Abstract
Dietary (n-3) PUFA reduce inflammation, an independent risk factor for cardiovascular disease. The antiinflammatory effects of docosahexaenoic acid (DHA) in hypertriglyceridemic men have not been previously reported, to our knowledge, and were the focus of this study. Hypertriglyceridemic men (n = 17 per group) aged 39–66 y, participated in a double-blind, randomized, placebo-controlled parallel study. They received no supplements for the first 8 d and then received either 7.5 g/d DHA oil (3 g DHA/d) or olive oil (placebo) for the last 90 d. Blood samples were collected from fasting men on study days −7, 0, 45, 84, and 91. DHA supplementation for 45 and 91 d decreased the number of circulating neutrophils by 11.7 and 10.5%, respectively (P < 0.05). It did not alter the circulating concentrations of other inflammatory markers tested within 45 d, but at 91 d it reduced (P < 0.05) concentrations of C-reactive protein (CRP) by 15%, interleukin-6 by 23%, and granulocyte monocyte-colony stimulating factor by 21% and DHA increased the concentration of antiinflammatory matrix metalloproteinase-2 by 7%. The number of circulating neutrophils was positively associated with the weight percent (wt %) of 20:4(n-6) in RBC lipids, and negatively to the wt % of 20:5(n-3) and 22:6(n-3). Concentrations of CRP and serum amyloid A were positively associated with the sum of SFA and negatively with the wt % of 18:1(n-9) and 17:0 in RBC lipids; CRP was also positively associated with the wt % of 20:2(n-6). The mean size of VLDL particles was positively associated with plasma concentrations of neutrophils and CRP. In conclusion, DHA may lessen the inflammatory response by altering blood lipids and their fatty acid composition.
Introduction
Cardiovascular disease (CVD)9 and stroke are the leading causes of death in the United States, accounting for 38% of all deaths (1). Inflammatory processes are important contributors to the development of atherogenesis as well as to the vulnerability of atherosclerotic lesions to rupture. The most extensively studied biomarker of inflammation in CVD is C-reactive protein (CRP) (2). Among apparently healthy men, the baseline level of inflammation, as assessed by the serum CRP, predicts long-term risk of a first myocardial infarction, ischemic stroke, peripheral vascular disease, and all-cause mortality (3). The association between CRP and CVD persists after adjustments for age, smoking, lipid levels, blood pressure, and diabetes (4–7). A meta-analysis of 22 published studies reported that the odds ratio for patients in the top one-third compared with the bottom one-third of serum CRP levels was 1.58 (8). Thus, CRP is an important factor that determines the risk for CVD and stroke.
In addition to CRP, an increase in the concentrations of other inflammatory markers, including the number of white blood cells (WBC), particularly neutrophils, serum amyloid A (SAA), nitric oxide (NO), inflammatory cytokines [interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor-α (TNFα)], and adhesion molecules [intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-Selectin] also appear to contribute to the development of atherosclerotic disease (9). Other proteins such as matrix metalloproteinase-2 (MMP-2), IL-10, and transforming growth factor-β have antiinflammatory properties that may attenuate the adverse effects of inflammatory factors (10–13).
We have previously reported that supplementing the diets of healthy men with docosahexaenoic acid [DHA; 22:6(n-3)] significantly reduced the number of circulating WBC, which was primarily due to the reduction in the number of circulating neutrophils (14). Conversely, supplementing diets with arachidonic acid [AA; 20:4(n-6)] significantly increased the number of circulating neutrophils (15). The mechanisms by which DHA and AA altered the number of circulating neutrophils are not understood. In this study, we attempted to understand the role of DHA.
An increase in the consumption of fish, marine oils, purified eicosapentaenoic acid [EPA; 20:5(n-3)] or DHA decreased the concentrations of inflammatory markers in some but not all studies. In particular, results from a number of epidemiological studies showed an inverse association between serum CRP concentrations and fish consumption (16–21). In another study, CRP concentration was negatively associated with the granulocyte DHA concentration (22). Supplementing human diets with DHA or DHA + EPA did not alter the serum concentration of CRP in many studies (23–29) but reduced it in others (30–32). Similar inconsistencies have been reported regarding the effects of dietary (n-3) PUFA on the concentrations of other inflammatory markers (33).
