas in a large colonoscopy-based case-control study, with a special interest in the intakes of subgroups of fruits and vegetables.
Materials and Methods
Study population.
Participants were part of the Tennessee Colorectal Polyp Study (TCPS),10 an ongoing colonoscopy-based case-control study being conducted in Nashville, Tennessee. Eligible participants, aged between 40 and 75 y were identified from patients scheduled for colonoscopy at the Vanderbilt Gastroenterology Clinic and the Veteran's Affairs Tennessee Valley Health System Nashville campus between February 1, 2003 and December 31, 2005. Excluded from our study were participants who had genetic colorectal cancer syndromes or a prior history of inflammatory bowel disease, adenomatous polyps, or any cancer other than nonmelanoma skin cancer. Among 4617 eligible participants, 3083 provided written informed consent (67%). Among those, 2678 (87%) completed a telephone interview. Participants were recruited prior to the colonoscopy and most were undergoing colonoscopy for screening purposes (52%). On the basis of the clinical colonoscopy and pathology findings, recruited participants were designated as polyp-free controls, cases with adenomatous polyps, or cases with other polyp types. To be designated as a control, the consenting participant must have had a complete colonoscopy reaching the cecum and be polyp free at colonoscopy. Included in this analysis were 874 adenomatous polyp cases and 1773 polyp-free controls. The study was approved by the Institutional Review Board of Vanderbilt Medical Center for the use of human subjects in research.
Dietary assessment.
Dietary assessment was conducted within 13 d after the colonoscopy. Dietary intake in the 12 mo prior to colonoscopy was ascertained using a validated 108-item semiquantitative FFQ, which was developed at Vanderbilt University specifically to capture diet in the southern United States (32). Briefly, food items were identified from the NHANES-III database using relevant groups. In a comparison of the pilot FFQ data with NHANES-III data, the energy-adjusted intakes were very similar (0.96, 0.92, and 1.01 for total fat, protein, and carbohydrate, respectively). Response categories were “never, rarely, 1/mo, 2–3/mo, 1/wk, 2–3/wk, 4–6/wk, 1/d, or 2+/d.” Participants were also asked about their usual serving size: small, medium, or large. Fruits and vegetables were classified according to their botanical taxonomy and phytochemical content to identify the fruits and vegetables that may be potentially rich sources of vitamins or bioactive components (33) (Supplemental Table 1). Fruit and vegetable intakes were normalized with the usual portion size and expressed in servings/wk. The FFQ was completed by 764 cases and 1525 controls. Participants with 14 or more missing items in the FFQ or with total caloric intake outside the range of 3349–17,585 kJ/d for males and 2512–14,654 kJ/d for females were excluded from the analysis. The final data for the analysis consisted of 764 cases and 1517 controls.
Statistical analysis.
We used chi-square statistics and Wilcoxon's rank sum test to evaluate case-control differences. Both restricted cubic spline regression and categorical regression were used with an unconditional logistic regression model to examine the association between dietary intake and adenoma risk. Energy intake adjustment was performed by entering energy intake into the model (34). Intakes of fruits or vegetables were mutually adjusted for in the model. Other potential confounders, including other dietary factors, were selected for evaluation in accordance with previous studies (22,25–28,31). The final model adjusted for age, sex, race, study location, BMI, smoking status, regular alcohol consumption (5 or more drinks/wk for at least 12 mo in a row), nonsteroidal antiinflammatory drug use, physical exercise, educational attainment, household income, family history of colorectal cancer in a first-degree relative, and red/processed meat intake (including hamburgers, cheeseburgers, beef, pork, and their regular products). The colinearity among the covariates was also analyzed. P-values of <0.05 (2-sided) were considered significant. All calculations and computations were performed using R package version 2.4.0 (35).
Results
Selected demographic characteristics and risk factors of the participants were compared between cases and controls. Compared with controls, adenoma cases were older and more likely to be male, smokers, alcohol drinkers, physically inactive, and of lower educational attainment and lower social economic status. Cases also had higher daily intakes of total energy and red/processed meat and had lower BMI. A similar proportion of cases and controls underwent colonoscopy for screening purposes (Table 1). No strong correlations were found among the covariates and the distributions of all the intakes were markedly skewed (data not shown).
TABLE 1.
