Abstract
Humans cannot synthesize biotin and thus must obtain this vitamin from exogenous sources. The intestine is exposed to 2 sources of biotin: a dietary source and a bacterial source, which is normal microflora of the large intestine. Dietary protein-bound biotin is converted to free biotin prior to absorption. Absorption of free biotin in the small and large intestine involves a saturable and Na+-dependent carrier-mediated process that is shared with panthothenic acid and lipoate. For this reason, the involved transport system is referred to as the sodium-dependent multivitamin transporter (SMVT); in humans, it is designated as hSMVT. The hSMVT system has been cloned, demonstrated to be exclusively expressed at the apical membrane of enterocytes, and shown, by means of gene-specific short interfering RNA, to be the main biotin uptake system that operates in human intestinal epithelial cells. The 5′-regulatory region of the hSMVT gene has also been cloned and characterized both in vitro and in vivo. Further, the human intestinal biotin uptake process was adaptively up-regulated in biotin deficiency via a transcriptionally mediated mechanism(s) that involves Kruppel-like factor 4 sites. Studies on cell biology of hSMVT have shown a region in the cytoplasmic C-terminal domain of the polypeptide to be essential for its targeting to the apical membrane domain of epithelial cells. Intracellular trafficking of the hSMVT protein appears to involve distinct trafficking vesicles that require an intact microtubules network and the motor protein dynein for their mobility.
Introduction
The water-soluble vitamin biotin is required for normal cellular functions, growth, and development. The vitamin (a carboxyl carrier) acts as a cofactor for 5 carboxylases; 4 are located in the mitochondria and 1 in the cytoplasm (reviewed in 1–3). These carboxylases play a critical role in the intermediate metabolism of gluconeogenesis, fatty acid synthesis, and amino acid catabolism (1–3). Recent studies have suggested an additional role for biotin in the regulation of gene expression (reviewed in 4), with both stimulation (as in the case of the insulin receptor, glucokinase, and human thiamin transporter-2) and suppression (as in the case of hepatic phosphoenolpyruvate carboxykinase) being reported. In addition, a role for biotin in normal immune functions (5–7) and cell proliferation (8,9) has been cited. Thus, it is not surprising that deficiency of this essential micronutrient leads to a variety of clinical abnormalities. These include growth retardation, neurological disorders, and dermatological abnormalities (reviewed in 10). The incidence of biotin-deficiency and suboptimal levels have been reported with increased frequency in recent years and occurs in patients on long-term parenteral nutrition (11,12), in patients with inborn errors of biotin metabolism (3,10), and in those on long-term therapy with anticonvulsant agents (13,14). Suboptimal levels of biotin have been reported in a substantial number of alcoholics (15,16), in women during pregnancy (17), in patients with inflammatory bowel disease (18,19), and in patients with seboric dermatitis and Leiner's disease (20,21).
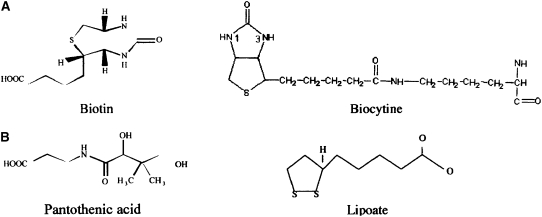
In nature, the biotin molecule (Fig. 1A; pKa = 4.5) exists in the form of 8 stereoisomers, but only the D-biotin isomer is biologically active. Microorganisms (like certain bacteria and yeast) and plant cells, but not mammalian cells, can synthesize biotin endogenously (1). Biotin is widely distributed in foodstuffs, with organ meat (like liver and kidney), egg yolks, some vegetables, and cow milk representing good sources for the vitamin (22,23).
FIGURE 1 .
Chemical structure of biotin and related compounds.
Digestion and absorption of biotin
General characteristics of the human intestinal biotin uptake process.