Differences in the amount and duration of the fish oils used, the ratio between EPA and DHA, the composition of basal diet, the inflammatory status of study participants, and the sensitivity of the assay methods used may have caused differences between the results from previous studies with (n-3) PUFA and inflammation. We recently reported the effects of DHA (in the absence of EPA) supplementation on fasting and postprandial lipids in hypertriglyceridemic men (34,35). This study was conducted with hypertriglyceridemic men, because they have been shown to have increased inflammation and are at increased risk for CVD (36). Furthermore, we controlled the diet, amount, and duration of supplementation with DHA or placebo and used highly sensitive methods to determine the concentrations of inflammatory markers. Here, we report the effects of DHA supplementation on circulating markers of inflammation (numbers of WBC and concentrations of CRP, SAA, MMP-2, NO, and cytokines) from the same study. We hypothesized that: 1) DHA supplementation will increase the (n-3):(n-6) PUFA ratio of RBC lipids and that will be negatively associated with the number of circulating neutrophils and the concentration of CRP; and 2) the reduction in circulating neutrophils will be related to the diminished concentration of granulocyte colony stimulating factor (G-CSF) and granulocyte monocyte-colony stimulating factor (GM-CSF) that regulate the growth and differentiation of these cells. In addition, we determined the associations among the plasma concentrations of inflammatory markers and plasma lipids and lipoproteins, as well as between inflammatory markers and RBC fatty acids.
Subjects and Methods
Participants.
Details regarding the study design and participants have been previously published (34,35). Moderately hyperlipidemic but otherwise healthy men (39–66 y) participated in this study. Potential participants regularly taking antiinflammatory medications, including steroids, as well as those taking antihypertensives, non-sulfonyl urea medications for diabetes mellitus, or drugs that alter serum triacylglycerols and HDL cholesterol (HDL-C) levels (i.e. fibrates and niacin) were excluded. Also excluded were consumers of illegal substances, >5 drinks of alcohol per week, more than one fish meal per week, and those taking supplements of fish oil, flaxseed oil, or vitamin C or E. One drink of alcohol was considered to be 142 mL of standard wine, 340 mL of beer, 35 mL of 80-proof liquor, or 10 mL of pure alcohol. Clinical chemistry and hematology panels for all qualified participants were in the normal ranges with the exception of blood lipids. All selected participants had serum CRP concentrations of 1–10 mg/L; fasting serum triglyceride concentrations of 150–400 mg/dL (1.70–4.53 mmol/L), total cholesterol < 300 mg/dL (7.78 mmol/L), LDL cholesterol (LDL-C) < 220 mg/dL (5.69 mmol/L), and BMI between 22 and 35 kg/m2. Seventeen participants in each group completed the study.
Study design.
The study protocol was approved by the Institutional Review Boards of the University of California Davis and the Veterans Administration Medical Center, Mather, CA. It is listed in the government Clinical Trials by the identifier NCT00728338 (37). The experimental design was a double-blind, placebo-controlled parallel study with 2 metabolic periods: baseline (first 8 d) and intervention (last 90 d). During the intervention period, participants supplemented their diets with either placebo or DHA capsules. The DHA group received 7.5 g/d DHA oil (DHA 3.0 g/d and no EPA) that is produced in the microalga Crypthecodinium cohinii (Martek Biosciences). The placebo group received 7.5 g/d of olive oil. The dose and sources of DHA and placebo oils were based on published reports and on our previous DHA study (38). Participants were instructed not to change their usual diets and activity levels throughout the study. Usual dietary intakes were estimated by 3 unannounced 24-h dietary recalls obtained by telephone using a multi-pass interview method during each of the metabolic periods. One of the recalls was on a weekend day and the other 2 were on weekdays. Dietary intake data were collected and analyzed using the Nutrition Data System for Research software (version 2005, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
To provide uniformity in the composition of diets between the subjects and blood draw days, the metabolic kitchen provided all 3 meals on the day before each blood draw. The energy intake was adjusted for body height, body weight, age, and the estimated physical activity of the participants using the Mifflin-St. Jeor equation and appropriate activity factors (39). The test diet that was weighed and served on the day before each blood draw (pretest diet) did not differ in composition or total energy intake between the 2 groups (not shown). For these days, mean energy intake for the 2 groups was 10,450 ± 240 kJ; mean intakes for fat, carbohydrates, and proteins were 82, 340, and 100 g/d, respectively. SFA, monounsaturated fatty acids (MUFA), and PUFA provided 11.1, 10.1, and 8.8% of total energy, respectively.
Blood samples were drawn on study d −7 and 0 (baseline), d 45 (middle intervention), and d 84 and 91 (end intervention) following a 12-h fast. Plasma, sera, and RBC were prepared and stored as previously reported (34,35). Concentrations of WBC, CRP, SAA, MMP-2, and NO were determined on d −7, 0, 45, 84, and 91 and those of G-CSF, GM-CSF, IL-6, and a number of other cytokines were determined only on study d 0 and 91. For the variables tested on all blood draw days, baseline represents the mean of study d −7 and 0, middle intervention represents study d 45, and end intervention represents the mean of study d 84 and 91.
WBC count and RBC fatty acid analysis.
For each blood draw, a complete and differential cell count was performed using a Cell Dyn 3200 Hematology analyzer (Abbott Laboratories). Details regarding lipid extraction and fatty acid analysis have been previously published (34).
Inflammatory markers.
