Abstract
Ligands of the aryl hydrocarbon receptor (AhR) include the environmental xenobiotic 2,3,7,8 tetrachlorodibenzo(p)dioxin (TCDD), polycyclic aryl hydrocarbons, and the dietary compounds 3, 3′-diindolylmethane (DIM), a condensation product of indol-3-carbinol found in Brassica vegetables, and the phytoalexin resveratrol (RES). The AhR and its cofactors regulate the expression of target genes at pentameric (GCGTG) xenobiotic responsive elements (XRE). Because the activation of cyclooxygenase-2 (COX-2) expression by AhR ligands may contribute to inflammation and tumorigenesis, we investigated the epigenetic regulation of the COX-2 gene by TCDD and the reversal effects of DIM in MCF-7 breast cancer cells. Results of DNA binding and chromatin immunoprecipitation (ChIP) studies documented that the treatment with TCDD induced the association of the AhR to XRE harbored in the COX-2 promoter and control CYP1A1 promoter oligonucleotides. The TCDD-induced binding of the AhR was reduced by small-interfering RNA for the AhR or the cotreatment with synthetic (3-methoxy-4-naphthoflavone) or dietary AhR antagonists (DIM, RES). In time course ChIP studies, TCDD induced the rapid (15 min) occupancy by the AhR, the histone acetyl transferase p300, and acetylated histone H4 (AcH4) at the COX-2 promoter. Conversely, the cotreatment of MCF-7 cells with DIM (10 μmol/L) abrogated the TCDD-induced recruitment of the AhR and AcH4 to the COX-2 promoter and the induction of COX-2 mRNA and protein levels. Taken together, these data suggest that naturally occurring modulators of the AhR such as DIM may be effective agents for dietary strategies against epigenetic activation of COX-2 expression by AhR agonists.
Introduction
The aryl hydrocarbon receptor (AhR)5 is a ligand-dependent transcription factor (1), which is activated by a variety of structurally diverse xenobiotic and dietary bioactive food compounds (2). Upon activation, the AhR translocates to the nucleus and binds to xenobiotic responsive elements (XRE) harbored in promoters of target genes, including genes involved in detoxification, inflammation, and cancer (2–6). Diet is a primary vehicle of exposure to environmental AhR agonists such as polycyclic aryl hydrocarbons (PAH) (7) and dioxins (8), of which 2,3,7,8 tetrachlorodibenzo(p)dioxin (TCDD) is the prototype. In addition, diet comprises many naturally occurring ligands of the AhR, including curcumin (9), quercetin, and kaempferol (10,11). Of the many phytochemicals found in Brassica vegetables, acid-catalyzed metabolism of indole-3-carbinol (I3C) generates the condensation product 3, 3′-diindolylmethane (DIM), which exhibits antitumorigenic properties (12). Both I3C and DIM are ligands of the AhR, although they bind to the AhR with lower affinity compared with TCDD (13). Similarly, resveratrol (RES; trans-3,5,4′-trihydroxystilbene), a polyphenol found in wine and other sources, has antagonistic activity on the AhR (14) and has been shown to inhibit TCDD-induced expression of CYP1A1 (15).
Increased levels of cyclooxygenase-2 (COX-2) have been associated with inflammation and the etiology of a variety of tumors, including breast cancer (16–18). Therefore, the regulation of COX-2 is a viable target for dietary prevention strategies. Recently, we reported that the promoter activity of the COX-2 gene was induced by AhR ligands (5) and this activation was paralleled by increased binding of the AhR to XRE in the COX-2 promoter. The transcriptional activation of the COX-2 gene has been linked to changes in chromatin modifications, including histone acetylation by histone acetyl transferases (19,20). Similarly, the recruitment of the AhR to target promoter genes such as CYP1A1 has been associated with the recruitment of transcription coactivators and chromatin modifications (4,21). However, it is not known whether the binding of the AhR to the COX-2 promoter recruits coactivators and if this is associated with chromatin reorganizations at the COX-2 gene. Therefore, we hypothesized that the recruitment of the AhR to the COX-2 promoter induces chromatin modifications that activate COX-2 transcription, whereas dietary compounds that target the AhR may exert antagonistic effects and prevent the activation of COX-2 expression. To test this hypothesis, we challenged MCF-7 cells with the AhR ligand TCDD and examined the kinetics of recruitment of the AhR and its cofactor p300 to the COX-2 promoter along with changes in acetylated histone H4 (AcH4). We also examined the antagonistic effects of DIM on AhR and AcH4 recruitment to the COX-2 promoter and COX-2 expression. We conclude that DIM is an effective dietary agent to prevent epigenetic activation of COX-2 expression by AhR agonists.
Materials and Methods
Cell culture and reagents.
