Abstract
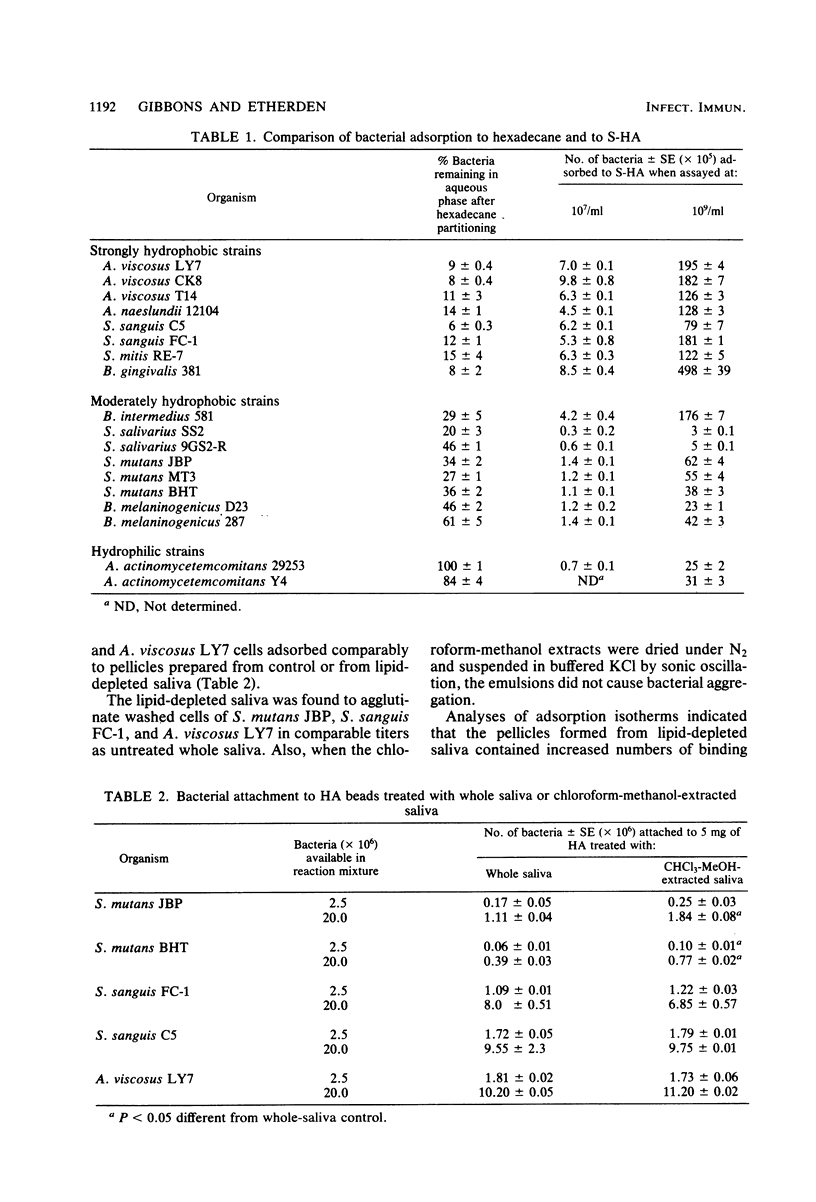
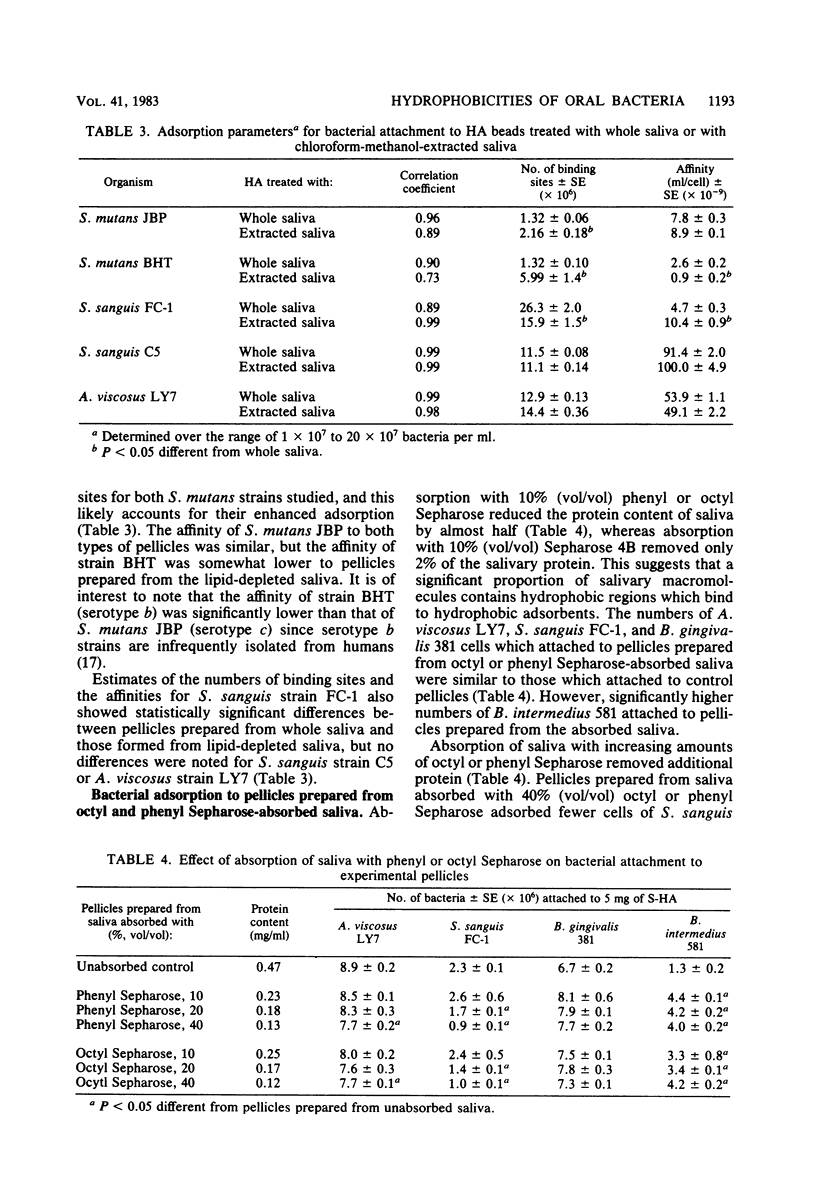
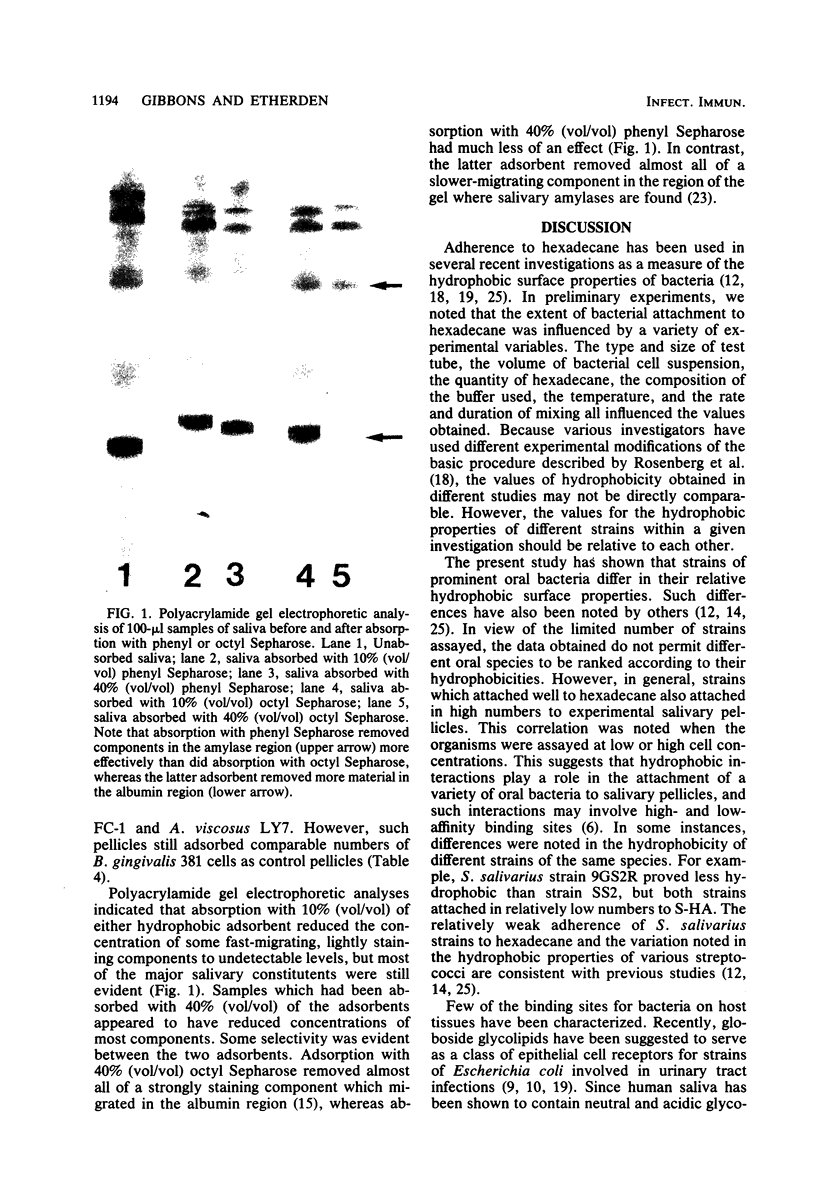
Oral bacteria were found to differ in their surface hydrophobicities as determined by their ability to adsorb to hexadecane. Strains of Actinomyces viscosus, A. naeslundii, Streptococcus sanguis, S. mitis, and Bacteroides gingivalis proved highly hydrophobic. Strains of B. intermedius, S. salivarius, S. mutans, and B. melaninogenicus were less hydrophobic, whereas strains of Actinobacillus actinomycetemcomitans were hydrophilic. An overall correlation was noted between the adsorption of bacteria to hexadecane and their numbers which attached to experimental salivary pellicles formed on hydroxyapatite surfaces. This suggests that hydrophobic bonding plays an important role in this process. Pellicles prepared from saliva which had been extracted with chloroform-methanol to remove lipids adsorbed comparable numbers of S. sanguis and A. viscosus and increased numbers of S. mutans. Analyses of adsorption isotherms indicated that pellicles formed from lipid-depleted saliva contained increased numbers of binding sites for the S. mutans strains studied, and this likely accounts for their enhanced adsorption. Absorption of saliva with 10% octyl or phenyl Sepharose reduced the protein content of saliva by almost half, but the numbers of bacteria which attached to pellicles prepared from such absorbed saliva were similar to or higher than those