Plasma concentrations of E-Selectin, ICAM-1, VCAM-1, IL-1β, IL-2, IL-6, IL-8, and IL-10, TNFα, TNF-R1 and R2, IL-6 soluble receptor, G-CSF, and GM-CSF were determined using the Bioplex Suspension Array system (Bio-Rad Laboratories) and the reagent beads (Biosource International) following the manufacturer's instructions. Concentrations of serum CRP were measured by a high-sensitivity chemiluminescent assay (Immulite, Siemens Medical Solutions Diagnostics). Concentrations of plasma MMP-2 and serum SAA were analyzed by ELISA assays (R&D Systems; Biological). The plasma concentration of NO was assessed by determining its oxidation products nitrite and nitrate using the Total Nitric Oxide colorimetric assay kit (R&D Systems).
Statistical analysis.
SAS version 9.1.3 (40) was used for statistical analysis. The SAS proc mixed was used to fit repeated measures, mixed model with a first-order autoregressive covariance structure among the repeated measures (41). Diet, time, and the interaction are the fixed effects and subjects within diets are the random effect. Single degree of freedom contrasts were used to compare the baseline with the middle and end intervention means, and the middle with the end means within diets using 1-tailed tests; P-values were Bonferroni corrected for multiple comparisons. Results shown are the mean ± SEM. P < 0.05 (P < 0.016 after Bonferroni correction) was considered significant. Associations between plasma concentrations of inflammatory markers and those of plasma lipids and lipoproteins and RBC fatty acids were determined using data from both study groups to calculate the Kendall's R.
Results
Circulating WBC.
DHA supplementation decreased the number of circulating WBC by 7.2 and 6.4%, respectively, at the middle and end of the intervention compared with the start of the study (P < 0.05; Table 1). The decrease in WBC in the DHA group was primarily due to a decrease in the number of neutrophils, which were reduced by 11.7% at the middle of the intervention and by 10.5% at the end (P < 0.05). The number of circulating lymphocytes, monocytes, eosinophils, and basophils was not altered by DHA supplementation. In the placebo group, concentrations of all cell types remained constant throughout the study.
TABLE 1.
DHA supplementation decreases the number of circulating neutrophils in hypertriglyceridemic men1
| Intervention
|
||||
|---|---|---|---|---|
| Cell type2 | Treatment | Baseline | Middle | End |
| n x 10−9 | ||||
| WBC | DHA | 5.66 ± 0.33a | 5.25 ± 0.23b | 5.30 ± 0.30b |
| Placebo | 6.64 ± 0.39 | 6.73 ± 0.48 | 6.49 ± 0.48 | |
| Neutrophils | DHA | 3.23 ± 0.20a | 2.85 ± 0.17b | 2.89 ± 0.19b |
| Placebo | 3.87 ± 0.30 | 3.97 ± 0.37 | 3.72 ± 0.28 | |
| Lymphocytes | DHA | 1.67 ± 0.12 | 1.63 ± 0.11 | 1.68 ± 0.12 |
| Placebo | 1.93 ± 0.11 | 1.91 ± 0.12 | 1.92 ± 0.12 | |
| Monocytes | DHA | 0.50 ± 0.04 | 0.51 ± 0.04 | 0.48 ± 0.04 |
| Placebo | 0.58 ± 0.04 | 0.59 ± 0.04 | 0.59 ± 0.04 | |
| Eosinophils | DHA | 0.19 ± 0.03 | 0.19 ± 0.02 | 0.18 ± 0.03 |
| Placebo | 0.19 ± 0.03 | 0.19 ± 0.03 | 0.20 ± 0.03 | |
| Basophils | DHA | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| Placebo | 0.07 ± 0.00 | 0.07 ± 0.01 | 0.07 ± 0.00 | |
Data are means ± SEM, n = 17. Baseline represents the mean from study d −7 and 0, middle intervention represents study d 45, and end intervention represents mean of study d 84 and 91. Labeled DHA means without a common letter differ; P < 0.01.
Circulating CRP, MMP-2, SAA, and NO concentrations.
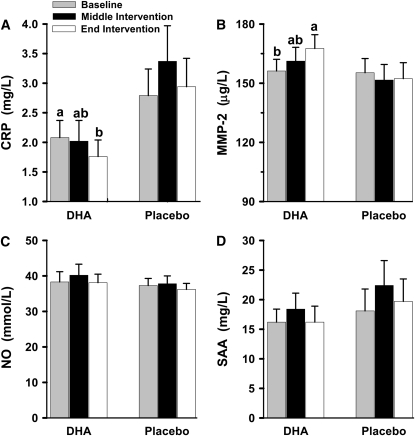
DHA supplementation for 45 d (middle intervention) did not significantly alter circulating concentrations of all 4 markers compared with their concentrations before the start of DHA supplementation (baseline). At the end of the intervention (mean d 84 and 91), there was a significant 15% reduction in the concentration of serum CRP and a significant 7% increase in the concentration of plasma MMP-2 when compared with their concentrations at baseline (Fig. 1). DHA supplementation for 91 d did not alter serum concentrations of SAA and plasma NO. Concentrations of these markers did not change in the placebo group during the study.
