Abstract
We examined the cross-sectional association between plasma 25-hydroxyvitamin D [25(OH)D] and markers of the insulin resistant phenotype. Plasma 25(OH)D concentrations were measured in 808 nondiabetic participants of the Framingham Offspring Study. Outcome measures included fasting and 2-h post 75-g oral glucose tolerance test (OGTT) glucose and insulin; these were used to calculate the homeostatic model assessment-insulin resistance (HOMA-IR) and insulin sensitivity index (ISI0,120). We also measured plasma adiponectin, triacylglycerol, and HDL cholesterol concentrations as markers of the insulin-resistant phenotype. After adjusting for age, sex, BMI, waist circumference, and current smoking status, plasma 25(OH)D concentration was inversely associated with fasting plasma glucose and insulin concentrations, and HOMA-IR. Compared with the participants in the lowest tertile category of plasma 25(OH)D, those in the highest tertile category had a 1.6% lower concentration of fasting plasma glucose (P-trend = 0.007), 9.8% lower concentration of fasting plasma insulin (P-trend = 0.001), and 12.7% lower HOMA-IR score (P-trend < 0.001). After adjusting for age and sex, plasma 25(OH)D was positively associated with ISI0,120, plasma adiponectin, and HDL cholesterol and inversely associated with plasma triacylglycerol, but these associations were no longer significant after further adjustment for BMI, waist circumference, and current smoking status. 25(OH)D and 2-h post-OGTT glucose were not associated. Among adults without diabetes, vitamin D status was inversely associated with surrogate fasting measures of insulin resistance. These results suggest that vitamin D status may be an important determinant for type 2 diabetes mellitus.
Introduction
Vitamin D deficiency is frequently observed in U.S. adults, in particular the elderly (1). Low vitamin D status can be caused by a number of factors, including insufficient cutaneous synthesis (due to limited sunlight exposure or aging), inadequate intake and absorption of vitamin D, obesity, or darker skin (2,3). People living at higher latitudes (>35°) are especially at increased risk for vitamin D deficiency, because from November through February most of the UVB radiation from sunlight, which is required for cutaneous vitamin D synthesis, is absorbed by the atmosphere and does not reach the earth's surface (4).
There is accumulating evidence to suggest that poor vitamin D status may play a role in the development of type 2 diabetes mellitus (DM)7 (5,6). Cross-sectional studies have consistently demonstrated that blood 25-hydroxyvitamin D [25(OH)D] concentrations are lower in patients with DM (7–11). Prospective studies have showed that lower vitamin D intakes or lower 25(OH)D concentrations might increase the risk of type 2 DM (12,13).
Insulin resistance is a recognized precursor in the development of type 2 DM. Although a few observational studies have examined the relationship between 25(OH)D and insulin resistance represented by the homeostatic model assessment of insulin resistance (HOMA-IR) or insulin sensitivity derived from hyperglycemic clamp (11,14), no study to our knowledge has used measures from the oral glucose tolerance test (OGTT), which is commonly used for the diagnosis of type 2 DM, to assess insulin resistance. In the current study, we tested the hypothesis that plasma 25(OH)D concentrations are inversely associated with insulin resistance among nondiabetic adults using surrogate markers of the insulin resistant phenotype derived from fasting and post-oral glucose challenge measures. Using the OGTT measures, we examined the relations between 25(OH)D and 2-h post-OGTT glucose and insulin sensitivity index (ISI0,120). Compared with surrogate measures of insulin resistance based on fasting glucose and insulin, these measures based on 2-h post-OGTT are thought to better reflect the body's overall glycemic control and insulin resistance (15–17).
Research Design and Methods
Study population.
The Framingham Study was initiated in 1948 as a longitudinal, population-based study of cardiovascular disease. In 1971, 5135 offspring of original participants of the study and spouses of the offspring were recruited to participate in the Framingham Offspring Study. The participants were essentially all Caucasian. Members of the Framingham Offspring Study have returned approximately every 4 y for a physical examination, questionnaires, laboratory tests, and assessment of cardiovascular and other risk factors (18). During the 7th examination cycle (between 1998 and 2001), a total of 3539 participants underwent a standardized medical history and physical examination. We excluded individuals with diabetes based on previous diagnosis, a fasting plasma glucose level ≥7.0 mmol/L (126 mg/dL), or current use of insulin or hypoglycemic agents (n = 455). We also excluded those without fasting glucose or insulin measures (n = 273) or information on BMI (n = 8). After exclusions, a total of 2803 participants were eligible for the present investigation.
