Abstract
Glycine is a precursor of purines, protein, glutathione, and 1-carbon units as 5,10-methylenetetrahydrofolate. Glycine decarboxylation through the glycine cleavage system (GCS) and glycine-serine transformation by serine hydroxymethyltransferase (SHMT) require pyridoxal 5′-phosphate (PLP; active form of vitamin B-6) as a coenzyme. The intake of vitamin B-6 is frequently low in humans. Therefore, we determined the effects of vitamin B-6 restriction on whole-body glycine flux, the rate of glycine decarboxylation, glycine-to-serine conversion, use of glycine carbons in nucleoside synthesis, and other aspects of 1-carbon metabolism. We used a primed, constant infusion of [1,2-13C2]glycine and [5,5,5-2H3]leucine to quantify in vivo kinetics in healthy adults (7 males, 6 females; 20–39 y) of normal vitamin B-6 status or marginal vitamin B-6 deficiency. Vitamin B-6 restriction lowered the plasma PLP concentration from 55 ± 4 nmol/L (mean ± SEM) to 23 ± 1 nmol/L (P < 0.0001), which is consistent with marginal deficiency, whereas the plasma glycine concentration increased (P < 0.01). SHMT-mediated conversion of glycine to serine increased from 182 ± 7 to 205 ± 9 μmol·kg−1·h−1 (P < 0.05), but serine production using a GCS-derived 1-carbon unit (93 ± 9 vs. 91 ± 6 μmol·kg−1·h−1) and glycine cleavage (163 ± 11 vs. 151 ± 8 μmol·kg−1·h−1) were not changed by vitamin B-6 restriction. The GCS produced 1-carbon units at a rate (∼140–170 μmol·kg−1·h−1) that greatly exceeds the demand for remethylation and transmethylation processes (∼4–7 μmol·kg−1·h−1). We conclude that the in vivo GCS and SHMT reactions are quite resilient to the effects of marginal vitamin B-6 deficiency, presumably through a compensatory effect of increasing substrate concentration.
Introduction
Glycine has many roles in human metabolism, including as a substrate in purine and protein synthesis, as a precursor of glutathione, and as a source and acceptor of 1-carbon units. The mitochondrial glycine cleavage system (GCS)10 cleaves glycine to CO2, ammonia, and a 1-carbon unit in the methylene (-CH2-) state as 5,10-methylenetetrahydrofolate (5,10-methyleneTHF) (1). Serine hydroxymethyltransferase (SHMT) reversibly transfers a 1-carbon group from 5,10-methyleneTHF to glycine, forming tetrahydrofolate (THF) and serine. Serine is the major 1-carbon source for homocysteine remethylation (2).
Vitamin B-6 in the form of pyridoxal 5′-phosphate (PLP) serves as a coenzyme in many reactions of human intermediary metabolism. With respect to 1-carbon metabolism, PLP is required as a coenzyme for glycine decarboxylase of the GCS, cytosolic, and mitochondrial forms of SHMT, and cystathionine β-synthase and cystathionine γ-lyase in the transsulfuration system. Marginal vitamin B-6 deficiency, as reflected by plasma PLP concentrations between 20 and 30 nmol/L (3), occurs frequently (4) and has been associated with coronary artery disease (5–7), stroke (8), and elevated risk of Alzheimer's disease (9). The mechanisms responsible for such linkages between vitamin B-6 status and chronic disease have not been established and often do not involve severity of deficiency associated with hyperhomocysteinemia.
Alterations of glycine metabolism at various levels of vitamin B-6 deficiency have been shown in rats, with hepatic glycine concentration inversely proportional to vitamin B-6 intake (10,11). In another study with rats, vitamin B-6 deficiency yielded nearly a doubling of the plasma, liver, and muscle free glycine concentrations (12). Plasma glycine increased 29% in healthy participants after 4 wk of a vitamin B-6–restricted diet that provided <0.5 mg/d vitamin B-6 (13) and increased 26% after 3 wk of 0.16 mg/d vitamin B-6 (14). We hypothesized that the increase in glycine concentration during marginal vitamin B-6 deficiency is due to reduced activity of the vitamin B-6–dependent GCS. The quantitative importance of the GCS is implied by the fact that loss-of-function mutations affecting genes encoding the 4 enzymes of the GCS cause accumulation of glycine to pathological concentrations, leading to nonketotic hyperglycinemia or glycine encephalopathy (15,16). Predictions of the effects of various degrees of vitamin B-6 deficiency on glycine metabolism are complicated, because a deficiency also leads to reduced activity of other PLP-dependent enzymes in glycine metabolism, including SHMT (13,17), serine-pyruvate/alanine-glyoxylate aminotransferase (18–21), and the GCS. Changes in the activities of these enzymes could affect the concentration and turnover of glycine in tissues and plasma.
The nutritional importance of glycine in nucleoside synthesis has not been adequately determined. In de novo purine synthesis, a single molecule glycine is incorporated to form carbon-4, carbon-5, and nitrogen-7 of the purine ring, whereas carbon-2 and carbon-8 derive from 1-carbon units transferred from 10-formylTHF (22). Thymidylate is formed from deoxyuridine monophosphate and the 1-carbon unit of 5,10-methyleneTHF (23). Because the GCS is a major source of cellular 5,10-methyleneTHF production, we hypothesized that the synthesis of purines and thymidylate could be impaired under conditions of marginal deficiency.
In an initial report regarding the in vivo kinetics of glycine-based metabolic reactions (24), we provided data from 5 healthy volunteers who underwent primed, constant infusion with [1,2-13C2]glycine and [2H3]leucine to examine aspects of glycine metabolism in adequate vitamin B-6 status. We found a high rate of serine synthesis (193 μmol·kg−1·h−1) from glycine via SHMT in healthy men and women and a similarly high rate of glycine cleavage (24). These findings show quantitatively the extent to which glycine is a major substrate in serine synthesis and that the GCS is responsible for the production of 1-carbon units as 5,10-methyleneTHF at a high rate. Thus, this study showed that the mitochondrial GCS has a major quantitative role in the production of 5,10-methyleneTHF for 1-carbon metabolism. We report here further data from that study as well as the sensitivity of glycine metabolism and related reactions in 1-carbon metabolism to marginal vitamin B-6 deficiency in 13 healthy female and male volunteers while considering genotypes with respect to polymorphisms related to 1-carbon metabolism. The data from the predepletion infusion from 2 of the male participants were included in a preliminary report of the assessment of glycine kinetics using protocol (24).
Methods
Materials
[1,2-13C2]Glycine, l-[5,5,5-2H3]leucine, and sodium [13C]bicarbonate were purchased from Cambridge Isotopes Laboratories. The parenteral solutions of these compounds were prepared in isotonic saline, filter sterilized, and analyzed to ensure lack of pyrogenicity and microbial contamination.
