Abstract
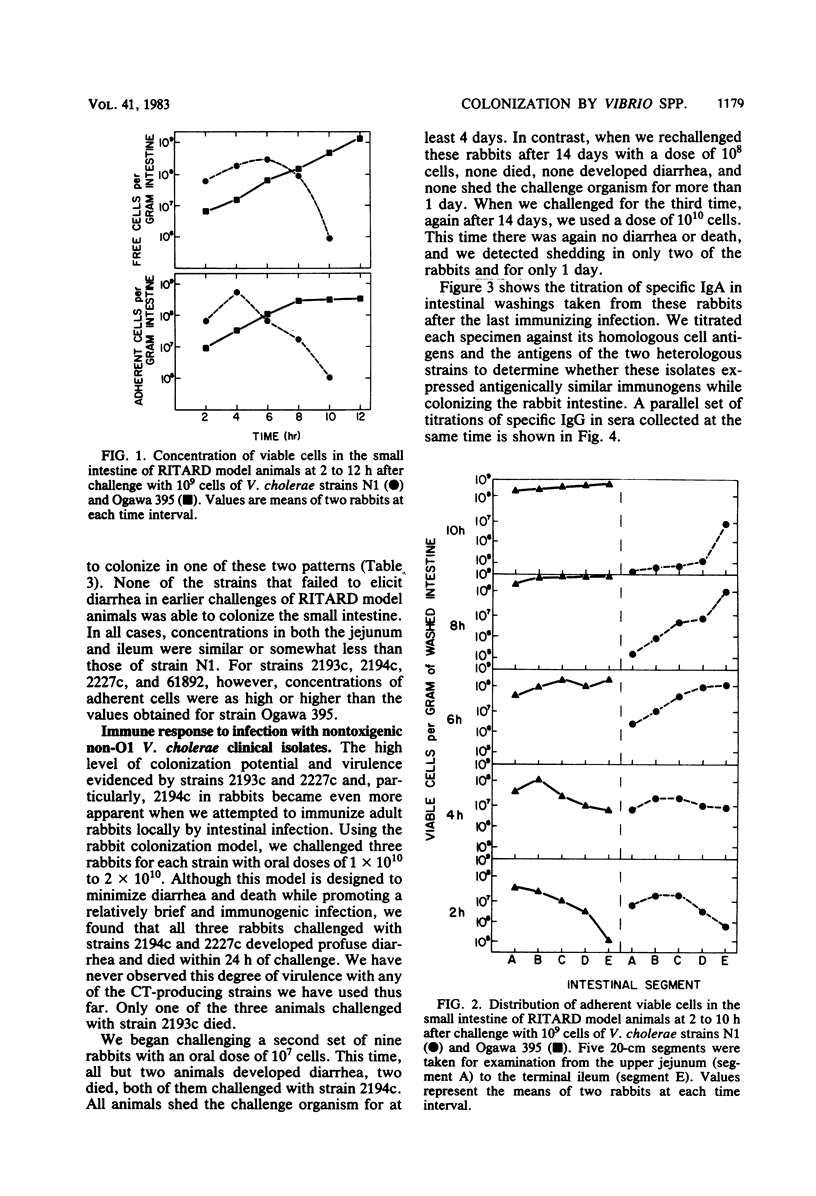
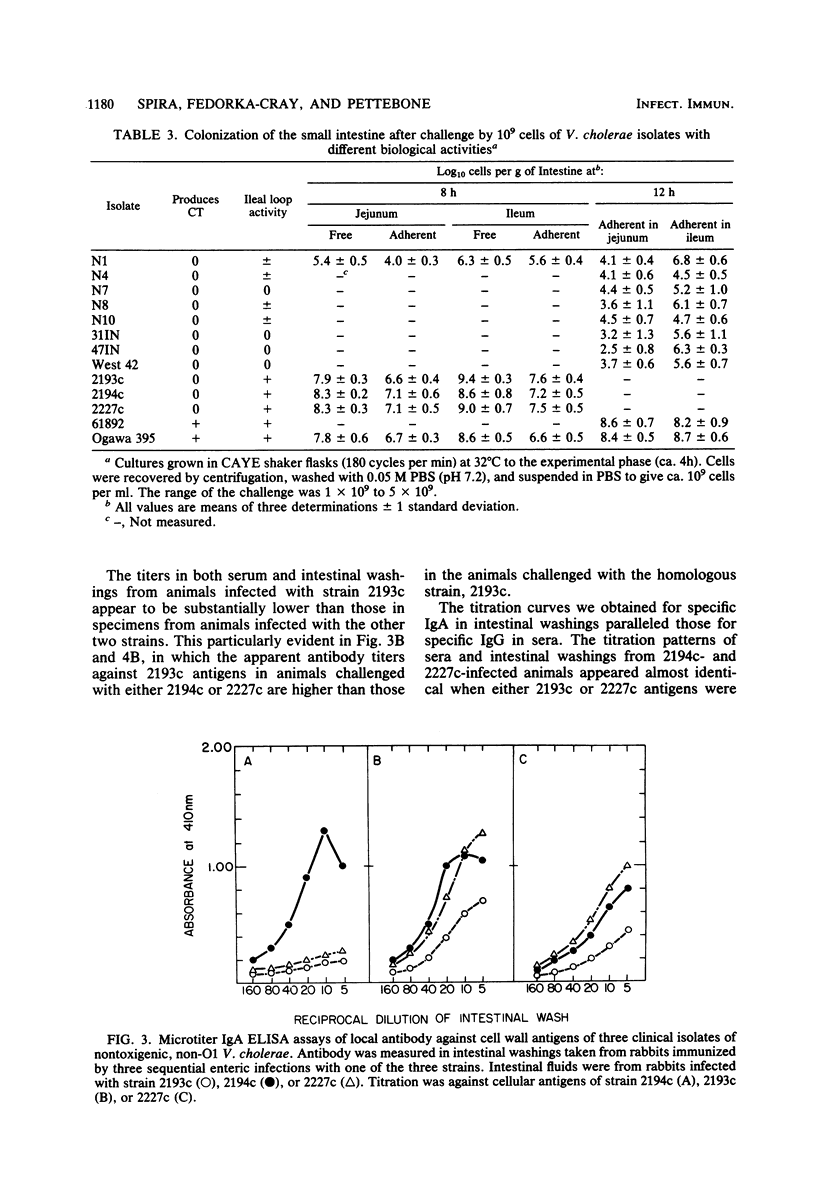
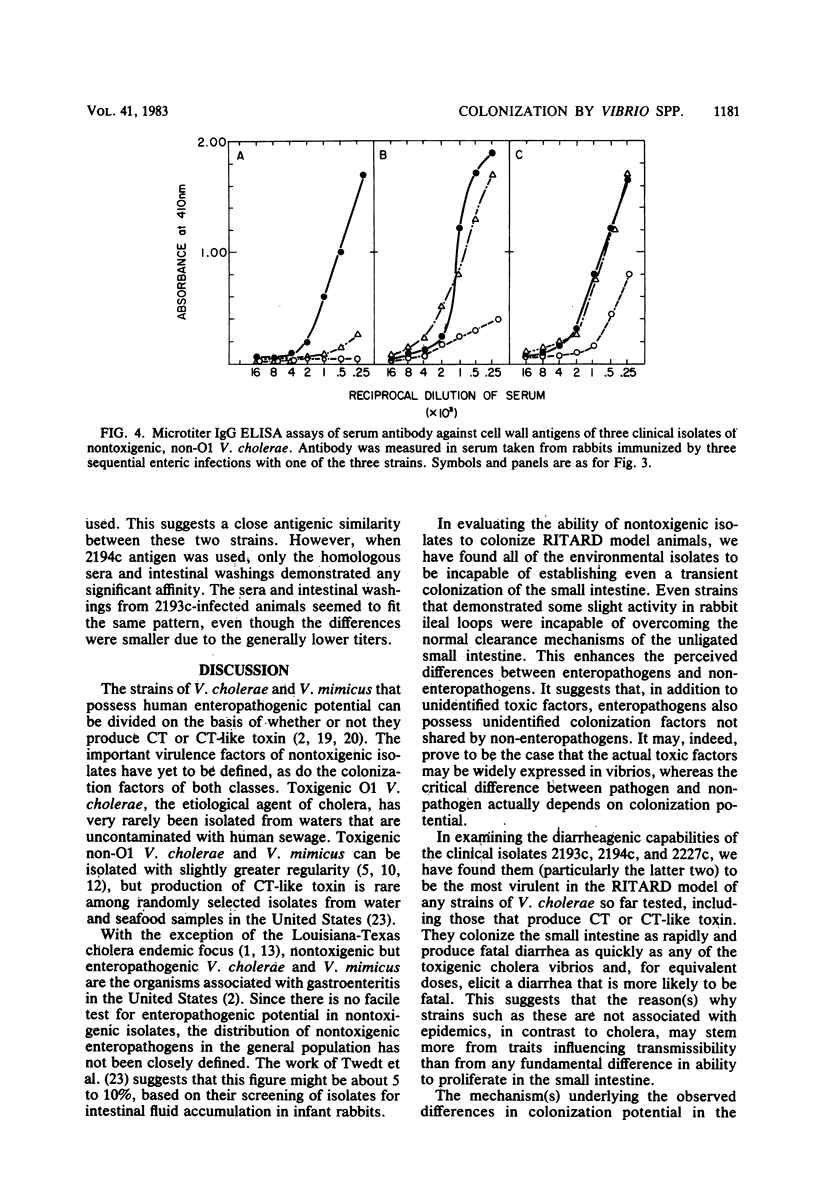
We examined the capability of 12 isolates of non-cholera toxin-producing O1 and non-O1 Vibrio cholerae to colonize the small intestine of adult rabbits and cause diarrhea. Using the removable intestinal tie-adult rabbit diarrhea model, we found that eight environmental isolates that showed no or marginal biological activity in other diarrhea models (rabbit ileal loop, infant rabbit, and suckling mouse) appeared to be incapable of attaching to and colonizing, even transiently, the small intestinal mucosa of animals with normal clearance mechanisms. In contrast, three clinical isolates attached, proliferated rapidly, and colonized mucosal surfaces of the entire small intestine within 8 h of challenge. This led to diarrhea with strikingly high rates of mortality compared with that of rabbits given similar challenges doses with strains of O1 V. cholerae that produce cholera toxin and Vibrio mimicus, which produces a toxin similar to cholera toxin. We have further demonstrated that multiple exposures to enteric infection by these strains elicited local and serum antibodies that reacted strongly with cell surface antigens of the homologous strain and showed a high degree of cross-reactivity against the cell surface antigens of the two heterologous strains. The enteric infections appeared to engender protection against subsequent infection as well, as evidenced by reduced incidence of diarrhea and duration of fecal shedding of the challenge organism upon subsequent challenges.