As part of an ancillary study, plasma 25(OH)D concentrations were measured in 808 of the 2803 eligible participants from September 1998 to May 1999 at the 7th offspring examination. With the exception of a small difference in age and sex distribution, there were no significant differences between participants who had a plasma 25(OH)D measure (n = 808) and those who were eligible but did not have a plasma 25(OH)D measure (n = 1995) with respect to BMI, waist circumference, physical activity, smoking, alcohol consumption, and calcium intake. Those who had a plasma 25(OH)D measure are more likely to be male (49.8 vs. 42.8%; P < 0.05) and younger (59.6 vs. 60.8 y; P < 0.05) compared with those who did not.
The institutional review boards for human research at Boston University and Tufts Medical Center approved the study protocol and procedures.
OGTT procedures.
A 75-g OGTT was administered during the 7th examination in a subsample of participants based on their OGTT results at the 5th examination cycle. All subjects with glucose intolerance [fasting plasma glucose, 6.1–6.9 mmol/L (110–125 mg/dL) or 2-h post-OGTT plasma glucose, 7.8–11.0 mmol/L (140–199 mg/dL)] at the 5th examination were offered the OGTT at the 7th examination. In addition, a subset of participants with normal glucose tolerance at the 5th examination [fasting plasma glucose <6.1 mmol/L (110 mg/dL) and 2-h post-OGTT plasma glucose <7.8 mmol/L (140 mg/dL)] were randomly selected to have an OGTT during the 7th examination using a block selection procedure based on sex and quintile categories of fasting plasma glucose. Among the 808 participants with plasma 25 (OH)D measures, 290 participated in the OGTT and thereby had 2-h post-OGTT glucose and insulin measures. Within the subset with plasma 25(OH)D measures (n = 808), those who had OGTT (n = 290) had a higher prevalence of impaired fasting glucose, defined as fasting plasma glucose ≥5.6 mmol/L (100mg/dL) (52.1 vs. 31.7%; P = 0.01) and higher BMI (28.5 vs. 27.5; P < 0.01) and waist circumference (100.4 vs. 96.7 cm; P < 0.01) than those who did not (n = 518). Those differences are expected, because participants with impaired glucose tolerance were over-represented among those who were offered OGTT at the 7th examination.
Laboratory methods.
Fasting blood samples were drawn after an 8- to 10-h overnight fast. Fasting plasma glucose and 2-h post-OGTT plasma glucose were measured with a hexokinase reagent kit (A-gent glucose test, Abbott Laboratories). Glucose assays were performed in duplicate; intra-assay CV was <3% (19). Fasting and 2-h post-OGTT plasma insulin were measured with an insulin assay specific to insulin and had no cross-reactivity with proinsulin or insulin split-products (Linco Research); the assay CV was <6.8% (19). Plasma adiponectin concentrations were measured by ELISA (R&D Systems); intra-assay CV was 5.8%. Fasting plasma lipid measures included enzymatic measurement of triacylglycerol (20) and the measurement of the HDL-cholesterol fraction after precipitation of LDL and VLDL cholesterol with dextran sulfan magnesium (21). Plasma 25(OH)D concentration was determined by RIA (Diasorin); total CV for control plasma 25(OH)D of 36 and 137 nmol/L were 8.5 and 13.2%, respectively (22). All the participants included in this study had plasma 25(OH)D and fasting glucose measurements (n = 808); of those, 805 had fasting insulin, 558 had plasma adiponectin, 806 had triacylglycerol and HDL cholesterol measurements, and 290 had 2-h post-OGTT glucose and insulin measures.
Surrogate measures of insulin resistance and sensitivity.
We used the HOMA-IR as a surrogate measure of insulin resistance and ISI0,120 as a surrogate measure of insulin sensitivity. HOMA-IR was calculated using the following formula (23):
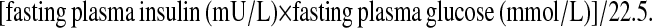 |
Higher values of HOMA-IR indicate greater insulin resistance.
