Abstract
Recommendations for vitamin A intake and liver stores are based on maintaining normal vision. We propose that higher levels may be required to maintain normal innate immune function. To test this hypothesis, we conducted an 8-wk residential study among 36 healthy Bangladeshi men with low vitamin A stores. Subjects were randomized to receive vitamin A (240 mg in 4 doses) or placebo during study wk 2 and 3. They received 2 vaccines during wk 5 and vitamin A stores were estimated by isotopic dilution at wk 8. The serum concentration of the chemokine interferon-γ–induced protein 10, a component of T-helper 1 (Th1) response, increased significantly after supplementation and was positively and significantly associated with vitamin A stores. Blood concentrations of natural killer (NK) and NK T-cells, which have anticancer and antiviral activity, were positively associated with stores (P < 0.05), as was monocyte oxidative burst (P < 0.05), a marker of bacterial killing ability. However, serum interleukin (IL)-6 and IL-17, cytokines that regulate the antibacterial Th17 response, were significantly and negatively associated with stores, as was production of the regulatory cytokine IL-10 by whole-blood cultures stimulated with bacterial lipopolysaccharide. In summary, vitamin A stores were positively associated with several measures of innate immune activity across a broad range of stores, suggesting that vitamin A enhances protection against diverse pathogens even at concentrations above those needed to maintain normal vision. The negative association of stores with serum IL-6 and IL-17 suggests that not all protective responses are similarly enhanced by vitamin A.
Introduction
Several aspects of innate immunity are compromised by clinical and subclinical vitamin A deficiency. Vitamin A is essential in maintaining the integrity of the epithelial barrier, the first line of defense against many infections. Vitamin A also modulates monocyte and granulocyte development and transcription of cytokines involved in inflammation (1). In vitro experiments and animal studies suggest the important regulatory role of vitamin A in myeloid cell differentiation and function (2,3). Vitamin A supplementation enhanced phagocytic activity and the production of reactive oxygen by Kupffer cells and monocytes in vitamin A-sufficient rats (4) and restored normal phagocytosis and generation of reactive oxygen by neutrophils in vitamin A-deficient rats (5). Several rodent studies have also demonstrated that vitamin A deficiency decreases natural killer (NK)7 cell number and lytic activity (6,7). In vitro experiments show that retinoic acid, the active metabolite of vitamin A, inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNFα) production (8,9) while enhancing interleukin (IL)-1β (8,10) responses in monocytes and macrophages.
Human studies of vitamin A and innate immune function are limited and most investigated cytokine response as an indirect measure of monocyte and macrophage functions. Vitamin A supplementation among patients with common variable immune deficiency with low serum retinol concentrations resulted in increased IL-10 and decreased neopterin (a metabolic product of activated macrophages) and TNFα in plasma, although a parallel control group without vitamin A intervention was not included (11). In contrast, a vitamin A supplementation trial in pregnant women at risk of deficiency showed an increased index of inflammation measure by mitogen-induced interferon-γ (IFNγ)/IL-10 and TNFα/IL-10 ratios during pregnancy and postpartum (12), although these cytokines may have been produced by T lymphocytes rather than cells of the innate immune system. Vitamin A supplementation does not affect markers of the acute phase responses such as C-reactive protein (CRP), serum amyloid A, and α1-acid glycoprotein (AGP) among children at risk of vitamin A deficiency (13) as well as children with documented subclinical or clinical vitamin A deficiency (14), although serum retinol concentrations are often lower in children with an active acute phase response, as recently demonstrated (15). Even though the effects of vitamin A supplementation on innate immunity are not well described from human studies, meta-analysis has shown that vitamin A supplementation leads to reduced child mortality (16–18), suggesting that vitamin A may improve innate immune function, the first line of defense against pathogenic microorganisms.
Serum retinol has limitations as an indicator of vitamin A status, particularly when liver stores are adequate, and in such situations, assessment of vitamin A stores using stable isotope dilution is the best approach to assessing vitamin A status (19). To our knowledge, immune function has not been used in a human study to assess “adequacy” of vitamin A stores, although immune function clearly is responsive to vitamin A status. We hypothesized that liver vitamin A stores above the minimum recommended concentration of 0.070 μmol/g used to establish the recommended dietary allowance (RDA) based on maintenance of retinal function (20) may be needed to maintain innate immune function. We also postulated that these responses might correlate with vitamin A stores at low vitamin A concentrations and that a threshold might be present where markers of immune function would no longer improve, thus forming a plateau in a dose-response analysis. This “break point” in a dose-response curve might be useful in defining a future RDA for vitamin A based on maintenance of innate immune function. To test this hypothesis, we conducted a controlled dietary study that provided recommended concentrations of all major micronutrients and energy (except vitamin A) and used high-dose vitamin A sup-plementation in a double-blind, randomized, placebo-controlled trial in which we estimated the total body vitamin A pool size by deuterated-retinol-dilution technique (19). We also used immunization to induce a low-level inflammatory response during the study. A previous manuscript describes the effect of vitamin A on the adaptive immune response to immunization (21).
Materials and Methods
Study site and subjects.
This study was carried out at the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B); Dhaka, Bangladesh. The Institutional Review Board of the University of California, Davis and the Ethical Review Committee of ICDDR,B approved the study procedure. All participants provided informed written consent. Recruitment for the study has been described (21). Briefly, 36 healthy men (20–30 y of age) were recruited based on having low serum retinol (all were <1.22 μmol/L) and normal CRP (<5 mg/L). These criteria were used because an earlier study involving 1 of the present authors (19) demonstrated that the men recruited in this fashion had liver vitamin A stores near the minimum concentration used to define the RDA for vitamin A (0.070 μmol/g). Subjects also had normal clinical chemistry and hematology values, as described. Subjects lived near ICDDR,B during the study and came to the ICDDR,B grounds to stay at the Advanced Biomedical Research Unit for 12 h/d, 7 d/wk, where they received and consumed all meals during the 2-mo residential study period.
Study diet.
A low-vitamin A basal diet modeled on the traditional Bangladeshi diet that provided ∼40 μg/d retinol equivalent was used in this study, as described (21). The basal study diet complied with the RDA for total energy, other major vitamins, and minerals as evaluated from the unenriched single food items from the USDA and Bangladeshi nutrient databases as described previously (21). At the end of the study, all subjects were supplemented with 1 high dose (60 mg) of vitamin A.
Study design.