FIGURE 1 .
Effects of DHA supplementation on circulating concentrations of CRP (A), MMP-2 (B), NO (C), and SAA (D) in hypertriglyceridemic men. Baseline represents the mean from study d −7 and 0, middle intervention represents study d 45, and end intervention represents the mean from study d 84 and 91. Data are means ± SEM, n = 17. Means without a common letter differ, P < 0.05.
Inflammatory cytokines and adhesion molecules.
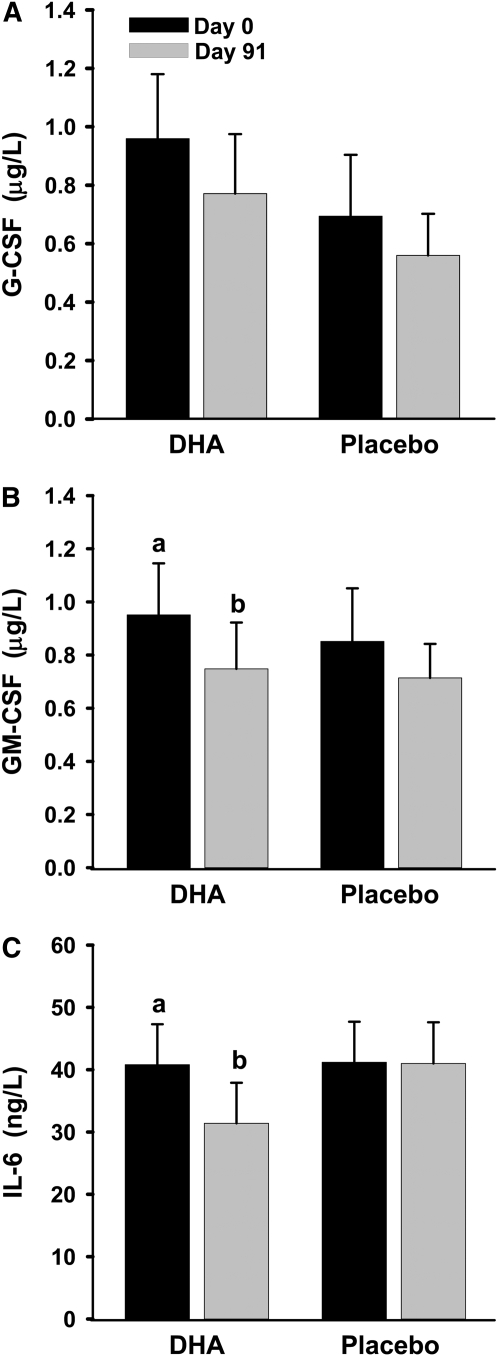
DHA supplementation for 91 d decreased the concentrations of GM-CSF and IL-6 by ∼20% and tended to decrease that of G-CSF (P = 0.18); concentrations of these markers did not change in the placebo group (Fig. 2). Plasma concentrations of other cytokines (IL-1β, IL-2, IL-8, IL-10, and TNFα) and adhesion molecules (ICAM-1, VCAM-1, and E-Selectin) in both the DHA and placebo groups did not change (not shown).
FIGURE 2 .
Effects of DHA supplementation on plasma concentrations of G-CSF (A), GM-CSF (B), and IL-6 (C) in hypertriglyceridemic men. Data are means ± SEM, n = 17, except IL-6, n = 11 for the DHA group. Means without a common letter differ, P < 0.05.
Associations between markers of inflammation, RBC fatty acids, and plasma lipids and lipoproteins.
Kendall's R indicating positive and negative associations between concentrations of inflammatory markers and RBC fatty acid composition and plasma concentrations of lipids and lipoproteins are shown (Tables 2 and 3). Both WBC and neutrophils showed strong positive associations with the concentrations of large VLDL and the mean size of VLDL particles; both also had significant negative associations with the concentrations of medium and small VLDL particles and the size of HDL particles (Table 3). Neutrophils displayed a significant positive association with the weight percent (wt %) of 20:4(n-6) in RBC lipids and negative associations with the wt % of 20:5(n-3) and 22:6(n-3), total (n-3) PUFA, the EPA:AA ratio, and the (n-3):(n-6) PUFA ratio in RBC lipids (Table 2).
TABLE 2.