Using post-glucose challenge data, ISI0,120 was calculated using the following formula (17,24):
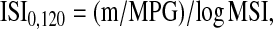 |
where m = [75,000 mg + (fasting glucose – 2-h post-OGTT glucose) × 0.19 × body weight (kg)]/120 min, which represents the glucose uptake rate in peripheral tissues (mg/min); mean plasma glucose (MPG) is the mean of fasting and 2-h post-OGTT glucose concentrations (mg/dL); m/MPG represents the metabolic clearance rate; and MSI (mean serum insulin) is the mean of fasting and 2-h post-OGTT insulin concentrations (mU/L). ISI0,120 reflects peripheral insulin resistance and glucose disposal and lower values indicate insulin resistance.
Dietary assessment.
Usual dietary intakes for the previous year were assessed using the 126-item semiquantitative Harvard FFQ (version 88GP) (25). The questionnaires were mailed to participants before the examination and they were asked to bring the completed questionnaire with them to their scheduled appointment. The FFQ consists of a list of foods with a standardized serving size and a selection of 9 frequency categories ranging from never or <1 serving/mo to >6 servings/d. Nutrient intakes were calculated at the Harvard Channing Laboratory by multiplying the frequency of consumption of each unit of food from the FFQ by the nutrient content of the specified portion. Separate questions about vitamin and mineral supplement use and type of breakfast cereal most commonly consumed were also included in the questionnaire. FFQ with reported energy intakes <2.51 MJ/d (600 kcal/d) for men and women, or >16.74 MJ/d (4000 kcal/d) for women or >17.57 MJ/d (4200 kcal/d) for men, or with >12 food items left blank were considered invalid. The FFQ has been shown to be valid for both nutrients and foods (25,26).
Covariate measurements.
Height and weight were measured while participants were standing. BMI was calculated as kg/m2. Additional covariate information included age, sex, current smoking status, and waist circumference, which was measured at the umbilicus while the participant was standing with the tape measure parallel to the floor.
Statistical analysis.
All statistical analyses were performed using SAS version 9.1 (SAS Institute). We used ANCOVA to display the differences in subject characteristics and outcome variables across tertile categories of plasma 25(OH)D. Because fasting insulin, HOMA-IR, triacylglycerol, and adiponectin were skewed, we used natural logarithmic transformations to normalize the distributions of these outcome variables. A multiple linear regression model treating plasma 25(OH)D concentration as a continuous variable was used to calculate the P-trend. The covariates included age, sex, BMI (continuous), waist circumference (continuous), and current smoking status (yes/no). Because adiposity is a strong determinant of outcomes and strongly influences 25(OH)D concentration, we included both BMI and waist circumference as covariates to limit the possibility of residual confounding by adiposity. To examine whether calcium intake is an effect modifier on the relationship between plasma 25(OH)D and outcomes, we tested the first-order interactions between plasma 25(OH)D and calcium intake. No interaction was found between these 2 variables. A P-value < 0.05 was considered significant. We did not conduct multiple comparisons adjustment for P-values, because we are testing one hypothesis and all the outcome variables are inter-correlated, reflecting insulin resistant phenotypes. We repeated the analyses described above with vitamin D intake as our exposure and energy intake as an additional covariate.
Results
A total of 402 men and 406 women with a mean age of 59.6 ± 0.3 y were included in the present analysis. The mean plasma 25(OH)D concentration was 47.4 ± 0.6 nmol/L (range, 5.5–127.3). Plasma 25(OH)D was positively associated with age, calcium intake, vitamin D intake, and vitamin D supplement use and inversely associated with waist circumference and BMI (Table 1). Current smokers tended to have lower plasma 25(OH)D concentrations. Plasma 25(OH)D concentrations were not related to sex, alcohol intake, total energy intake, or physical activity.
TABLE 1.