The placebo-controlled, double-blind, randomized intervention trial was conducted sequentially in 2 phases between May and October 2005 (18 subjects per residential phase). Briefly, participants received the proscribed diet for a 1-wk stabilization period (d 1–7) and were then randomized to receive vitamin A (4 × 60 mg retinol equivalent) or placebo (corn oil) (d 7, 12, 17, and 22). One wk later (d 29), participants were vaccinated with yellow fever virus (YFV) and tetanus toxoid (TT) vaccines. One wk after vaccination (d 36), a single dose (10 mg) of stable isotope-labeled vitamin A was given orally to estimate total body vitamin A pool size. To investigate the effect of supplementation on innate immunity, venous blood was obtained before (d 7) and 1 wk after (d 29) supplementation. To investigate the vaccine-mediated induction of innate responses and to estimate total body vitamin A pool size, venous blood was collected 1 wk (d 36) and 1 mo (d 57) after vaccination. The vitamin A and placebo groups will be referred to as the high vitamin A (VA) and low VA groups, respectively.
Serum retinol and vitamin A reserves.
Serum was stored at −80°C for the measurement of retinol by HPLC as described previously (22). Total body vitamin A pool size was estimated quantitatively by using the deuterated-retinol–dilution technique and the equation described by Furr et al. (23). The vitamin A concentration in liver was estimated as described (21).
Immunophenotyping of cells in whole blood.
A multitest 4-color reagent kit with Trucount tubes (BD Bioscience) was used to determine the absolute counts (per liter of whole blood) of NK (CD3-CD16+CD56+) and NKT (CD3+CD16+CD56+) cells in a 4-color flow cytometer and CELLQUEST software (FACSCalibur; BD Bioscience). Absolute counts of neutrophils, monocytes, and eosinophils were obtained from the complete blood count report using Excell 22 Hematology Analyzer (DANAM).
Whole blood oxidative burst assay.
Oxidative burst activity of monocytes and granulocytes was assessed by flow cytometry to measure the intensity of redox indicator dye di-chloro-fluorescence di-acetate (DCF-DA) as described earlier (24). DCF-DA, a nonfluorescence compound, can diffuse through the cell membrane. Activated monocytes and neutrophils generate reactive oxygen species (ROS) that react with DCF-DA to produce DCF, which is trapped inside the cell and emits fluorescence. Thus, the intensity of DCF is an indirect measure of the concentration of ROS generated in a cell following activation. In this experiment, DCF-DA (Sigma) was added into 100 μL heparinized blood at a final concentration of 200 μmol/L and incubated in a water bath for 30 min at 37°C in the dark. Phorbol-myristate-acetate (PMA) at 50 μg/L or 1 × 107 Escherichia coli (ATCC no. 8739; Molecular Probes) or PBS as negative control was added and incubated for another 30 min. Samples were then placed on ice, washed twice with ice-cold PBS, and RBC were lysed by ammonium chloride solution. For analysis, 50,000 cells were colleted in a flow cytometer and DCF signals were detected in the green-fluorescence (FL1). CELLQUEST software was used to identify monocytes and granulocytes by FS-SS gating. Percentage of DCF responder and geometric mean fluorescence intensity (MFI) of the gated individual cell populations were estimated.
Whole blood phagocytosis assay.
Phagocytic activity of monocytes and granulocytes was measured by flow cytometry as described previously (24). Briefly, 1 × 107 fluorescein isothiocyanate (FITC)-labeled E. coli (Molecular Probes) were added into 100 μL heparinized blood and incubated in a water bath for 30 min at 37°C in the dark. Samples were kept on ice and an equal volume of ethidium bromide (EtBr) (Invitrogen) was added to a final concentration of 200 μmol/L. EtBr acts as quenching agent that converts the green fluorescence of nonphagocytosed, FITC-labeled E. coli into red fluorescence while internalized E. coli remains green (25). The concentration of EtBr was preoptimized by evaluating the intensity of FL1, red-fluorescence, and EtBr fluorescence in the phagocytic cells with and without varying concentrations of EtBr. Cells were washed twice with ice-cold PBS and RBC were lysed by ammonium chloride solution. For analysis, 50,000 cells were colleted in a flow cytometer and FITC signals were detected in the FL1. CELLQUEST software was used to identify monocytes and granulocytes by FS-SS gating. Percentages of FITC responder and geometric MFI of the gated individual cell populations were estimated.
Whole blood LPS-induced inflammatory cytokine assay.
LPS-induced inflammatory cytokine assay was carried out as described previously (26). Briefly, 20% whole blood, diluted in 3000 USP/L heparin (BD Bioscience) containing Russ-10 media (27) was stimulated by LPS from E. coli 0111:B4 (Sigma) at a final concentration of 1 mg/L for 24 h at 37° and 5% CO2. Cell culture was carried out in 24-well, clear, flat-bottom ultra-low-attachment plates (Corning) that had eliminated any nonspecific activation of blood monocytes through plastic adherence. Cell-free culture supernatant was collected and stored in 1% protease inhibitor cocktail at −80°C. The inflammatory cytokine markers IL-1β, IL-12p40, TNFα, IL-10, IL-6, and IL-8 were measured by multiplexed luminex assay technology in Bio-Plex system (Bio-Rad Laboratories).
Serum cytokine, chemokine, and acute phase protein assay.
Serum IFNγ-induced-protein 10 (IP10; also termed CXCL10, also known as CCL11), Eotaxin, IL-6, IL-17, and TNFα were measured by multiplexed Luminex assay technology in Bio-Plex system (Bio-Rad Laboratories). Serum CRP was measured by solid-phase chemiluminescent immunometric assay with Immulite analyzer (Diagnostic Products) and AGP was measured by immunoturbidimetric assay using a kit (Kamiya Biomedical) and the Hitachi 902 Automatic Analyzer (Roche Diagnostics). Serum neopterin was measured using an EIA kit (Alpco Diagnostics) according to the manufacturer's instructions.
Statistical analysis.
Descriptive frequencies of the study variables were analyzed to assess data validity, distributions, and assumptions of normality and equal variance. Log10 transformations of data were made where needed. Statistical analyses were done in SigmaStat 3.1 (Systat Software) and SPSS 13.0 for windows (SPSS). As previously discussed (21), vitamin A pool size estimates were unreliable for 3 subjects. Data from these 3 subjects were not used in the analysis where the estimated total body vitamin A pool size was used (i.e. correlation and regression analyses). ANCOVA was used for comparisons between groups after supplementation, in which center-transformed presupplementation values were used as covariates. Two-way repeated-measures ANOVA and the post hoc test Holm-Sidak procedure were used for pair-wise multiple comparisons to compare vaccine-induced serum immune responses. Post hoc test was applied when study groups, days, or their interactions significantly differed. A between-groups comparison was done by Student's t test. The overall significance level of these tests was set at <0.05.