Significant Kendall's R between markers of inflammation and RBC fatty acids in hypertriglyceridemic men taking DHA supplements1
| Variable | WBC | Neutrophils | CRP | SAA | MMP-2 | NO |
|---|---|---|---|---|---|---|
| R (P-value) | ||||||
| 17:0 | −0.20 (0.022) | −0.26 (0.005) | −0.21 (0.015) | |||
| 18:0 | −0.21 (0.018) | |||||
| 18:1(n-9) | −0.33 (0.0001) | −0.34 (0.0002) | ||||
| 18:2(n-6) | −0.18 (0.05) | |||||
| 20:0 | −0.19 (0.04) | |||||
| 20:1(n-9) | −0.18 (0.04) | |||||
| 20:2(n-6) | 0.21 (0.02) | −0.22 (0.01) | ||||
| 20:3(n-9) | −0.24 (0.02) | |||||
| 20:3(n-6) | 0.21 (0.01) | |||||
| 20:4(n-6) | 0.19 (0.03) | |||||
| 20:5(n-3) | −0.19 (0.03) | |||||
| 22:0 | −0.23 (0.009) | |||||
| 22:6(n-3) | −0.19 (0.03) | |||||
| 24:0 | −0.22 (0.01) | |||||
| 24:1(n-9) | −0.23 (0.01) | |||||
| Total SFA | 0.18 (0.03) | 0.31 (0.0008) | −0.18 (0.04) | |||
| Total MUFA | −0.28 (0.002) | −0.32 (0.0005) | ||||
| Total (n-6) PUFA | ||||||
| Total (n-3) PUFA | −0.22 (0.01) | |||||
| EPA:AA ratio | −0.21 (0.02) | |||||
| (n-3):(n-6) PUFA ratio | −0.20 (0.02) | |||||
R were calculated between inflammatory markers and RBC fatty acid composition using 3 values (study d 0, 45, and 91) from each participant, n = 17.
TABLE 3.
Significant Kendall's R between markers of inflammation and plasma lipids and lipoproteins in hypertriglyceridemic men taking DHA supplements1
| Variable | WBC | Neutrophils | CRP | SAA | MMP-2 | NO |
|---|---|---|---|---|---|---|
| R (P-value) | ||||||
| Triglycerides, mmol/L | −0.015 (0.05) | |||||
| Total-C, mmol/L | −0.14 (0.008) | |||||
| HDL-C, mmol/L | −0.14 (0.01) | |||||
| LDL-C, mmol/L | −0.17 (0.001) | |||||
| NEFA, mmol/L | 0.16 (0.002) | |||||
| Apo B, μmol/L | 0.14 (0.009) | −0.12 (0.02) | ||||
| Apo CIII, μmol/L | −0.12 (0.02) | −0.17 (0.001) | ||||
| Lp (a), μmol/L | 0.12 (0.02) | −0.22 (0.01) | ||||
| Total-C:HDL-C ratio | −0.17 (0.001) | |||||
| LDL-C:HDL-C ratio | −0.19 (0.0003) | |||||
| Triglyceride:HDL-C ratio | 0.19 (0.03) | |||||
| LDL-C:Apo B ratio | −0.14 (0.01) | −0.14 (0.008) | −0.18 (0.0004) | |||
| HDL:Apo A ratio | −0.11 (0.05) | −0.14 (0.007) | −0.13 (0.02) | |||
| Large VLDL, nmol/L | 0.26 (<0.0001) | 0.29 (<0.0001) | 0.14 (0.007) | −0.17 (0.0009) | ||
| Medium VLDL, nmol/L | −0.15 (0.004) | −0.15 (0.005) | 0.25 (<0.0001) | 0.11 (0.03) | ||
| Small VLDL, nmol/L | −0.15 (0.004) | −0.16 (0.002) | 0.12 (0.02) | |||
| Small LDL, nmol/L | 0.12 (0.02) | −0.15 (0.004) | ||||
| Small HDL, nmol/L | 0.24 (<0.0001) | |||||
| Size VLDL, nm | 0.32 (<0.0001) | 0.33 (<0.0001) | 0.18 (0.0007) | −0.15 (0.003) | ||
| Size HDL, nm | −0.15 (0.008) | −0.15 (0.007) | ||||
R were calculated between inflammatory markers and plasma lipids and lipoproteins using 5 values (study d −7, 0, 45, 84, and 91) from each participant, n = 17.
The serum CRP concentration was positively associated with the wt % concentration of 20:2(n-6) and total SFA in RBC, plasma concentrations of nonesterified fatty acids (NEFA), large and medium VLDL particles, and the mean size of VLDL particles (Tables 2 and 3). Serum CRP was negatively associated with the wt % of 18:1(n-9), 17:0, the sum of MUFA, and the plasma concentrations of HDL-C and apolipoprotein (apo) CIII. Serum SAA exhibited associations similar to those noted for CRP; however, it had additional negative associations with RBC 20:3(n-9) and the plasma HDL:apo A ratio and positive associations with plasma concentrations of small HDL-C, apo B, and lipoprotein (a).
Because the plasma concentration of MMP-2 increased and that of CRP decreased following supplementation with DHA, they showed opposing associations with some of the fatty acids and lipids (total SFA, large VLDL, and mean size of VLDL). However, both were negatively associated with apo C III. Furthermore, MMP-2 was positively associated with the wt % of 20:3(n-6) in RBC lipids. The plasma concentrations of NO demonstrated an unusual response to changes in fatty acids and lipids. It was negatively associated with the wt % of several fatty acids [17:0, 18:0, 20:0, 22:0, 24:0, 20:1(n-9), 24:1(n-9), and 20:2(n-6)] in RBC lipids, and plasma concentrations of total- and LDL-C. It had a significant positive association with the concentration of medium-sized VLDL particles.