Human participants
Participants underwent a physical examination and were screened by standard clinical measures of hematological, hepatic, renal, and thyroid function. Medical history, dietary habits, and demographic data were assessed by a questionnaire. Of the 37 recruited healthy adult male and nonpregnant female volunteers, 23 met the following inclusion criteria: age between 20 and 40 y; no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; no vitamin, amino acid, or protein supplementation; no chronic consumption of a high-protein diet; and a BMI <28 kg/m2. All selected participants were in adequate nutritional status for serum folate (>7 nmol/L) and vitamin B-12 (>200 pmol/L), and plasma PLP (>30 nmol/L) and total homocysteine (<12 μmol/L).
Before the study, 6 of the 23 participants that passed screening withdrew because of personal reasons or scheduling problems. Three participants were withdrawn during intervention due to changes in health status and 1 withdrew for personal reasons. All participants gave written informed consent. The University of Florida Institutional Review Board and the General Clinical Research Center (GCRC) Scientific Advisory Committee reviewed and approved this protocol.
Dietary treatment
All meals were prepared by the Bionutrition Unit of the GCRC. Participants consumed nutritionally adequate meals with standardized composition for 2 d to minimize dietary variation immediately prior to the first infusion. Participants began consuming a vitamin B-6–restricted diet (<0.5 mg/d vitamin B-6) on the day after the first infusion and continued this regimen for 28 consecutive days (13,25). Participants consumed breakfast in the GCRC, were given a take-out lunch and snack to eat at their convenience, and returned to the GCRC to consume their evening meal. We compensated for vitamin and mineral inadequacies of the study diets (other than vitamin B-6) by administering custom supplements daily to the participants. Compliance with the dietary regimen was monitored by weekly measurements of plasma PLP as described below. After the second infusion day, participants were offered nutritionally adequate meals in the GCRC. An over-the-counter, multivitamin-multimineral supplement was provided to the participants after the vitamin B-6–restricted diet to facilitate restoration of normal vitamin B-6 status.
Analytical methods
Screening measurements.
Serum folate and vitamin B-12 were analyzed with the use of a chemiluminescence-based assay (Elecsys, Roche Diagnostics). Plasma PLP concentration was measured as the semicarbazone-derivative by reverse-phase HPLC with fluorescence detection (26). Plasma total homocysteine concentration was measured as the ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate derivative by reverse-phase HPLC with fluorescence detection (27). Selected single nucleotide polymorphisms (SNP) were determined in DNA purified from whole blood (QIAamp DNA Mini kit; Qiagen) using a high-level multiplex genotyping method based on matrix-assisted laser desorption/ionization time-of-flight MS (28).
GC-MS determination of amino acid isotopic enrichment and quantification.
Plasma free amino acids were isolated, derivatized, and analyzed by GC-MS in electron capture negative ionization mode as previously described (29). The relative abundance of specific ions was determined by selected-ion monitoring at the following mass:charge ratios: glycine (293–295), serine (518–521), leucine (349–352), and cystathionine (677–681). Isotopic enrichments are expressed as molar ratios (mol % excess) of labeled:nonlabeled isotopomers after correction for the natural abundance of stable isotopes, essentially as performed by Storch et al. (30). Glycine, serine, and cystathionine concentrations in fasting plasma samples of each infusion day were measured by this method except with the use of stable-isotope labeled internal standards: [13C2]glycine, [2H3]serine, and [2H4]cystathionine (Cambridge Isotope Laboratories). The calibration curve was derived from the abundances of unlabeled and labeled isotopomers.
13C isotopic enrichment of breath CO2.
Breath samples were collected in Exetainer tubes (Metabolic Solutions) and analyzed by isotope ratio-MS (Metabolic Solutions) to determine 13C isotopic enrichment of breath CO2. Total CO2 production rate (VCO2) was determined with the use of a metabolic cart (TrueMax 2400; ParvoMedics). Measurements were taken at 30-s intervals for ∼5 min until 4 consecutive time points differed by no more than ± 0.01 L/min.
Liquid chromatography-tandem MS analysis of monocyte DNA isotopic enrichment and methyldeoxycytidine concentration.
Monocytes were purified from whole blood using Vacutainer Sodium Heparin CPT tubes (Becton Dickinson) followed by magnetic labeling of the monocytes with human CD14 MicroBeads (Miltenyi Biotec) and isolation using a MACS Separation column (Miltenyi Biotec). DNA was purified from the isolated monocytes using a QIAamp DNA Mini kit (Qiagen). The concentration of DNA in each sample was quantified by the Qubit Quantitation Platform using Quant-iT fluorescence technology (Invitrogen). DNA was hydrolyzed using a recently developed digest mix containing 250 U Benzonase, 300 mU phosphodiesterase I, and 200 U alkaline phosphatase (31). Isotopic enrichment was determined by liquid chromatography-tandem MS with the use of a Luna 18(2) column (50 × 3.0 mm, 3 μm, Phenomenex) and a Thermo-Finnigan TSQ Quantum mass spectrometer in heated electrospray ionization mode (31) with the mass transitions reported earlier (23). We employed a modification of this procedure with the addition of [15N3]deoxycytidine and [15N3]methyldeoxycytidine as internal standards for the determination of methyldeoxycytidine (MdC) and deoxycytidine to calculate the percentage of methylation of deoxycytidine (MdC%) (32).
Infusion protocol
Participants were admitted to the GCRC on the evening before the infusion protocol and consumed no food or drinks, except water, between 2100 and the first blood draw. On the morning of the infusion, a catheter was inserted in the antecubital vein of each arm, 1 for the tracer infusion and 1 for blood collection. Fasting blood samples were taken 2 h before infusion (at ∼0700) for measurement of plasma PLP, serum folate and vitamin B-12, and plasma amino acid concentrations. Infusions were initiated at ∼0900 with a 5-min, ∼20-mL priming dose that delivered 9.26 μmol/kg of [1,2-13C2]glycine, 1.87 μmol/kg of [5,5,5-2H3]leucine, and 2.15 μmol/kg of NaH13CO3. The 9-h constant infusion followed immediately after the priming dose and delivered ∼20 mL infusion solution/h that contained 9.26 μmol/kg [1,2-13C2]glycine and 1.87 μmol/kg [5,5,5-2H3]leucine.
Blood samples were taken at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7.5, and 9 h of the infusion. They were placed immediately on ice and were centrifuged within 15 min after the blood draw (1500 × g; 10 min at 4°C). Plasma was stored in microcentrifuge tubes at −80°C.
Breath samples were collected into Exetainer tubes at times 0, 1, 2, 3, 4, 5, and 6 h of infusion to measure 13CO2 production. Measurements of the VCO2 were conducted at 0, 2, 4, 6, and 8 h of infusion. The participants received a nutritive formula hourly starting 2 h before infusion to maintain a fed state (30). This formula provided a balanced composition of amino acids at a rate based on requirements of 0.8 g protein·kg−1·d−1, which equals an hourly protein dose of ∼0.03 g/kg with 5.23 kJ·kg−1·d−1 for women and 5.44 kJ·kg−1·d−1 for men. The formula further provided an adequate energy intake according to the requirements of 126 kJ·kg−1·d−1 for women and 130 kJ·kg−1·d−1 for men.