Spearman rho correlation analysis was used to determine whether individual immune response variables were associated with vitamin A stores. Two-phase segmental linear regression was used to estimate the relationship between liver vitamin A concentration and functionally similar clusters of immune response variables and to identify possible “break points” at which 2 different regression lines with significantly different intersected slopes. Functionally similar immune responses were converted to Z-scores (mean of 0, SD of 1) and grouped together into the same regression. Our initial hypothesis was that a positive slope in the first line would be seen below the postulated break point at 0.070 μmol/g, the current recommendation for minimum adequate liver vitamin A stores, and this positive slope in the first line would continue to appear above 0.070 μmol/g to reach a critical point for starting a plateau phase (2nd line), i.e. additional increases in liver vitamin A stores would no longer contribute to “improved” immune function. Initial analysis revealed no such significant slope in the first line at liver vitamin A concentrations < 0.070 μmol/g, whereas significant positive slopes in the 2nd line (i.e. ≥0.070 μmol/g) were identified. In the further analysis, break-point was restricted to >0.070 μmol/g and under this condition, 2 line segments intersected at an unknown point that represented significant change in immune response to ensue above the current recommendation for liver vitamin A storage. Clusters used in this analysis were: 1) E. coli-induced oxidative burst and phagocytic activity of both monocytes and granulocytes; 2) LPS-induced whole blood proinflammatory cytokine responses (IL-1β, TNFα, IL-6, and IL-8); 3) serum acute phase responses (TNFα, IL-6, CRP, AGP, and neopterin); 4) absolute counts of NK and NKT cells per liter of whole blood; and 5) serum inflammatory cytokine (IL-17 and IL-6) responses involved in the generation of antiinflammatory (T regulatory cell) and proinflammatory (Th17 cell) T-cell in adaptive immunity. We used GraphPad Prism 5 for Windows (GraphPad Software) for this analysis.
Results
Baseline characteristics.
The age of study subjects in the low and high VA groups was 24.3 ± 2.70 and 23.5 ± 3.05 y, respectively, and the BMI for each group was 19.7 ± 1.6 and 19.7 ± 1.8 kg/m2, respectively. Subjects had normal hematological measurements at baseline. These data have been reported previously (21).
Vitamin A status.
Serum retinol at baseline did not differ between the intervention (1.49 ± 0.37 μmol/L) and control groups (1.52 ± 0.39 μmol/L). Vitamin A status improved following supplementation as indicated by increases in serum retinol and whole body vitamin A stores, as previously reported (21). The range of estimated liver stores in the low VA group was 0.077–0.084 μmol/g and in the high VA group it was 0.070–0.286 μmol/g.
Cellular responses.
The concentrations of innate immune cell types in peripheral blood were comparable in the 2 groups before supplementation and did not change significantly after supplementation (Table 1). Similarly, functional indicators of monocyte and granulocyte activity did not differ at baseline and did not change significantly after supplementation (Table 2). The concentration of monocytes, NKT cells, and NK cells in peripheral blood (Table 1), the oxidative burst activity (PMA induced ROS) of monocytes, and the phagocytic activity (for E. coli) of neutrophils (Table 2) all decreased significantly over time in both groups.
TABLE 1.
Distribution of the absolute number of cells primarily involved in innate immunity in men before and 1 wk after supplementation with placebo (low VA stores) or vitamin A (high VA stores)1
| Low VA store
|
High VA store
|
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| n x103/L whole blood | ||||
| Monocytes | 448 ± 47.8* | 312 ± 26.4 | 408 ± 38.1* | 286 ± 22.4 |
| Neutrophils | 5066 ± 343* | 4223 ± 293 | 4344 ± 225 | 4452 ± 428 |
| Eosinophils | 649 ± 101 | 642 ± 87.4 | 633 ± 117 | 498 ± 86.3 |
| NKT cells | 168 ± 32.8* | 124 ± 24.9 | 187 ± 23.2* | 147 ± 24.0 |
| NK cells | 756 ± 79.1* | 455 ± 51.0 | 729 ± 49.1* | 489 ± 53.8 |
Values are means ± SE, n = 18. *Different from postsupplementation (within group), P < 0.05.
TABLE 2.
Functional assessments of monocytes and granulocytes in men before and 1 wk after supplementation with placebo (low VA stores) or vitamin A (high VA stores)1
| Low VA store
|
High VA store
|
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Monocytes | MFI (% of responder cells) | |||
| PMA ROS | 203 ± 22.9* | 156 ± 11.0 | 211 ± 14.2* | 173 ± 10.1 |
| (67.7 ± 2.72) | (65.1 ± 1.77) | (70.3 ± 2.07) | (67.6 ± 1.35) | |
| E. coli ROS | 56.9 ± 4.58 | 53.7 ± 3.06 | 61.5 ± 3.95 | 59.9 ± 3.81 |
| (26.0 ± 2.50) | (26.0 ± 2.61) | (30.0 ± 2.14) | (29.3 ± 2.67) | |
| E. coli Phago | 1720 ± 163 | 1653 ± 211 | 1747 ± 156 | 1646 ± 197 |
| (82.61 ± 1.81) | (81.4 ± 2.18) | (83.6 ± 1.58) | (82.0 ± 1.68) | |
| Granulocytes | ||||
| PMA ROS | 2100 ± 134 | 2187 ± 81.7 | 2066 ± 178 | 2101 ± 76.6 |
| (98.7 ± 0.22) | (99.2 ± 0.13) | (97.9 ± 0.37) | (98.9 ± 0.17) | |
| E. coli ROS | 382 ± 50.3 | 332 ± 26.0 | 426 ± 52.3 | 374 ± 45.6 |
| (84.9 ± 2.32) | (87.2 ± 1.76) | (81.5 ± 3.15) | (87.8 ± 1.70) | |
| E. coli Phago | 5001 ± 461* | 4190 ± 444 | 5166 ± 494* | 4167 ± 421 |
| (97.9 ± 0.32) | (97.8 ± 0.42) | (97.9 ± 0.70) | (98.0 ± 0.28) | |
Values are means ± SE, n = 18. *Different from postsupplementation (within group), P < 0.05.