Characteristics of the participants by tertile categories of plasma 25(OH)D in the Framingham Offspring Study1
| Plasma 25(OH)D tertile category
|
||||
|---|---|---|---|---|
| T1 | T2 | T3 | P-trend | |
| Participants, n (%) | 270 (33.4) | 268 (33.2) | 270 (33.4) | |
| Plasma 25(OH)D, nmol/L | 30.2 (5.5–38.6) | 46.2 (38.7–53.2) | 63.5 (53.4–127.3) | |
| Age, y | 59.1 ± 9.2 | 59.5 ± 9.2 | 60.2 ± 9.2 | 0.03 |
| Women, n (%) | 140 (51.9) | 130 (48.5) | 136 (50.4) | 0.93 |
| Waist circumference,2cm | 100.7 ± 13.0 | 98.6 ± 12.9 | 94.9 ± 12.8 | <0.001 |
| BMI,2kg/m2 | 28.8 ± 5.1 | 28.1 ± 5.1 | 26.7 ± 5.1 | <0.001 |
| Current smoker,2n (%) | 41 (15.0) | 29 (10.8) | 22 (8.3) | <0.001 |
| Alcohol intake,2g/d | 11.8 ± 15.4 | 9.5 ± 15.2 | 10.1 ± 15.1 | 0.13 |
| Total energy intake,2kJ/d | 8057 ± 3098 | 8119 ± 3041 | 7715 ± 3042 | 0.43 |
| Calcium intake,3mg/d | 852 ± 473 | 1069 ± 465 | 1164 ± 465 | <0.001 |
| Vitamin D intake,3μg/d | 7.8 ± 7.4 | 11.9 ± 7.2 | 13.5 ± 7.2 | <0.001 |
| Vitamin D supplement user, n (%) | 73 (26.8) | 148 (55.5) | 168 (62.2) | <0.001 |
| Physical activity score,2MET-hours/d | 37.1 ± 6.7 | 38.0 ± 6.7 | 37.6 ± 6.9 | 0.28 |
Values are means ± SD, n (%), or medians (range).
Adjusted for age and sex.
Adjusted for age, sex, and total energy intake.
After being adjusted for age and sex, plasma 25(OH)D was inversely associated with fasting glucose, fasting insulin, HOMA- IR, and triacylglycerol and positively associated with ISI0,120, adiponectin, and HDL cholesterol (Table 2). After additional adjustment for BMI, waist circumference, and smoking, the inverse associations between 25(OH)D and fasting glucose, fasting insulin, and HOMA-IR were attenuated but remained significant; however, the associations with ISI0,120, adiponectin, triacylglycerol, and HDL cholesterol were no longer significant. The BMI and waist circumference were the main factors responsible for the attenuation. Compared with the participants in the lowest tertile category of plasma 25(OH)D, those in the highest tertile category had a 1.6% lower concentration of fasting glucose, 9.8% lower concentration of fasting insulin, and 12.7% lower HOMA-IR score. Plasma 25(OH)D and 2-h post-OGTT glucose were not significantly associated. Additional adjustment for physical activity did not affect any of the observed associations (results not shown).
TABLE 2.
Association between surrogate markers of glucose tolerance and insulin resistance and plasma 25(OH)D concentrations in the Framingham Offspring Study1
| Plasma 25(OH)D tertile category
|
|||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | β2 | P-trend | |
| Fasting plasma glucose (n = 808), mmol/L | |||||
| Model 1 | 5.44 (5.37–5.50) | 5.38 (5.32–5.45) | 5.27 (5.21–5.34) | −0.0047 | <0.001 |
| Model 23 | 5.45 (5.38–5.51) | 5.38 (5.32–5.44) | 5.27 (5.21–5.33) | −0.0050 | <0.001 |
| Model 34 | 5.41 (5.35–5.47) | 5.37 (5.31–5.43) | 5.32 (5.25–5.38) | −0.0028 | 0.007 |
| 2-h post-OGTT glucose (n = 290), mmol/L | |||||
| Model 1 | 7.05 (6.63–7.48) | 6.64 (6.20–7.08) | 6.68 (6.20–7.17) | −0.0076 | 0.33 |
| Model 23 | 7.10 (6.70–7.50) | 6.68 (6.27–7.10) | 6.58 (6.12–7.04) | −0.0122 | 0.10 |
| Model 34 | 6.97 (6.58–7.36) | 6.70 (6.31–7.10) | 6.74 (6.29–7.18) | −0.0051 | 0.49 |
