Abstract
In this study, our main objective was to determine whether energy restriction (ER) affects the rate of oxygen consumption of mice transiently or lastingly and whether metabolic rate plays a role in the ER-related extension of life span. We compared rates of resting oxygen consumption between C57BL/6 mice, whose life span is prolonged by ER, and the DBA/2 mice where it is not, at 6 and 23 mo of age, following 40% ER for 2 and 19 mo, respectively. Mice of the 2 strains that consumed food ad libitum (AL) had a similar body mass at the age of 4 mo and consumed similar amounts of food throughout the experiment; however, the body weight subsequently significantly increased (20%) in the C57BL/6 mice but did not increase significantly in the DBA/2 mice. The resting rate of oxygen consumption was normalized as per g body weight, lean body mass, organ weight, and per mouse. The resting rate of oxygen consumption at 6 mo was significantly higher in AL DBA/2 mice than the AL C57BL/6 mice for all of the criteria except organ weight. A similar difference in AL mice of the 2 strains was present at 23 mo when resting oxygen consumption was normalized to body weight. Resting oxygen consumption was lowered by ER in both age groups of each strain according to all 4 criteria used for normalization, except body weight in the C57BL/6 mice. The effect of ER on resting oxygen consumption was thus neither transient nor age or strain dependent. Our results suggest that ER-induced extension of life span occurs in the mouse genotype in which there is a positive imbalance between energy intake and energy expenditure.
Introduction
Reduction in the amount of dietary energy intake, also referred to as caloric restriction, has been repeatedly demonstrated to significantly prolong the life span of some laboratory strains of mice and rats compared with those who consumed food ad libitum (AL).5 Comparisons of a variety of age-related changes have been made between energy-restricted (ER) and AL rodents in an effort to identify the key physiological/biochemical processes that may mediate the life span extension effect of ER; however, in general, a vast majority of the age-related changes occurring in the AL subjects have been found to be either attenuated or retarded by ER (1,2). Consequently, the original goal of gaining insight into the nature of the mechanisms by which ER exerts its effect on aging has largely remained elusive. Retrospectively, it seems that the laboratory strains of rats and mice chosen for such comparisons are quite often those in which longevity is markedly extended by ER. The main limitation of such experimental models is that the age-related changes linked to the longevity extension effect of ER cannot be discerned from the more broad adaptations occurring in response to decreased energy intake. Thus, it may be useful to modify the conventional experimental design by the inclusion of a “negative” control, i.e. a strain whose life span is not prolonged by ER.
Despite repeated claims in the literature implying that ER extends the life span of virtually all species (3), there is considerable evidence that this effect is not universal. For instance, life span of the housefly is progressively shortened in proportion to the reductions in the amount of energy consumed by the AL flies (4,5). It was also recently shown that, contrary to some previous reports, if the amount of food consumption is properly quantified, ER has no life extension effect in Drosophila melanogaster (6). In cohorts of mice derived from wild-caught ancestors, Harper et al. (7) did not observe a significant extension of life span following ER. Notably, the effect of ER on longevity of different strains of inbred mice is also selective. For instance, whereas the life spans of C57BL/6 and B6D2F1 mice are extended by ∼25–30% in response to a 40% decrease in energy intake, the same regimen has no demonstrable effect on the longevity of DBA/2 mice (8), suggesting that genetic background is a factor in determining the longevity extension effect of ER. It would seem that the inclusion of the latter strain as a negative control may help in distinguishing between the age-related physiological/biochemical alterations, ostensibly associated with the mechanisms of life span prolongation by ER from those that are unrelated.
One hypothesis, initially proposed by Sacher (9), is that ER prolongs the life span of rodents by lowering the rate of metabolism. The rationale was that: 1) survival time of poikilotherms and some mammals can be markedly prolonged by lowering the rate of energy expenditure; 2) the total amount of oxygen consumed by the AL and ER subjects is quite comparable, whereas the life span of the former is considerably shorter; and 3) in feral rodents, body temperature drops notably under conditions of food scarcity. More recently, lowering of the core body temperature by 0.3–0.5°C has been found to extend the life span of transgenic mice of C57BL/6 genetic background by up to 20% (10,11).
Nevertheless, the issue of whether ER lowers the metabolic rate of animals has remained mired in controversy, with different studies reporting no effect, an elevation, a transient, or a prolonged decrease in the rate of metabolism in response to ER (reviewed in 1,12). However, a broader and arguably more central question is whether the mechanism of life span extension by ER is inextricably linked to a reduction in metabolic rate and vice versa. The present study addresses these issues by comparing the effect of relatively short- and long-term ER on the rate of oxygen consumption between the C57BL/6 mice, in which the life span is prolonged by ER, and the DBA/2 mice, in which it is not.