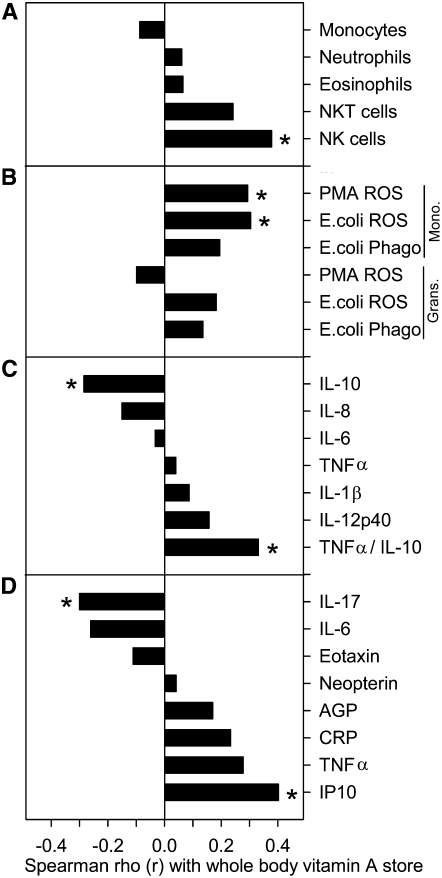
In contrast to direct comparisons made between the low and high VA groups, there were significant associations between vitamin A stores and several dependent variables using regression analysis. Total body vitamin A pool size correlated positively with absolute numbers of NK cells (r = 0.378; P < 0.05) and marginally with NKT cells (r = 0.242; P = 0.08) (Fig. 1A). Pool size also correlated positively with 2 measures of monocyte activity, oxidative burst in response to PMA at 50 μg/L (r = 0.293; P < 0.05) and 1 × 107 E. coli (r = 0.304; P < 0.05) as determined by the production of ROS measured indirectly by DCF fluorescence (Fig. 1B).
FIGURE 1 .
Spearman correlation between whole body vitamin A store and measures of innate immunity, n = 33. (A) Absolute counts (per liter of whole blood) of leukocytes primarily involved in the innate immunity. (B) Intensities of PMA (50 μg/L) and 1 × 107 E. coli-induced ROS as measure of oxidative burst activity and intensities of 1 × 107 E. coli-induced phagocytic activities of monocytes and granulocytes in heparinized blood. (C) Inflammatory cytokine responses in the culture supernatant of 20% whole blood stimulated by 1 mg/L LPS for 24 h. (D) Serum cytokine, chemokine, and acute phase protein responses. Very similar correlation coefficients were seen when these immune response variables were correlated with estimated liver vitamin A stores (data not shown). *P < 0.05.
LPS-induced proinflammatory cytokine secretion by whole blood cultures.
Cytokine secretion did not differ between the low and high VA groups at baseline although the high VA group tended to secrete less IL-6 (P = 0.12). Cytokine secretion did not change as a result of supplementation (Supplemental Table 1). However, vitamin A pool size correlated positively with the LPS-induced proinflammatory to antiinflammatory cytokine ratio, TNFα/IL-10 (r = 0.331; P < 0.05), and correlated negatively with IL-10 (r = −0.285; P < 0.05) (Fig. 1C).
Serum markers of immune activation.
Serum cytokine, chemokine, acute phase protein, and neopterin concentrations did not differ between the groups at baseline and most (5 of the 8 tested) did not change after supplementation. However, TNFα and IP10 both decreased in the low VA group but not the high VA group (P < 0.05). In contrast, the serum IL-6 concentration tended to decrease in the high VA compared to the low VA group (P = 0.08) (Table 3).
TABLE 3.
Serum markers in men before and 1 wk after supplementation with placebo (low VA stores) or vitamin A (high VA stores)1
| Low VA store
|
High VA store
|
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| IL-17, ng/L | 5.74 ± 1.14 | 5.03 ± 1.02 | 4.09 ± 1.09 | 3.55 ± 1.10 |
| IL-6, ng/L | 44.7 ± 28.7 | 51.3 ± 31.6 | 80.0 ± 43.3 | 26.3 ± 23.8 |
| Eotaxin, ng/L | 273 ± 29.2 | 281 ± 34.4 | 247 ± 23.4 | 269 ± 29.7 |
| Neopterin, nmol/L | 8.25 ± 0.41 | 8.55 ± 0.53 | 7.58 ± 0.57 | 7.97 ± 0.58 |
| AGP, g/L | 8.04 ± 0.42 | 8.20 ± 0.38 | 8.56 ± 0.47 | 8.78 ± 0.42 |
| CRP, mg/L | 1.18 ± 1.74 | 0.75 ± 0.49 | 0.73 ± 0.14 | 1.27 ± 0.32 |
| TNFα, ng/L | 17.0 ± 1.42* | 14.0 ± 1.41 | 14.7 ± 1.83 | 14.5 ± 1.69# |
| IP10, ng/L | 154 ± 31.9* | 113 ± 12.6 | 154 ± 29.4 | 182 ± 29.1# |
Values are means ± SE, n = 18. *Different from postsupplementation (within group), P < 0.05; #different from low VA after adjustment for presupplementation value, P < 0.05.
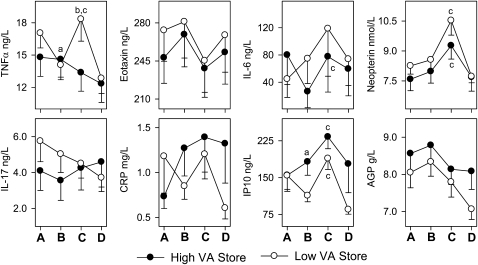
The serum TNFα concentration decreased by 18% in the low VA group but did not change in the high VA group after supplementation, suggesting that vitamin A supplementation blocked a decrease that would have occurred without supplementation. At 1 wk after supplementation, vitamin A pool size and serum TNFα tended to be positively correlated (r = 0.278; P = 0.06) (Fig. 1D). Serum TNFα and other markers of innate immunity were also measured 1 and 4 wk after immunization to gauge the effect of vitamin A supplementation on the response to the TT and YFV vaccines. In contrast to the positive association of vitamin A with serum TNFα prior to immunization, serum TNFα increased significantly 1 wk after immunization in the low VA group but not in the high VA group and then returned to baseline concentrations 3 wk later (Fig. 2). There was no such increase in the high VA group, suggesting a differential impact of vitamin A on basal compared with stimulated TNFα in these subjects.
FIGURE 2 .
Change in serum cytokine, chemokine, and acute phase protein concentrations in response to supplementation and vaccination. Legends in horizontal time lines. (A) Presupplementation, (B) 1 wk postsupplementation, (C) 1 wk postvaccination, and (D) 1 mo postvaccination. Between-group differences at B; a P < 0.05 (Table 3). Between-group difference at B, C, and D; bP < 0.05. Within-group difference at B, C, and D; c P < 0.05.