Blood samples were collected before the infusion (0-time) to determine the natural isotopic abundance of monocyte DNA. Because monocytes appear ∼1 d after synthesis in circulation and have a half-life of ∼2 d, blood samples were collected after 2, 2.5, 3, 3.5, and 4 d of each infusion procedure for monocyte isolation and determination of DNA enrichment. MdC% was determined in monocytes collected prior to each infusion (0-time) before and after vitamin B-6 restriction.
Kinetic principles and analysis
As reported previously (24), the combined use of [1,2-13C2]glycine and [2H3]leucine allowed us to determine quantitative aspects of glycine metabolism, including its overall flux and rates of decarboxylation, direct glycine-to-serine interconversion by SHMT (monitored by the formation of [13C2]serine), and the contribution of glycine-derived 1-carbon units as 5,10-methyleneTHF in serine formation via SHMT. The latter is indicated by the formation of [13C1]serine, because the glycine tracer is decarboxylated and catabolized via the GCS whereas the original 13C-labeled 2-carbon of glycine is transferred to THF to yield 5,10-methyleneTHF. [2H3]Leucine was included to evaluate any nutritional effects on protein turnover as indicated by leucine flux (13,33).
Plateau enrichments (Ep) for all infused amino acid tracers were calculated as the mean of the isotopic enrichments for the ∼1.5–9-h time points for the infused [13C2]glycine and [2H3]leucine tracers. Ep of all labeled metabolic products were determined by fitting enrichment data to single exponential curves defined by the equation
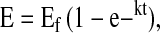 |
where E is the enrichment at time t (h), Ef the enrichment at infinity (i.e. Ep), and k the rate constant (h−1) from the fitted curve (34). Data were fit to a single exponential regression equation using the “exponential rise to maximum” function of SigmaPlot 2002 (version 8.02; SPSS).
Steady-state kinetics of amino acid tracers were calculated using standard equations (35), including correction for overestimation of intracellular enrichment from plasma enrichment data (30,34,35), as discussed below. The flux of an amino acid is the rate of appearance of that amino acid from endogenous production (de novo synthesis and protein breakdown), absorption, and the tracer infusion and is calculated from the Ep of the corresponding amino acid tracer. Specifically, the flux (Q) of leucine (QLeu) in the plasma pool is calculated as:
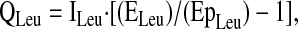 |
where ILeu is the [2H3]leucine infusion rate, ELeu is the enrichment of the [2H3]leucine tracer, and EpLeu is the Ep of [2H3]leucine in plasma. The Ep of plasma leucine was not corrected for overestimation of intracellular enrichment, consistent with previous studies using leucine flux as a relative indicator of protein turnover (13,33,36).
Glycine Q (QGly) was calculated from plasma [13C2]glycine enrichment after correcting for the difference between the intracellular and plasma [13C2]glycine enrichment. This prediction of intracellular [13C2]glycine enrichment (Ep'Gly) was accomplished by multiplying the observed plasma [13C2]glycine enrichment by a correction factor of 0.4 derived from previous glycine tracer infusion studies in humans (37,38).
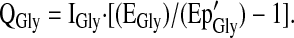 |
The flux values for labeled metabolic products, i.e. serine M+1 and serine M+2, were estimated by assuming a serine Q (QSer) of 271 μmol·kg−1·h−1 at normal vitamin B-6 status and QSer = 281 μmol·kg−1·h−1 at marginal vitamin B-6 deficiency (13).
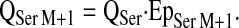 |
The synthesis rates of metabolic products derived from the infused tracers were calculated from the flux and the Ep of the infused tracer after correction for intracellular isotopic dilution (13). The rate of serine M+1 synthesis, which was indicative of serine synthesis using a glycine-derived 1-carbon unit, was thus calculated as:
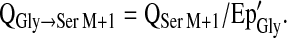 |
The rate of serine M+2 synthesis, reflecting direct conversion of glycine to serine, was calculated in analogous fashion.
The rate of production of 13CO2 served as a measure of the whole-body rate of decarboxylation of the glycine tracer. This was measured in standard fashion as in amino acid oxidation studies (30,39). In this procedure, the rate of 13CO2 release (V13CO2, in units of μmol·h−1·kg−1 body weight) and the rate of glycine catabolism via decarboxylation (CGly) (μmol·h−1·kg−1 body weight) were calculated as follows:
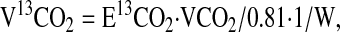 |
where E13CO2 is breath 13CO2 enrichment plateau and 0.81 is the assumed fraction of CO2 release from the body pool of bicarbonate and W is body weight (39).
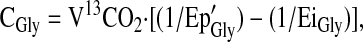 |
where Ep′Gly is the Ep of plasma [13C2]glycine corrected for intracellular overestimation and EiGly is the enrichment of the infused glycine tracer.
The fraction of glycine flux occurring via glycine decarboxylation was calculated as
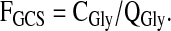 |
The area under the curve (AUC) of nucleoside enrichment over time (infusion day until d 4 postinfusion, i.e. over 5 d) was determined using the trapezoidal method to compare the rate of glycine and 1-carbon incorporation in nucleoside synthesis before and after vitamin B-6 restriction. The maximum enrichment (Emax) also is reported for each deoxynucleoside.
Statistical analysis
In all tracer measurements, isotopic enrichments are expressed as ratio of labeled:nonlabeled isotopomers after correction for the natural abundance of stable isotopes. The study was designed based on power calculations assuming variance equivalent to that observed in previous studies of homocysteine remethylation (13). The power of the study was >80% for detecting a difference of >25% in measured fluxes with 14 participants with an assumed SD of 25–30%. All data were presented as means ± SEM. An unpaired t test was performed to determine differences in baseline characteristics between men and women. A paired t test was used for assessment of differences between normal vitamin B-6 status and marginal vitamin B-6 deficiency. Two-factor ANOVA was performed to determine interactions between kinetic measurements and SNP. Data were analyzed using Microsoft Office Excel 2007, SigmaStat 3.0, and SPSS 16.0.
Results
Nutritional status and baseline characteristics.
The 13 participants who completed the intervention had normal nutritional status for vitamin B-6, folate, and vitamin B-12 at baseline (Table 1). Genetic polymorphism analysis (Table 1) that was conducted to facilitate possible inferences regarding any observed atypical kinetic patterns or metabolic profiles showed genotype distributions consistent with those reported previously (40).
TABLE 1.