Discussion
In this study of hypertriglyceridemic men, we showed that DHA, in the absence of EPA, significantly reduced the number of circulating neutrophils and serum CRP and GM-CSF and increased the concentration of MMP-2. The maximum effect of DHA on the number of circulating granulocytes was attained within 45 d, whereas significant effects on serum CRP and MMP-2 were found at 91 d. DHA supplementation also tended to reduce the G-CSF concentration (P = 0.18). These data suggest that reduction in the number of circulating neutrophils may be due to the decreased concentrations of GM-CSF and G-CSF. Plasma concentrations of triglycerides, VLDL, size of VLDL, and the wt % of 20:4(n-6) in RBC lipids were the best positive predictors of neutrophil numbers and the wt % of (n-3) PUFA in RBC lipids was the best negative predictor for the number of circulating neutrophils (Tables 2 and 3). Reduction in the number of circulating neutrophils after DHA supplementation in this study is consistent with results from our previous DHA study (14). The reduction in circulating neutrophils in our current study was approximately one-half of that found in the earlier study (21%). The amount of DHA used in our previous study was twice that of the amount used in this study. Thus, the reduction in the number of circulating neutrophils seems to be a function of the amount of DHA used. A positive association between the number of circulating neutrophils with the wt % of 20:4(n-6) in RBC is consistent with our earlier results in which we reported that AA supplementation increased the number of circulating neutrophils in healthy men (15).
Although the sum of (n-3) PUFA in RBC was negatively associated with the circulating number of neutrophils, the best negative associations for CRP and SAA were with wt % concentrations of 18:1(n-9) and a minor SFA (17:0) in RBC lipids. Individually, wt % of none of the RBC SFA had a positive association with plasma CRP and SAA concentrations, but the sum of all SFA had strong positive associations for these 2 markers (Table 2). The lack of a significant effect of individual SFA may be because of their weak effects individually, but collectively, this effect became significant. It is unclear why 17:0 acted differently than the rest of SFA. CRP concentrations were also positively associated with plasma concentrations of NEFA, large and medium VLDL, and the mean size of VLDL particles. DHA supplementation significantly increased wt % of both EPA and DHA in RBC lipids and did not change the wt % of 18:1(n-9) (34). The lack of a negative association between RBC (n-3) PUFA and serum CRP and SAA may be because SAA concentration did not change and the reduction in CRP was modest after DHA supplementation. Higher amount or longer duration of DHA supplementation and an increased number of study participants may reveal a negative association between RBC DHA and serum CRP.
Our results showing a decrease in serum CRP following DHA supplementation are at variance with results from several other studies that reported no effects of (n-3) PUFA on serum CRP (23–29). In our study, we used DHA 3 g/d. This amount did not alter CRP within 45 d of supplementation but decreased it significantly by 91 d (Fig. 1). Therefore, the discrepancy of our results with those of other investigators may be due to a shorter supplementation period of 6 wk or less (23,24,28) and study subjects with low CRP and/or low intake of (n-3) PUFA (26,27,29). Our results are consistent with those of previous studies with fish or fish oils (19,30–32). One of these was an epidemiological study in which CRP concentration was negatively associated with (n-3) PUFA intake (19) and the other 3 were intervention studies (30–32). One of the intervention studies involved fish oil supplementation for 6 mo for patients with rheumatoid arthritis (31), whereas the others involved supplementation for only 5 (30) and 8 (32) wk. The amounts of (n-3) PUFA in the 5-wk study was only 1.3 or 2.6 g/d, but this study was conducted with women receiving hormone replacement therapy, which may have increased the response to (n-3) PUFA. The 8-wk intervention study had a much higher intake of (n-3) PUFA (14 g/d sardine oil plus 180 g/wk oily fish). Furthermore, the basal diet in this study was high in antiinflammatory fatty acids [30% PUFA from walnuts, flaxseed, or canola oil high in (n-3) PUFA, 40% from olive oil, and 30% from SFA]. The (n-3) PUFA provided only 0.7% of energy for the basal diet in our study. Our data suggest that relatively elevated CRP and consumption of at least 3 g of (n-3) PUFA daily for >6 wk may be needed to detect any effects of (n-3) PUFA on CRP. Furthermore, the health status of the study subjects and the fatty acid composition of the basal diet are important in determining the effects of (n-3) PUFA.
