Abstract
In studies of marginal biotin deficiency induced experimentally in adults, increased urinary excretion of 3-hydroxyisovaleric acid (3HIA), which likely reflects decreased activity of the biotin-dependent enzyme β-methylcrotonyl-CoA carboxylase, and decreased activity of the biotin-dependent enzyme propionyl-CoA carboxylase (PCC) in peripheral blood lymphocytes have been validated as indices of biotin status. About half of pregnant women excrete increased amounts of urinary 3HIA. However, interpretation of urinary 3HIA excretion rates is problematic, because renal function is altered by pregnancy per se. In a recent pilot study, activity of PCC in peripheral blood lymphocytes was decreased in 18 of 22 pregnant women. In 4 of 4 pregnant women with decreased PCC activity, biotin supplementation caused increased PCC activity by a mean of 95%. Taken together, such studies provide evidence that a substantial proportion of pregnant women are marginally biotin deficient. In mice, degrees of biotin deficiency that are metabolically similar to those seen in pregnant women are very teratogenic. Moreover, in mice, a marginal degree of biotin deficiency in the dam causes a much more severe degree of deficiency in the fetus. These observations further raise concerns that biotin deficiency does occur and does cause human birth defects.
Teratogenesis related to nutrient deficiencies
Fetal malformations are a tragic and all too common event in the United States. For example, neural tube defects occur in ∼1 in 1000 births of first pregnancies, leading to ∼4000 affected pregnancies per year (1). Butterworth and Bendich (1) have speculated that a substantial number of birth defects are potentially related to nutrient deficiencies or may be preventable by nutrient supplementation. The hypothesis concerning a teratogenic role for additional and multiple nutrient deficiencies is supported by several studies (1). As reported by Czeizel and Dudás (2,3), women were given prenatal multivitamin supplements (which included folic acid as well as biotin); other women were given placebos. The incidence of major birth defects was smaller in the supplemented group (14.7 major birth defects per 1000 births) compared with the placebo group (28.3 per 1000), with a relative risk of 1.8 (CI = 1.23 – 3.09).
With regard to biotin, my posthoc analysis of the data from this classical study (2,3) suggested that biotin deficiency might be teratogenic in humans. Czeizel and Dudás observed that folic acid supplementation prior to and during pregnancy reduced the incidence of grouped neural tube defects from 6 in the ∼2000 women of the mineral supplement control group to zero in the ∼2000 women who received folic acid in a multivitamin supplement. This reduction was significant (P = 0.02) by Fisher's test. Of note, the vitamin supplement also included biotin. In analogy with grouping of all neural tube defects based on studies showing that folic acid deficiency causes a spectrum of neural tube defects in animals, we were guided by the results of our mouse studies to group isolated cleft palate with limb shortening. Multivitamin supplementation reduced the combined incidence of cleft palate and limb shortening from 7 to 1. This reduction approaches classical significance by Fisher's test (P = 0.07). According to the Centers for Disease Control and Prevention, the condition with highest prevalence from 1991 to 2001 was orofacial clefts, occurring in approximately 1 in every 1000 births (4) and affecting ∼6800 infants annually (5).
The etiology of orofacial clefts is complex, including multiple genetic and environmental factors. Although orofacial clefts are the subject of numerous studies, the genetic and other etiologic factors contributing to orofacial birth defects in humans remain largely unknown. Only ∼10% of orofacial clefts occur with other types of birth defects; the remainder are isolated, nonsyndromic birth defects (4). The nonsyndromic form is likely due to gene-environment interactions. Use of animal models, in particular mouse models, has led to important recent advances in our understanding of these disorders (6).
Teratogenesis caused by biotin deficiency
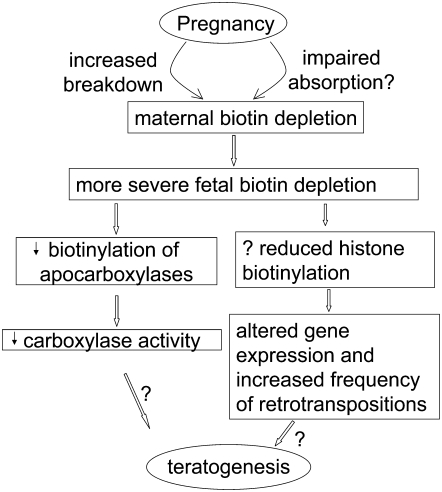
Although biotin deficiency severe enough to produce hair loss, dermatitis, or central nervous system dysfunction has never been reported in human pregnancy, several observations raise concern that marginal degrees of biotin deficiency might be teratogenic in humans (7). This discussion provides an overview of current knowledge and findings concerning biotin status during human pregnancy and attempts to relate potential mechanisms of teratogenesis caused by biotin deficiency (Fig. 1).