Humans and other mammals cannot synthesize biotin and thus must obtain the vitamin from exogenous sources via intestinal absorption. The human intestine is exposed to 2 sources of biotin: a dietary source, and a bacterial source, which is normal microflora of the large intestine. Bioavailability of dietary biotin differs from one food source to another and ranges from 5% to close to 100% (22). Dietary biotin exists in protein-bound and free forms (24). Protein-bound forms of biotin are digested to free biotin prior to absorption in the small intestine. Digestion is first performed by the action of gastrointestinal proteases and peptidases, leading to the generation of biocytin (biotinyl-L-lysine; Fig. 1A) and biotin-short peptides (25). The enzyme biotinidase then liberates free biotin from biocytin and biotin-short peptides (25). The latter step is essential for efficient absorption and optimal bioavailability of dietary protein-bound biotin (25,26). Mutations in biotinidase, which occur in the autosomal recessive disorder of biotinidase deficiency (27), lead to impairment in the bioavailability of dietary protein-bound biotin (25).
Uptake of free biotin by human small intestinal epithelial cells occurs via an efficient Na+-dependent, carrier-mediated mechanism that saturates at the micromolar range (reviewed in 28–30). The carboxyl group of the valeric acid moiety of the biotin molecule must be free for its proper recognition by the involved mechanism (31). Because transport across the highly polarized human intestinal epithelial cells represents movement of biotin across 2 structurally and functionally different membrane domains, i.e., the brush border membrane (BBM)4 and the basolateral membrane (BLM) domains, understanding the mechanisms involved in each transport step is important. This was addressed using purified BBM vesicles and BLM vesicles isolated by established procedures from the small intestine of human organ donors. Biotin uptake by human intestinal BBM vesicles occurred via a carrier-mediated system that is Na+ gradient-dependent and is capable of moving the substrate against a concentration gradient (32). Higher biotin transport was found in the proximal compared with the distal small intestine (33). The biotin transport event across the human intestinal BBM was sensitive to the inhibitory effect of the anticonvulsant drugs carbamazepine and primidone (34). Studies with human intestinal BLM vesicles showed that biotin transport across the BLM was via a carrier-mediated mechanism, but the system was Na+-independent and electrogenic in nature (35).
An important characteristic of the human intestinal biotin uptake process is that it is also utilized by 2 other structurally and functionally unrelated nutrients, namely pantothenic acid and lipoate (Fig. 1B; 36, 37). Pantothenic acid is a water-soluble vitamin that plays a role in the synthesis of coenzyme A and acryl carrier proteins. Lipoate is a potent intracellular and extracellular antioxidant and also plays a role in redox cycling of other antioxidants (e. g., vitamins C and E) and in regulating the glutathione cellular level. It is for the above reason that the involved transport system is referred to as the sodium-dependent multi-vitamin transport system (SMVT); in humans it is referred to as hSMVT.
Regarding the bacterial source of biotin, the normal microflora of the large intestine synthesize and release into the intestinal lumen a substantial amount of free biotin (38). The relative contribution of this source of biotin toward total human biotin nutrition, however, is not well defined. In vivo studies have shown that the human large intestine is capable of absorbing installed biotin (39). The mechanism involved in the large intestinal biotin absorption process was investigated using cultured human colonic epithelial NCM460 cells, with results showing the existence of an efficient Na+ -dependent, carrier-mediated process (36). This process was again shared with pantothenic acid and lipoate. However, the abundantly available (and negatively charged) short-chain fatty acids acetate and butyrate, which are also produced by the normal microflora of the large intestine, did not interfere with the colonic biotin uptake process (36).
Molecular identity of the intestinal biotin transport system.
Knowledge about the molecular identity of the human intestinal Na+-dependent biotin uptake system (hSMVT) has increased substantially following cloning of the system by Prasad et al. (40). The hSMVT protein (635 amino acids) is the product of the SLC5A6 gene, which is located on chromosome 2p23 and consists of 17 exons (41). The protein showed considerable homology with members of the Na+:glucose family of transporters (41); it also showed sequence homology, at both the nucleotide and the amino-acid levels, with SMVT of a number of other mammalian species. The hSMVT polypeptide is predicted to have 12 trans-membrane domains and have a number of potential post-translational modification sites (i.e., phosphorylation and glycosylation sites); also, both the amino- and the carboxy- terminals of the polypeptide are oriented toward the cell interior (40,41). Functional characterization of the hSMVT in heterologus systems showed high Na+ dependence and utilization by pantothenic acid and lipoate (40,41); it also showed uptake kinetic parameters similar to those of the native intestinal biotin uptake process.