which attached to control pellicles. These observations suggest that saliva does not contain unique highly hydrophobic salivary macromolecules which serve as essential pellicle receptors for the bacteria studied. The data obtained are consistent with the view that hydrophobic bonding together with interactions between complementary molecules are involved in bacterial attachment to salivary pellicles on teeth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Leffler H. Glycosphingolipids of human urinary tract epithelial cells as possible receptors for adhering Escherichia coli bacteria. Scand J Infect Dis Suppl. 1980;Suppl 24:144–147. [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Enzymatic modification of bacterial receptors on saliva-treated hydroxyapatite surfaces. Infect Immun. 1982 Apr;36(1):52–58. doi: 10.1128/iai.36.1.52-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Etherden I. Concentration-dependent multiple binding sites on saliva-treated hydroxyapatite for Streptococcus sanguis. Infect Immun. 1983 Jan;39(1):280–289. doi: 10.1128/iai.39.1.280-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miörner H., Johansson G., Kronvall G. Lipoteichoic acid is the major cell wall component responsible for surface hydrophobicity of group A streptococci. Infect Immun. 1983 Jan;39(1):336–343. doi: 10.1128/iai.39.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I. Further studies of human serum albumin in oral fluid. Helv Odontol Acta. 1972 Apr;16(1):22–26. [PubMed] [Google Scholar]