Characteristics of study participants in the TCPS, 2003–20051
| Case | Control | P-value | |
|---|---|---|---|
| n (%) | 764 (33) | 1517 (67) | |
| Age, y | 59.6 (54.9, 65.2) | 56.7 (51.7, 63.1) | <0.001 |
| Female, % | 23 | 44 | <0.001 |
| White, % | 86 | 89 | 0.013 |
| Study sites, % | <0.001 | ||
| Veteran's Affairs | 51 | 35 | |
| Academic | 49 | 65 | |
| Reason for colonoscopy, % | 0.120 | ||
| Screening | 52 | 52 | |
| Family history | 10 | 13 | |
| Diagnosis/follow-up/bleeding/other | 38 | 35 | |
| Education, % | <0.001 | ||
| High school or less | 66 | 55 | |
| College or higher | 34 | 45 | |
| Household Income, % | <0.001 | ||
| <30,000 | 38 | 25 | |
| 30,0000 – <50,000 | 22 | 22 | |
| ≥50,000 | 40 | 53 | |
| NSAID2 use, % | 0.957 | ||
| Current | 52 | 54 | |
| Former | 7 | 7 | |
| Never | 41 | 39 | |
| Smoking status, % | <0.001 | ||
| Current | 28 | 12 | |
| Former | 39 | 37 | |
| Never | 33 | 51 | |
| Regular alcohol consumption, % | <0.001 | ||
| Current | 21 | 17 | |
| Former | 28 | 23 | |
| Never | 51 | 60 | |
| Regular physical activity past 10 y, % | 50 | 58 | <0.001 |
| Family history of colorectal cancer, % | 14 | 13 | 0.887 |
| BMI, kg/m2 | 27.9 (24.7, 31.3) | 27.3 (23.8, 30.9) | <0.001 |
| Median dietary intake | |||
| Red/processed meat, servings/wk | 1.74 (1.03, 2.81) | 1.50 (0.80, 2.39) | <0.001 |
| Total energy, kJ/d | 6745 (5229, 8441) | 6506 (5112, 8185) | 0.064 |
| Total fruit, servings/wk | 6.7 (2.6, 12.5) | 8.7 (4.0, 14.2) | <0.001 |
| Total vegetable, servings/wk | 23.0 (15.0, 33.2) | 24.1 (16.4, 34.9) | 0.029 |
Values are medians (interquartile range) or percent.
NSAID, nonsteroidal antiinflammatory drug.
The overall estimates of the 2 methods were similar; thus, we only report the results from the spline regression (Table 2; Supplemental Fig. 1). Colorectal adenoma risk was inversely related to increased intakes of total fruits, berries, fruit juice, and green leafy vegetables. An additional analysis of fruit juice found that total fruits, fruits excluding fruit juice, and fruit juice alone were all significantly inversely associated with adenoma risk (data not shown).
TABLE 2.
Association of fruit and vegetable intake with the risk of colorectal adenomas in the TCPS, 2003–20051–4
| Intake tertile
|
|||
|---|---|---|---|
| T1 (Lower) | T2 OR (95% CI) | T3 (Upper) OR (95% CI) | |
| Total fruit | 1.0 (ref) | 0.75 (0.62–0.91) | 0.66 (0.51–0.86) |
| Total vegetable | 1.0 (ref) | 0.99 (0.82–1.19) | 0.94 (0.72–1.22) |
| Fruit/vegetable | 1.0 (ref) | 0.98 (0.97–1.00) | 0.75 (0.58–0.97) |
| Juice | 1.0 (ref) | 0.87 (0.78–0.98) | 0.72 (0.56–0.92) |
| Citrus fruit | 1.0 (ref) | 0.90 (0.76–1.07) | 0.83 (0.63–1.10) |
| Berries | 1.0 (ref) | 0.92 (0.87–0.97) | 0.64 (0.47–0.87) |
| Tree fruit | 1.0 (ref) | 0.92 (0.80–1.05) | 0.83 (0.64–1.08) |
| Melon | 1.0 (ref) | 1.04 (0.95–1.14) | 1.10 (0.86–1.39) |
| Other fruits | 1.0 (ref) | 1.00 (0.91–1.11) | 0.95 (0.70–1.28) |
| Cruciferous vegetables | 1.0 (ref) | 0.96 (0.85–1.09) | 0.94 (0.73–1.20) |
| Legumes | 1.0 (ref) | 0.97 (0.83–1.14) | 0.95 (0.74–1.24) |
| Alliums | 1.0 (ref) | 0.99 (0.88–1.13) | 0.98 (0.76–1.27) |
| Green leafy vegetables | 1.0 (ref) | 0.92 (0.77–1.09) | 0.74 (0.58–0.96) |
| Other vegetables | 1.0 (ref) | 1.00 (0.82–1.21) | 0.94 (0.74–1.19) |
| β-Carotene rich | 1.0 (ref) | 0.92 (0.80–1.07) | 0.83 (0.64–1.07) |
| Lutein rich | 1.0 (ref) | 0.90 (0.76–1.07) | 0.84 (0.66–1.08) |
| Lycopene rich | 1.0 (ref) | 0.84 (0.69–1.02) | 0.83 (0.66–1.06) |
| Vitamin C rich | 1.0 (ref) | 0.89 (0.77–1.04) | 0.83 (0.64–1.09) |
Adjusted for age, sex, race, study location, BMI, smoking status, alcohol consumption, NSAID use, physical exercise, education level, family income, family history of colorectal cancer (CRC) in a first-degree relative, and red/processed meat intake in addition to total energy intake.