Demographic information and baseline characteristics of 13 men and women who completed study intervention1
| Men | Women | |
|---|---|---|
| n | 7 | 6 |
| Race distribution, n | ||
| African-American | 1 | 1 |
| Asian | 0 | 1 |
| Caucasian | 4 | 3 |
| Hispanic | 2 | 1 |
| Age, y | 24 ± 2 | 26 ± 3 |
| BMI, kg/m2 | 25 ± 1 | 24 ± 1 |
| Plasma PLP, nmol/L | 63 ± 4 | 46 ± 6* |
| Serum folate, nmol/L | 34 ± 3 | 28 ± 3 |
| Serum vitamin B-12, pmol/L | 371 ± 45 | 388 ± 57 |
| Plasma total homocysteine, μmol/L | 8.7 ± 0.5 | 7.1 ± 0.6 |
| SNP,23n:n:n | ||
| MTHFR 677C > T | 2: 4: 1 | 2: 4: 0 |
| MTHFR 1298A > C | 5: 2: 0 | 5: 0: 1 |
| MTR 2756A > G | 3: 4: 0 | 5: 1: 0 |
| MTRR 66A > G | 2: 5: 0 | 2: 2: 2 |
| CBS 844ins68 | 3: 4: 0 | 6: 0: 0 |
| CBS 699C > T | 3: 3: 1 | 1: 5: 0 |
| BHMT 742G > A | 3: 4: 0 | 4: 1: 1 |
| SHMT 1420C > T | 5: 2: 0 | 3: 2: 1 |
Values are means ± SEM or n. *Different from men, P < 0.05 (unpaired t test).
Distribution given as n of wild type:n of heterozygotes:n of homozygotes.
Abbreviations: MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; CBS, cystathionine β-synthase; BHMT, betaine homocysteine methyltransferase.
Plasma PLP, amino acid concentrations, and monocyte DNA methylation.
After 28 d of dietary vitamin B-6 restriction, the plasma PLP concentration decreased into the range of marginal deficiency (20–30 nmol/L) from 55 ± 4 nmol/L to 23 ± 1 nmol/L (P < 0.001), with a final range of 18–29 nmol/L (Table 2). During the 28-d period of vitamin B-6 restriction, the compliance of participants was monitored weekly by measuring plasma PLP concentrations. Marginal vitamin B-6 deficiency was reached after 22 ± 2 d. Serum folate and plasma total homocysteine concentrations did not change from vitamin B-6 restriction. Serum vitamin B-12 concentrations decreased (P < 0.01) but remained in the normal range. Plasma glycine (P < 0.01) and cystathionine (P < 0.001) concentrations increased, whereas plasma serine, cysteine, and methionine concentrations remained constant. Monocyte DNA showed a trend toward lower MdC% after vitamin B-6 restriction (P = 0.09).
TABLE 2.
Plasma or serum PLP, folate, vitamin B-12, and amino acid concentrations of 1-carbon metabolism and relative extent of monocyte DNA methylation in healthy men and women at baseline and after 28 d of moderate vitamin B-6 restriction1
| Baseline | Restricted | |
|---|---|---|
| B vitamins | ||
| Plasma PLP, nmol/L | 55 ± 4 | 23 ± 1** |
| Serum folate, nmol/L | 31 ± 2 | 35 ± 6 |
| Serum vitamin B-12, pmol/L | 379 ± 34 | 334 ± 30* |
| Amino acids in plasma | ||
| Total homocysteine, μmol/L | 6.7 ± 0.3 | 6.9 ± 0.4 |
| Total cysteine, μmol/L | 237 ± 33 | 237 ± 29 |
| Glycine, μmol/L | 306 ± 20 | 339 ± 18* |
| Serine, μmol/L | 96 ± 5 | 97 ± 6 |
| Methionine, μmol/L | 29 ± 1 | 29 ± 1 |
| Cystathionine, nmol/L | 99 ± 5 | 169 ± 15** |
| Methylation of deoxycytidine in monocyte DNA, MdC as % of total deoxycytidine | 4.48 ± 0.03 | 4.41 ± 0.04 |
Values are means ± SEM, n = 13. *Different from baseline, P < 0.01; **P < 0.001 (paired t test).
In vivo amino acid kinetics.
Primed, constant infusion with 9.26 μmol·kg−1·h−1 [13C2]glycine and 1.87 μmol·kg−1·h−1 [2H3]leucine yielded quantifiable time course plots of plasma enrichment of the infused stable isotope-labeled amino acids and the glycine-derived metabolic products for all participants (Fig. 1). The whole-body leucine flux (106 μmol·kg−1·h−1) and glycine flux (445–450 μmol·kg−1·h−1) did not change as a function of vitamin B-6 restriction (Table 3). The absence of a change in leucine flux indicates that this level of vitamin B-6 restriction did not alter the rate of protein turnover.
FIGURE 1 .
Plasma enrichment of infused amino acids (A) and metabolic products (B) in healthy men and women before and after 28 d of vitamin B-6 restriction during a primed, constant infusion with 9.26 μmol·kg−1·h−1 [13C2]glycine and 1.87 μmol·kg−1·h−1 [2H3]leucine. Values are means ± SEM, n = 13.
TABLE 3.
Plasma isotopic Ep of stable isotope-labeled amino acids and their metabolic products and the corresponding whole-body flux values in healthy men and women at baseline and after 28 d of moderate vitamin B-6 restriction1
| Ep
|
Whole-body Q
|
|||
|---|---|---|---|---|
| Baseline | Restricted | Baseline | Restricted | |
| mol % excess | μmol·kg−1·h−1 | |||
| Infused amino acids | Qinfused AA | |||
| [2H3]leucine | 1.75 ± 0.06 | 1.80 ± 0.09 | 106 ± 42 | 106 ± 72 |
| [13C2]glycine | 5.21 ± 0.29 | 5.17 ± 0.19 | 448 ± 203 | 446 ± 163 |
| Metabolic products | QGly→Ser M+x | |||
| [13C1]serine (Ser M+1) | 0.70 ± 0.06 | 0.67 ± 0.05 | 93 ± 94 | 91 ± 64 |
| [13C2]serine (Ser M+2) | 1.37 ± 0.05 | 1.50 ± 0.08 | 182 ± 75 | 205 ± 95* |
| QGly→CO2 | ||||
| 13CO2 | 0.046 ± 0.002 | 0.043 ± 0.004 | 163 ± 116 | 151 ± 86 |
Values are means ± SEM, n = 13. *Different from baseline, P = 0.02 (paired t test).
QLeu.
QGly.
Serine synthesis via SHMT using a glycine-derived 1-carbon unit.
Serine synthesis from glycine via SHMT.
Generation of CO2 from glycine catabolism.
The total rate of serine synthesis via SHMT, as calculated from the appearance of [13C2]serine from infused [13C2]glycine, increased and comprised 41 ± 1% and 47 ± 2% of the whole-body glycine flux before and after vitamin B-6 restriction, respectively (P < 0.05). The rate of SHMT-mediated serine synthesis using a glycine cleavage-derived 1-carbon unit (as reflected by a 13C-labeled 1-carbon unit coupled to an unlabeled glycine to yield [13C1]serine) constituted 51 ± 4% and 45 ± 7% of the total glycine-to-serine conversion before and after vitamin B-6 restriction, respectively. Under the conditions of this protocol, the enrichment of [13C1]methionine (i.e. M+1, which reflected homocysteine remethylation with a glycine-derived 13C-methyl group) was typically below the limit of precise measurement (<0.2 mol % excess).