MCF-7 breast cancer cells were obtained from the American Type Culture Collection. Cells were maintained in DMEM-F12 (Sigma) supplemented with 10% fetal bovine serum (FBS) (Hyclone) and penicillin (100 kU/L)/streptomycin (100 mg/L) (Sigma) at 37°C, 95% relative humidity, and 5% CO2. TCDD was supplied by the National Cancer Institute, Division of Cancer Biology, Chemical and Physical Carcinogenesis Branch and distributed by Midwest Research Institute under a contract to National Cancer Institute (64 CFR 72090, 64 CFR 28205). The 3-methoxy-4-naphthoflavone (3M4NF) was synthesized by E. A. Mash (University of Arizona, Tucson, AZ). RES and DIM were purchased from Biomol. Treatments were carried out in DMEM containing 0.5% FBS and control treatments contained either dimethyl sulfoxide or ethanol vehicle. All other chemicals and cell culture media were from Sigma.
DNA-protein binding (pull-down) assay.
The binding of nuclear proteins to selected oligonucleotides was evaluated as described previously (5). Briefly, nuclear extracts were prepared using the NE-PER Nuclear and Cytoplasmic Extraction reagents (Pierce Biotechnology), quantitated using the BCA Protein Assay kit (Pierce Biotechnology), and incubated with biotin-labeled, double-stranded DNA oligonucleotides and streptavidin agarose beads. Following dissociation, bound nuclear proteins were separated by SDS-PAGE and analyzed by Western blot analysis with an antibody against the AhR (Santa Cruz Biotechnology no. sc-5579). Sequences of biotin-labeled oligonucleotides (Sigma) were: COX-2/XRE1, sense: 5′-CTGTCCCGACGTGACTTCCTC-3′, antisense: 5′-GAGGAAGTCACGTC GGGACAG-3′; CYP1A1: sense, 5′-CGGCT CTTGTCACGCAACTCCGAGCTCA-3′; antisense: 5′-TGAGCTCGGAGTTGCGTG AGAAGAGCCG-3′ (5).
Small inhibitory RNA for AhR.
Small inhibitory RNA (siRNA) duplexes for the AhR (siAhR) were synthesized by Dharmacon using the sequences previously described (AhR: 5′-UACUUCCACCUCAGUUGGCTT-3′ and 3′-TTAUGAAGGUGGAGUCAACCG-5′) (22). The siRNA duplexes were transfected into MCF-7 cells using Lipofectamine 2000 according to the protocol provided by the manufacturer (Invitrogen). Nontargeting siRNA (siCON) and siRNA for human glyceraldehyde-3-phosphate (GADPH) (siGADPH) were purchased from Dharmacon. Following transfection, the media was replaced with DMEM (10% FBS) and cells were allowed to grow for 48 h. Cells were then treated with either a vehicle control or TCDD and harvested either to prepare total cell lysates for Western blot analysis or nuclear extracts for DNA protein-binding assays.
Chromatin immunoprecipitation assay and quantitative real-time PCR for chromatin immunoprecipitation studies.
Following treatment and formaldehyde cross-linking, MCF-7 cells were harvested and the chromatin immunoprecipitation (ChIP) assay was performed using the EZ ChIP kit according to the manufacturer's protocol (Millipore). Chromatin was immunoprecipitated with antibodies against the AhR (Biomol no. SA-210), p300 (Millipore no. 05-257), AcH4 (Millipore no. 06-598), or IgG as a negative control. Input and bound DNA were purified using the Qiagen Nucleotide Removal kit and amplified by quantitative real-time PCR (qPCR) using the SYBR Green PCR Reagents kit as described by the manufacturer (Applied Biosystems). Briefly, reactions were run at a final volume of 25 μL consisting of the following master mix: 12.5 μL of 2× SybrGreen buffer, 1 μL each of forward (5′-CAGCCTATTAAGCGTCGTCAC-3′) and reverse (5′-CCGTGTCTGGTCTGTACGTCT-3′) primers that flank the COX-2 XRE-1, nuclease free water, and 5 μL DNA purified from the ChIP assay. The standard curve was generated using a plasmid containing the COX-2 promoter. Bound DNA was normalized to input DNA.
RNA isolation and qPCR for COX-2 mRNA.
Total cellular RNA was extracted using Tri-Reagent (Molecular Research Center) according to the manufacturer's instructions. After synthesis of the first-strand cDNA by RT (Fermentes), qPCR was performed with the following primers: (COX-2 mRNA forward) 5′-AGGGTTGCTGGTGGTAGGAA -3′ and (COX-2 mRNA reverse) 5′- GGTCAATGGAAGCCTGTGATACT-3′. We used the 18S ribosomal RNA amplification product as an internal standard. The COX-2 standard curve was generated with the plasmid pSG5-COX-2 containing a cDNA for the human COX-2 gene.
Statistical analysis.
Statistical analysis was performed using Statview from the SAS Institute. Densitometry after Western blotting was performed using Kodak ID Image Analysis Software. Data from factorial experiments were analyzed by 2-way ANOVA. Post hoc multiple comparisons were run using Fisher's protected least significant different test. Data are presented as means ± SE and differences were considered significant at P ≤ 0.05.
Results
siRNA for AhR inhibits binding to the COX-2 promoter.