Materials and Methods
Animals.
All experiments were conducted in adherence with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Southern California Institutional Animal Care and Use Committee. Forty male DBA/2J and 40 C57BL/6J male mice, 8–9 wk of age, were obtained from the Jackson Laboratory and subsets of 28 DBA/2J and 31 C5BL/6J mice were used for measurement of rates of oxygen consumption. Upon arrival at the University of Southern California vivarium, they were housed individually (one mouse per cage) in polycarbonate cages with mesh tops. Prior to the initiation of ER, all mice consumed a standard NIH-31 diet AL and were kept on a 12-h-light/-dark cycle, with the light phase beginning at 0600.
At 14 wk of age, one-half of the mice from each strain were placed under the ER regimen, which entailed a 10% decrease in the amount of food consumed by the mice during the first week, followed by 20% in wk 2, and 40% in wk 3 and thereafter. The ER mice were fed a vitamin-fortified NIH-31 diet designed to equalize per mouse the amount of essential nutrients ingested to that of the AL mice consuming the standard NIH-31 diet (13). The nutrient composition of the standard and fortified NIH-31 diets has been recently described by Ritz et al. (14). The amount of food consumed by the AL mice was monitored throughout the study to maintain the level of food intake of the ER mice at 60% of the amount consumed by the AL mice. The rate of resting oxygen consumption was measured in separate age groups (6 or 23 mo) of the AL and ER mice of each strain (n = 6–8).
Measurement of oxygen consumption.
Mice were placed in a polycarbonate cylindrical respiration chamber (713 cm3) and desiccated air, with carbon dioxide removed, was passed through the chamber at a flow rate of 500 mL·min−1. The change in oxygen concentration was determined at 5-s intervals using an Oxzilla Dual Absolute and Differential Oxygen analyzer (Sable Systems International). The rate of oxygen consumption was calculated over a 10- to 30-min period when the mouse was asleep, as indicated by inactivity, decreased rate of oxygen consumption, and stabilization of the recording line.
Prior to the measurement of oxygen consumption, the feeding time of the ER mice was shifted from 0800–1000 to 1600–1700 to preclude its effect on oxygen consumption. Two mice, 1 of each strain, were tested per day and the sequence of testing was alternated. Rates of resting oxygen consumption were normalized as per mouse, unit (g) body mass, fat-free/lean body mass, and organ weight (liver + kidney + heart + brain). Body weights were determined immediately before the mice were placed in the respiration chamber. Fat-free mass/lean body mass was calculated by subtracting 8 times the epididymal white adipose mass (EWAM) from the body mass, as described by Bishop and Hill (15).
Statistical analysis.
Body weight was considered in a 3-way, repeated-measures ANOVA, with strain and diet as between-groups factors, and age as a within-groups factor. Weights of the different tissues and measures of body composition, as well as rates of resting respiration (i.e. per whole mouse or per g body mass, lean body mass, and organ weight) were analyzed separately using 3-way analyses with strain, age, and diet as inter-group factors. Planned comparisons (individual contrasts) between the 2 strains, the AL and ER groups, and the 2 age groups were made using single degree-of-freedom F-tests and the error term from the overall analysis. The effect of ER on rate of respiration, standardized by the different criteria, was confirmed with regression-based adjustments using ANCOVA. In these analyses, respiration rate per mouse was analyzed considering either body mass, lean body mass, or organ weight as a covariate. Significance was defined as P < 0.05. Values given in the text are means ± SEM. All statistical analyses were performed using Systat (version 8.0, SPSS).
Results
Effect of strain, age, and ER on the amount of food intake, body weight, and organ weights.
The amount of food consumed by the mice was regularly monitored from 2 to 16 mo of age. The amounts consumed by the AL C57BL/6 and DBA/2 mice were quite similar and showed little variation as a function of age (mean food intake = 3.99 ± 0.1 g/d). From 4 to 23 mo of age, mice of both strains on the ER regimen were provided with 60% of this amount (2.4 ± 0.05 g/d).