- Perers L., Andåker L., Edebo L., Stendahl O., Tagesson C. Association of some enterobacteria with the intestinal mucosa of mouse in relation to their partition in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):308–316. doi: 10.1111/j.1699-0463.1977.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Qureshi J. V., Goldner M., Riche W. H., Hargreaves J. A. Streptococcus mutans serotypes in young schoolchildren. Caries Res. 1977;11(3):141–152. doi: 10.1159/000260260. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Perry A., Bayer E. A., Gutnick D. L., Rosenberg E., Ofek I. Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect Immun. 1981 Jul;33(1):29–33. doi: 10.1128/iai.33.1.29-33.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany A., Slomiany B. L., Mandel I. D. Lipid composition of human parotid saliva from light and heavy dental calculus-formers. Arch Oral Biol. 1981;26(2):151–152. doi: 10.1016/0003-9969(81)90087-x. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A., Mandel I. D. Lipid composition of human submandibular gland secretion from light and heavy calculus formers. Arch Oral Biol. 1980;25(11-12):749–751. doi: 10.1016/0003-9969(80)90129-6. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J. C., Keller P. J. An electrophoretic analysis of the protein components of human parotid saliva. Arch Oral Biol. 1968 Oct;13(10):1213–1222. doi: 10.1016/0003-9969(68)90077-0. [DOI] [PubMed] [Google Scholar]