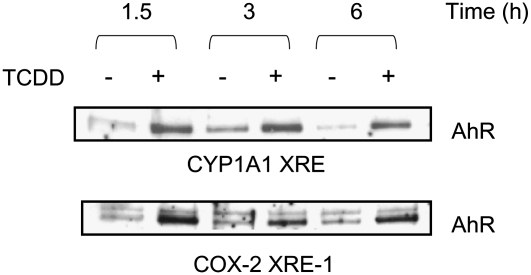
AhR ligands modulate transcription at target promoters by inducing the recruitment of the AhR and associated cofactors to XRE (21). Time course experiments (Fig. 1) indicated that the treatment of MCF-7 cells with TCDD induced the binding of the AhR as early as 1.5 h to XRE-1 in the COX-2 and control CYP1A1 (CYP1A1 XRE) oligonucleotides harboring the consensus XRE sequence 5′-GCGTG-3′. Compared with later time points (3 and 6 h), AhR binding to the COX-2 XRE-1 was higher at 1.5 h after treatment with TCDD. Therefore, the latter time point was used in subsequent binding studies.
FIGURE 1 .
TCDD stimulates the binding of the AhR to the XRE-1 of the COX-2 promoter. The DNA protein-binding assay was performed with nuclear extracts harvested from MCF-7 cells cultured in the presence or absence (vehicle) of TCDD (100 nmol/L) for 1.5, 3, or 6 h and control CYP1A1 XRE and COX-2 XRE-1 oligonucleotides.
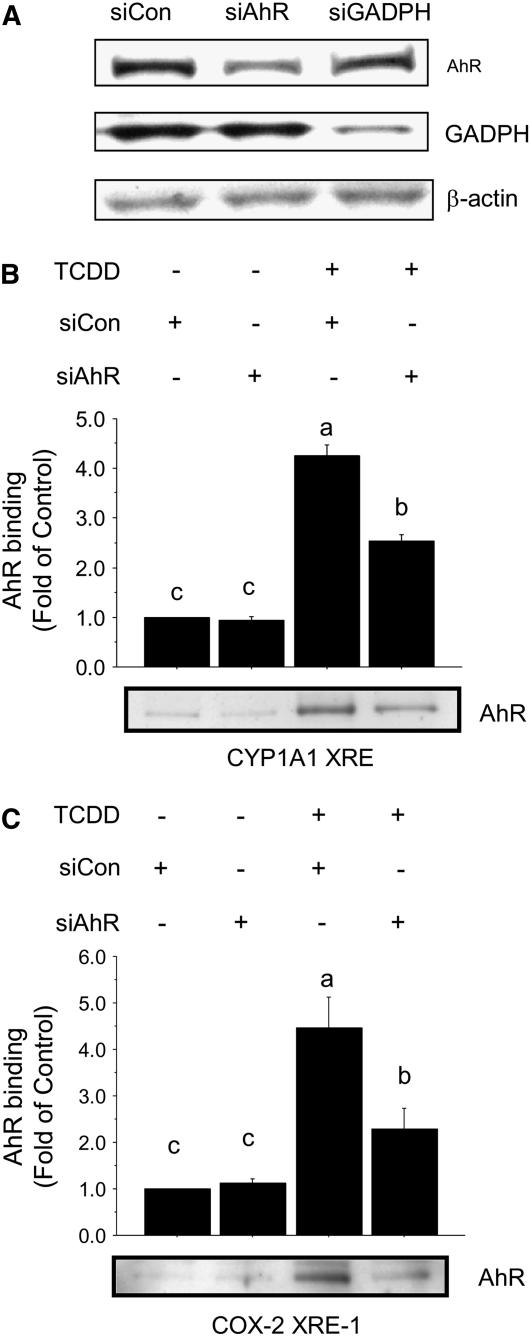
To further confirm that TCDD directed the recruitment of the AhR to the COX-2 XRE-1, we transfected MCF-7 cells with AhR-specific siRNA duplexes as described previously (22). siCON and siGADPH were used as negative and positive controls (Fig. 2A-C). The transfection of MCF-7 cells with siAhR reduced AhR protein levels and the binding of the AhR to the COX-2 XRE (Fig. 2B) and CYP1A1 XRE control oligonucleotides (Fig. 2C) (P < 0.01). Together, these results established that the COX-2 promoter harbors a putative XRE and the binding of the AhR to this sequence may be a target for treatment with siRNA for the AhR or AhR antagonists.
FIGURE 2 .
siRNA for the AhR antagonizes the recruitment of the AhR to COX-2 XRE. MCF-7 cells were transfected with siCON, siAhR, or siGADPH. (A) Whole-cell lysates were analyzed for AhR or GADPH protein levels. β-actin was used as an internal control for equal loading. (B,C) Nuclear extracts were harvested from MCF-7 cells transfected with either siCON or siAhR and treated in the presence or absence (vehicle) of TCDD (100 nmol/L). The DNA protein-binding assay was performed with control CYP1A1 XRE (B) or COX-2 XRE-1 oligonucleotides (C). Bars are means ± SE of triplicate measurements from 2 independent experiments. Means without a common letter differ, P < 0.05.