FIGURE 1 .
Relationship of biotin deficiency during pregnancy to teratogenesis. Biotin is catabolized at an increased rate during pregnancy and absorption from the intestine may be impaired, leading to maternal biotin depletion. Fetal biotin deficiency is more severe than maternal, leading to reduced biotinylation of biotin-dependent carboxylases and reduced carboxylase activity. Further, biotin deficiency may reduce histone biotinylation, which many alter gene expression and cause an increased frequency of retrotranspositions. The contribution of these 2 actions to teratogenesis is under study in several laboratories worldwide.
Biotin deficiency is teratogenic in several animal species at degrees of deficiency that produce no physical findings in pregnant animals, including chickens, turkeys, and mice (8). Biotin deficiency causes cleft lip and cleft palate and impairs skeletal long bone growth in mice. Weak human placental transport of biotin may predispose to human fetal biotin deficiency. Studies from our laboratory and others (9,10) provided evidence that the transport of biotin across the human placenta is slow and does not generate a substantial maternal-to-fetal gradient.
Reduced activity of the biotin-dependent enzymes acetyl-CoA carboxylase (ACC)4 I and II and propionyl-CoA carboxylase (PCC) can cause alterations of lipid metabolism and might theoretically lead to impaired synthesis of PUFA and prostaglandins. Arachidonic acid [20:4(n-6)] deficiency and prostaglandin deficiency are teratogenic. For example, the teratogenic effects of glucocorticoids or phenytoin (Dilantin), which cause cleft palate in susceptible strains of mice (11,12) act, at least in part, via arachidonic acid deficiency and prostaglandin deficiency. Acting through phospholipase A2-inhibitory protein, glucocorticoids and phenytoin inhibit the phospholipase A2-mediated release of arachidonic acid from membrane phospholipids (13). This deficiency of arachidonic acid leads to deficient synthesis of prostaglandin products in the cyclooxygenase pathway (e.g., prostaglandin E2) that are needed for proper palatal plate growth, elevation, and fusion. Arachidonic acid administered subcutaneously to the mouse dam reduces the incidence of glucocorticoid and phenytoin teratogenesis by half. Similar reduction in teratogenesis is caused by arachidonic acid provided in fetal culture (14). Moreover, cyclooxygenase inhibitors (e.g., indomethacin, aspirin, phenylbutazone) at high doses in fetal culture cause cleft palate directly; at lower doses, they reverse the ameliorating effects of arachidonic acid (15). Studies by Watkins et al. (16) have shown that the skeletal defects in biotin deficient chicks are caused by derangements of (n-6) fatty acid metabolism, particularly reduced metaphyseal prostaglandin E2. Effects on bone and cartilage fatty acid composition are likely to be relevant to mammals in general and to the human fetus in particular. In biotin-deficient infants (17) and biotin-deficient rats (18,19), abnormalities in (n-6) fatty acid composition and metabolism have been reported. Moreover, in a dietary interaction study conducted in rats (20), supplementation of PUFA almost completely prevented the cutaneous manifestations of biotin deficiency.
Effects on gene expression could be acting synergistically or in place of effects on carboxylase activity to mediate the teratogenic effects of biotin deficiency. As reported by Zempleni (21), biotin deficiency decreases the abundance of K12-biotinylated histone H4 (K12BioH4) and K9-biotinylated histone H2A (K9BioH2A) in human and animal retrotransposons. Decreased abundance of biotinylated histones at these loci increases the transcriptional activity of retrotransposons, the production of viral particles, and the frequency of retrotranspositions and chromosomal abnormalities. He hypothesized that genomic instability in biotin-deficient mice and humans may account for fetal malformations.
Whatever the mechanisms at the cellular and molecular level, Zempleni and Mock (8) have reviewed the strong evidence, including the pioneering observations of Watanabe, that maternal biotin deficiency is highly teratogenic in mice at degrees of deficiency that produce no signs or symptoms in the mouse dam.