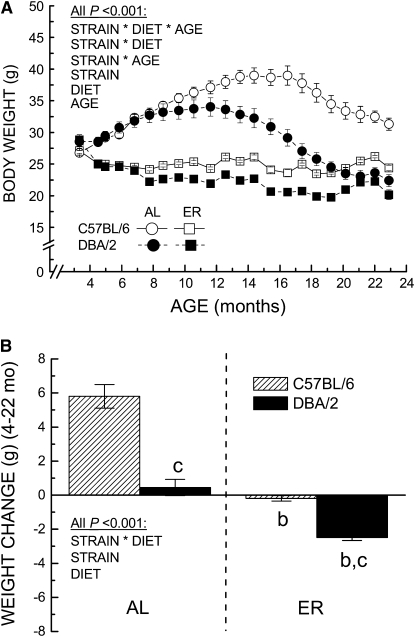
ANOVA in body weights of the mice indicated a 3-way interaction among strain, diet, and age (P < 0.001). This interaction was driven by inter-strain differences in the effect of age on weight under both dietary regimens (Fig. 1A,B). Prior to the initiation of ER at 14 wk of age, the body weights of the 2 strains of mice were nearly equal. Subsequently, the AL mice had a significant increase in body weight that was equivalent in both strains until 8–10 mo. However, the pattern of weight gain and loss thereafter differed significantly in the AL mice of each strain. The C57BL/6 mice continued to gain weight up to the age of 15–16 mo, succeeded by a gradual decline (Fig. 1A). In contrast, the body weights of the DBA/2 mice remained stable from 8–13 mo of age and then declined steadily until the age of 21 mo. After the age of 15 mo, the C57BL/6 mice on the AL regimen were 33–42% heavier than the age-matched DBA/2 mice. The mean body weight of AL C57BL/6 mice from 4 to 22 mo of age was 5.8 ± 0.69 g, or 20% higher than at 4.5 mo of age. In contrast, the mean weight of the DBA/2 mice for the same period was <2% higher than their weight at 4.5 mo of age (Fig. 1B).
FIGURE 1 .
Effect of strain and energy intake on body weight (A) and weight change between 4.5 and 22 mo (B) of DBA/2 and C57BL/6 mice as a function of age. In A, values are means ± SEM of the mice surviving from groups of 20 at the indicated time points (n = 10−20). In B, weight change was calculated as the mean of the monthly weight for each mouse minus the weight at 4.5 mo. Values are means ± SEM, n = 19–20. Letters indicate differences, P < 0.05: (b) different from AL in the same strain; (c) different from C57BL/6 fed the same diet.
After the imposition of ER, the body weight of mice was significantly decreased, with the 2 strains maintaining nearly equivalent weights until 7 mo of age; thereafter, the body weight of the C57BL/6 mice on the ER regimen remained relatively unchanged, whereas it showed a small but significant decline in the DBA/2 mice maintained on ER. At 15 mo of age, i.e. ∼12 mo after the imposition of 40% ER, the C57BL/6 mice weighed 14% more than the DBA/2 mice. The magnitude of the difference in the body weight between the AL and ER mice was quite similar (−33% in DBA/2 and −38% in C57BL/6) at this age, although the mean difference in body weight was 43% greater in the C57BL/6 mice (−14.6 ± 0.87 g) compared with the DBA/2 mice (−10.2 ± 0.92 g). After 15 mo of age, the mean differences in body weights between the AL and ER groups became smaller in both strains, although this trend was more pronounced in the DBA/2 mice, in which the body weights of the AL mice became nearly equal to the ER mice around 21 mo of age. Compared with their weight at 4.5 mo, the DBA/2 mice receiving the ER regimen lost 2 ± 0.18 g or ∼10% of their mean body weight over 4–22 mo, whereas the C57BL/6 mice body weights did not change (Fig. 1B). A noteworthy observation was that the body weights of the DBA/2 mice receiving the AL regimen and of the C57BL/6 mice receiving the ER regimen showed little net change during adult life.
Effect of ER on fat content and organ weights.