The rate of 13CO2 release corresponded to total CO2 generation in glycine catabolism at 163 ± 11 μmol·kg−1·h−1 before and 151 ± 8 μmol·kg−1·h−1 after the vitamin B-6–restricted diet. This also indicated that the glycine-derived generation of CO2, which we assumed to be primarily via the GCS, accounted for 37 ± 2% of whole body glycine flux during adequate vitamin B-6 status and for 34 ± 2% at marginal vitamin B-6 deficiency.
Labeling of monocyte DNA from glycine.
We measured deoxyguanosine (dG) M+2 and deoxyadenosine (dA) M+2 enrichment in DNA from monocytes after infusion with [13C2]glycine to determine purine de novo synthesis. The AUC for dG M+2 and dA M+2 enrichment from direct incorporation of glycine in purine synthesis did not change in response to vitamin B-6 restriction (Table 4). The enrichment of dG M+1, dA M+1, thymidylate M+1, and MdC M+1 that would be derived from the incorporation of a glycine-derived 13C-1-carbon unit was below the detection limit.
TABLE 4.
AUC and Emax for dG M+2 and dA M+2 enrichment in monocyte DNA over the 5-d period following infusion with 9.26 μmol·kg−1·h−1 [13C2]glycine in healthy men and women at baseline and after 28 d of moderate vitamin B-6 restriction12
| Baseline | Restricted | |
|---|---|---|
| dG M+2 | ||
| AUC, mol % excess over 5 d | 0.28 ± 0.04 | 0.35 ± 0.04 |
| Emax, mol % excess | 0.10 ± 0.01 | 0.13 ± 0.01* |
| dA M+2 | ||
| AUC, mol % excess over 5 d | 0.34 ± 0.05 | 0.38 ± 0.04 |
| Emax, mol % excess | 0.13 ± 0.01 | 0.14 ± 0.01 |
Values are means ± SEM, n = 13. *Different from baseline, P = 0.02 (paired t test).
Tissue Ep'gly was predicted to be 2.08 and 2.07 mol % excess before and after vitamin B-6 restriction, respectively.
Kinetic consequences of genetic polymorphisms.
No clear relationships were discerned between the measured fluxes and the polymorphisms evaluated in view of the small number of participants in this analysis. Because this study provided a direct measurement of SHMT flux (i.e. QGly →Ser M+2) and the kinetic consequences of SHMT 1420C > T polymorphism had not been examined previously to our knowledge, we classified glycine-to-serine fluxes by SHMT 1420C > T genotype. The glycine-to-serine synthesis rate before and after vitamin B-6 restriction was 191 ± 7 and 204 ± 12 in participants with the SHMT 1420CC genotype (n = 8), 162 ± 17 and 198 ± 16 in participants with the SHMT 1420CT genotype (n = 4), and 179 and 239 in 1 participant with the SHMT 1420TT genotype. There was no significant interaction between genotypes and the glycine-to-serine synthesis rate.
Discussion
Vitamin B-6 and in vivo fluxes of GCS, SHMT, and transsulfuration.
We hypothesized that glycine cleavage would be reduced based on consistent increases in plasma and urinary glycine concentrations in vitamin B-6–restricted human participants (13,14), as also seen in this study (Table 2), and from analysis of tissues of vitamin B-6–depleted rats (10–12). A study in vitamin B-6–depleted rats had significantly lower 14CO2 production after injection of [14C]glycine. In vitro studies using liver and kidney homogenates of vitamin B-6–depleted rats confirmed decreased glycine decarboxylation and thus lowered maximum velocity for GCS during vitamin B-6 deficiency (18,41). The quantitative importance of the mitochondrial GCS in glycine metabolism is illustrated by the fact that GCS accounted for more than one-third of whole-body glycine flux under the conditions of this protocol at both normal vitamin B-6 status and marginal vitamin B-6 deficiency. Generation of 13CO2 from the [13C2]glycine infusion was not significantly lowered by dietary vitamin B-6 restriction. The qualitative change in glycine cleavage rate was variably affected by vitamin B-6 restriction. Using the SD of 29 μmol·kg−1·h−1 as the criterion of change, 3 of the 13 participants showed a decrease, 2 increased, and 8 exhibited no change in the rate of glycine cleavage.
The reduced plasma PLP and increased plasma glycine and cystathionine concentrations in these participants and in previous human trials (13,14) and rat studies (10–12) indicate that this extent of dietary vitamin B-6 restriction (i.e. to the range of 20–30 nmol/L plasma PLP) leads to functional changes in vitamin B-6–dependent metabolic processes. Plasma PLP and amino acid concentrations are reflective of their liver concentrations (11). Vitamin B-6 restriction induces cellular PLP depletion as demonstrated in human erythrocytes (25) and animal tissues (10–12,17,18) and affects intracellular compartments indicated by lowered enzyme activity in both cytosolic and mitochondrial SHMT (11). Cystathionine serves as a biomarker of vitamin B-6 deficiency because of the high susceptibility of cystathionine γ-lyase to reduced vitamin B-6 status (10,42).
Dietary vitamin B-6 restriction did not alter whole-body glycine flux or rate of glycine cleavage, which we suggest is due to a concurrent increase in tissue glycine concentration. The Michaelis constant (Km) of glycine decarboxylase in the GCS is 6 mmol/L (43), which is much higher than the concentration of free glycine in tissues. The total free glycine concentration in human adult liver has been reported to be 2.48 μmol/g wet weight (∼2.48 mmol/L) (44) with a predicted compartmentalization of 0.83 mmol/L in cytosol and 1.86 mmol/L in mitochondria (45). Michaelis-Menten principles predict that the rate of mitochondrial glycine decarboxylation and concurrent generation of 5,10-methyleneTHF by the GCS would be a sensitive function of mitochondrial glycine concentration because of the high glycine Km value for the GCS and comparatively lower typical mitochondrial glycine concentration (i.e. [gly] < < Km). Therefore, increases in tissue glycine in marginal vitamin B-6 deficiency would tend to counteract the effect of PLP depletion (and resulting reduction in maximum velocity) on glycine decarboxylase activity, thereby maintaining adequate glycine cleavage flux well into the range of marginal vitamin B-6 status.