In a study from our group in CD-1 mice (22), dam biotin status was controlled by feeding diets with varying egg white content. This and other animal studies described here were individually approved by the Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
Although no overt signs of deficiency appeared in the dams, biotin excretion decreased and 3-hydroxyisovaleric acid (3HIA) excretion increased with increasing egg white concentrations; the rates of cleft palate and limb hypoplasia approached 100% at egg white concentrations >5%. The following 3 control diets were used: 1) unpurified rodent diet, 2) 0% egg white, and 3) a 25% egg-white diet supplemented with enough biotin to occupy all the biotin-binding sites of avidin and still provide excess free biotin. All 3 groups had similar low rates of malformations (<3%).
Fetal biotin status correlated significantly with maternal biotin status as judged by hepatic biotin and PCC activity; however, PCC activity in deficient fetuses was reduced to ∼20% of deficient dams. In a subsequent investigation of the mechanism of reduced carboxylase activity in fetuses and dams (23), a 5% egg-white diet produced the expected high incidence of malformations without overt signs of deficiency in the dams. In deficient dams, hepatic holocarboxylase abundances for hepatic ACC, pyruvate carboxylase, PCC, and β-methylcrotonyl-CoA carboxylase (MCC) were only half that of sufficient dams; in deficient fetuses, hepatic holocarboxylase abundances were <10% of sufficient fetuses. For ACC, PCC, MCC, and holocarboxylase synthetase, mRNA abundances were not different between deficient and sufficient fetuses or dams. The observed reductions in biotinylated carboxylase activity and mass coexisting with normal gene expression for the carboxylases support a mechanism in which maternal biotin deficiency results in a lack of adequate biotin to biotinylate apocarboxylases in the fetus, despite the normal expression of genes coding for the apocarboxylases and holocarboxylase synthetase. The relative preservation of maternal carboxylase activities suggests that the limited amount of biotin available to biotinylate proteins is sequestered in the dam liver. Unlike its ability to scavenge several other micronutrients, the mouse fetus appears to be an inefficient biotin parasite of the dam.
Studies of biotin status during normal human gestation
Concerns about health effects of biotin deficiency on the mother and on the fetus have led to several studies by our laboratory of biotin status during human gestation. All human studies were individually approved by the institutional review board of the University of Arkansas for Medical Sciences and were carried out according to the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects. Results of studies measuring biotin concentrations in plasma using bioassays have conflicted (8). The conflicts likely arose from both analytical and pharmacokinetic problems (8). Moreover, the plasma concentration of biotin is not a very early or sensitive indicator of marginal biotin deficiency (24).
Three indicators of marginal biotin status have been validated in healthy men and nonpregnant women in whom biotin deficiency was induced experimentally by egg white consumption (24,25): 1) urinary excretion of biotin; 2) urinary excretion of 3HIA that reflects reduced activity of MCC; and 3) PCC activity in peripheral blood lymphocytes. When asymptomatic, marginal biotin deficiency was induced in adult volunteers by consuming a diet high in raw egg white for 3 wk (24) or 4 wk (25), biotin excretion decreased to frankly abnormal values in ∼80% of these subjects. In 3 similar studies (24–26), 3HIA excretion was abnormally increased in ∼90% of the subjects after 3 or 4 wk of egg white feeding. In 2 similar studies, lymphocyte PCC activity was abnormally decreased in all subjects by 4 wk (26,27). Further, based on rat studies, lymphocyte PCC activity does indeed reflect whole body biotin status (28). On the basis of these studies, urinary 3HIA and lymphocyte PCC appear to be the best indicators of marginal biotin deficiency.
Several studies provide evidence that a marginal degree of biotin deficiency develops in a substantial proportion of women during normal pregnancy. In a cross-sectional study (29), 3HIA excretion was increased in both early (median = 17 wk) and late (median = 36 wk) gestation in pregnancy. However, the urinary excretion of biotin increased during late pregnancy (29). In a longitudinal study (30), urinary excretion of 3HIA was increased (P = 0.0001) in both early (median = 10 wk) and late (median = 36 wk) gestation in pregnancy, confirming the results of the cross-sectional study. The urinary excretion of biotin was less in late pregnancy than in early pregnancy or normal controls. The urinary ratio of the inactive catabolite bisnorbiotin to biotin was increased in both early and late pregnancy, suggesting accelerated biotin catabolism.
Is the increased 3HIA excretion observed in pregnant women an effect of pregnancy per se (e.g., an effect of pregnancy on protein turnover, amino acid metabolism, or renal handling of organic acids), rather than reflecting marginal biotin deficiency? This question was addressed in a randomized, placebo-controlled, single-blinded trial of biotin supplementation (31). Twenty-six healthy pregnant women with increased 3HIA excretion were randomized either to 300 μg biotin (1.2 μmol = 10 times the recommended dietary allowance) for 2 wk or to placebo. The urinary excretion of 3HIA decreased in every woman who was treated with biotin; the mean 3HIA excretion for the placebo group did not change. The difference between biotin treatment and placebo was significant (P < 0.003) at both early (median = 13.5 wk) and late (median = 30.5 wk) gestation.