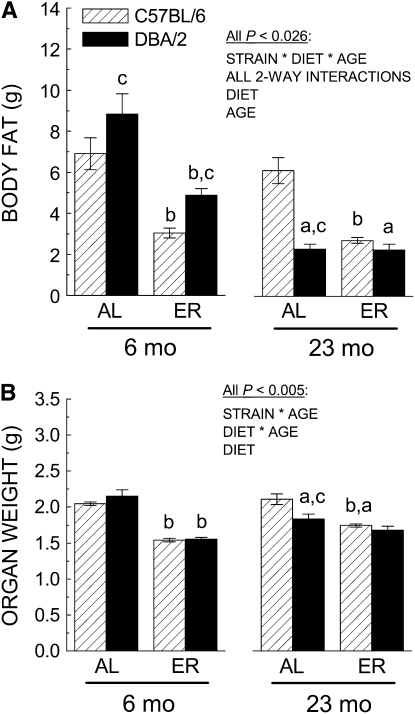
Four different criteria were used for normalizing the rate of oxygen consumption, namely per mouse, per g body weight, per g fat-free/lean body mass (calculated based on the EWAM), and per g of the combined weight of heart, kidney, liver, and brain. EWAM and organ weights were determined at 6 and 23 mo of age in conjunction with the measurements of oxygen consumption. At 6 mo of age, when the 2 strains had similar body weights under the AL regimen, the EWAM was 28% higher in the DBA/2 than in the C57BL/6 mice (Fig. 2A). In contrast, in the 23-mo-old mice, the EWAM was 1.6 times greater in the C57BL/6 mice than in DBA/2 mice. At 6 mo of age, a nearly equivalent decrease in EWAM was evident in the ER group for each strain. However, by 23 mo of age, the EWAM of the AL- and ER-maintained DBA/2 mice was similar, whereas EWAM of the C57BL/6 mice under ER was 56% lower than the AL group. Analysis of the EWAM data indicated an interaction of age, strain, and diet (P < 0.026).
FIGURE 2 .
Effect of mouse strain, age, and diet on body fat (A) and organ weight (B). Data are presented as the means ± SEM, n = 11–20 in both panels. (a) Significantly different from the 6 mo group of the same strain and diet (effect of age); (b) significantly different from the AL group of the same strain and age (effect of ER); (c) significantly different from the C57BL/6 group of the same age and diet (effect of strain), with P < 0.05.
Organ weight was lower in the ER mice than in the AL mice at 6 mo of age and this effect was similar in both strains of mice (Fig. 2B). However, at 23 mo of age, organ weights of the AL and ER groups differed significantly only in the C57BL/6 mice. The absence of an effect of ER on organ weight of the DBA/2 mice at 23 mo was attributable to an age-related decrease in organ weight in the AL DBA/2 mice. A similar age-related decrease in organ weight did not occur in the C57BL/6 mice. Analysis of the organ weight data yielded an age × strain × diet interaction that neared but did not reach significance (P < 0.06).
In addition to the combined organ weights, the weights of the individual organs were also determined in 11–14 mice of each strain, age, and diet condition (Table 1). During the period from 6 to 23 mo, the individual organ weights of AL C57BL/6 mice were unchanged for heart and liver but had small, albeit significant, increases for kidney and brain. In contrast, in the AL DBA/2 mice, there was a significant age-related loss of weight in kidney, live, and brain, but not the heart. The effect of ER on the weights of the individual organs was quite similar in the DBA/2 and C57BL/6 mice at 6 mo of age. At this age, the mean weights of heart, kidney, and liver were 15–39% lower in mice receiving the ER regimen than their AL counterparts. The difference in brain weight for the 6-mo AL and ER groups was much smaller (<8%) and only significant in the DBA/2 mice. In the C57BL/6 mice, the ER-related differences in the individual weights of heart, kidney, and liver were also evident at 23 mo of age. As also suggested by the data for the combined organ weight, the differences in the heart and kidney weights between the ER and AL mice at 23 mo of age were relatively smaller in DBA/2 than in the C57BL/6 mice and there was no significant effect of ER on the liver weight of the DBA/2 mice. All ANOVA performed on the individual tissues revealed a main effect for the factors of diet and strain (P < 0.001). The analysis for the liver weight yielded an interaction of strain, diet, and age (P < 0.004).
TABLE 1.