The serum IP10 concentration did not differ between the low and high VA groups on d 7, before supplementation (Table 3). However, by d 29 (1 wk after supplementation), serum IP10 had decreased by 29% (P < 0.05) in the low VA group (Table 3), resulting in a difference with the high VA group (P < 0.05; Table 3). Vitamin A pool size and serum IP10 were positively correlated (r = 0.402; P < 0.05) 1 wk after supplementation (Fig. 1D). IP10 increased significantly in both the high and low VA groups 1 wk after immunization (Fig. 2) and concentrations tended to remain elevated in the high compared with the low VA group after 1 mo immunization (P = 0.09). Thus, improving vitamin A status significantly increased serum IP10 concentration, although both groups responded with transient increases following immunization.
The serum IL-6 concentration did not change significantly in the low VA group between d 7 and 29 (15% increase) but decreased by 67% in the high VA group over the same period, resulting in a difference between the groups that was marginally significant (P = 0.08) after supplementation (Table 3). Vitamin A pool size and serum IL-6 tended to be negatively correlated (r = −0.261; P = 0.07) (Fig. 1D). The serum IL-6 concentration increased after immunization in the high VA group (Fig. 2).
Significant changes in serum IL-17 were not seen as a result of supplementation or in association with immunization (Table 3; Fig. 2). However, vitamin A pool size and serum IL-17 were negatively correlated (r = −0.301; P < 0.05) (Fig. 1D).
The high and low VA groups did not differ in serum eotaxin, CRP, AGP, and neopterin, and there were no significant associations with vitamin A stores (Table 3; Figs. 1 and 2). Serum eotaxin decreased transiently after immunization (P < 0.05), as might be expected for a chemokine associated with the Th2 response, whereas serum neopterin increased after immunization (Fig. 2), consistent with increased macrophage activation that would be present in a Th1 response to a live-virus vaccine.
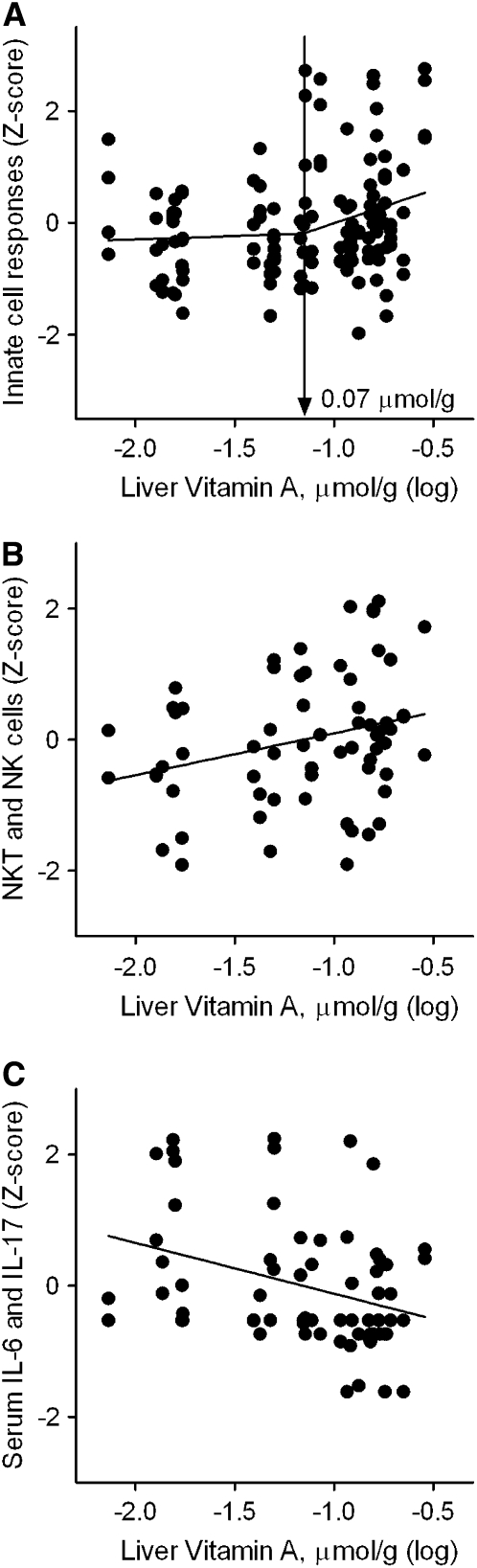
Association of innate immune response with liver vitamin A stores: segmented linear regression.
Simple linear regression and break-point regression analyses were performed with groupings of similar immune response variables to characterize associations with estimated liver vitamin A stores and to identify inflection points in these associations that might indicate a critical value of liver vitamin A stores for maintaining immune function. Only one such break-point was detected at 0.070 μmol/g in the association between liver vitamin A stores and measures of phagocyte activation (Z-score–normalized, E. coli-induced oxidative burst and phagocytic activity of monocytes and granulocytes) (Fig. 3A). There was a significant, positive association above the break-point [slope (β) ± SE = 1.19 ± 0.54; P < 0.05] but not below. No linear associations or break-points were detected for LPS-induced whole blood proinflammatory cytokine responses (IL-1β, TNFα, IL-6, and IL-8) or serum markers of inflammation (TNFα, IL-6, CRP, AGP, and neopterin). However, Z-score–normalized absolute numbers of NK and NKT cells when grouped together had a positive relationship [slope (β) ± SE] (0.64 ± 0.28; P < 0.05; Fig. 3B) with liver stores, whereas Z-score–normalized serum IL-6 and IL-17 examined together had a significant negative relationship ([slope (β) ± SE] (−0.78 ± 0.27; P < 0.01; Fig. 3C) with liver vitamin A stores. No break-points were seen in these latter 2 analyses.
FIGURE 3 .
Relationship between liver vitamin A concentrations and logical groupings of innate immune measures (n = 33). (A) E .coli-induced oxidative burst and phagocytic activity of monocytes and granulocytes. (B) Absolute counts of NK and NKT cells per liter of whole blood. (C) Serum IL-6 and IL-17 concentrations. The Z-score–normalized responses for each group were plotted and the break-point estimates obtained from the 2-phase segmental linear regression model as described in “Materials and Methods.”
Discussion
RDAs are defined as the level of intake that will prevent signs of deficiency in ∼97.5% of the population. For this purpose, vitamin A “deficiency” has been defined as diminished retinal sensitivity to light (20). However, immune function is also affected by vitamin A status (1) and might be used to define a different RDA for vitamin A, one to prevent immunodeficiency. A difficulty with this approach is that there are many ways to measure immune function, as recently discussed (28). Thus, one might find different associations of vitamin A with immune function depending on the immunologic indicator selected for analysis.