Fruits and vegetables were mutually adjusted for in the models.
The OR were estimated by using the fitted spline regression model with the lowest tertile as the reference group.
The estimates were computed with n = 764 cases and n = 1517 controls.
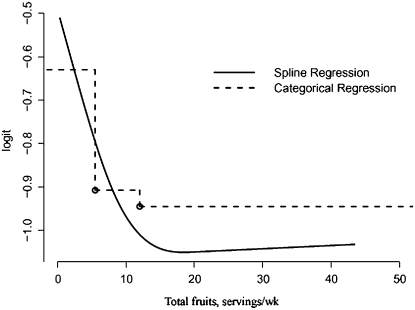
Adenoma risk decreases as total fruit intake increases from low to moderate, but the risk does not decrease further with an additional increase of total fruit intake (Fig. 1). The spline regression falls apart at the upper tertile of categorical model, indicating an underestimated reduction of the risk for upper compared with lower tertile. The spline regression also found a nonlinear association for total fruit intake (Fig. 1). In addition, spline regression had narrower 95% CI relative to categorical regression (data not shown).
FIGURE 1 .
Association between total fruit intake and adenoma risk in categorical and spline models. Spline regression demonstrated a nonlinear relationship between risk and fruit intake, whereas the categorical regression provided a step function of the intake with an underestimate of risk reduction in the higher intake range. Estimates were based on n = 764 cases and n = 1517 controls.
Discussion
In this study, we found that intakes of total fruits, particularly berries and fruit juices, and green leafy vegetables were inversely associated with risk for colorectal adenomas. Fruit and vegetable intake has been associated with a decreased risk for colorectal adenomas in some (24–28,36,37) but not all previous studies (29–31). Interestingly, 3 of the 4 previously noted cohort studies reported high intake of total fruits reduced adenoma risk (22,23,38). In the Nurses' Health Study (NHS), fruit consumption was inversely related to the risk of colorectal adenomas, whereas high intake of vegetables did not substantially change the risk (22). In the Health Professionals' Follow-up Study conducted in men, frequent consumption of fruits, but not vegetables, was associated with reduced prevalence of adenomas (38). Another follow-up study also observed that higher fruit intake reduced the growth of colorectal adenoma (23). Most recently in a case-control study, a diet high in fruits and low in meats reduced colorectal adenoma risk (24). Overall, these previous studies supported our findings that vegetables and particularly fruits may protect against the initiation of colorectal adenomas. It is possible that because vegetables, unlike fruits, are often consumed in cooked dishes, the cooking process could cause significant loss of antioxidant activity (39) and, thus, fruit intake provides more protection.
No study has evaluated the association between adenoma risk and berry intake alone (23). A number of studies have suggested the potential chemopreventive properties of berries (40,41). Berries possess much more potent antioxidant activity compared with other fruits and vegetables (41). A clinical trial found that berry powder caused an approximate 50% regression rate of rectal polyps (40). Two case-control studies have looked at the relationship between fruit juice drinking and adenoma risk with a protective result in one study and no association in the other (27,31). The disparity between our results and previous reports may be ascribable to the differences in fruit availability and/or eating habits from different regions (42). Our study has been the only study conducted in southern states.