Effect of ER on weights of organs in C57BL/6 and DBA/2 mice of different ages1
| 6 mo C57BL/6
|
6 mo DBA/2
|
23 mo C57BL/6
|
23 mo DBA/2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AL | ER | Difference | AL | ER | Difference | AL | ER | Difference | AL | ER | Difference | |
| g | % | g | % | g | % | g | % | |||||
| Heart | 0.14 ± 0.01 | 0.10 ± 0.01b | −24 | 0.15 ± 0.03 | 0.13 ± 0.02b,c | −15 | 0.14 ± 0.01 | 0.10 ± 0.03b | −30 | 0.13 ± 0.02 | 0.10 ± 0.04a,b | −19 |
| Kidney | 0.36 ± 0.02 | 0.24 ± 0.04b | −33 | 0.58 ± 0.12c | 0.35 ± 0.02b,c | −39 | 0.41 ± 0.06a | 0.30 ± 0.02a,b | −29 | 0.48 ± 0.10a,c | 0.39 ± 0.05b,c | −19 |
| Liver | 1.09 ± 0.10 | 0.76 ± 0.07b | −30 | 1.01 ± 0.20 | 0.70 ± 0.02b | −31 | 1.12 ± 0.16 | 0.91 ± 0.08a,b | −19 | 0.83 ± 0.13a,c | 0.82 ± 0.18a,c | −02 |
| Brain | 0.46 ± 0.02 | 0.44 ± 0.02b | −06 | 0.41 ± 0.01c | 0.37 ± 0.03b,c | −08 | 0.49 ± 0.02 a | 0.45 ± 0.03b | −09 | 0.39 ± 0.02a,c | 0.37 ± 0.02b,c | −05 |
| Skeletal muscle | 2.66 ± 0.23 | 2.23 ± 0.15b | −16 | 2.06 ± 0.27c | 1.88 ± 0.24c | −09 | 2.42 ± 0.17 a | 2.05 ± 0.16b | −15 | 1.82 ± 0.39a,c | 1.65 ± 0.34a,c | −09 |
Values are mean weight ± SD. aDifferent from 6 mo group of same strain and diet, P < 0.05; bDifferent from AL group of the same strain and age, P < 0.05; cDifferent from C57BL/6 of the same diet and age, P < 0.05.
The muscles from both hind-limbs were pooled and used as a surrogate indicator of skeletal muscle mass. In the AL mice at 6 mo of age, the hind-limb muscle mass was 29% greater in the C57BL/6 mice than in the DBA/2 mice, whereas by 23 mo, the 2 strains had a similar (10–12%) decrease in muscle mass. In the ER mice, at both 6 and 23 mo of age, the muscle weight of C57BL/6 was significantly lower (−15 to −16%) compared with the corresponding AL mice; however, ER did not affect muscle mass in DBA/2 mice. ANOVA on muscle mass revealed a main effect of age and a strain × diet interaction (P < 0.025).
Effect of age, strain, and ER on rate of resting oxygen consumption.
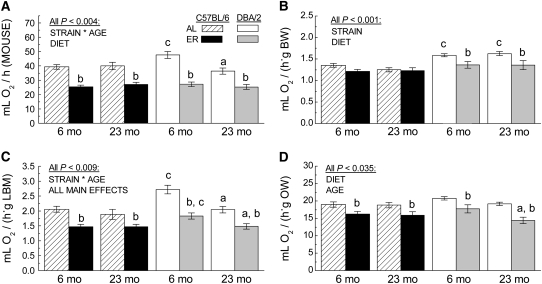
The resting rates of oxygen consumption were measured in different groups at 6 or 23 mo of age and normalized according to 4 different criteria: 1) per mouse (Fig. 3A); 2) units (g) of body weight (Fig. 3B); 3) lean body mass (Fig. 3C); and 4) organ weight (Fig. 3D). At 6 mo of age, the resting rate of oxygen consumption per mouse was 21% higher in AL DBA/2 mice than in the AL C57BL/6 mice. The relatively higher rate of oxygen consumption in the AL DBA/2 mice was also evident when data were expressed in units (g) of body weight (17%), lean body mass (32%), and organ weight (10%; P = 0.15).
FIGURE 3 .
Rate of resting oxygen consumption in C57BL/6 and DBA/2 mice at 6 or 23 mo of age, respectively, after 2 or 19 mo on the AL or ER regimen. Resting oxygen consumption of each mouse was normalized as: per mouse (A), body weight (B), lean body mass (C), and organ weight (D). All values are means ± SEM, n = 6–8 in each panel. (a) Significantly different from the 6-mo group of the same strain and diet (effect of age); (b) significantly different from the AL group of the same strain and age (effect of ER); (c) significantly different from the C57BL/6 group of the same age and diet (effect of strain), with P < 0.05.
Regardless of the criterion used for normalization, there was no apparent effect of age on oxygen consumption in the AL C57BL/6 mice. On the other hand, the rate of oxygen consumption decreased during the period from 6–23 mo in the AL DBA/2 mice when normalized as per mouse or lean body mass. According to these 2 criteria, oxygen consumption in the AL DBA/2 mice declined with age to the extent that it reached the level of the AL C57BL/6 mice by 23 mo. A similar pattern of age-related decline contributed to a main effect of age for oxygen consumption/g organ weight (P = 0.035). An age-related decline was not evident for the DBA/2 mice when oxygen consumption was standardized per g body weight. This discrepancy most probably reflected a disproportionate age-related loss in the body weight relative to the lean body mass or organ weight in these mice.