The hSMVT system appears to be the main, if not the only, biotin uptake system in human intestinal epithelial cells (42). This conclusion is based on recent studies utilizing gene specific short interfering RNA to selectively knock down the endogenous hSMVT system of human intestinal epithelial Caco-2 cells. Results of these studies showed that pretreatment with the hSMVT-specific siRNA led to a specific and marked reduction in the level of hSMVT mRNA and protein, and to a severe inhibition of carrier-mediated biotin uptake (42).
Regulation of the intestinal biotin absorption process
Transcriptional activity of the hSMVT gene.
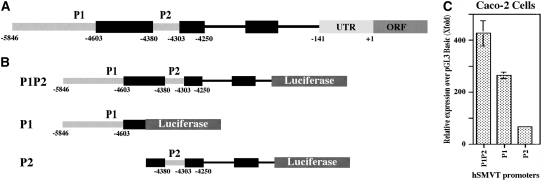
The 5′-regulatory region of the hSMVT gene has been recently cloned and characterized (43,44). Two distinct and functional promoters (P1 and P2; Fig. 2A) were identified, and both were TATA-less, CAAT-less, contained highly GC-rich sites, and had multiple putative regulatory cis-elements. Functionality was assessed by fusing the promoters to the Firefly luciferase reporter gene (Fig. 2B), followed by transfection into human intestinal epithelial Caco-2 cells. hSMVT P1 was much more active than P2 in these cells (Fig. 2C). The minimal region required for basal activity of hSMVT P1 was encoded by a sequence between -5846 and -5313, whereas that of P2 was encoded by a sequence between -4417 and -4244 (relative to the translation initiation codon). Mutation of specific cis-regulatory elements in the minimal region [Kruppel-like factor 4 (KLF-4) and activator protein-2] led to a decrease in promoter activity. The trans-acting factors KLF-4 and activator protein-2 were indeed found to interact with these identified cis-regulatory elements, as shown by studies using oligonucleotide competition and antibody super-shift assays. Activity of the hSMVT promoter (using an integrated hSMVT promoter-luciferase construct) was confirmed in vivo in transgenic mice to establish the physiological relevance of the in vitro promoter studies described above (44). Studies with the transgenic mice also showed that the pattern of expression of the hSMVT promoter in different mice tissues was similar to the pattern of expression of the endogenous mouse SMVT message.
FIGURE 2 .
Diagrammatic representation of the hSMVT promoters P1 and P2 (A), fusion constructs of the hSMVT promoters with luciferase reporter gene (B), and activity of the hSMVT promoters in human intestinal epithelial Caco-2 cells (C). In panel C, values are means ± SEM, n = 3, with the data adapted from (44) with the permission of the APS.
Adaptive regulation of the human intestinal biotin uptake process.
The intestinal biotin uptake process is adaptively regulated by substrate levels in both humans and animal models (31,45). Biotin deficiency leads to a substantial and specific up-regulation in biotin uptake by human intestinal epithelial Caco-2 cells, and the effect is mediated via a marked increase (258%) in the Vmax of the vitamin uptake process (31). This up-regulation in biotin uptake was also associated with a marked induction in protein and mRNA levels of hSMVT (46). The increase in hSMVT mRNA levels was not because of an increase in RNA stability, rather it was the result of an induction in the activity of the hSMVT promoter (46). A biotin deficiency-responsive region that confers the response to biotin deficiency was mapped to a 103-bp region within the hSMVT promoter (46). This region contained KLF4 sites, whose mutation led to abrogation in hSMVT promoter response to biotin deficiency (46).
Differentiation-dependent regulation of biotin uptake by human intestinal epithelial cells.
The human intestinal biotin uptake process appears to undergo differentiation-dependent regulation. This conclusion is based on recent observations obtained using the human-derived intestinal epithelial Caco-2 cells (a good model for studying differentiation aspects of intestinal epithelia). Biotin uptake and level of expression of hSMVT mRNA and protein, as well as activity of the hSMVT promoter, was higher in postconfluent (differentiated) Caco-2 cells than in preconfluent (undifferentiated) Caco-2 cells (J. Reidling and H. M. Said, unpublished data). These findings point to the possible involvement (at least in part) of a transcriptional regulatory mechanism(s) in this type of regulation of the intestinal biotin uptake process. Further studies are required in this area.