| Fasting plasma insulin (n = 805), pmol/L | |||||
| Model 1 | 97.4 (91.8–103.3) | 91.2 (85.9–96.7) | 79.2 (74.7–84.0) | −0.00545 | <0.001 |
| Model 23 | 97.8 (92.2–103.7) | 91.0 (85.8–96.5) | 79.1 (74.6–83.9) | −0.0055 | <0.001 |
| Model 34 | 92.9 (88.2–97.9) | 89.9 (85.3–94.6) | 83.8 (79.6–88.3) | −0.0028 | 0.001 |
| HOMA-IR (n = 805) | |||||
| Model 1 | 3.59 (3.34–3.86) | 3.30 (3.06–3.55) | 2.74 (2.55–2.95) | −0.00705 | <0.001 |
| Model 23 | 3.61 (3.36–3.88) | 3.29 (3.06–3.54) | 2.74 (2.55–2.94) | −0.0072 | <0.001 |
| Model 34 | 3.38 (3.17–3.61) | 3.24 (3.04–3.45) | 2.95 (2.77–3.15) | −0.0037 | <0.001 |
| ISI0,120 (n = 290) | |||||
| Model 1 | 21.6 (20.2–22.9) | 23.2 (21.8–24.6) | 23.4 (21.8–24.9) | 0.0383 | 0.13 |
| Model 23 | 21.4 (20.1–22.7) | 23.1 (21.8–24.4) | 23.6 (22.2–25.1) | 0.0616 | 0.03 |
| Model 34 | 21.9 (20.7–23.1) | 23.0 (21.8–24.2) | 23.0 (21.6–24.4) | 0.0233 | 0.31 |
| Plasma adiponectin (n = 558), mg/L | |||||
| Model 1 | 8.4 (7.7–9.1) | 8.3 (7.6–9.1) | 10.0 (9.1–11.1) | 0.00455 | 0.002 |
| Model 23 | 8.4 (7.9–9.0) | 8.6 (7.9–9.3) | 9.6 (8.8–10.5) | 0.0034 | 0.007 |
| Model 34 | 8.7 (8.1–9.3) | 8.5 (7.9–9.2) | 9.2 (8.5–10.1) | 0.0020 | 0.12 |
| Plasma triacylglycerol (n = 678),6mmol/L | |||||
| Model 1 | 1.36 (1.27–1.45) | 1.23 (1.15–1.31) | 1.16 (1.09–1.24) | −0.00335 | 0.002 |
| Model 23 | 1.37 (1.28–1.46) | 1.23 (1.15–1.31) | 1.16 (1.09–1.24) | −0.0035 | 0.001 |
| Model 34 | 1.31 (1.23–1.39) | 1.22 (1.14–1.30) | 1.21 (1.14–1.29) | −0.0012 | 0.24 |
| Plasma HDL cholesterol (n = 678),6mmol/L | |||||
| Model 1 | 1.35 (1.30–1.40) | 1.38 (1.33–1.44) | 1.44 (1.39–1.49) | 0.0026 | 0.002 |
| Model 23 | 1.33 (1.29–1.38) | 1.37 (1.33–1.42) | 1.43 (1.38–1.47) | 0.0026 | 0.001 |
| Model 34 | 1.36 (1.31–1.40) | 1.38 (1.33–1.43) | 1.40 (1.36–1.45) | 0.0014 | 0.07 |
Values for fasting glucose, 2-h post-OGTT glucose, ISI0,120, and HDL are adjusted means (95% CI). Values for fasting insulin, HOMA-IR, adiponectin, and triacylglycerol are adjusted geometric means (95% CI).
Linear regression coefficient (slope) relating the insulin resistant phenotype to plasma 25(OH)D as a continuous variable.
Adjusted for age and sex.
Adjusted for age, sex, current smoking status (yes/no), BMI (continuous), and waist circumference (continuous).
Analysis of linear trend based on natural logarithmic transformations of fasting insulin, HOMA-IR, adiponectin, and triacylglycerol.
Excluded those using cholesterol lowering medications.
We repeated these analyses using vitamin D intake as our exposure, but it was not significantly associated with any of our markers of insulin resistance after adjusting for age and sex (results not shown).
Discussion
Vitamin D deficiency has been suspected as a risk factor for impaired glucose tolerance and diabetes among adults (10,11,27). Recent studies have suggested that poorer vitamin D status might increase the risk of type 2 DM (12,13). However, the pathways by which vitamin D could affect the risk of type 2 DM are not clear. In the current study, we found that among adults without diabetes, plasma 25(OH)D concentration was inversely associated with fasting glucose and fasting measures of insulin resistance such as fasting insulin and HOMA-IR.