The relatively higher rate of resting oxygen consumption of the AL DBA/2 mice at 6 mo than at 23 mo of age contributed to a strain × age interaction (in the absence of a 3-way interaction) when ANOVA were performed on values per mouse or lean body mass (all P < 0.004). Although a similar pattern was evident per unit of organ weight, the effect was relatively small and only the main effect of age was detectable (P < 0.035). Analysis of the rate of oxygen consumption/g body weight yielded a main effect of strain (P < 0.001), which is consistent with the relatively higher oxygen consumption in the DBA/2 than in the C57BL/6 mice at both ages.
According to all 4 criteria used for standardization, ER significantly decreased the rate of resting oxygen consumption in the DBA/2 mice at both 6 and 23 mo of age (Fig. 3A–D). The magnitude of the decrease ranged from 14% in the young mice and 20% in the old mice/g body weight, and 43 and 35%, respectively, per mouse. The ER-induced decrease in oxygen consumption in the C57BL/6 mice at 6 and 23 mo of age was, respectively, 36% and 32% per mouse, 29% and 19% per g lean body mass, and 15% and 26% per g organ weight.
ANOVA of the rates of oxygen consumption, expressed as per mouse, units of lean body mass or organ weight, indicated a main effect of diet (Ps < 0.035) but not of diet × strain or age × diet × strain interactions (all P > 0.16). With 1 exception, these results were confirmed in analyses using lean body mass or organ weight as covariates for regression-based adjustment of oxygen consumption per mouse. A diet × strain interaction was detected (P = 0.01) in the analysis of oxygen consumption with body weight as a covariate, whereas this interaction was not significant following the conventional ANOVA on oxygen consumption per g body weight.
Discussion
The C57BL/6 mice, which exhibit an ER-related prolongation of life span, and the DBA/2 mice, which do not, were compared at 6 and 23 mo of age following the imposition of a 40% ER regimen for 2 and 19 mo, respectively, to address the twin issues of whether ER lowers the resting rate of metabolism either transiently or stably and whether lowering of the metabolic rate is intrinsically linked to an extension of life span. Understanding the relationship among metabolic rate, ER, and longevity is deemed significant because of its relevance to the oxidative stress hypothesis of aging, which proposes that aerobic respiration entails the production of reactive oxygen species, perturbation of the redox state, and infliction of macromolecular oxidative damage, leading to the senescence-associated attrition of physiological fitness (reviewed in 16).
Resting metabolic rate accounts for ∼50–70% of daily energy expenditure in homeotherms and is measured either as metabolic heat production or more frequently as rate of oxygen consumption or carbon dioxide production (17). Because ER decreases the body mass and fat content, comparisons of rates of oxygen consumption between AL and ER rodents are generally normalized as a function of lean body mass or some other surrogate of metabolically active mass; nevertheless, the nature of the relationship between ER and oxygen consumption has remained controversial (discussed in 12,18,19). Retrospectively, it seems that the controversy primarily stems from the assumptions made and criteria used for the estimation of the respective metabolically active, lean body masses of the AL and ER groups. Often, lean body mass is calculated as an exponent of the body mass or as body mass minus the fat content. However, the procedures for the measurement of fat/lean body mass by different investigators not only vary but also differ in the degree of accuracy and/or sensitivity (20,21). Although the fat-free/lean body mass is more representative of metabolically active tissues, it also has several limitations, as indicated by the results of the present and previous studies (12,22). Foremost, ER-related losses in the weights of different tissues and organs lack uniformity. For instance, in the present study, the effect of ER on the weights of different organs ranged between −6 and −33% in C57BL/6 mice and ∼8 to −39% in DBA/2 mice at 6 mo of age, after 2 mo on the ER regimen, with the rank order: brain < skeletal muscle < heart < liver < kidney. Each of these organs/tissues is known to have a distinct rate of oxygen consumption (23; reviewed in 24). Furthermore, the pattern of ER-related differences in organ weights varied with age and differed in the 2 strains of mice. Thus, comparisons of the rates of oxygen consumption between ER and AL mice based on the assumption that the weights of different tissues constituting the fat-free lean body mass are affected uniformly rather than variably by ER can lead to erroneous inferences.