TCDD-induced kinetics of AhR and p300 recruitment, and histone-4 acetylation at the COX-2 promoter.
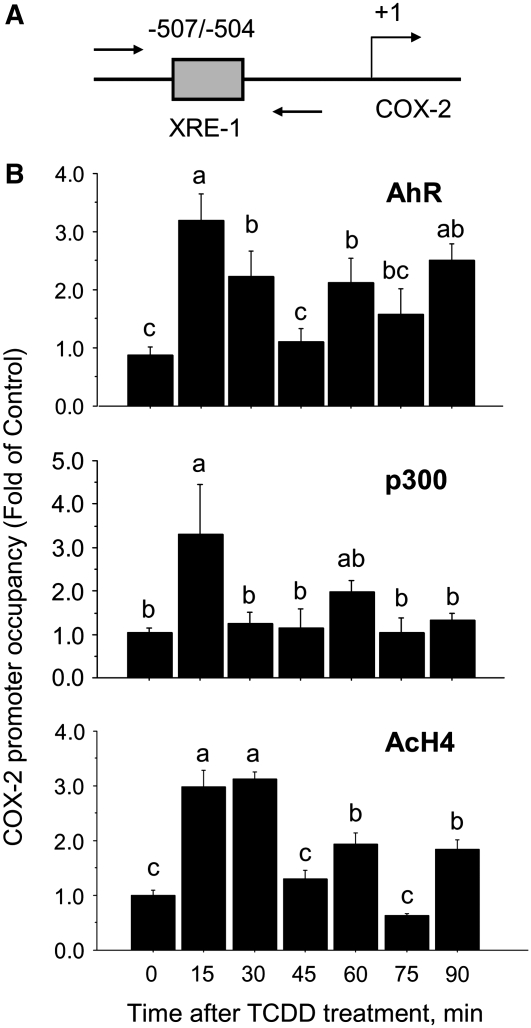
Using the ChIP assay, we examined in MCF-7 cells treated with TCDD the association of the AhR with the COX-2 promoter region harboring the XRE-1 (Fig. 3A). These experiments revealed that the occupancy of the AhR was influenced by the time of exposure to TCDD (Fig. 3B). Compared with control time 0, the recruitment of the AhR increased by ∼200% (P < 0.01) within 15 min and declined to near-control levels after 45 min. Subsequent increases (∼100-130%; P < 0.01) in AhR binding occurred at 60 and 90 min post-treatment with TCDD.
FIGURE 3 .
Kinetics of recruitment of AhR/p300 and AcH4. ChIP assays and qPCR were used to test the time-dependent recruitment of the AhR, p300, and AcH4 to the COX-2 promoter using primers flanking the COX-2 XRE-1 (A). (B) MCF-7 cells were treated with TCDD (100 nmol/L) for 0, 15, 30, 45, 60, 75, and 90 min. The ChIP assay was performed with antibodies for the AhR, p300, and AcH4. Bars are means ± SE of triplicate measurements from 2 independent experiments. Means without a common letter differ, P < 0.05.
Because p300 is a coactivator that interacts with the AhR (21), we examined the recruitment of p300 to the XRE-1 in the COX-2 promoter. Within 15 min, the treatment with TCDD stimulated an increase (∼200%; P < 0.01) in p300 occupancy at the COX-2 promoter. However, the recruitment of p300 was reduced to near basal levels at 30 min. An additional increase in p300 recruitment occurred at 60 min, although this accumulation did not differ compared with time 0 (Fig. 3B). Because the cofactor p300 possesses histone acetyl transferase activity, we monitored the recruitment of AcH4 to the COX-2 promoter. The association of AcH4 followed a cyclical pattern similar to that of AhR and p300 (Fig. 3B). The treatment with TCDD induced the occupancy of AcH4 at 15 and 30 min (∼200%; P < 0.01) and was followed by a significant (P < 0.01), albeit smaller, increase (∼80%) in AcH4 association at 60 and 90 min. These cumulative results indicated that binding of the AhR at the COX-2 promoter was coordinated with the recruitment of its cofactor p300 and acetylation of histone-H4.
DIM antagonizes the binding of the AhR to the COX-2 promoter.