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. Cholera--a possible endemic focus in the United States. N Engl J Med. 1980 Feb 7;302(6):305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Yamamoto K., Takeda Y., Miwatani T. Production of cholera-like enterotoxin by a Vibrio cholerae non-O1 strain isolated from the environment. Infect Immun. 1981 Oct;34(1):90–97. doi: 10.1128/iai.34.1.90-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DIXON J. M. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. doi: 10.1002/path.1700790116. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981 Oct;34(1):215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Moseley S. L., Falkow S. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect Immun. 1981 May;32(2):661–667. doi: 10.1128/iai.32.2.661-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J., Lockman H., Colwell R. R., Joseph S. W. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 1979 Jan;37(1):91–103. doi: 10.1128/aem.37.1.91-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Peterson J. W., Sarles H. E., Jr, Romanko M., Martin D., Hafkin B. Cholera on the Texas Gulf Coast. JAMA. 1982 Mar 19;247(11):1598–1599. [PubMed] [Google Scholar]
- Kusama H., Craig J. P. Production of Biologically Active Substances by Two Strains of Vibrio cholerae. Infect Immun. 1970 Jan;1(1):80–87. doi: 10.1128/iai.1.1.80-87.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. M., Nematollahi W. P., Hill W. E., McCardell B. A., Twedt R. M. Virulence of three clinical isolates of Vibrio cholerae non-O-1 serogroup in experimental enteric infections in rabbits. Infect Immun. 1981 Aug;33(2):616–619. doi: 10.1128/iai.33.2.616-619.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Still C. S., Isaäcson M., Koornhof H. J., Appelbaum P. C., Scragg J. N. Pathogenic mechanisms of a non-agglutinable Vibrio cholerae strain: demonstration of invasive and enterotoxigenic properties. Infect Immun. 1977 Nov;18(2):542–545. doi: 10.1128/iai.18.2.542-545.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Huda S., Neogi P. K., Daniel R. R., Spira W. M. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol. 1980 Jan;11(1):35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B. Kinetics of early cholera infection in the removable intestinal tie-adult rabbit diarrhea model. Infect Immun. 1982 Mar;35(3):952–957. doi: 10.1128/iai.35.3.952-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twedt R. M., Madden J. M., Hunt J. M., Francis D. W., Peeler J. T., Duran A. P., Hebert W. O., McCay S. G., Roderick C. N., Spite G. T. Characterization of Vibrio cholerae isolated from oysters. Appl Environ Microbiol. 1981 Jun;41(6):1475–1478. doi: 10.1128/aem.41.6.1475-1478.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]