Pilot PCC Study
The validation of lymphocyte PCC as an index of biotin status afforded us the opportunity to conduct a pilot study assessing biotin status in pregnant women. Lymphocyte PCC activities were decreased below the lower limit of normal controls (P < 0.0001; nonparametric Kruskal-Wallis test), both early (n = 10 of 12 subjects) and late (n = 8 of 10 subjects) in pregnancy.
Pilot Intervention Study
Our demonstration that the majority of pregnant women have an abnormally low PCC is certainly striking, but it might not be evidence of pervasive biotin deficiency. Rather, low PCC could be the consequence of pregnancy per se. We have conducted a limited pilot intervention study. In this open-label study (i.e., not placebo controlled), we screened 5 pregnant women. The PCC activity of 4 women fell well below contemporaneous normal controls. These 4 women successfully completed the biotin supplementation (300 μg daily for 2 wk). The PCC activity increased substantially in each subject; the mean increase was 95%. These data provide preliminary evidence that reduced PCC activity does indeed reflect marginal biotin deficiency and that marginal biotin deficiency is common in normal human gestation.
Taken together, the lines of evidence presented above suggest that marginal biotin deficiency develops frequently during normal gestation and that the deficiency is severe enough to produce metabolic derangements in women. However, the degree of deficiency is not severe enough to produce the classic cutaneous and behavioral manifestations of biotin deficiency and goes unrecognized. Whether the degree of deficiency is severe enough to cause birth defects remains to be investigated.
Other articles in this supplement include references (21, 32–34).
Published as a supplement to The Journal of Nutrition. Presented as part of the symposium “Advances in Understanding of the Biological Role of Biotin at the Clinical, Biochemical, and Molecular Level” given at the 2008 Experimental Biology meeting on April 7, 2008, in San Diego, CA. The symposium was sponsored by the American Society for Nutrition and supported by an educational grant from Mead Johnson Nutritionals. The symposium was chaired by Donald Mock and Hamid M. Said.
Supported by the NIH, DK- 36823 and the UAMS GCRC, M01RR14288.
Author disclosures: D. M. Mock, no conflicts of interest.
Abbreviations used: ACC, acetyl-CoA carboxylase; 3HIA, 3-hydroxyisovaleric acid; MCC, β-methylcrotonyl-CoA carboxylase; PCC, propionyl-CoA carboxylase.
References
- 1.Butterworth CE, Jr., Bendich A. Folic acid and the prevention of birth defects. In: McCormick DB, Bier DM, Goodridge AG, editors. Annual Reviews of Nutrition. Palo Alto, CA: Annual Reviews Inc.; 1996. [DOI] [PubMed]
- 2.Czeizel AE. Prevention of congenital abnormalities by periconceptional multivitamin supplementation. BMJ. 1993;306:1645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. [DOI] [PubMed] [Google Scholar]
- 4.Marazita ML. Genetic etiologies of facial clefting. In: Mooney M, Siegel M, editors. Understanding craniofacial anomalies: The etiopathogenesis of craniosynostosis and facial clefting. New York: Wiley; 2002. p. 147–62.
- 5.Office of Pollution Prevention ToxinsThe Cost of Illness Handbook. In: Bouwes N, editor. Chapter iii3 Cost of Cleft Lip and Palate: US Environmental Protection Agency; 2006.