In the present study, we have examined the effect of vitamin A status on innate (reported here) and adaptive (reported previously) immune function (21) using 2 approaches. First, we identified changes within treatment groups (i.e. vitamin A vs. placebo) before and after supplementation. This approach is useful for identifying the effect of the intervention but has the disadvantage of considering vitamin A status as a bimodal variable. For this reason, we also assessed vitamin A status using stable isotope dilution to have a continuous variable reflecting vitamin A stores for each individual. This approach is also useful, because it provides a means to compare our data to the RDA, which uses a value of 0.070 μmol/g for liver vitamin A stores to define the RDA (20). Estimated liver stores for subjects in the present study ranged from 10% (0.007 μmol/g) to 400% (0.286 μmol/g) of this target concentration.
The present study found a significant, positive correlation between vitamin A stores and peripheral blood NK cells and a similar, marginally significant association for NKT cells. Our data agree with previous observations that vitamin A supports NK cell number and function (6,7,29,30). NK cells develop in the bone marrow and have diverse biological activities, being particularly important to human health for their anticancer activity and ability to identify and kill virus-infected cells before the development of an adaptive immune response (31). NKT cells also have anticancer activity (32). It is thus plausible that the increased risk of cancer with vitamin A deficiency may result at least in part from immunodeficiency rather than solely from a direct effect on cancer cells (33). In addition, treatment of some viral infections, particularly measles (34), with vitamin A improves clinical outcomes and augmented NK protection could be responsible. However, vitamin A supplementation does not always improve outcomes associated with viral infections (35,36). Further studies to confirm and evaluate the effect of vitamin A on NK and NKT cell function in humans are needed to expand the current findings.
Whereas a significant association of vitamin A stores with monocyte or granulocyte numbers was not seen in the present study, although animal studies suggested that such differences might be found (37,38), there was a positive association between vitamin A stores and production of reactive oxygen by stimulated monocytes. This finding is consistent with a study that found a lower production of reactive oxygen in vitamin A-deficient than control rats and also found that this defect was corrected with vitamin A supplements (5). The implication of these findings is that vitamin A may improve bacterial killing by activated monocytes.
Interestingly, although there was a break-point in the association of vitamin A stores with production of reactive oxygen, the slope was greater above 0.070 μmol/g, suggesting improvement above the current threshold set for the determination of the RDA. This was also the pattern for measures of adaptive immunity previously reported from this study (21). In addition, the positive association of vitamin A stores with NK and NK T-cell showed a positive association across the entire range of vitamin A stores in the present study. Together, these findings suggest continued “improvements” in immune function with continuing increases in vitamin A stores above the cut-off level used for defining the RDA. The absence of a break-point and plateau for all of these measures of immune function was unexpected and contrasts with the underlying assumption for determining the RDA that postulates a threshold will be reached where a deficiency disease is “cured” and nutritional status becomes “adequate.” This observation suggests that a different approach may be needed in using immunologic endpoints for evaluating nutritional status.
In this study, we measured serum concentrations of molecules that mediate communication between cells of the immune system. One of these molecules is the chemokine IP10, which is induced by IFNγ-producing Th1 cells in many cell types (39–41). Serum IP10 increased in response to vitamin A supplementation and was positively associated with liver vitamin A stores. The magnitude of this increase is biologically important, because immunization with the TT and YFV vaccines, which induces significant immune activation, increased serum IP10 by a similar amount in these subjects. Elevated serum IP10 concentrations are seen in Yellow Fever patients (42) and the IP10 response in this study may be due largely to the Th1-inducing effects of the live-virus YFV vaccine (43,44), although a role for the inactivated TT vaccine is also plausible. The increased serum IP10 in the high VA group is interesting, because, in addition to being a chemotactic factor for inflammatory cells, IP10 also promotes IFNγ responses by Th1 cells (45). This finding supports our previous observation that normal vitamin A status helps promote a Th1 response relative to the response in deficient mice (46). Conversely, several studies showed that vitamin A promotes Th2 and inhibits Th1 responses (1,27). Differences in findings among studies may indicate that vitamin A can have differing effects on T cell endpoints depending on the nature of the underlying innate immune response that is driving T-helper (Th) cell development.
IL-6 is a proinflammatory cytokine that can promote the development of Th17 cells, which protect against extracellular bacterial infections and promote autoimmune inflammation in animal studies (47). In the present study, the high VA group had marginally lower serum IL-6 (P = 0.08). Vitamin A stores tended to be negatively associated with IL-6 (P = 0.07) and were negatively associated with IL-17 (P < 0.05). In addition, normalized serum IL-6 and IL-17 concentrations analyzed together were negatively associated with vitamin A stores (P < 0.005). This result is reminiscent of recent animal studies showing that retinoic acid inhibits the generation of Th17 (which produce IL-17) cells by IL-6 (48,49). These findings suggest that vitamin A may diminish Th17 development in humans, which suggests that high dietary vitamin A could decrease the risk or severity of autoimmune disease but also suggests that Th17-mediated protective responses (e.g. against Klebsiella pneumoniae and Candida albicans) might also be diminished (47). In vivo data from human or mouse studies would be needed to evaluate these possibilities and illustrate the balance between protective and destructive responses mediated by the immune system. Such factors need to be considered when evaluating the impact of nutritional interventions on infectious and chronic diseases in human populations.
IL-10 is a regulatory cytokine that diminishes the development of proinflammatory immune responses, including responses mediated by both innate and adaptive immune cells (50). A negative correlation (P < 0.05) was seen between vitamin A stores and LPS-stimulated IL-10 from whole-blood cultures, presumably representing TNFα production primarily by innate immune cells stimulated by LPS. This finding is reminiscent of results from the present study showing a similar negative association of vitamin A stores with TT-specific IL-10 production by T cells (21) and a mouse study in which vitamin A deficiency increased the number of IL-10–producing T cells (46). Overproduction of IL-10 could thus represent a mechanism by which vitamin A deficiency downregulates or impairs some immune responses.
TNFα is a proinflammatory cytokine produced by macrophages as well as Th1 cells (51). Serum TNFα did not change following vitamin A supplementation in the present study, although the placebo group decreased. Vitamin A stores and the LPS-stimulated TNFα:IL-10 ratio were significantly and positively correlated. This proinflammatory:antiinflammatory ratio was examined because it is a useful way to represent the balance between such responses and because a previous study reported a marginally significant increase in this ratio as a result of vitamin A supplementation in a human intervention trial using data from whole blood cultures stimulated with a T-cell mitogen (phytohemaglutinin) (12). Whereas these previous data suggest that vitamin A supports production of TNFα, perhaps by supporting the Th1 response, in vitro studies show that retinoic acid suppresses LPS-induced TNFα production in murine macrophages (8,52,53) as well as cord blood mononuclear cells (54). In agreement with these findings, serum TNFα significantly increased after immunization in the low VA group but not in the high VA group, perhaps as a result of stimulation of innate immune cells via toll-like receptors by components of the live-virus YFV vaccine. The reason for these different results is not clear, but results from the present study are consistent with vitamin A enhancing TNFα production by Th1 cells and suppressing TNFα production by innate immune cells. Further work is needed to evaluate this supposition and its impact on disease resistance.
