Abstract
Recent studies have identified a number of forms of muscular dystrophy, termed dystroglycanopathies, which are associated with loss of natively glycosylated α–dystroglycan. Here we identify a new animal model for this class of disorders in Sphynx and Devon Rex cats. Affected cats displayed a slowly progressive myopathy with clinical and histologic hallmarks of muscular dystrophy including skeletal muscle weakness with no involvement of peripheral nerves or CNS. Skeletal muscles had myopathic features and reduced expression of α–dystroglycan, while β–dystroglycan, sarcoglycans, and dystrophin were expressed at normal levels. In the Sphynx cat, analysis of laminin and lectin binding capacity demonstrated no loss in overall glycosylation or ligand binding for the α-dystroglycan protein, only a loss of protein expression. A reduction in laminin-α2 expression in the basal lamina surrounding skeletal myofibers was also observed. Sequence analysis of translated regions of the feline dystroglycan gene (DAG1) in affected cats did not identify a causative mutation, and levels of DAG1 mRNA determined by real-time QRT-PCR did not differ significantly from normal controls. Reduction in the levels of glycosylated α–dystroglycan by immunoblot was also identified in an affected Devon Rex cat. These data suggest that muscular dystrophy in Sphynx and Devon Rex cats results from a deficiency in α-dystroglycan protein expression, and as such may represent a new type of dystroglycanopathy where expression, but not glycosylation, is affected.
Keywords: Dystroglycan, Muscular Dystrophy, Feline, Neuromuscular, Sphynx, Devon Rex, Glycosylation
1. Introduction
Muscular dystrophies are a diverse group of inherited myopathies that have been reported in a variety of species including humans [1-5], dogs [6-8], cats [9,10], mice [11-13], hamsters [14] and chicken [15]. The most common human forms of muscular dystrophy (MD) result from mutations of the gene coding for dystrophin [4]. Other relatively common forms of MD result from mutations in genes encoding dystrophin-associated glycoproteins within the dystrophin-glycoprotein (DAG) complex [16]. The DAG complex is a multimeric transmembrane protein complex that links the cytoskeleton to the extracellular matrix and is thought to confer structural stability to the sarcolemma during contraction [17].
Dystroglycan is a central component of the DAG complex. The dystroglycan protein is composed of α and β subunits that are derived from a single mRNA via post-translational cleavage [18,19]. α-dystroglycan (α-DG) is an extracellular membrane-associated protein that binds to a variety of extracellular matrix proteins including laminin-α2 [19], while β-dystroglycan (β-DG) is a transmembrane protein that binds strongly to α-DG via its extracellular domain and to dystrophin (and utrophin) via its intracellular domain [20]. As such, dystroglycan is a critical component of the DAG complex with regards to maintaining membrane integrity in skeletal muscle, as well as in other tissues, including brain and heart. Indeed, loss of dystroglycan, specifically in skeletal muscle, causes muscular dystrophy in mice [21], akin to observations with loss of other members of the DAG complex, including dystrophin. Dystroglycan may also serve important roles in cell signaling, as it can mediate activation of a number of signal transduction pathways [22-26].
Although mutations of the dystroglycan gene (DAG1) have not been identified in any human muscular dystrophies to date, glycosylation of α-DG, which is essential for the binding of extracellular matrix proteins, including laminin, to the dystroglycan protein has been shown to be altered in multiple forms of the disease. This group of neuromuscular disorders display varying severity of dystrophic changes in skeletal muscle and can include severe brain malformation and structural eye abnormalities [3,5]. Reported syndromes include Walker-Warburg syndrome (WWS), muscle-eye-brain disease (MEB), Fukuyama congenital muscular dystrophy (FCMD), congenital muscular dystrophy type 1C (MDC1C) and type 1D, and limb girdle muscular dystrophy type 2K and type 2I (LGMD2I). Six genes have been identified as giving rise to this spectrum of neuromuscular disorders, including POMT1 (protein O-mannosyltransferase 1), POMT2 (protein O-mannosyltransferase 2), POMGnT1 (UDP-GlcNAc:N-acetylglucosaminyltransferase), FKTN (fukutin), FKRP (fukutin-related protein), and LARGE (N-acetylclucosaminyltransferase-like protein) [27-35]. In almost all cases, reduced or absent levels of natively glycosylated α-DG have been demonstrated, either by immunolabelling or immunoblot analysis of skeletal muscle, using glycosylation-dependent monoclonal antibodies [28,31,33-39]. In contrast, both underglycosylated α-DG polypeptide and β-DG, which is co-translated with α-DG from the DAG1 gene [18], are expressed at normal levels [39]. Typically, both clinical and pathological findings correlate with the extent of loss of native α-DG expression resulting from its under-glycosylation and loss of laminin binding in affected tissues.