One approach to mitigate these complications may be that a subset of organs, whose weights are either relatively little or similarly affected by ER, are used as a surrogate of metabolic mass. In the present study, the weight of the brain was found to be only slightly reduced by ER, whereas the losses in the weights of liver, kidney, and heart tended to be quite comparable (Table 1). Moreover, on the basis of a comparison among 10 different indicators of metabolic mass to normalize the metabolic rates of AL and ER Fischer 344 rats, Greenberg and Boozer (25) inferred that combined weights of heart, liver, kidney, and the brain provided the most accurate estimate of metabolic mass. Accordingly, in the present study, the aggregated weight of brain, heart, liver, and kidney was included among the 4 different criteria used to standardize the rate of oxygen consumption. These organs are thought to be responsible for the majority of energy use during resting conditions (24,26).
Results of this study indicated that according to all 4 criteria used for normalization, namely per mouse, body mass, fat-free/lean body mass, and organ weights, the rate of resting oxygen consumption, determined under conditions of physical inactivity, was lowered in both strains of mice and in both the young and the old mice after the imposition of ER for 2 or 19 mo. This finding implies that the effect of chronic ER on resting metabolic rate in these mice is long-lasting rather than transitory. Additional evidence that ER lowers the metabolic rate of mice chronically is that the daily mean core body temperature, a direct correlate of the rate of oxygen consumption, is lowered by 0.9–1.2°C in both C57BL6 and DBA/2 mice at 17–18 mo of age, after 13–14 mo on the 40% ER regimen (27). It is an established physiological precept that ∼95% of the energy expended by the body is derived from the reactions of oxygen with organic molecules and metabolic heat production is dependent upon the combined rates of cellular metabolism (17). Thus, a decrease in the core body temperature in response to ER, which has been observed widely (28–32) and over which there is relatively little disagreement, also supports the notion that ER tends to lower metabolic activity.
Although it is frequently supposed that ER ubiquitously extends life span of various mammalian and invertebrate species, there are several demonstrations to the contrary (4–8,33), suggesting that this phenomenon is selective and not universal. Thus, the question arises: why is longevity extended in some, but not other genotypes? Results of this study indicate that ER lowers the metabolic rate in both strains of mice, whereas the longevity prolongation effect is observed only in the C57BL/6 strain (8,33,34). This finding does not imply that metabolic rate has no effect on the life span of the mice; rather, the issue being addressed is whether lowering of the metabolic rate invariably results in an extension of life span or whether ER-related extension of life span is due to the lowering of metabolic rate. A major difference between the 2 strains of mice used here was that although the AL mice of both strains consumed a similar amount of food/energy throughout life and had a similar body weight at 4–5 mo of age, the C57BL/6 mice gained and maintained more weight than the DBA/2 during adult life. During 4 to 23 mo of age, the C57BL/6 mice on the AL regimen sustained a mean body weight that was 20% (∼6 g) higher than their weight at 4.5 mo, whereas the gain in mean body weight in the DBA/2 mice was insignificant. It is worth noting that, when considered over the period of adult life, the net change in body weight of the C57BL/6 mice on the ER regimen was equivalent to that of the AL DBA/2 mice (Fig. 1B). A plausible explanation of this inter-strain difference in energy balance under the AL regimen could be that compared with C57BL/6 mice, the DBA/2 mice have a relatively higher rate of energy expenditure, as indicated by the resting rate of oxygen consumption observed here and the core body temperature (27). Our previous studies have shown that the rate of cellular respiration, measured in vitro, is higher in the DBA/2 than C57BL/6 mice (35). Thus, a major difference between the AL and ER mice and between the 2 strains is that the ER regimen tends to eliminate the positive energy imbalance in the C57BL/6 mice, indicated by gain in weight, whereas the energy balance remains relatively neutral in the AL DBA/2 mice.
A general question raised by the present study is why the life span is extended by ER in C57BL/6 but not in the DBA/2 mice. Although no cause and effect relationship can be established on the basis of this study, the results suggest some hypothetical possibilities. One is that chronic energy imbalance and the consequent gain in body mass and fat content result in relatively more severe age-related metabolic abnormities and oxidative stress in the AL than in the ER mice and this effect is more evident in the C57BL/6 because of the greater energy imbalance in this strain. There exists an incontrovertible body of evidence that chronic nutritional overloading and adiposity are associated with a variety of homeostatic perturbations, including the elevation of oxidative stress, dysregulation of insulin production and signaling, impairment of mitochondrial function, and release of inflammatory mediators by adipose tissue and liver (reviewed in 36,37). Our previous studies have shown that ER lowers the level of oxidative stress, as indicated by the glutathione:glutathione disulfide ratio and the amount of protein mixed disulfides (38,39). Furthermore, the age-associated decline in the glutathione:glutathione disulfide ratio was greater in the C57BL/6 mice than in the DBA/2 mice (35).