Neopterin is produced by activated macrophages and our results showing an increase in neopterin after TT and YFV immunization are consistent with other observations after YFV immunization (55). This is also consistent with the postvaccine increase in the Th1 chemokine IP10 in this study, as well as the mirror-image decrease in eotaxin, a Th2 chemokine. Although these responses were not associated with vitamin A status, they do show the value of examining serum markers of immune activation in conjunction with immunization to examine the effect of nutritional status on innate immune function.
In conclusion, this study shows that vitamin A stores are associated with higher circulating concentrations of NK cells, which protect against viral infections and kill cancer cells, higher production of ROS by stimulated monocytes, which suggests greater protection against bacterial infections, higher serum concentrations of the chemokine IP10, which may indicate a greater potential response to pathogens that elicit Th1 responses, and lower serum IL-6 and IL-17 concentrations, which suggest a lower potential response to pathogens that elicit Th17 responses. This study also indicates that the effect of vitamin A stores on these responses extends above the 0.070 μmol/g threshold for maintenance of normal retinal function and these associations do not exhibit an obvious threshold. These findings suggest that vitamin A status may need to be considered as a continuous variable when thinking about the role of the immune system in resisting infection or mediating chronic inflammatory disease, rather than as a discrete variable where status is either deficient or adequate. Although these studies were conducted in adults, similar effects may be seen in infants and young children, but age-specific factors (e.g. immune system maturity and breast-feeding status) may modify the effect of vitamin A supplementation in infants and young children.
Supplementary Material
Acknowledgments
We thank the following persons for their help and expertise: Xiaowen Jiang for estimating cytokines by multiplexed luminex assay, Alina Wettstein for measuring serum retinol, Emmanuel Aklamati for carrying out isotopic retinol measurements, Janet M. Peerson for advice on statistical analysis, and Protim Sarker for conducting flow analysis and helping during in vitro method development. We also thank Dr. M. Jobayer Chisti and Dr. Kazi Jamil for monitoring health of the study subjects at ICDDR,B. Dhaka, Bangladesh.
Supported by the USDA CRIS project no. 5306-51530-013-00D and Specific Cooperative Agreement no. 58-5306-4-034F with International Centre for Diarrheal Disease Research, B Dhaka, Bangladesh. On-campus doctoral training of S. M. A. at University of California Davis was supported by NIH Research grant no. D43 TW01267 funded by the Fogarty International Center and the National Institute of Child Health and Human Development.
Author disclosures: S. M. Ahmad, M. J. Haskell, R. Raqib, and C. B. Stephensen, no conflicts of interest.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AGP, α-acid glycoprotein; CRP, C-reactive protein; DCF-DA, di-chloro-fluorescence di-acetate; DRD, deuterated retinol dilution; EtBr, ethidium bromide; FITC, fluorescein isothiocyanate; ICDDR,B, International Centre for Diarrheal Disease Research, Bangladesh; IFNγ, interferon-γ; IL, interleukin; IP10, interferon-γ–induced protein 10; LPS, lipopolysaccharide; MFI, mean fluorescence intensity; NK, natural killer cell; PMA, phorbol myristate acetate; RDA, recommended dietary allowance; ROS, reactive oxygen species; Th1, T-helper type 1; Th2, T-helper type 2; Th17, T-helper 17; TNFα, tumor necrosis factor α; TT, tetanus toxoid; VA, vitamin A; YFV, yellow fever virus.
References
- 1.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res. 2004;297:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang YJ, Xu TR, Lu B, Mymin D, Kroeger EA, Dembinski T, Yang X, Hatch GM, Choy PC. Cyclooxygenase expression is elevated in retinoic acid-differentiated U937 cells. Biochim Biophys Acta. 2003;1633:51–60. [DOI] [PubMed] [Google Scholar]
- 4.Hoglen NC, Abril EA, Sauer JM, Earnest DL, McCuskey RS, Lantz RC, Mobley SA, Sipes IG. Modulation of Kupffer cell and peripheral blood monocyte activity by in vivo treatment of rats with all-trans-retinol. Liver. 1997;17:157–65. [DOI] [PubMed] [Google Scholar]
- 5.Twining SS, Schulte DP, Wilson PM, Fish BL, Moulder JE. Vitamin A deficiency alters rat neutrophil function. J Nutr. 1997;127:558–65. [DOI] [PubMed] [Google Scholar]
- 6.Ross AC, Stephensen CB. Vitamin A and retinoids in antiviral responses. FASEB J. 1996;10:979–85. [PubMed] [Google Scholar]
- 7.Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr. 1999;129:1510–7. [DOI] [PubMed] [Google Scholar]
- 8.Mathew JS, Sharma RP. Effect of all-trans-retinoic acid on cytokine production in a murine macrophage cell line. Int J Immunopharmacol. 2000;22:693–706. [DOI] [PubMed] [Google Scholar]
- 9.Mehta K, McQueen T, Tucker S, Pandita R, Aggarwal BB. Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J Leukoc Biol. 1994;55:336–42. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto S, Hayashi S, Yoshida S, Kujime K, Maruoka S, Matsumoto K, Gon Y, Koura T, Horie T. Retinoic acid differentially regulates interleukin-1beta and interleukin-1 receptor antagonist production by human alveolar macrophages. Leuk Res. 1998;22:1057–61. [DOI] [PubMed] [Google Scholar]
- 11.Aukrust P, Muller F, Ueland T, Svardal AM, Berge RK, Froland SS. Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur J Clin Invest. 2000;30:252–9. [DOI] [PubMed] [Google Scholar]
- 12.Cox SE, Arthur P, Kirkwood BR, Yeboah-Antwi K, Riley EM. Vitamin A supplementation increases ratios of proinflammatory to anti-inflammatory cytokine responses in pregnancy and lactation. Clin Exp Immunol. 2006;144:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood BR, Tomkins AM, Gyapong JO. Vitamin A supplementation, morbidity, and serum acute-phase proteins in young Ghanaian children. Am J Clin Nutr. 1995;62:434–8. [DOI] [PubMed] [Google Scholar]
- 14.Semba RD, Muhilal, West KP Jr, Natadisastra G, Eisinger W, Lan Y, Sommer A. Hyporetinolemia and acute phase proteins in children with and without xerophthalmia. Am J Clin Nutr. 2000;72:146–53. [DOI] [PubMed] [Google Scholar]
- 15.Kongsbak K, Wahed MA, Friis H, Thilsted SH. Acute-phase protein levels, diarrhoea, Trichuris trichiura and maternal education are predictors of serum retinol: a cross-sectional study of children in a Dhaka slum, Bangladesh. Br J Nutr. 2006;96:725–34. [PubMed] [Google Scholar]
- 16.Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta-analysis. JAMA. 1993;269:898–903. [PubMed] [Google Scholar]
- 17.Bates CJ. Vitamin A. Lancet. 1995;345:31–5. [DOI] [PubMed] [Google Scholar]
- 18.Glasziou PP, Mackerras DE. Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ. 1993;306:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haskell MJ, Mazumder RN, Peerson JM, Jones AD, Wahed MA, Mahalanabis D, Brown KH. Use of the deuterated-retinol-dilution technique to assess total-body vitamin A stores of adult volunteers consuming different amounts of vitamin A. Am J Clin Nutr. 1999;70:874–80. [DOI] [PubMed] [Google Scholar]
- 20.Panel on Micronutrients, Institute of Medicine (U.S.). Food and Nutrition Board: DRI, dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: a report of the Panel on Micronutrients. National Academy Press, Washington, DC; 2001.