To date, 4 studies have examined intake of green leafy vegetables; 2 showed protective effects (22,27) and 2 observed no associations (30,31). The protective effects of green leafy vegetables in our study were very similar to the NHS (22). Our finding may also suggest that only some subgroups of vegetables are protective or more protective than others, because the protection by total vegetable intake was not significant (16). Folate's central function is in maintaining DNA integrity; it is rich in green leafy vegetables and has benefits in cancer prevention when administered prior to the existence of preneoplastic lesions (19). However, studies have found increased colorectal neoplasia when folate is administered after lesions are present (43). A recent clinical trial also did not observe risk reduction by folate supplementation for participants with a recent history of colorectal adenomas (44).
Antioxidants or other phytochemicals may have cancer-inhibitory effects in an early stage of carcinogenesis (45,46), whereas they may stimulate the growth during tumor promotion (46) (19). This may explain the results from randomized clinical trials in which adenoma recurrence, not primary adenoma, were used as the endpoint (15–18). Undetected early adenoma precursor lesions may have already been present in the participants' mucosa in these studies (44). Therefore, the time course may not be long enough for specific dietary components to function (19). This possibility could contribute to the NHS finding in which the risk reduction by fruits and vegetables was more pronounced in incident adenomas relative to prevalent adenomas (22). This potential time period issue is also supported by the observation that fruit intake had no significant effects on colorectal carcinoma risk in both the NHS and the Health Professionals' Follow-up Study (47), but fruit intake reduced adenoma risk in the same 2 cohorts (22,38).
The present study has several strengths. Participants were excluded with prior history of adenomatous polyps, a condition that may relate to a change in dietary habits. We included only controls undergoing a full colonoscopy throughout colon and rectum; thus, the potential contamination of cases in the control group is not a major concern. The FFQ was developed specifically to capture diet in the southern United States. Furthermore, most participants were recruited before the colonoscopy that defined their case or control status. Thus, controls were not any less likely to participate in the study than cases. In this study, spline regression provided more complete information about the association in addition to a more appropriately estimated odds ratio (OR) and better fit for skewed data. By contrast, regression of average risk on average exposures as with categorical regression can inaccurately present the exposure effects if those effects are nonlinear within categories (48) as demonstrated in our study. There are also some concerns or limitations in the study. Dietary intake was assessed a few days after the diagnosis; therefore, recall bias may be a concern. The maximum intake frequency included on the FFQ was ≥2 times/d. It is possible that, if a higher threshold exists, we were unable to distinguish it. However, only a very small fraction of participants reported such high consumption for a particular item. Additionally, the ability to distinguish higher intake levels would not have changed the observed findings but, instead, would have improved the effect size. In the study, controls were slightly more likely to undergo a colonoscopy due to family history. However, there were no appreciable differences in effect estimates in stratified analysis by colonoscopy indication. Selection bias is another potential concern, but we have found that age, sex, study site, and the reasons for the colonoscopy did not differ substantially between persons who did or did not consent to participate in the study. Although we adjusted for many confounding factors, residual confounding may still arise when there were measurement errors in confounding factors (16,22).
In conclusion, the findings from this study suggest that an increase in total fruit intake and certain fruit and vegetable intake may reduce the risk for colorectal adenomas. The inverse associations between intake of berries and green leafy vegetables and adenoma risk are relatively new and should be evaluated in other study populations.
Supplementary Material
Acknowledgments
The authors thank Brandy Venuti for helpful technical comments in the preparation of this manuscript and Theresa A. Scott for assistance with the graphics.
Supported by grants from NIH: P50CA95103 and R01CA97386. The project described was supported by grants P50CA95103 and R01CA97386 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the NIH.
Author disclosures: H. Wu, Q. Dai, M. J. Shrubsole, R. M. Ness, D. Schlundt, W. E. Smalley, H. Chen, M. Li, Y. Shyr, and W. Zheng, no conflicts of interest.
Supplemental Table 1 and Supplemental Figure 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: NHS, Nurses' Health Study; OR, odds ratio; TCPS, Tennessee Colorectal Polyp Study.
References
- 1.Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523–6. [DOI] [PubMed] [Google Scholar]
- 2.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–39. [DOI] [PubMed] [Google Scholar]
- 3.American Institute for Cancer Research/World Cancer Research Fund. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 2007.