We also have shown that this marginal level of vitamin B-6 deficiency has little effect on in vivo flux in SHMT-dependent generation of 1-carbon units from serine (13) despite ∼40% reduction in SHMT activity measured in vitro in lymphocytes from the same human participants (13). Also, vitamin B-6 restriction in rats causes substantial reductions in rat liver SHMT activity assayed in vitro over a comparable stage of deficiency (11). Increases in tissue glycine concentration would tend to maintain serine production during low vitamin B-6 status, which is consistent with the increased flux of serine synthesis from glycine observed here (Table 3). In the present study, we examined SHMT flux in the direction of glycine-to-serine synthesis, concurrent with the conversion of 5,10-methyleneTHF to THF. Our previous studies (13,33,42) employed protocols designed to examine serine as a source of 1-carbon units and, thus, examined consequences of the SHMT reaction in the serine-to-glycine direction. Our calculation of serine synthesis rate in this protocol assumes a QSer of 271 μmol·kg−1·h−1 at normal vitamin B-6 status and 281 μmol·kg−1·h−1 at marginal vitamin B-6 deficiency derived from a previous study (13). We used this approach because a simultaneous determination of serine and glycine flux was not feasible within the design of this tracer protocol. Limitations of resources and limits imposed by blood sampling guidelines precluded the use of additional infusions that would be necessary for determination of QSer in each participant before and after vitamin B-6 depletion. We recognize that individual variability is overlooked when using QSer as a constant in the calculation of glycine-to-serine kinetics. Also, the QSer determined in the Davis et al. study (13) was measured in participants fed an amino acid-free but energy-adequate nutritive formula, whereas the nutritive formula in the present study provided hourly amino acids, including 19.4 μmol·kg−1·h−1 leucine, 10.4 μmol·kg−1·h−1 serine, and 9.4 μmol·kg−1·h−1 glycine. The provision of dietary amino acids in the present study accelerated amino acid turnover as reflected by the whole-body leucine flux (106 ± 4 μmol·kg−1·h−1) compared with 80 ± 3 μmol·kg−1·h−1 reported by Davis et al. (13). The variability of response in participants fed a protein-free or amino acid-containing formula, however, did not change, as shown by the SEM of both studies, which suggests that interindividual differences were negligible or that any such differences did not complicate out kinetic analysis with participants in the fed state. The estimate of whole-body glycine flux in the present study (448 ± 20 μmol·kg−1·h−1) is consistent with a report (46) of 458 ± 45 μmol·kg−1·h−1 that also was determined in the fed state. Whole-body glycine flux at the food-deprived state was 240 ± 22 μmol·kg−1·h−1 and did not change during glucose infusion (238 ± 24 μmol·kg−1·h−1) (39). We acknowledge a potential underestimation of the serine synthesis rate in the present study. This would imply an even higher contribution of serine synthesis to whole-body glycine flux. No studies, to our knowledge, have so far investigated whole body QSer in the fed state.
This tracer protocol was performed in participants in the fed state to accelerate pathways by substrate loads (30), but hormonal variations would likely also influence these pathways. For example, insulin causes increased transsulfuration by transcriptionally upregulating cystathionine β-synthase production (47), whereas glucagon activates GCS by a phosphorylation-mediated, cell-signaling mechanism (48,49). The in vivo relationship of hormonal status and metabolic fluxes is poorly understood, especially as influenced by vitamin B-6 status.
We used measurements of breath 13CO2 enrichment to quantify the glycine cleavage rate. However, 13CO2 might be generated by several processes other than the glycine decarboxylase activity of the GCS after [13C2]glycine infusion, as discussed previously (24). 13CO2 also could have been formed from both carbons of [13C2]glycine through pathways of folate or pyruvate metabolism in addition to glycine decarboxylation in the GCS. The 2-carbon of glycine is transferred on THF, forming 5,10-methyleneTHF in the GCS. 5,10-MethyleneTHF can be reduced to 10-formylTHF and 10-formylTHF dehydrogenase converts 10-formylTHF to THF and CO2 (29). The reaction catalyzed by 10-formylTHF dehydrogenase might have contributed partially to 13CO2 formation after [13C2]glycine infusion. Pyruvate metabolism also can yield CO2 formation indirectly from glycine. After conversion of glycine to serine, serine is transformed to pyruvate by the vitamin B-6–dependent serine dehydratase. Pyruvate can enter the tricarboxylic acid cycle either as oxaloacetate or acetyl-CoA. The decarboxylation of pyruvate by pyruvate dehydrogenase to acetyl-CoA and CO2 also could contribute to 13CO2 formation in this protocol. However, 10-formylTHF dehydrogenase, pyruvate dehydrogenase, and the tricarboxylic acid cycle are further downstream in the metabolism where labeling of compounds becomes increasingly diluted. Moreover, we propose that CO2 formation through the GCS is likely to be quantitatively greater than CO2 generation in the folate or pyruvate metabolism, because glycine is the direct substrate for GCS.
Quantitative aspects of glycine metabolism.
For each glycine molecule processed, the GCS produces 1 molecule of 5,10-methyleneTHF and CO2. The high rate of glycine cleavage and, thus, 5,10-methyleneTHF production (163 μmol·kg−1·h−1 in normal vitamin B-6 status and 151 μmol·kg−1·h−1 in marginal deficiency), greatly exceeds the demand for 1-carbon units in remethylation and transmethylation processes. As reported earlier, total homocysteine remethylation was not impaired by vitamin B-6 restriction (7.0 μmol·kg−1·h−1 at normal vitamin B-6 status, 6.4 μmol·kg−1·h−1 at marginal vitamin B-6 deficiency) (13). In the present study, the incorporation of GCS-derived 5,10-methyleneTHF accounted for 45% (∼92 μmol·kg−1·h−1) of serine synthesis. This implies that ∼60% of GCS-derived 1-carbon units enter serine synthesis while the other 40% enter other aspects of 1-carbon metabolism, including nucleoside synthesis, homocysteine remethylation, and S-adenosylmethionine-dependent methylation reactions. In yeast experiments, GCS-yielded 5,10-methyleneTHF was primarily utilized to synthesize serine (50). As shown in mathematical modeling, glycine and serine are major sources of 1-carbon units released by GCS and SHMT. Model predictions of a very high flux of the GCS (glycine decarboxylase) reaction (45) are consistent with the observations of this study. Our previous stable isotopic studies showed that serine is the major 1-carbon donor for remethylation reactions (29). The high serine synthesis rate (∼182 μmol·kg−1·h−1) observed here exceeds the demand of 1-carbon units for remethylation and transmethylation processes (∼7 μmol·kg−1·h−1) (13). Mitochondrial serine production in humans has been shown to support gluconeogenesis (45). The high rate of whole-body production of 5,10-methyleneTHF and serine in this study would contribute to glucose formation as well as nucleoside synthesis.
Glycine in nucleoside synthesis.
Quinlivan et al. (23) demonstrated with infusion of [3-13C]serine and [13C5]methionine the feasibility of using monocyte DNA analysis as a tool for examining nutritional and genetic variables affecting synthesis of the deoxynucleotide precursors. In that study (23), the peak enrichment of deoxynucleosides in monocyte DNA occurred at 3 d postinfusion. The AUC for the appearance of doubly labeled (M+2) dG and dA in monocyte DNA after [13C2]glycine infusion reflects relative rates of de novo purine synthesis, monocyte synthesis, and turnover. The absence of impairment of vitamin B-6 restriction on dA and dG M+2 AUC values indicates that these processes were not affected by this level of vitamin B-6 deficiency. We did note a significant decrease in the Emax of dG M+2 but not of its AUC. However, this result needs careful interpretation, because 11 of 13 participants showed Emax of dG M+2 at the last time point of blood sampling, i.e. 4 d postinfusion and the full time course could not be evaluated. However, we anticipate no further increase in purine enrichment after 4 d postinfusion. The fact that, with [13C2]glycine infusion in the present study, no peak was observed at 3 d postinfusion might indicate that the turnover of the precursor pool of bone marrow glycine is slower than the turnover of the 1-carbon donors serine and methionine (23). De novo synthesis accounts for 90% of nucleotide generation (51,52). Mathematical modeling suggests the rates of purine and pyrimidine synthesis are constant even when extracellular glycine or serine concentrations are altered. This is due to rebalancing of glycine and serine pools by the reversible reaction of SHMT (45,50). The incorporation of 1-carbon units into purines through the 10-formylTHF-dependent transformylase reactions could not be detected, because the M+1 enrichments of dA and dG were below the detection limit. Baggott et al. (22) observed incorporation of glycine-derived, 1-carbon units in purine synthesis after a much larger oral dose of [2-13C1]glycine. In the present study, dietary vitamin B-6 restriction did not change whole-body glycine flux or plasma glycine enrichment, and thus tissue enrichment, leading us to the assumption that nucleoside synthesis is not altered in marginal vitamin B-6 deficiency.