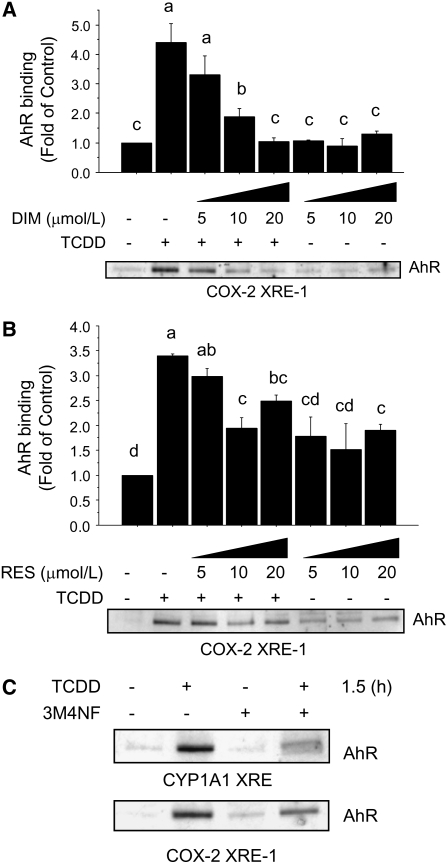
Previous studies have investigated the chemopreventative effects of synthetic and naturally occurring compounds that target the AhR (13,21). However, to date, no studies have characterized the effects of DIM on activation of the COX-2 gene by the AhR. The results of DNA pull-down experiments indicated that the treatment with DIM alone did not alter the basal binding of the AhR to the COX-2 promoter (Fig. 4A), but it inhibited TCDD-induced association of the AhR in a dose-dependent fashion (20 > 10 > 5 μmol/L; P < 0.01). In parallel experiments, only concentrations of 10 and 20 μmol/L RES were effective at reducing the TCDD-induced binding of the AhR to the COX-2 promoter oligonucleotide (Fig. 4B) but were less effective than equimolar doses of DIM (P < 0.01). Interestingly, the treatment with RES alone increased the association of the AhR with the COX-2 oligonucleotide. Using the synthetic AhR antagonist 3M4NF as a positive control for DIM and RES, we found that 3M4NF reduced the TCDD-induced binding of the AhR to the COX-2 and CYP1A1 promoter oligonucleotides (Fig. 4C). These cumulate data indicated that at equimolar concentrations DIM was more effective than RES in antagonizing the recruitment of the AhR to the COX-2/XRE-1.
FIGURE 4 .
Differential effects of DIM and RES on binding of AhR to COX-2 XRE-1. The DNA protein-binding assay was performed with nuclear extracts harvested from MCF-7 cells cultured for 1.5 h with or without TCDD (100 nmol/L) plus DIM (A) or RES (B). Bars are means ± SE of triplicate measurements from 2 independent experiments. Means without a common letter differ, P < 0.05. (C) Control binding of the AhR to CYP1A1 XRE or COX-2 XRE-1 after treatment of MCF-7 cells with TCDD, 3M4NF (100 nmol/L), or their combination for 1.5 h.
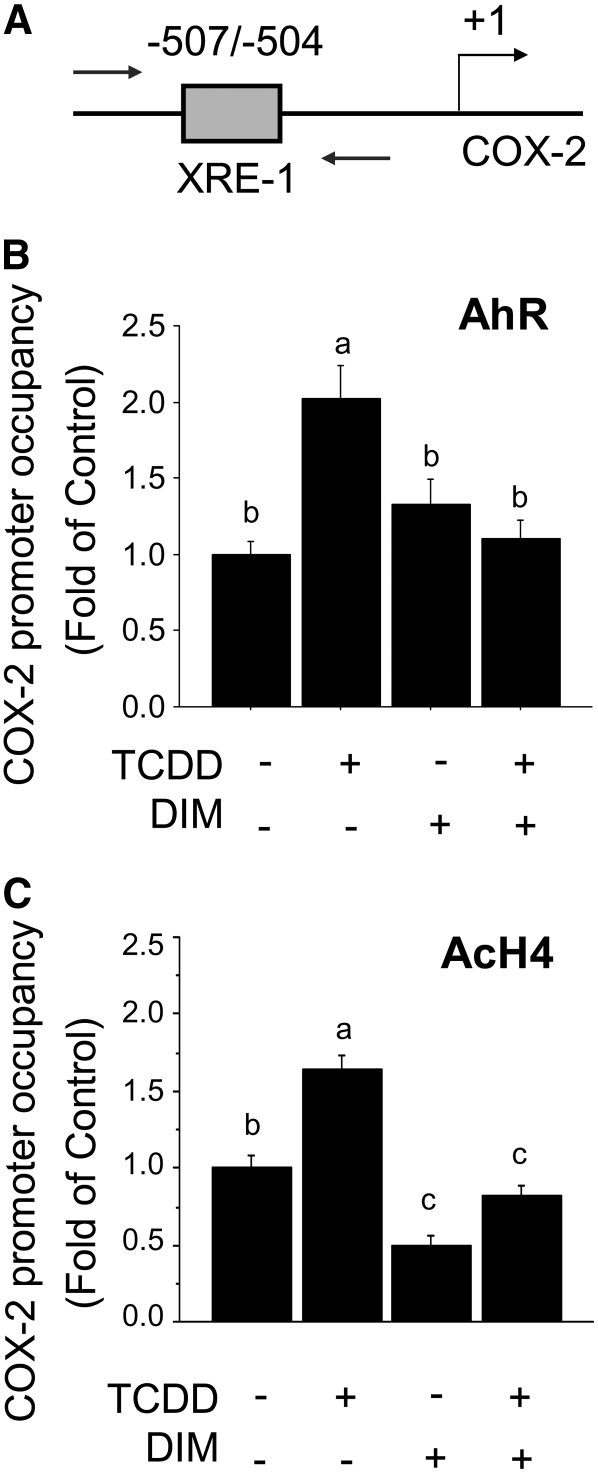
DIM reduces AhR-induced COX-2 expression and AhR/AcH4 recruitment.