There are several plausible mechanisms by which vitamin D status may affect insulin sensitivity. First, decreased vitamin D concentrations result in elevated concentrations of parathyroid hormone (PTH). Elevated PTH in turn affects insulin sensitivity by regulating the intracellular free calcium concentrations in target cells (28,29). Studies have shown that increased PTH concentrations were associated with impaired glucose tolerance and decreased insulin sensitivity (30,31). Second, vitamin D may play a role in insulin action by stimulating the expression of insulin receptor and thereby enhancing insulin responsiveness for glucose transport (32,33). Finally, vitamin D has a modulating effect on the immune system (34,35). Poorer vitamin D status might induce a higher inflammatory response, which is associated with insulin resistance (36,37). However, a recent report from the Framingham Offspring Study did not support the hypothesis that vitamin D status is associated with inflammation (38).
The inverse association between plasma 25(OH)D and fasting glucose is consistent with previous epidemiologic studies (11,39). Further, consistent with data from non-Hispanic Whites in the NHANES III (11), plasma 25(OH)D was not associated with 2-h post-OGTT glucose in our study. However, 2 smaller studies (n < 150) found that circulating 25(OH)D concentrations were inversely related to 30-min or 1-h postchallenge glucose during OGTT (27,40). Inconsistent results between studies may be attributed to different populations studied and different time points of glucose measures during OGTT. To our knowledge, the current study is the first conducted in a nondiabetic U.S. cohort to examine the relationship between plasma 25(OH)D concentrations and 2-h postchallenge measures of glucose.
Insulin resistance and impaired β-cell function are the 2 main defects that drive the development of type 2 DM. There is ample evidence that vitamin D plays a role in insulin synthesis and secretion from pancreatic β-cells in both humans and animal models (6). In contrast, the information on the role of vitamin D in insulin resistance is limited (11,14,41). Chiu et al. (14) observed that serum 25(OH)D concentrations were positively associated with insulin sensitivity, as determined from the 3-h hyperglycemic clamp in healthy glucose-tolerant subjects. In a double-blind, randomized, controlled trial designed for bone-related outcomes, Pittas et al. (41) found that among participants with impaired fasting glucose at baseline, those who took combined calcium-vitamin D supplements had a lower increase in HOMA-IR. However, in the normal fasting glucose subgroup, there was no difference in the change of HOMA-IR between treatment and placebo groups. We found that plasma 25(OH)D concentration was inversely associated with fasting insulin and HOMA-IR, but plasma 25(OH)D and ISI0,120 were not associated. Because only a small subset of subjects (n = 290) was available for this analysis, we might have limited power to detect a weak association between plasma 25(OH)D and ISI0,120. With a sample size of 290, we have a statistical power of 88.3% to detect a 15% difference in ISI0,120 between the lowest and highest tertile categories of plasma 25(OH)D. But if the desired difference is lowered to 10%, we have only 55.6% power to detect it. On the other hand, compared with fasting insulin and HOMA-IR, ISI0,120 tends to reflect peripheral insulin resistance and β-cell function. The different properties of the biomarkers offer a potential explanation for the inconsistency regarding their associations with 25(OH)D.
Adiponectin is one of the several adipokines produced by mature adipocytes. Adiponectin favors glucose uptake and free fatty acids oxidation in muscle and inhibits gluconeogenesis in liver, thereby improving insulin sensitivity (37). It is well established that plasma adiponectin levels are lower in obese individuals. To date, several cross-sectional studies have related various aspects of diet, such as dietary fiber, glycemic index, and load, and a Mediterranean diet pattern to plasma adiponectin (42–44). To our knowledge, this is the first study to examine the relationship between plasma 25(OH)D and adiponectin concentrations. There are several reasons that justify examining the relationship. First, because vitamin D receptor has been identified in preadipocytes (45,46), it is possible that 1,25(OH)2D, the active form of this vitamin, may regulate the adiponectin gene expression. Second, as a hormone, 1,25(OH)2D is thought to play a role in regulating the production of tumor necrosis factor-α (35), which is one of the proposed factors affecting the synthesis of adiponectin (47–49). According to our results, a 5.7% increase in adiponectin concentrations was observed among individuals in the higher tertile category of plasma 25(OH)D compared with those in the lowest. Although after adjustment for potential confounders the observed association was not significant (P = 0.12), we were unable to exclude a possible association between plasma 25(OH)D and adiponectin concentrations. With the smaller subset of subjects with both adiponectin and 25(OH)D measurements (n = 558), perhaps we had limited power to detect the modest association between these 2 variables.