- 6.Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet. 2002;61:248–56. [DOI] [PubMed] [Google Scholar]
- 7.Said HM. Biotin bioavailability and estimated average requirement: why bother? Am J Clin Nutr. 1999;69:352–3. [DOI] [PubMed] [Google Scholar]
- 8.Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med. 2000;223:14–21. [DOI] [PubMed] [Google Scholar]
- 9.Karl PI, Fisher SE. Biotin transport in microvillous membrane vesicles, cultured trophoblasts and the isolated perfused cotyledon of the human placenta. Am J Physiol. 1992;262:C302–8. [DOI] [PubMed] [Google Scholar]
- 10.Schenker S, Hu Z, Johnson RF, Yang Y, Frosto T, Elliott BD, Henderson GI, Mock DM. Human placental biotin transport: Normal characteristics and effect of ethanol. Alcohol Clin Exp Res. 1993;17:566–75. [DOI] [PubMed] [Google Scholar]
- 11.Goldman AS. Biochemical mechanism of phenytoin-induced cleft palate. Adv Biosci. 1986;56:247–50. [Google Scholar]
- 12.Piddington RL, Goldman AS. Palatal development and the arachidonic acid cascade. Prog Clin Biol Res. 1985;171:295–306. [PubMed] [Google Scholar]
- 13.Katsumata M, Gupta C, Goldman AS. Glucocorticoid receptor 1B: mediator of anti-inflammatory and teratogenic functions of both glucocorticoids and phenytoin. Arch Biochem Biophys. 1985;243:385–95. [DOI] [PubMed] [Google Scholar]
- 14.Kay ED, Goldman AS, Daniel JC. Common biochemical pathway of dysmorphogenesis in murine embryos: use of the glucocorticoid pathway by phenytoin. Teratog Carcinog Mutagen. 1990;10:31–9. [DOI] [PubMed] [Google Scholar]
- 15.Montenegro MA, Palomino H. Induction of cleft palate in mice by inhibitors of prostaglandin synthesis. J Craniofac Genet Dev Biol. 1990;10:83–94. [PubMed] [Google Scholar]
- 16.Watkins BA, Bain SD, Newbrey JW. Eicosanoic fatty acid reduction in the tibiotarsus of biotin-deficient chicks. Calcif Tissue Int. 1989;45:41–6. [DOI] [PubMed] [Google Scholar]
- 17.Mock DM, Johnson SB, Holman RT. Effects of biotin deficiency on serum fatty acid composition: Evidence for abnormalities in humans. J Nutr. 1988;118:342–8. [DOI] [PubMed] [Google Scholar]
- 18.Kramer TR, Briske-Anderson M, Johnson SB, Holman RT. Effects of biotin deficiency on polyunsaturated fatty acid metabolism in rats. J Nutr. 1984;114:2047–52. [DOI] [PubMed] [Google Scholar]
- 19.Mock DM, Mock NI, Johnson SB, Holman RT. Effects of biotin deficiency on plasma and tissue fatty acid composition: Evidence for abnormalities in rats. Pediatr Res. 1988;24:396–403. [DOI] [PubMed] [Google Scholar]
- 20.Mock DM. Evidence for a pathogenic role of ω6 polyunsaturated fatty acid in the cutaneous manifestations of biotin deficiency. J Pediatr Gastroenterol Nutr. 1990;10:222–9. [DOI] [PubMed] [Google Scholar]
- 21.Zempleni J. Sodium-dependent multivitamin transporter is regulated at the chromatin level by histone biotinylation in human Jurkat lymphoblastoma cells. J Nutr. 2008;138:163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr. 2003;133:2519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sealey W, Stratton SL, Hansen DK, Mock DM. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr. 2005;135:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr. 1997;65:951–8. [DOI] [PubMed] [Google Scholar]
- 25.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr. 2002;76:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stratton SL, Bogusiewicz A, Mock MM, Mock NI, Wells AM, Mock DM. Lymphocyte propionyl-CoA carboxylase and its activation by biotin are sensitive indicators of marginal biotin deficiency in humans. Am J Clin Nutr. 2006;84:384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mock DM, Henrich CL, Carnell N, Mock NI, Swift L. Lymphocyte propionyl-CoA carboxylase and accumulation of odd-chain fatty acid in plasma and erythrocytes are useful indicators of marginal biotin deficiency. J Nutr Biochem. 2002;13:462–70. [DOI] [PubMed] [Google Scholar]
- 28.Mock DM, Mock NI. Lymphocyte propionyl-CoA carboxylase activity is an early and sensitive indicator of biotin deficiency in rats, but urinary excretion of 3-hydroxyisopropionic acid is not. J Nutr. 2002;132:1945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mock DM, Stadler DD. Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr. 1997;16:252–7. [DOI] [PubMed] [Google Scholar]
- 30.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr. 1997;127:710–6. [DOI] [PubMed] [Google Scholar]
- 31.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr. 2002;75:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mock DM, Said HM. Introduction to Advances in Understanding of the Biological Role of Biotin at the Clinical, Biochemical, and Molecular Level. J Nutr. 2008;138:152–3. [DOI] [PubMed] [Google Scholar]
- 33.Said HM. Cell and molecular aspects of the human intestinal biotin absorption process. J Nutr. 2008;138:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckett D. Biotin sensing at the molecular level. J Nutr. 2008;138:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]