Cell biology of hSMVT in epithelial cells
Using live cell confocal imaging and human intestinal epithelial Caco-2 cells and canine renal epithelial MDCK cells, membrane targeting and intracellular trafficking mechanisms of the hSMVT protein (fused to green fluorescent protein; i.e., hSMVT-GFP) have been the subject of a recent investigation (47). Expression of the hSMVT protein was exclusively confined to the apical membrane domain of these epithelial cells. The cytoplasmic C-terminal tail of the hSMVT polypeptide was essential for targeting the protein to the apical membrane domain of these cells. Intracellular trafficking of the hSMVT protein was critically dependent on an intact microtubule network and involved distinct trafficking vesicles. A role for the minus-end directed microtubule motor protein dynein in regulating the polarized delivery of hSMVT protein to the apical cell surface has also been reported (47).
Concluding remarks
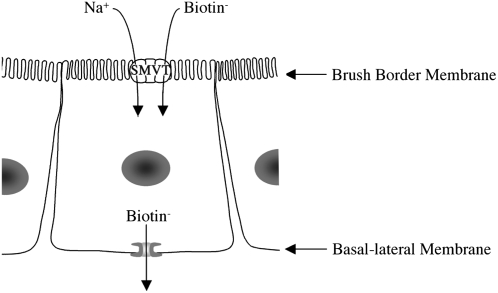
Substantial progress has been made in recent years regarding the physiology and cell and/or molecular biology aspects of the human (mammalian) intestinal biotin absorption process. It is clear now that the human intestinal biotin uptake process involves carrier-mediated systems at both the apical BBM and BLM domains of the polarized enterocyte (Fig. 3), that the hSMVT system mediates the transport event at the apical BBM (the rate limiting step), and that the biotin uptake process is also utilized by pantothenic acid and lipoate. Considerable knowledge regarding regulation of the intestinal biotin uptake process and the mechanisms involved has also been gained in recent years. In addition, knowledge of intracellular trafficking and membrane targeting of the hSMVT protein has begun to emerge. Further studies, however, are needed to further our understanding of the intestinal biotin uptake process at the integrated whole animal level in vivo, and of the structural-functional requirements of the systems involved.
FIGURE 3 .
Current understanding of how biotin is transported across human intestinal epithelial cells.
Other articles in this supplement include references (48–51).
Presented as part of the symposium “Advances in Understanding of the Biological Role of Biotin at the Clinical, Biochemical, and Molecular Level” given at the 2008 Experimental Biology meeting on April 7, 2008, in San Diego, CA. The symposium was sponsored by the American Society for Nutrition and supported by an educational grant from Mead Johnson Nutritionals.
Supported by the Department of Veterans Affairs and the NIH (grants DK 56061 and DK58057).
Author disclosures: H. M. Said, no conflicts of interest.
Abbreviatons used: BBM, brush border membrane; BLM, basolateral membrane; KLF4, Kruppel-like factor 4; P1 and P2, promoters 1 and 2; SMVT, sodium-dependent multivitamin transporter.
References
- 1.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr. 2002;22:221–39. [DOI] [PubMed] [Google Scholar]
- 2.Mock D. Biotin: Physiology, dietary sources and requirements. In: CaballeroB, Allen L, Prentice A, editors. Encyclopedia of human nutrition; 2nd ed; London: Academic Press; 2004.
- 3.Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomna. Annu Rev Nutr. 1986;6:317–43. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin. J Nutr Biochem. 2003;14:680–90. [DOI] [PubMed] [Google Scholar]
- 5.Rabin BS. Inhibition of experimentally induced autoimmunity in rats by biotin deficiency. J Nutr. 1983;113:2316–22. [DOI] [PubMed] [Google Scholar]
- 6.Baéz-Saldaña A, Díaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr. 1998;67:431–7. [DOI] [PubMed] [Google Scholar]
- 7.Petrelli F, Moretti P, Campanati G. Studies on the relationships between biotin and the behavior of B and T lymphocytes in the guinea pig. Experientia. 1981;37:1204–6. [DOI] [PubMed] [Google Scholar]
- 8.Dakshinamurti K, Chalifour LE, Bhullar RJ. Requirement for biotin and the function of biotin in cells in culture. In: Dakshinamurti K, Bhagavan HN, editors. Biotin. New York: Academy of Science; 1985. p. 38–55. [DOI] [PubMed]
- 9.Matnhey KC, Griffin JB, Zempleni J. Biotin supply affects expression of biotin transporters, biotinylation of carboxylases, and metabolism of interleukin-2 in Jurkat cells. J Nutr. 2002;132:2316–22. [DOI] [PubMed] [Google Scholar]
- 10.Wolf B. Disorders of biotin metabolism. In: Scriver CR, Beaudet AL, Aly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill Medical Publishing Division; 2001. p. 3935–62.