- 4.Satia-Abouta J, Galanko JA, Martin CF, Ammerman A, Sandler RS. Food groups and colon cancer risk in African-Americans and Caucasians. Int J Cancer. 2004;109:728–36. [DOI] [PubMed] [Google Scholar]
- 5.Chiu BC, Ji BT, Dai Q, Gridley G, McLaughlin JK, Gao YT, Fraumeni JF Jr, Chow WH. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:201–8. [PubMed] [Google Scholar]
- 6.Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369–75. [DOI] [PubMed] [Google Scholar]
- 7.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–33. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi S, Favero A, La Vecchia C, Negri E, Conti E, Montella M, Giacosa A, Nanni O, Decarli A. Food groups and risk of colorectal cancer in Italy. Int J Cancer. 1997;72:56–61. [DOI] [PubMed] [Google Scholar]
- 9.Ghadirian P, Lacroix A, Maisonneuve P, Perret C, Potvin C, Gravel D, Bernard D, Boyle P. Nutritional factors and colon carcinoma: a case-control study involving French Canadians in Montreal, Quebec, Canada. Cancer. 1997;80:858–64. [DOI] [PubMed] [Google Scholar]
- 10.McCullough ML, Robertson AS, Chao A, Jacobs EJ, Stampfer MJ, Jacobs DR, Diver WR, Calle EE, Thun MJ. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control. 2003;14:959–70. [DOI] [PubMed] [Google Scholar]
- 11.Michels KB, Giovannucci E, Chan AT, Singhania R, Fuchs CS, Willett WC. Fruit and vegetable consumption and colorectal adenomas in the Nurses' Health Study. Cancer Res. 2006;66:3942–53. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Tsubono Y, Nakaya N, Ogawa K, Kurashima K, Kuriyama S, Hozawa A, Nishino Y, Shibuya D, et al. Fruit and vegetable consumption and risk of colorectal cancer in Japan: the Miyagi Cohort Study. Public Health Nutr. 2005;8:309–14. [DOI] [PubMed] [Google Scholar]
- 13.Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: the Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2000;152:1081–92. [DOI] [PubMed] [Google Scholar]
- 14.Wark PA, Weijenberg MP, van 't Veer P, van Wijhe G, Luchtenborg M, van Muijen GN, de Goeij AF, Goldbohm RA, van den Brandt PA. Fruits, vegetables, and hMLH1 protein-deficient and -proficient colon cancer: the Netherlands cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14:1619–25. [DOI] [PubMed] [Google Scholar]
- 15.Hartman TJ, Yu B, Albert PS, Slattery ML, Paskett E, Kikendall JW, Iber F, Brewer BK, Schatzkin A, et al. Does nonsteroidal anti-inflammatory drug use modify the effect of a low-fat, high-fiber diet on recurrence of colorectal adenomas? Cancer Epidemiol Biomarkers Prev. 2005;14:2359–65. [DOI] [PubMed] [Google Scholar]
- 16.Lanza E, Hartman TJ, Albert PS, Shields R, Slattery M, Caan B, Paskett E, Iber F, Kikendall JW, et al. High dry bean intake and reduced risk of advanced colorectal adenoma recurrence among participants in the polyp prevention trial. J Nutr. 2006;136:1896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson DJ, Sandler RS, Haile R, Tosteson TD, Greenberg ER, Grau M, Baron JA. Fat, fiber, meat and the risk of colorectal adenomas. Am J Gastroenterol. 2005;100:2789–95. [DOI] [PubMed] [Google Scholar]
- 18.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, Shike M, Weissfeld J, Burt R, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342:1149–55. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich CM, Potter JD. Folate and cancer: timing is everything. JAMA. 2007;297:2408–9. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer R, McShane L, Wargovich M, Burt R, Kikendall W, Lawson M, Lanza E, Schatzkin A. The effect of a low-fat, high fiber, fruit and vegetable intervention on rectal mucosal proliferation. Cancer. 2003;98:1161–8. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC. Diet and cancer: one view at the start of the millennium. Cancer Epidemiol Biomarkers Prev. 2001;10:3–8. [PubMed] [Google Scholar]
- 22.Michels KB, Giovannucci E, Chan AT, Singhania R, Fuchs CS, Willett WC. Fruit and vegetable consumption and colorectal adenomas in the Nurses' Health Study. Cancer Res. 2006;66:3942–53. [DOI] [PubMed] [Google Scholar]