- 21.Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. Men with low vitamin A stores respond adequately to primary yellow fever and secondary tetanus toxoid vaccination. J Nutr. 2008;138:2276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI Jr, Gammon RB Jr. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr. 1994;60:388–92. [DOI] [PubMed] [Google Scholar]
- 23.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR III, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr. 1989;49:713–6. [DOI] [PubMed] [Google Scholar]
- 24.Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, Williams F, Butterworth DE. Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. J Appl Physiol. 1998;84:1252–9. [DOI] [PubMed] [Google Scholar]
- 25.Saresella M, Roda K, Speciale L, Taramelli D, Mendozzi E, Guerini F, Ferrante P. A flow cytometric method for the analysis of phagocytosis and killing by polymorphonuclear leukocytes. Ann N Y Acad Sci. 1997;832:53–61. [DOI] [PubMed] [Google Scholar]
- 26.Hermann C, von Aulock S, Graf K, Hartung T. A model of human whole blood lymphokine release for in vitro and ex vivo use. J Immunol Methods. 2003;275:69–79. [DOI] [PubMed] [Google Scholar]
- 27.Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002;168:4495–503. [DOI] [PubMed] [Google Scholar]
- 28.Albers R, Antoine JM, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd B, Samartin S, Sanderson IR, Van Loo J, et al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr. 2005;94:452–81. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z, Murasko DM, Ross AC. The role of vitamin A in natural killer cell cytotoxicity, number and activation in the rat. Nat Immun. 1994;13:29–41. [PubMed] [Google Scholar]
- 30.Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol. 1996;80:S63–72. [DOI] [PubMed] [Google Scholar]
- 31.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. [DOI] [PubMed] [Google Scholar]
- 32.Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102:886–98. [DOI] [PubMed] [Google Scholar]
- 34.Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005;CD001479. [DOI] [PMC free article] [PubMed]
- 35.Wiysonge CS, Shey MS, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2005;CD003648. [DOI] [PubMed]
- 36.Bresee JS, Fischer M, Dowell SF, Johnston BD, Biggs VM, Levine RS, Lingappa JR, Keyserling HL, Petersen KM, et al. Vitamin A therapy for children with respiratory syncytial virus infection: a multicenter trial in the United States. Pediatr Infect Dis J. 1996;15:777–82. [DOI] [PubMed] [Google Scholar]
- 37.Seguin-Devaux C, Hanriot D, Dailloux M, Latger-Cannard V, Zannad F, Mertes PM, Longrois D, Devaux Y. Retinoic acid amplifies the host immune response to LPS through increased T lymphocytes number and LPS binding protein expression. Mol Cell Endocrinol. 2005;245:67–76. [DOI] [PubMed] [Google Scholar]
- 38.Twining SS, Schulte DP, Wilson PM, Fish BL, Moulder JE. Retinol is sequestered in the bone marrow of vitamin A-deficient rats. J Nutr. 1996;126:1618–26. [DOI] [PubMed] [Google Scholar]
- 39.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–204. [DOI] [PubMed] [Google Scholar]
- 40.Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I. Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol. 2004;150:910–6. [DOI] [PubMed] [Google Scholar]
- 41.Herder C, Hauner H, Kempf K, Kolb H, Skurk T. Constitutive and regulated expression and secretion of interferon-gamma-inducible protein 10 (IP-10/CXCL10) in human adipocytes. Int J Obes (Lond). 2006;31:403–10. [DOI] [PubMed]
- 42.ter Meulen J, Sakho M, Koulemou K, Magassouba N, Bah A, Preiser W, Daffis S, Klewitz C, Bae HG, et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis. 2004;190:1821–7. [DOI] [PubMed] [Google Scholar]
- 43.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, McCarthy K, Johnson C, Ermak T, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006;103:6694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangur V, Simons FE, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. FASEB J. 1998;12:705–13. [DOI] [PubMed] [Google Scholar]
- 46.Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr. 2004;134:2660–6. [DOI] [PubMed] [Google Scholar]
- 47.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. [DOI] [PubMed] [Google Scholar]
- 48.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal Th-17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. [DOI] [PubMed]
- 49.von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 51.Bleijs DA, de Waal-Malefyt R, Figdor CG, van Kooyk Y. Co-stimulation of T cells results in distinct IL-10 and TNF-alpha cytokine profiles dependent on binding to ICAM-1, ICAM-2 or ICAM-3. Eur J Immunol. 1999;29:2248–58. [DOI] [PubMed] [Google Scholar]
- 52.Kim BH, Kang KS, Lee YS. Effect of retinoids on LPS-induced COX-2 expression and COX-2 associated PGE(2) release from mouse peritoneal macrophages and TNF-alpha release from rat peripheral blood mononuclear cells. Toxicol Lett. 2004;150:191–201. [DOI] [PubMed] [Google Scholar]
- 53.Motomura K, Sakai H, Isobe H, Nawata H. Effects of retinoids on the production of tumour necrosis factor-alpha and nitric oxide by lipopolysaccharide-stimulated rat Kupffer cells in vitro: evidence for participation of retinoid X receptor signalling pathway. Cell Biochem Funct. 1997;15:95–101. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Allen C, Ballow M. Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol. 2007;27:193–200. [DOI] [PubMed] [Google Scholar]
- 55.Reinhardt B, Jaspert R, Niedrig M, Kostner C, L'Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56:159–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.