- 11.Forbes GM, Forbes A. Micronutrient status in patients receiving home parenteral nutrition. Nutrition. 1997;13:941–4. [DOI] [PubMed] [Google Scholar]
- 12.Mock DM, DeLorimer AA, Liebman WM. Biotin deficiency: an unusual complication of parenteral alimentation. N Engl J Med. 1981;304:820–3. [DOI] [PubMed] [Google Scholar]
- 13.Krause KH, Berlit P, Bonjour JP. Impaired biotin status in anticonvulsant therapy. Ann Neurol. 1982;12:485–6. [DOI] [PubMed] [Google Scholar]
- 14.Krause KH, Bonjour J, Berlit P, Kochen W. Biotin status of epileptics. Ann N Y Acad Sci. 1985;447:297–313. [DOI] [PubMed] [Google Scholar]
- 15.Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholics: I Aetiological role of aneurin and other B-complex vitamins. BMJ. 1964;2:1290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonjour JP. Vitamins and alcoholism. Int J Vitam Nutr Res. 1980;50:425–40. [PubMed] [Google Scholar]
- 17.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr. 1997;127:710–6. [DOI] [PubMed] [Google Scholar]
- 18.Urabe K. Decreased plasma biotin levels in patients with Crohn's disease. Jpn J Gastroenterol. 1986;83:307–9. [PubMed] [Google Scholar]
- 19.Fernandez-Banares F, Lacruz AA, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bound disease. Am J Gastroenterol. 1989;84:744–8. [PubMed] [Google Scholar]
- 20.Nisenson A. Seborrhoeic dermatitis of infants and Leiner's disease: A biotin deficiency. J Pediatr. 1957;51:537–48. [DOI] [PubMed] [Google Scholar]
- 21.Messaritakis J, Katlamis C, Karabula C, Matsaniotis N. Generalized seborrhoeic dermatitis: clinical and therapeutic data of 25 patients. Arch Dis Child. 1975;50:871–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combs GF. Biotin. In: The vitamins: fundamental aspects in nutrition and health. San Diego (CA): Acdemic Press; 1992. p. 329–43.
- 23.Hardinge MG, Crooks H. Lesser known vitamins in foods. J Am Diet Assoc. 1961;38:240–5. [PubMed] [Google Scholar]
- 24.Lampen JO, Bahler GP, Peterson WH. The occurrence of free and bound biotin. J Nutr. 1942;23:11–21. [Google Scholar]
- 25.Wolf B, Heard GS, Secor-McVoy JR, Raetz HM. Biotinidase deficiency: the possible role of biotinidase in the processing of dietary protein-bound biotin. J Inherit Metab Dis. 1984;7:121–2. [DOI] [PubMed] [Google Scholar]
- 26.Said HM, Thuy LP, Sweetman L, Schatzman B. Transport of the biotin dietary derivative biocytin (N-biotinyl-L-lysine) in rat small intestine. Gastroenterology. 1993;104:75–9. [DOI] [PubMed] [Google Scholar]
- 27.Blanton SH, Pandya A, Landa BL, Javaheri R, Xia XJ, Nance WE, Pomponio RJ, Norrgard KJ, Swango KL, et al. Fine mapping of the human biotinidase gene and haplotype analysis of five common mutations. Hum Hered. 2000;50:102–11. [DOI] [PubMed] [Google Scholar]
- 28.Said HM. Recent advances in carrier-mediated absorption of water-soluble vitamins. Annu Rev Physiol. 2004;66:419–46. [DOI] [PubMed] [Google Scholar]
- 29.Said HM, Rose R, Seetharam B. Intestinal absorption of water-soluble vitamins: cellular and molecular aspects. In: Barrett KE, Donowitz M. Gastrointestinal transport: molecular physiology. San Diego (CA): Academic Press; 2000. p. 35–76.