To conclude, results of this study suggest that ER indeed lowers the rate of oxygen consumption in mice chronically rather than transiently, irrespective of its effect on life span. Although the amount of energy (food) consumption is similar in the 2 strains of mice, the C57BL/6 mice, whose longevity is extended by ER, have a relatively low metabolic rate and gain weight with age, whereas the DBA/2 mice have a higher metabolic rate, do not show a gain in the mean weight during adult life, and their longevity is not extended by ER. Taken together, these results suggest that ER-induced prolongation of life span occurs in a genotype that displays a positive imbalance between energy intake and expenditure. We provisionally hypothesize that the longevity extension effect occurs only when the implementation of ER serves to offset such an energy imbalance.
Supported by the grant R01 AG13563 from the NIH-National Institute on Aging.
Author disclosures: R. S. Sohal, M. Ferguson, B. H. Sohal, and M. J. Forster, no conflicts of interest.
Abbreviations used: AL, ad libitum; ER, energy restriction; EWAM; epididymal white adipose mass.
References
- 1.Masoro EJ. Caloric restriction: a key to understanding and modulating aging. Amsterdam: Elsevier; 2002.
- 2.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield (IL): Charles C Thomas; 1988.
- 3.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. [DOI] [PubMed] [Google Scholar]
- 4.Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 2004;18:1591–3. [DOI] [PubMed] [Google Scholar]
- 5.Mockett RJ, Cooper TM, Orr WC, Sohal RS. Effects of caloric restriction are species-specific. Biogerontology. 2006;7:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacher GA. Life table modifications and life prolongation. In: Finch E, Hayflick L, editors. Handbook of the biology of aging. New York: Van Nostrand; 1977. p. 582–638.
- 10.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–8. [DOI] [PubMed] [Google Scholar]
- 11.Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000;29:946–68. [DOI] [PubMed] [Google Scholar]
- 13.Witt WM, Brand CD, Atwood VG, Soave OA. A nationally supported study on caloric restriction of rodents. Lab Anim (NY). 1989;18:37–43. [Google Scholar]
- 14.Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr. 2008;138:2269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop SC, Hill WG. Effects of selection on growth, body composition, and food intake in mice. III. Correlated responses: growth, body composition, food intake and efficiency and catabolism. Genet Res. 1985;46:57–74. [DOI] [PubMed] [Google Scholar]
- 16.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–86. [DOI] [PubMed] [Google Scholar]
- 17.Guyton AC, Hall JE. Textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders; 2006.
- 18.Speakman JR, van Acker A, Harper EJ. Age-related changes in the metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–75. [DOI] [PubMed] [Google Scholar]
- 19.Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev. 2005;126:783–93. [DOI] [PubMed] [Google Scholar]
- 20.Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond). 2006;30:1322–31. [DOI] [PubMed] [Google Scholar]
- 21.Speakman JR. Body composition analysis in animals: a handbook of non-destructive methods. Cambridge (UK): Cambridge University Press; 2001.
- 22.Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs HA. Body size and tissue respiration. Biochim Biophys Acta. 1950;4:249–69. [DOI] [PubMed] [Google Scholar]
- 24.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney J, editor. Energy metabolism: tissue determinants and cellular corollaries. New York: Raven; 1992. p. 61–77.
- 25.Greenberg JA, Boozer CN. Metabolic mass, metabolic rate, caloric restriction, and aging in male Fischer 344 rats. Mech Ageing Dev. 2000;113:37–48. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–58. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech Ageing Dev. 2007;128:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rikke BA, Johnson TE. Lower body temperature as a potential mechanism of life extension in homeotherms. Exp Gerontol. 2004;39:927–30. [DOI] [PubMed] [Google Scholar]
- 30.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter JE. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. [DOI] [PubMed] [Google Scholar]
- 31.Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci USA. 1996;93:4159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy PH, Leakey JEA, Pipkin JL, Turturro A, Hart RW. The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ Res. 1997;73:242–8. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci USA. 1976;73:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp Gerontol. 2008;43:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–94. [DOI] [PubMed] [Google Scholar]
- 38.Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35:626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]