Plasma concentrations of several other markers, including cytokines (IL-β, IL-2, IL-8, IL-10, and TNFα), adhesion molecules (ICAM-1, VCAM-1, and E-Selectin), SAA, and NO did not change in our study. Failure to detect effects of DHA on these markers may be due to their low circulating concentrations and low sensitivity of the assay methods used. Our results showing a reduction in circulating IL-6 following DHA supplementation are consistent with those of 2 other recent studies that found either a significant reduction or a trend toward reduction in IL-6 concentration (30,32). Compared with the lack of effect of DHA on plasma concentrations of IL-1β and TNFα in our current study, DHA supplementation (6 g/d, 90 d) reduced the lipopolysaccharide-stimulated production of both these cytokines by 40–45% in our previous study (38). This inconsistency may be due to the differences in the dose of DHA and its effects on basal compared with stimulated levels of these cytokines.
In contrast to the decline in the circulating concentrations of inflammatory markers CRP and IL-6, concentration of the antiinflammatory protein MMP-2 was significantly increased with DHA supplementation. MMP-2 expression has been reported to be upregulated in heart failure (42,43); however, recent studies show that it attenuated inflammation in several tissues (10–13). We believe this increase in MMP-2 caused by DHA is due to an increased antiinflammatory response rather than an increase associated with heart failure. Our results differ from those of a study with dogs in which fish oil supplementation decreased MMP-2 expression in knee synovia (44). Future studies are needed to understand the antiinflammatory role of MMP-2 and regulation of its expression by (n-3) PUFA.
Overall, RBC concentrations of SFA and (n-6) PUFA, the plasma concentration of large VLDL particles, and the mean size of VLDL particles were positively associated with several markers of inflammation; wt % concentrations of 18:1(n-9) and (n-3) PUFA in RBC lipids were negatively associated with some of the markers of inflammation reported here. These findings are generally consistent with the proinflammatory effects of saturated and (n-6) PUFA and the antiinflammatory effects of (n-3) PUFA and MUFA. However, our findings of associations between markers of inflammation and a minor fatty acid (17:0) and size of VLDL particles are novel and need to be confirmed in future studies.
Our finding of a reduction in the concentration of CRP in response to DHA supplementation is comparable to the 15–25% reduction in CRP caused by statins (45,46) and may have clinical relevance. Furthermore, we anticipate a larger reduction in CRP with continued intake of DHA. Results from our DHA studies reported here and previously (34,35) indicate that DHA supplementation may improve cardiovascular health in several ways. DHA decreases the concentrations of fasting and postprandial triglycerides, small dense LDL particles, remnant-like chylomicron particles, and inflammatory markers and increases the concentrations of large HDL and LDL particles, antiinflammatory markers, and the (n-3) index.
Acknowledgments
We thank Drs. Ellen Bonnel and Leslie Woodhouse and their staff at Western Human Nutrition Research Center for the coordination of the study and analysis of blood samples. We are also grateful to Dr. Edward Nelson and Eileen Bailey (Martek Biosciences) for donating the DHA capsules and for analyzing the fatty acid compositions of RBC lipids.
Supported in part by the USDA grant number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Reference to a company or product name does not imply approval or recommendation of the product by the U.S. Department of Agriculture to the exclusion of others that may be suitable.
Author disclosures: D. S. Kelley, D. Siegel, D. M. Fedor, Y. Adkins, and B. E. Mackey, no conflicts of interest.
Abbreviations used: AA, arachidonic acid; apo, apolipoprotein; CRP, C-reactive protein; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte monocyte-colony stimulating factor; HDL-C, HDL cholesterol; ICAM, intracellular adhesion molecule; IL, interleukin; LDL-C, LDL cholesterol; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; MUFA, monounsaturated fatty acid; NEFA, nonesterified fatty acid; NO, nitric oxide; SAA, serum amyloid A; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; WBC, white blood cell; wt %, weight percent.
References
- 1.AHA. Heart disease and stroke statistics: 2007 update. AHA. Sept 25, 2007 [cited 2008 2 Sept]. Available from: www.americanheart.org.
- 2.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA. Screening for cardiovascular risk with C-reactive protein. 2007. [cited 2008 2 Sept]. Available from: www.utdonline.com.