- 30.Said HM, Seetheram P. Intestinal absorption of water-soluble vitamins. In: Johnson L, editor. Physiology of the G.I. tract. San Diego (CA): Elsever; 2006. p. 1791–1826.
- 31.Ma TY, Dyer DL, Said HM. Human intestinal cell line Caco-2: a useful model for studying cellular and molecular regulation of biotin uptake. Biochim Biophys Acta. 1994;1189:81–8. [DOI] [PubMed] [Google Scholar]
- 32.Said HM, Redha R, Nylander W. A carrier-mediated, Na+ gradient-dependent transport system for biotin in human intestinal brush border membrane vesicles. Am J Physiol. 1987;253:G631–6. [DOI] [PubMed] [Google Scholar]
- 33.Said HM, Nylander W, Redha R. Biotin transport in human intestine: site of maximum transport and effect of pH. Gastroenterology. 1988;95:1312–7. [DOI] [PubMed] [Google Scholar]
- 34.Said HM, Redha R, Nylander W. Biotin transport in the human intestine: inhibition by anticonvulsant drugs. Am J Clin Nutr. 1989;49:127–31. [DOI] [PubMed] [Google Scholar]
- 35.Said HM, Redha R. Biotin transport in basolateral membrane vesicles of human intestine. Gastroenterology. 1988;94:1157–63. [DOI] [PubMed] [Google Scholar]
- 36.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by the human colonic epithelial cells NCM460: A carrier-mediated process shared with pantothenic acid. Am J Physiol. 1998;275:C1365–71. [DOI] [PubMed] [Google Scholar]
- 37.Said HM. Cellular uptake of biotin: mechanisms and regulation. J Nutr. 1999;129:490S–3S. [DOI] [PubMed] [Google Scholar]
- 38.Wong OM, Edmonds CJ, Chadwick VS. Vitamins. In: The large intestine: its role in mammalian nutrition and homeostasis. New York: Wiley 1981. p. 157–66.
- 39.Sorrell MF, Frank O, Thomson AD, Aquino A, Baker H. Absorption of vitamins from the large intestine. Nutr Rep Int. 1971;3:143–8. [Google Scholar]
- 40.Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+ -dependent multivitamin transporter. Arch Biochem Biophys. 1999;366:95–106. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Huang W, Fei YJ. Human placental Na+-dependent multivitamin transporter. J Biol Chem. 1999;274:14875–83. [DOI] [PubMed] [Google Scholar]
- 42.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol. 2003;285:G73–7. [DOI] [PubMed] [Google Scholar]
- 43.Dey S, Subramanian VS, Chatterjee NS, Rubin SA, Said HM. Characterization of the 5′ regulatory region of the human sodium-dependent multivitamin transporter, hSMVT. Biochim Biophys Acta. 2002;1574:187–92. [DOI] [PubMed] [Google Scholar]
- 44.Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am J Physiol Cell Physiol. 2007;292:C1305–12. [DOI] [PubMed] [Google Scholar]
- 45.Said HM, Mock DM, Collins J. Regulation of intestinal biotin transport in the rat: effect of biotin deficiency and supplementation. Am J Physiol. 1989;256:G306–11. [DOI] [PubMed] [Google Scholar]
- 46.Reidling J, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol. 2007;292:G275–81. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian VS, Marchant JS, Said HM. Mechanisms of intracellular trafficking and membrane targeting of the human sodium-dependent multivitamin transporter (hSMVT) in polarized intestinal epithelial cells. Gastroenterology. 2007;132:A583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mock DM, Said HM. Introduction to Advances in Understanding of the Biological Role of Biotin at the Clinical, Biochemical, and Molecular Level. J Nutr. 2008;138:152–3. [DOI] [PubMed] [Google Scholar]
- 49.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in mice. J Nutr. 2008;138:154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zempleni J, Gralla M, Camporeale G, Hassan YI. Sodium-dependent multivitamin transporter gene is regulated at the chromatin level by histone biotinylation in human Jurkat lymphoblastoma cells. J Nutr. 2008;138:163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckett D. Biotin sensing at the molecular level. J Nutr. 2008;138:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]