Amino acid flux.
Marginal vitamin B-6 restriction did not affect whole-body glycine or leucine flux. The whole body glycine flux in these men and women was 448 μmol·kg−1·h−1 and 446 μmol·kg−1·h−1 at normal vitamin B-6 status and at marginal vitamin B-6 deficiency. The major contributor to whole-body glycine flux is whole-body protein breakdown (53). The whole-body glycine flux can be maintained by an increased endogenous glycine synthesis at low but adequate protein intake (53). We have not examined the in vivo kinetics of glycine transamination by serine-pyruvate/alanine-glyoxylate aminotransferase. Further pathways utilizing glycine are the formation of serine, shown here to be ∼40% of glycine flux, and glutathione, protein synthesis, and gluconeogenesis. Intracellular dilution of glycine enrichment results from glyoxylate metabolism, de novo synthesis, protein degradation, and interconversion from serine. The QLeu, an essential amino acid, remained constant and indicated that this level of vitamin B-6 restriction did not alter whole-body protein turnover.
In conclusion, this study has yielded novel information about several aspects of human glycine and serine metabolism, the role of glycine as a major source of 5,10-methyleneTHF for folate-dependent 1-carbon metabolism, and the contribution of glycine in nucleoside synthesis. Within the statistical power of our study, we have demonstrated the surprising resiliency of these processes to low vitamin B-6 intake, presumably through a compensatory effect of increasing substrate concentration. The mechanism by which this marginal level of vitamin B-6 deficiency is associated with risk of chronic disease remains to be clarified.
Supported by NIH grant R01 DK072398 (to J. F. G.), General Clinical Research Center grant M01-RR00082, and Program Project Grant is 5PO1DK058398-08 (to C. B. N.).
Author disclosures: Y. Lamers, J. Williamson, M. Ralat, E. P. Quinlivan, L. R. Gilbert, C. Keeling, R. D. Stevens, C. B. Newgard, P. M. Ueland, K. Meyer, A. Fredriksen, P. W. Stacpoole, and J. F. Gregory III, no conflicts of interest.
Abbreviations used: AUC, area under the curve; CGly, rate of glycine catabolism via decarboxylation; dA, deoxyadenosine; dG, deoxyguanosine; Emax, maximum enrichment; Ep, plateau enrichment; Ep'Gly, intracellular [13C2] glycine enrichment; GCRC, General Clinical Research Center; GCS, glycine cleavage system; I, infusion rate; Km, Michaelis constant; MdC, methyldeoxycytidine; MdC%, percentage of methylation of deoxycytidine; 5,10-methyleneTHF, 5,10-methylenetetrahydrofolate; Q, measurement of flux (e.g. glycine flux = QGly); PLP, pyridoxal 5′-phosphate; SHMT, serine hydroxymethyltransferase; SNP, single nucleotide polymorphism; THF, tetrahydrofolate; VCO2, rate of CO2 production.
References
- 1.Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973;1:169–87. [DOI] [PubMed] [Google Scholar]
- 2.Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood). 2004;229:988–95. [DOI] [PubMed] [Google Scholar]
- 3.Leklem JE. Vitamin B-6: a status report. J Nutr. 1990;120 Suppl 11:1503–7. [DOI] [PubMed] [Google Scholar]
- 4.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1446–54. [DOI] [PubMed] [Google Scholar]
- 5.Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, Pizzolo F, Faccini G, Beltrame F, et al. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79:992–8. [DOI] [PubMed] [Google Scholar]
- 6.Lin PT, Cheng CH, Liaw YP, Lee BJ, Lee TW, Huang YC. Low pyridoxal 5′-phosphate is associated with increased risk of coronary artery disease. Nutrition. 2006;22:1146–51. [DOI] [PubMed] [Google Scholar]
- 7.Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97:437–43. [DOI] [PubMed] [Google Scholar]
- 8.Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke. 2003;34:e51–4. [DOI] [PubMed] [Google Scholar]
- 9.Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ. Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology. 2002;58:1471–5. [DOI] [PubMed] [Google Scholar]
- 10.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JF III. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr. 2006;136:2141–7. [DOI] [PubMed] [Google Scholar]
- 11.Scheer JB, Mackey AD, Gregory JF III. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr. 2005;135:233–8. [DOI] [PubMed] [Google Scholar]
- 12.Swendseid ME, Villalobos J, Friedrich B. Free amino acids in plasma and tissues of rats fed a vitamin B6-deficient diet. J Nutr. 1964;82:206–8. [DOI] [PubMed] [Google Scholar]
- 13.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF III. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr. 2005;81:648–55. [DOI] [PubMed] [Google Scholar]
- 14.Park YK, Linkswiler H. Effect of vitamin B6 depletion in adult man on the plasma concentration and the urinary excretion of free amino acids. J Nutr. 1971;101:185–91. [DOI] [PubMed] [Google Scholar]
- 15.Hiraga K, Kochi H, Hayasaka K, Kikuchi G, Nyhan WL. Defective glycine cleavage system in nonketotic hyperglycinemia. Occurrence of a less active glycine decarboxylase and an abnormal aminomethyl carrier protein. J Clin Invest. 1981;68:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearl PL, Capp PK, Novotny EJ, Gibson KM. Inherited disorders of neurotransmitters in children and adults. Clin Biochem. 2005;38:1051–8. [DOI] [PubMed] [Google Scholar]
- 17.Martinez M, Cuskelly GJ, Williamson J, Toth JP, Gregory JF III. Vitamin B-6 deficiency in rats reduces hepatic serine hydroxymethyltransferase and cystathionine beta-synthase activities and rates of in vivo protein turnover, homocysteine remethylation and transsulfuration. J Nutr. 2000;130:1115–23. [DOI] [PubMed] [Google Scholar]
- 18.Runyan TJ, Gershoff SN. The effect of vitamin B6 deficiency in rats on the metabolism of oxalic acid precursors. J Biol Chem. 1965;240:1889–92. [PubMed] [Google Scholar]
- 19.Gershoff SN, Mayer AL, Kulczycki LL. Effect of pyridoxine administration on the urinary excretion of oxalic acid, pyridoxine, and related compounds in mongoloids and nonmongoloids. Am J Clin Nutr. 1959;7:76–9. [DOI] [PubMed] [Google Scholar]
- 20.Ribaya JD, Gershoff SN. Interrelationships in rats among dietary vitamin B6, glycine and hydroxyproline. Effects of oxalate, glyoxylate, glycolate, and glycine on liver enzymes. J Nutr. 1979;109:171–83. [DOI] [PubMed] [Google Scholar]