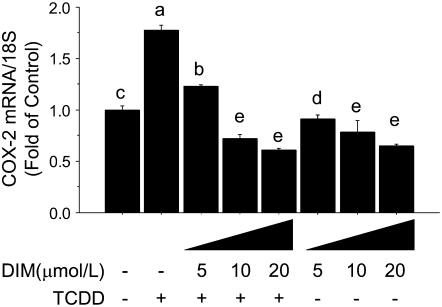
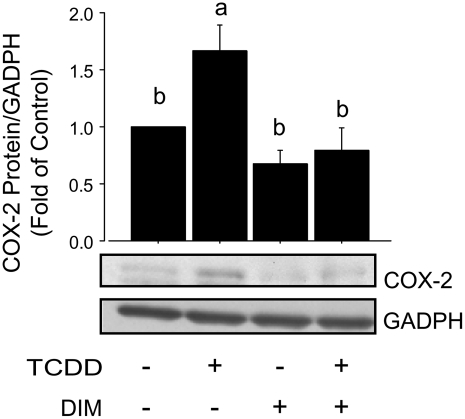
In ChIP experiments, we observed that the cotreatment with DIM reduced (P < 0.01) the TCDD-dependent recruitment of the AhR and AcH4 to the COX-2 XRE-1 (Fig. 5). To test the effects of DIM on COX-2 expression, we used qPCR to monitor changes in COX-2 mRNA levels. The treatment with DIM reduced both basal (10 and 20 > 5 μmol/L; P < 0.01) and TCDD-induced (10 and 20 > 5 μmol/L, P < 0.01) COX-2 mRNA levels (Fig. 6). Moreover, the cotreatment with DIM inhibited the TCDD-induced accumulation of COX-2 protein, as measured by Western blot analysis (Fig. 7). Taken together, these results indicated that DIM inhibited AhR and AcH4 recruitment to the COX-2 promoter and antagonized the stimulation of COX-2 mRNA and protein expression induced by the AhR-ligand, TCDD.
FIGURE 5 .
DIM prevents TCDD-induced recruitment of the AhR and AcH4 at the COX-2 promoter. ChIP assay and qPCR were used to test the effects of DIM (10 μmol/L) on recruitment of the AhR(A) and AcH4 (B) to the COX-2 XRE-1. Breast cancer MCF-7 cells were treated with TCDD (100 nmol/L), DIM, or their combination for 1.5 h. Bars are means ± SE of triplicate measurements from 4 independent experiments. Means without a common letter differ, P < 0.05.
FIGURE 6 .
DIM reduces TCDD-induced COX-2 mRNA expression. Levels of COX-2 mRNA were measured by qPCR and normalized against 18S ribosomal RNA following treatment of MCF-7 cells for 1.5 h with TCDD (100 nmol/L) alone or in combination with DIM (5, 10, and 20 μmol/L). Bars are means ± SE of triplicates from 2 independent experiments. Means without a common letter differ, P < 0.05.
FIGURE 7 .
DIM decreases TCDD-induced COX-2 protein levels. Western blot analyses were performed for COX-2 and GADPH (control) in cell lysates obtained from MCF-7 cells treated for 1.5 h with TCDD (100 nmol/L) in the presence or absence of DIM (10 μmol/L). Bars are means ± SE of triplicate measurements from 2 independent experiments. Means without a common letter differ, P < 0.05.
Discussion
Through diet and environmental pollution, humans are exposed to a wide variety of AhR ligands, including PAH, dioxins, natural compounds, and pharmaceuticals (2,23). Previous studies by other laboratories (24) and our own group (5) have documented that PAH and the dioxin compound TCDD induced the transcription of COX-2. Both the overexpression of COX-2 (25) and activation of the AhR (26) have been recognized to play a key role in the etiology of mammary tumorigenesis. Therefore, the present study addressed how AhR agonists may increase the expression of the proinflammatory COX-2 gene and the protective effects of dietary AhR antagonists.
The binding of the AhR to a core sequence (GCGTG) harbored in the COX-2 and control CYP1A1 promoters was induced by TCDD. Conversely, the transfection with siRNA for the AhR reduced AhR binding to COX-2 oligonucleotides. In addition, the association of the AhR with the COX-2 promoter and control CYP1A1 oligonucleotides was reduced by the cotreatment with the synthetic AhR antagonist 3M4NF. Our data corroborated those of previous studies documenting repression by 3M4NF of AhR nuclear translocation and COX-2 expression in human lung fibroblasts treated with cigarette smoke extracts (27). In ChIP experiments with MCF-7 cells, the TCDD-induced association of the AhR with the COX-2 promoter occurred rapidly (∼15 min). The increased occupancy of the AhR was paralleled by enhanced recruitment of p300 and AcH4. Patterns of rapid (∼15-30 min) recruitment of AhR, p300, and AcH4 have been reported in previous studies for the CYP1A1 promoter (21). Previous studies have documented increased binding of p300 and acetylation of histone H4 at the COX-2 promoter under diabetic conditions (19) or following the exposure of human bronchial epithelial cells to exhaust particles (20). Therefore, the coordinated recruitment of the AhR, p300, and histone H4 acetylation may play an important role in the activation of COX-2 expression.