- 23.Almendingen K, Hofstad B, Vatn MH. Dietary habits and growth and recurrence of colorectal adenomas: results from a three-year endoscopic follow-up study. Nutr Cancer. 2004;49:131–8. [DOI] [PubMed] [Google Scholar]
- 24.Austin GL, Adair LS, Galanko JA, Martin CF, Satia JA, Sandler RS. A diet high in fruits and low in meats reduces the risk of colorectal adenomas. J Nutr. 2007;137:999–1004. [DOI] [PubMed] [Google Scholar]
- 25.Macquart-Moulin G, Riboli E, Cornee J, Kaaks R, Berthezene P. Colorectal polyps and diet: a case-control study in Marseilles. Int J Cancer. 1987;40:179–88. [DOI] [PubMed] [Google Scholar]
- 26.Benito E, Cabeza E, Moreno V, Obrador A, Bosch FX. Diet and colorectal adenomas: a case-control study in Majorca. Int J Cancer. 1993;55:213–9. [DOI] [PubMed] [Google Scholar]
- 27.Witte JS, Longnecker MP, Bird CL, Lee ER, Frankl HD, Haile RW. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol. 1996;144:1015–25. [DOI] [PubMed] [Google Scholar]
- 28.Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res. 1990;81:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H. Colorectal adenomas and diet: a case-control study. Colorectal Adenoma Study Group. Dig Dis Sci. 2001;46:86–95. [DOI] [PubMed] [Google Scholar]
- 30.Mathew A, Peters U, Chatterjee N, Kulldorff M, Sinha R. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int J Cancer. 2004;108:287–92. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Warner SA, Elmer PJ, Fosdick L, Randall B, Bostick RM, Grandits G, Grambsch P, Louis TA, Wood JR, et al. Fruits, vegetables, and adenomatous polyps: the Minnesota Cancer Prevention Research Unit case-control study. Am J Epidemiol. 2002;155:1104–13. [DOI] [PubMed] [Google Scholar]
- 32.Buchowski MS, Schlundt DG, Hargreaves MK, Hankin JH, Signorello LB, Blot WJ. Development of a culturally sensitive food frequency questionnaire for use in the Southern Community Cohort Study. Cell Mol Biol (Noisy-le-grand). 2003;49:1295–304. [PubMed] [Google Scholar]
- 33.Smith SA, Campbell DR, Elmer PJ, Martini MC, Slavin JL, Potter JD. The University of Minnesota Cancer Prevention Research Unit vegetable and fruit classification scheme (United States). Cancer Causes Control. 1995;6:292–302. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998.
- 35.R project. [cited 2008]. Available from: http://cran.r-project.org/.
- 36.Kune GA, Kune S, Read A, MacGowan K, Penfold C, Watson LF. Colorectal polyps, diet, alcohol, and family history of colorectal cancer: a case-control study. Nutr Cancer. 1991;16:25–30. [DOI] [PubMed] [Google Scholar]
- 37.Sandler RS, Lyles CM, Peipins LA, McAuliffe CA, Woosley JT, Kupper LL. Diet and risk of colorectal adenomas: macronutrients, cholesterol, and fiber. J Natl Cancer Inst. 1993;85:884–91. [DOI] [PubMed] [Google Scholar]
- 38.Platz EA, Giovannucci E, Rimm EB, Rockett HR, Stampfer MJ, Colditz GA, Willett WC. Dietary fiber and distal colorectal adenoma in men. Cancer Epidemiol Biomarkers Prev. 1997;6:661–70. [PubMed] [Google Scholar]
- 39.Ismail A, Lee WY. Influence of cooking practice on antioxidant properties and phenolic content of selected vegetables. Asia Pac J Clin Nutr. 2004;13:S162. [Google Scholar]
- 40.Stoner GD, Wang LS, Zikri N, Chen T, Hecht SS, Huang C, Sardo C, Lechner JF. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44:701–5. [Google Scholar]
- 42.Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am J Med. 2006;119:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44:10–25. [DOI] [PubMed] [Google Scholar]
- 44.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. [DOI] [PubMed] [Google Scholar]
- 45.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84. [DOI] [PubMed] [Google Scholar]
- 46.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. [DOI] [PubMed] [Google Scholar]
- 47.Michels KB, Giovannucci E, Joshipura KJ, Rosner BA, Stampfer MJ, Fuchs CS, Colditz GA, Speizer FE, Willet WC. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740–52. [DOI] [PubMed] [Google Scholar]
- 48.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.