In our population sample, the plasma 25(OH)D concentration was associated with its previously identified determinants (2–4, 50–52). We observed that it was positively associated with age, vitamin D intake, vitamin D supplement use, and calcium intake. The positive association between age and plasma 25(OH)D concentrations was due to increased vitamin D supplement use among older participants in the Framingham Offspring Study. Calcium intake is significantly associated with plasma 25(OH)D, because milk, an important dietary source of calcium, is also the main food source for vitamin D in the American diet. We observed that plasma 25(OH)D concentrations were inversely associated with BMI and waist circumference, which is thought to be due to a greater capacity for sequestering vitamin D in fat stores, resulting in lower circulating concentrations of plasma 25(OH)D (50). We also observed that smokers had lower circulating plasma 25(OH)D concentrations (51,52), but the reason for this association is not known. Sunlight is another very important determinant of plasma 25(OH)D concentrations. We did not have a direct measure of sunlight exposure in our population sample, but the month of the study blood draw is a useful surrogate measure and correlates strongly with plasma 25(OH)D. Except for vitamin D intake and month of blood draw, we included these determinants as potential confounders (age, BMI, and smoking) or modifiers (calcium intake) of the association between 25(OH)D and the markers of insulin resistant phenotype. We did not consider vitamin D intake or month of blood draw as potential cofounders in these analyses for 2 reasons. First, we know of no information to suggest that there are effects of vitamin D intake or sunlight on insulin resistance that might be independent of plasma 25(OH)D concentrations. Second, given the strong causal association between both vitamin D intake and sunlight exposure and 25(OH)D, we felt that adjusting for these determinants of 25(OH)D could result in overadjustment.
Some limitations need to be noted. First, random measurement error arising from the use of single measures of glucose, insulin, and 25(OH)D to characterize the usual status of these factors may have attenuated the observed associations. Second, we have limited power to detect differences in 2-h post-OGTT glucose and ISI0,120 because of the small sample size. Third, a causal relationship between vitamin D and insulin resistant phenotypes cannot be assessed in our study, because the cross-sectional study design cannot separate the timing of exposure and outcomes.
In conclusion, among individuals without diabetes, we showed that higher vitamin D status is inversely associated with fasting glycemia and fasting markers of insulin resistance. Our findings suggest a pathophysiologic mechanism that at least in part may explain the inverse association between vitamin D status and incident type 2 DM seen in observational studies. Further study of potential mechanisms in the setting of randomized trials with vitamin D in participants at higher risk for type 2 DM is needed.
Supported in part by the USDA agreement 58-1950-7-707 and support from the Framingham Heart Study of the National Heart Lung and Blood Institute of the NIH (contract N01-HC-25195). Dr. Meigs was supported by a Career Development Award from the American Diabetes Association and by NIDDK K24 DK080140. Dr. McKeown was supported in part by a scientist development award from the AHA. Dr. Pittas was supported by National Institute of Diabetes and Digestive and Kidney Disease grants R01DK076092 and R21DK078867. Dr. Economos was supported by Beverage Institute for Health and Wellness. Dr. Booth was supported by National institute of Aging (AG14759).
Author disclosures: E. Liu, A. G. Pittas, C. D. Economos, S. L. Booth, N. M. McKeown, and P. F. Jacques, no conflict of interest. J. B. Meigs has consulting agreements with GlaxoSmithKline, sanofi-aventis, Interleukin Genetics, Kalypsis, and Outcomes Science.