The current findings suggest that the AhR-mediated activation of COX-2 expression may be an important molecular target for dietary intervention. Dietary compounds that have been investigated for their role as putative AhR ligands include naringenin, flavone, catechin, quercetin, kaempferol (10,11), RES (14,28), and red clover isoflavones (29). We examined the effects of DIM, a derivative of I3C found in cruciferous vegetables, and found that DIM inhibited the TCDD-induced binding of the AhR to the COX-2 promoter, with no effect on basal AhR recruitment. In ChIP experiments, we confirmed that the cotreatment with DIM (10 μmol/L) reduced the TCDD-induced occupancy of the AhR and AcH4 to the COX-2 promoter. Moreover, the cotreatment with DIM repressed the TCDD-induced expression of COX-2 mRNA and protein. One important question is whether or not DIM may reach sufficient levels in human serum to exert antagonistic effects against the AhR. Unfortunately, the information concerning the achievable levels of DIM in human is scarce. Nevertheless, previous investigations indicated that the concentrations of DIM and RES that were effective in this study (∼10 μmol/L) were similar to those used in previous investigations (28) and approximated dietary levels of phytochemicals (30) used to inhibit AhR activation by dioxin (31). Moreover, the compound DIM was the only I3C-derived product detectable in plasma samples of women receiving oral doses of 400-1200 mg I3C (32). Interestingly, a previous investigation of breast cancer MCF-7 cells reported that at higher concentrations (100 μmol/L), the treatment with DIM exerted agonistic effects and stimulated AhR recruitment to the CYP1A1 promoter and CYP1A1 mRNA expression (21). In contrast, in the current study, the treatment of MCF-7 cells with lower doses of DIM (5-10 μmol/L) did not change basal occupancy of the AhR at the COX-2 promoter while lowering basal COX-2 mRNA levels.
Our results complement those of in vivo investigations of RES, which reduced COX-2 expression in mammary (33) and esophageal (34) tumors and prevented mammary cancers in rats induced by the AhR-ligand 7,12-dimethylbenz[a]anthracene (35). Interestingly, we observed that DIM at equimolar concentrations (5-20 μmol/L) was more effective than RES at antagonizing the TCDD-induced recruitment of the AhR. In fact, RES increased basal AhR binding to the COX-2 promoter. The latter result could be due to the higher affinity of RES for the AhR (14,36) compared with that of DIM (13). These cumulative data point to relative binding affinity for the AhR and dose as important factors in determining whether natural dietary AhR ligands may exert agonistic or antagonistic effects.
In summary, our results document that the exposure to dietary AhR ligands resulting from xenobiotic sources such as the dioxin prototype, TCDD, induce COX-2 expression. Because constitutive activation of the AhR may contribute to mammary tumorigenesis (26), the present study illustrates a mechanism for upregulation of COX-2 expression through an AhR-dependent pathway and offers a potential target for prevention and treatment with naturally occurring dietary AhR antagonists. Whereas contradictory results from animal studies have raised caution against the use of DIM for cancer prevention in humans (37), this study suggests that DIM may exert important preventative properties against epigenetic activation of COX-2 expression by AhR agonists, including dioxins and PAH, found in processed foods and cooked meats. We are currently investigating the effects of AhR recruitment on other chromatin remodeling events such as histone phosphorylation and methylation at the COX-2 gene and the preventative effects of DIM against AhR-dependent COX-2 activation in preclinical models.
Acknowledgments
We thank E. A. Mash (Southwest Environmental Health Sciences Synthetic Core Facility, University of Arizona, Tucson, AZ) for synthesis of 3M4NF.
Supported in part by the Graduate Training Program in Toxicology and Toxicogenomics T32 ES-07091-24, NIEHS-NIH, Kraft American Society for Nutrition Predoctoral Fellowship (S. C. D.), and grants from the Arizona Biomedical Research Commission (100116, 0819, D. F. R.), and Susan G. Komen Breast Cancer Foundation (BCTR0707643, D. F. R.).
Author disclosures: S. C. Degner, A. J. Papoutsis, O. Selmin, and D. F. Romagnolo, no conflicts of interest.
Abbreviations used: AcH4, acetylated histone-H4; AhR, aryl hydrocarbon receptor; ChIP, chromatin immunoprecipitation assay; COX-2, cyclooxygenase-2; DIM, 3,3′-Diindolylmethane; FBS, fetal bovine serum; GADPH, glyceraldehyde-3-phosphate; I3C, indole-3-carbinol; 3M4NF, 3-methoxy-4-naphthoflavone; PAH, polycyclic aryl hydrocarbon; qPCR, quantitative real-time PCR; RES, resveratrol; siAhR, small inhibitory RNA duplexes for the AhR; siCON, nontargeting small inhibitory RNA; siGADPH, small inhibitory RNA for human glyceraldehyde-3-phosphate; siRNA, small inhibitory RNA; TCDD, 2,3,7,8 tetrachlorodibenzo(p)dioxin; XRE, xenobiotic responsive element.