- 21.Rowsell EV, Snell K, Carnie JA, Al-Tai AH. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969;115:1071–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baggott JE, Gorman GS, Tamura T. 13C enrichment of carbons 2 and 8 of purine by folate-dependent reactions after [13C]formate and [2-13C]glycine dosing in adult humans. Metabolism. 2007;56:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinlivan EP, Davis SR, Shelnutt KP, Henderson GN, Ghandour H, Shane B, Selhub J, Bailey LB, Stacpoole PW, et al. Methylenetetrahydrofolate reductase 677C->T polymorphism and folate status affect one-carbon incorporation into human DNA deoxynucleosides. J Nutr. 2005;135:389–96. [DOI] [PubMed] [Google Scholar]
- 24.Lamers Y, Williamson J, Gilbert LR, Stacpoole PW, Gregory JF III. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1,2-13C2]glycine and [2H3]leucine. J Nutr. 2007;137:2647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coburn SP, Ziegler PJ, Costill DL, Mahuren JD, Fink WJ, Schaltenbrand WE, Pauly TA, Pearson DR, Conn PS, et al. Response of vitamin B-6 content of muscle to changes in vitamin B-6 intake in men. Am J Clin Nutr. 1991;53:1436–42. [DOI] [PubMed] [Google Scholar]
- 26.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr. 1985;342:277–84. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2. [PubMed] [Google Scholar]
- 28.Meyer K, Fredriksen A, Ueland PM. High-level multiplex genotyping of polymorphisms involved in folate or homocysteine metabolism by matrix-assisted laser desorption/ionization mass spectrometry. Clin Chem. 2004;50:391–402. [DOI] [PubMed] [Google Scholar]
- 29.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF III. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–9 (Erratum 2004; 286: E674). [DOI] [PubMed] [Google Scholar]
- 30.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol. 1988;255:E322–31. [DOI] [PubMed] [Google Scholar]
- 31.Quinlivan EP, Gregory JF III. DNA digestion to deoxyribonucleoside: a simplified one-step procedure. Anal Biochem. 2008;373:383–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlivan EP, Gregory JF III. DNA methylation determination by liquid chromatography - tandem mass spectrometry using novel biosynthetic [U-15N]deoxycytidine and [U-15N]methyldeoxycytidine internal standards. Nucleic Acids Res. 2008;36:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuskelly GJ, Stacpoole PW, Williamson J, Baumgartner TG, Gregory JF III. Deficiencies of folate and vitamin B6 exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab. 2001;281:E1182–90. [DOI] [PubMed] [Google Scholar]
- 34.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947–55. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe RR. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley Liss; 1992.
- 36.Gregory JF III, Cuskelly GJ, Shane B, Toth JP, Baumgartner TG, Stacpoole PW. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am J Clin Nutr. 2000;72:1535–41. [DOI] [PubMed] [Google Scholar]
- 37.Arends J, Schafer G, Schauder P, Bircher J, Bier DM. Comparison of serine and hippurate as precursor equivalents during infusion of [15N]glycine for measurement of fractional synthetic rates of apolipoprotein B of very-low-density lipoprotein. Metabolism. 1995;44:1253–8. [DOI] [PubMed] [Google Scholar]
- 38.Cryer DR, Matsushima T, Marsh JB, Yudkoff M, Coates PM, Cortner JA. Direct measurement of apolipoprotein B synthesis in human very low density lipoprotein using stable isotopes and mass spectrometry. J Lipid Res. 1986;27:508–16. [PubMed] [Google Scholar]
- 39.Robert JJ, Bier DM, Zhao XH, Matthews DE, Young VR. Glucose and insulin effects on the novo amino acid synthesis in young men: studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism. 1982;31:1210–8. [DOI] [PubMed] [Google Scholar]
- 40.Fredriksen A, Meyer K, Ueland PM, Vollset SE, Grotmol T, Schneede J. Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum Mutat. 2007;28:856–65. [DOI] [PubMed] [Google Scholar]
- 41.Runyan TJ, Gershoff SN. Glycine metabolism in vitamin B6-deficient and deoxypyridoxine-treated rats. J Nutr. 1969;98:113–8. [DOI] [PubMed] [Google Scholar]
- 42.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF III. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr. 2006;136:373–8. [DOI] [PubMed] [Google Scholar]
- 43.Fujiwara K, Motokawa Y. Mechanism of the glycine cleavage reaction. Steady state kinetic studies of the P-protein-catalyzed reaction. J Biol Chem. 1983;258:8156–62. [PubMed] [Google Scholar]
- 44.Ryan WL, Carver MJ. Free amino acids of human foetal and adult liver. Nature. 1966;212:292–3. [DOI] [PubMed] [Google Scholar]
- 45.Nijhout HF, Reed MC, Lam SL, Shane B, Gregory JF III, Ulrich CM. In silico experimentation with a model of hepatic mitochondrial folate metabolism. Theor Biol Med Model. 2006;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gersovitz M, Bier D, Matthews D, Udall J, Munro HN, Young VR. Dynamic aspects of whole body glycine metabolism: influence of protein intake in young adult and elderly males. Metabolism. 1980;29:1087–94. [DOI] [PubMed] [Google Scholar]
- 47.Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem. 2002;277:42912–8. [DOI] [PubMed] [Google Scholar]
- 48.Jois M, Hall B, Brosnan JT. Stimulation of glycine catabolism in isolated perfused rat liver by calcium mobilizing hormones and in isolated rat liver mitochondria by submicromolar concentrations of calcium. J Biol Chem. 1990;265:1246–8. [PubMed] [Google Scholar]
- 49.Jois M, Hall B, Fewer K, Brosnan JT. Regulation of hepatic glycine catabolism by glucagon. J Biol Chem. 1989;264:3347–51. [PubMed] [Google Scholar]
- 50.McNeil JB, Bognar AL, Pearlman RE. In vivo analysis of folate coenzymes and their compartmentation in Saccharomyces cerevisiae. Genetics. 1996;142:371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berthold HK, Crain PF, Gouni I, Reeds PJ, Klein PD. Evidence for incorporation of intact dietary pyrimidine (but not purine) nucleosides into hepatic RNA. Proc Natl Acad Sci USA. 1995;92:10123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boza JJ, Jahoor F, Reeds PJ. Ribonucleic acid nucleotides in maternal and fetal tissues derive almost exclusively from synthesis de novo in pregnant mice. J Nutr. 1996;126:1749–58. [DOI] [PubMed] [Google Scholar]
- 53.Gibson NR, Jahoor F, Ware L, Jackson AA. Endogenous glycine and tyrosine production is maintained in adults consuming a marginal-protein diet. Am J Clin Nutr. 2002;75:511–8. [DOI] [PubMed] [Google Scholar]