Abbreviations used: DM, diabetes mellitus; HOMA-IR, homeostatic model assessment of insulin resistance; ISI0,120, insulin sensitivity index; MPG, mean plasma glucose; OGTT, oral glucose tolerance test; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 2.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66:929–36. [DOI] [PubMed] [Google Scholar]
- 3.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf). 2005;62:265–81. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MA, Kimlin MG. Vitamin D, aging, and the 2005 Dietary Guidelines for Americans. Nutr Rev. 2006;64:410–21. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–57. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietschmann P, Schernthaner G, Woloszczuk W. Serum osteocalcin levels in diabetes mellitus: analysis of the type of diabetes and microvascular complications. Diabetologia. 1988;31:892–5. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, Arcaro G. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf). 2006;65:593–7. [DOI] [PubMed] [Google Scholar]
- 9.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. [DOI] [PubMed] [Google Scholar]
- 10.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–8. [DOI] [PubMed] [Google Scholar]
- 11.Scragg R, Sowers M, Bell C. Third National Health and Nutrition Examination S. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. [DOI] [PubMed] [Google Scholar]
- 12.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6. [DOI] [PubMed] [Google Scholar]
- 13.Mattila C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J, Reunanen A. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. [DOI] [PubMed] [Google Scholar]
- 14.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. [DOI] [PubMed] [Google Scholar]
- 15.Piche ME, Lemieux S, Perusse L, Weisnagel SJ. High normal 2-hour plasma glucose is associated with insulin sensitivity and secretion that may predispose to type 2 diabetes. Diabetologia. 2005;48:732–40. [DOI] [PubMed] [Google Scholar]
- 16.Leiter LA, Ceriello A, Davidson JA, Hanefeld M, Monnier L, Owens DR, Tajima N, Tuomilehto J. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27 Suppl B:S42–56. [DOI] [PubMed] [Google Scholar]
- 17.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB Sr, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes. 2005;54:3252–7. [DOI] [PubMed] [Google Scholar]
- 18.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 19.Meigs JB, Dupuis J, Herbert AG, Liu C, Wilson PW, Cupples LA. The insulin gene variable number tandem repeat and risk of type 2 diabetes in a population-based sample of families and unrelated men and women. J Clin Endocrinol Metab. 2005;90:1137–43. [DOI] [PubMed] [Google Scholar]
- 20.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 22.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–9. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 24.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–84. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 27.Baynes KC, Boucher BJ, Feskens EJ, Kromhout D. Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia. 1997;40:344–7. [DOI] [PubMed] [Google Scholar]
- 28.Naveh-Many T, Silver J. Vitamin D and the parathyroid. 2nd ed. London: Elsevier; 2004.
- 29.Christakos S, Gabrielides C, Rhoten WB. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989;10:3–26. [DOI] [PubMed] [Google Scholar]
- 30.Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP, Saad MF. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49:1501–5. [DOI] [PubMed] [Google Scholar]
- 31.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–55. [DOI] [PubMed] [Google Scholar]
- 32.Maestro B, Davila N, Carranza MC, Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–30. [DOI] [PubMed] [Google Scholar]
- 33.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–91. [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–62. [DOI] [PubMed] [Google Scholar]
- 35.Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11–20. [PubMed] [Google Scholar]
- 36.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. [DOI] [PubMed] [Google Scholar]
- 37.Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35. [DOI] [PubMed] [Google Scholar]
- 38.Shea MK, Booth SL, Massaro JM, Jacques PF, D‘Agostino RB Sr, Dawson-Hughes B, Ordovas JM, O’Donnell CJ, Kathiresan S, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Need AG, O'Loughlin PD, Horowitz M, Nordin BE. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf). 2005;62:738–41. [DOI] [PubMed] [Google Scholar]
- 40.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239–45. [DOI] [PubMed] [Google Scholar]
- 41.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. [DOI] [PubMed] [Google Scholar]
- 42.Mantzoros CS, Williams CJ, Manson JE, Meigs JB, Hu FB. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am J Clin Nutr. 2006;84:328–35. [DOI] [PubMed] [Google Scholar]
- 43.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005;28:1022–8. [DOI] [PubMed] [Google Scholar]
- 44.Qi L, Meigs JB, Liu S, Manson JE, Mantzoros C, Hu FB. Dietary fibers and glycemic load, obesity, and plasma adiponectin levels in women with type 2 diabetes. Diabetes Care. 2006;29:1501–5. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Lee DK, Choi E, Lee JW. Identification of a functional vitamin D response element in the murine Insig-2 promoter and its potential role in the differentiation of 3T3–L1 preadipocytes. Mol Endocrinol. 2005;19:399–408. [DOI] [PubMed] [Google Scholar]
- 46.Kamei Y, Kawada T, Kazuki R, Ono T, Kato S, Sugimoto E. Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3–L1 preadipocytes. Biochem Biophys Res Commun. 1993;193:948–55. [DOI] [PubMed] [Google Scholar]
- 47.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. [DOI] [PubMed] [Google Scholar]
- 48.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–55. [DOI] [PubMed] [Google Scholar]
- 49.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin: a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80. [DOI] [PubMed] [Google Scholar]
- 50.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–23. [DOI] [PubMed] [Google Scholar]
- 51.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–6. [DOI] [PubMed] [Google Scholar]
- 52.Szulc P, Garnero P, Claustrat B, Marchand F, Duboeuf F, Delmas PD. Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab. 2002;87:666–74. [DOI] [PubMed] [Google Scholar]