- 4.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. [DOI] [PubMed] [Google Scholar]
- 9.Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr. 2005;93:3–9. [DOI] [PubMed] [Google Scholar]
- 10.Matsusaka H, Ikeuchi M, Matsushima S, Ide T, Kubota T, Feldman AM, Takeshita A, Sunagawa K, Tsutsui H. Selective disruption of MMP-2 gene exacerbates myocardial inflammation and dysfunction in mice with cytokine-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H1858–64. [DOI] [PubMed] [Google Scholar]
- 11.Corry DB, Rishi K, Kanellis J, Kiss A, Song LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643–7. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543–9. [DOI] [PubMed] [Google Scholar]
- 14.Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids. 1998;33:559–66. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Mackey BE, Kyle D. Effects of dietary arachidonic acid on human immune response. Lipids. 1997;32:449–56. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26:1362–8. [DOI] [PubMed] [Google Scholar]
- 17.Fredrikson GN, Hedblad B, Nilsson JA, Alm R, Berglund G, Nilsson J. Association between diet, lifestyle, metabolic cardiovascular risk factors, and plasma C-reactive protein levels. Metabolism. 2004;53:1436–42. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–11. [DOI] [PubMed] [Google Scholar]
- 19.Niu K, Hozawa A, Kuriyama S, Ohmori-Matsuda K, Shimazu T, Nakaya N, Fujita K, Tsuji I, Nagatomi R. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr. 2006;84:223–9. [DOI] [PubMed] [Google Scholar]
- 20.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–60. [DOI] [PubMed] [Google Scholar]
- 21.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, Stefanadis C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–4. [DOI] [PubMed] [Google Scholar]
- 22.Madsen T, Skou HA, Hansen VE, Fog L, Christensen JH, Toft E, Schmidt EB. C-reactive protein, dietary n-3 fatty acids, and the extent of coronary artery disease. Am J Cardiol. 2001;88:1139–42. [DOI] [PubMed] [Google Scholar]
- 23.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–81. [DOI] [PubMed] [Google Scholar]
- 24.Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr. 2003;77:300–7. [DOI] [PubMed] [Google Scholar]
- 25.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr. 2003;89:517–22. [DOI] [PubMed] [Google Scholar]
- 26.Geelen A, Brouwer IA, Schouten EG, Kluft C, Katan MB, Zock PL. Intake of n-3 fatty acids from fish does not lower serum concentrations of C-reactive protein in healthy subjects. Eur J Clin Nutr. 2004;58:1440–2. [DOI] [PubMed] [Google Scholar]
- 27.Madsen T, Christensen JH, Schmidt EB. C-reactive protein and n-3 fatty acids in patients with a previous myocardial infarction: a placebo-controlled randomized study. Eur J Nutr. 2007;46:428–30. [DOI] [PubMed] [Google Scholar]
- 28.Sanders TA, Gleason K, Griffin B, Miller GJ. Influence of an algal triacylglycerol containing docosahexaenoic acid (22: 6n-3) and docosapentaenoic acid (22: 5n-6) on cardiovascular risk factors in healthy men and women. Br J Nutr. 2006;95:525–31. [DOI] [PubMed] [Google Scholar]
- 29.Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS, Tapsell LC, Noakes M, Clifton PA, et al. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr. 2007;97:749–57. [DOI] [PubMed] [Google Scholar]
- 30.Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem. 2003;14:513–21. [DOI] [PubMed] [Google Scholar]
- 31.Sundrarjun T, Komindr S, Archararit N, Dahlan W, Puchaiwatananon O, Angthararak S, Udomsuppayakul U, Chuncharunee S. Effects of n-3 fatty acids on serum interleukin-6, tumour necrosis factor-alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. J Int Med Res. 2004;32:443–54. [DOI] [PubMed] [Google Scholar]
- 32.Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Harman SM. High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res. 2008;40:199–205. [DOI] [PubMed] [Google Scholar]
- 33.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley DS, Siegel D, Vemuri M, Chung GH, Mackey BE. Docosahexaenoic acid supplementation decreases remnant-like particle-cholesterol and increases the (n-3) index in hypertriglyceridemic men. J Nutr. 2008;138:30–5. [DOI] [PubMed] [Google Scholar]
- 35.Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. 2007;86:324–33. [DOI] [PubMed] [Google Scholar]
- 36.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov [Internet]. USDA; Western Human Nutrition Research Center. Bethesda (MD): National Library of Medicine (US). 2003–2005. Effect of docosahexenoic acid (22:6n-3, DHA) supplementation on risk factors for cardiovascular disease in hyperlipidemic men [cited 2008 Dec 2]. Available from http://clinicaltrials.gov/show/NCT00728338 NLM Identifier: NCT00728338.
- 38.Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, Yu R, Chandra RK, Mackey BE. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999;34:317–24. [DOI] [PubMed] [Google Scholar]
- 39.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 40.SAS Institute. SAS online Doc 9.1.3 ed. Cary (NC): SAS Institute, Inc.; 2004.
- 41.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS system for mixed models. 2nd ed. Cary (NC): SAS Institute, Inc.; 2006.
- 42.Carlyle WC, Jacobson AW, Judd DL, Tian B, Chu C, Hauer KM, Hartman MM, McDonald KM. Delayed reperfusion alters matrix metalloproteinase activity and fibronectin mRNA expression in the infarct zone of the ligated rat heart. J Mol Cell Cardiol. 1997;29:2451–63. [DOI] [PubMed] [Google Scholar]
- 43.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1229–35. [DOI] [PubMed] [Google Scholar]
- 44.Hansen RA, Harris MA, Pluhar GE, Motta T, Brevard S, Ogilvie GK, Fettman MJ, Allen KG. Fish oil decreases matrix metalloproteinases in knee synovia of dogs with inflammatory joint disease. J Nutr Biochem. 2008;19:101–8. [DOI] [PubMed] [Google Scholar]
- 45.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 46.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–5. [DOI] [PubMed] [Google Scholar]