References
- 1.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–34. [DOI] [PubMed] [Google Scholar]
- 3.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. [DOI] [PubMed] [Google Scholar]
- 4.Ko HP, Okino ST, Ma Q, Whitlock JP Jr. Dioxin-induced CYP1A1 transcription in vivo: the aromatic hydrocarbon receptor mediates transactivation, enhancer-promoter communication, and changes in chromatin structure. Mol Cell Biol. 1996;16:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degner SC, Kemp MQ, Hockings JK, Romagnolo DF. Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer MCF-7 cells: repressive effects of conjugated linoleic acid. Nutr Cancer. 2007;59:248–57. [DOI] [PubMed] [Google Scholar]
- 6.Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res. 2006;66:2224–32. [DOI] [PubMed] [Google Scholar]
- 7.Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin Immunol. 2001;98:2–10. [DOI] [PubMed] [Google Scholar]
- 8.Liem AK, Fürst P, Rappe C. Exposure of populations to dioxins and related compounds. Food Addit Contam. 2000;17:241–59. [DOI] [PubMed] [Google Scholar]
- 9.Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56:197–206. [DOI] [PubMed] [Google Scholar]
- 10.Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340:715–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Puppala D, Gairola CG, Swanson HI. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis. 2007;28:639–47. [DOI] [PubMed] [Google Scholar]
- 12.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–9. [DOI] [PubMed] [Google Scholar]
- 13.Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–36. [DOI] [PubMed] [Google Scholar]
- 14.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–90. [PubMed] [Google Scholar]
- 15.Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh YJ. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–13. [DOI] [PubMed] [Google Scholar]
- 16.Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–5. [PubMed] [Google Scholar]
- 17.Davies G, Salter J, Hills M, Martin LA, Sacks N, Dowsett M. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res. 2003;9:2651–6. [PubMed] [Google Scholar]
- 18.Oliveira VM, Piato S, Silva MA. Correlation of cyclooxygenase-2 and aromatase immunohistochemical expression in invasive ductal carcinoma, ductal carcinoma in situ, and adjacent normal epithelium. Breast Cancer Res Treat. 2006;95:235–41. [DOI] [PubMed] [Google Scholar]
- 19.Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091–7. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Bromberg PA, Samet JM. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol. 2007;37:232–9. [DOI] [PubMed] [Google Scholar]
- 21.Hestermann EV, Brown M. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol Cell Biol. 2003;23:7920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelrahim M, Smith R III, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol Pharmacol. 2003;63:1373–81. [DOI] [PubMed] [Google Scholar]
- 23.Fattore E, Fanelli R, Turrini A, di Domenico A. Current dietary exposure to polychlorodibenzo-p-dioxins, polychlorodibenzofurans, and dioxin-like polychlorobiphenyls in Italy. Mol Nutr Food Res. 2006;50:915–21. [DOI] [PubMed] [Google Scholar]
- 24.Miller ME, Holloway AC, Foster WG. Benzo-[a]-pyrene increases invasion in MDA-MB-231 breast cancer cells via increased COX-II expression and prostaglandin E2 (PGE2) output. Clin Exp Metastasis. 2005;22:149–56. [DOI] [PubMed] [Google Scholar]
- 25.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–9. [DOI] [PubMed] [Google Scholar]
- 26.Schlezinger JJ, Liu D, Farago M, Seldin DC, Belguise K, Sonenshein GE, Sherr DH. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–87. [DOI] [PubMed] [Google Scholar]
- 27.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;289:L391–9. [DOI] [PubMed] [Google Scholar]
- 28.Gouédard C, Barouki R, Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol. 2004;24:5209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medjakovic S, Jungbauer A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J Steroid Biochem Mol Biol. 2008;108:171–7. [DOI] [PubMed] [Google Scholar]
- 30.Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun YY, Hudson EA, Manson MM. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007;28:1274–304. [DOI] [PubMed] [Google Scholar]
- 31.Ashida H, Fukuda I, Yamashita T, Kanazawa K. Flavones and flavonols at dietary levels inhibit a transformation of aryl hydrocarbon receptor induced by dioxin. FEBS Lett. 2000;476:213–7. [DOI] [PubMed] [Google Scholar]
- 32.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–81. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12 dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–54. [PubMed] [Google Scholar]
- 34.Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–6. [DOI] [PubMed] [Google Scholar]
- 35.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amakura Y, Tsutsumi T, Nakamura M, Kitagawa H, Fujino J, Sasaki K, Toyoda M, Yoshida T, Maitani T. Activation of the aryl hydrocarbon receptor by some vegetable constituents determined using in vitro reporter gene assay. Biol Pharm Bull. 2003;26:532–9. [DOI] [PubMed] [Google Scholar]
- 37.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]