In a previous report [40], six closely related Devon Rex cats afflicted with a slowly progressive congenital muscle disease were described. Physical findings included passive ventroflexion of the head and neck, head bobbing, dorsal protrusion of the scapulae, megaesophagus, generalized appendicular weakness and fatigability. Signs became evident at 3 to 23 weeks-of-age and then usually progressed slowly or remained static. Plasma levels of creatine kinase (CK) and aspartate aminotransferase were not elevated, and only mild changes were evident using needle electromyography. Histologic examination of tissues from affected cats showed changes indicative of muscular dystrophy, with neither peripheral nerve, spinal cord or cardiac involvement, and normal staining for dystrophin. Four of the six cats died suddenly of laryngospasm after obstruction of the pharynx or larynx with food. Earlier genetic studies [41] had established that this condition was inherited in an autosomal recessive fashion, although there was considerable variation in the severity of clinical signs amongst affected cats.
In this study we investigated two young Sphynx cats, a breed closely related to the Devon Rex, presenting with slowly progressive signs of neuromuscular weakness, abnormal electromyograms and mild myopathic changes within muscle biopsy specimens. Compared to control feline muscle, both Sphynx cats had decreased levels of natively glycosylated α-DG on immunostaining and immunoblotting of skeletal muscle. Sequence and mRNA analyses of DAG1 revealed no abnormalities compared to control normal cats, suggesting the muscular dystrophy of Sphynx cats is not due to a defect in α-DG protein. Surprisingly, the extent of glycosylation and the lectin binding glycosylation profile of α-DG was unchanged in affected animals. α-DG protein expression, however, was reduced, both relative to total cellular protein and relative to β-DG. α-DG protein from Sphynx cats showed no deficit in laminin binding if protein levels were normalized to those found in non-dystrophic animals. These data suggest that the muscular dystrophy occurring in the Sphynx and Devon Rex cats is due to a defect in α-DG protein expression or turnover and represents a novel animal model for an expanding group of dystroglycanopathies described in humans.
2. Methods and Materials
2.1 Animals
Case 1
Three 15 month-old male castrated Sphynx cat littermates were presented to the Veterinary Medical Teaching Hospital (VMTH), University of California, Davis with a history of progressive generalized weakness that had been present since 10 to 16 weeks-of-age. Two additional male littermates were unaffected, as were the sire and dam. Physical findings included an inability to jump, passive ventroflexion of the head and neck, difficulty swallowing, dorsal protrusion of the scapulae, decreased muscle mass and fatigability that occurred following short periods of activity. The cats would commonly assume a “chipmunk” posture or rest their heads on objects to compensate for their cervical muscle weakness (Fig 1). The most severely affected cat was investigated further including a complete blood count, serum chemistry (including CK activity), urinalysis, brain magnetic resonance imaging (MRI) and cisternal cerebrospinal fluid (CSF) analysis. Electromyography (EMG) and muscle and nerve biopsies were performed under general inhalation anesthesia. Disease features remained static, as reported by the owner. All cats died within a three-month period, approximately one year after examination. Although necropsies were not performed, death was assumed to be the result of aspiration or choking.
Figure 1. Sphynx cats with muscular dystrophy showing postures consistent with generalized muscle weakness.
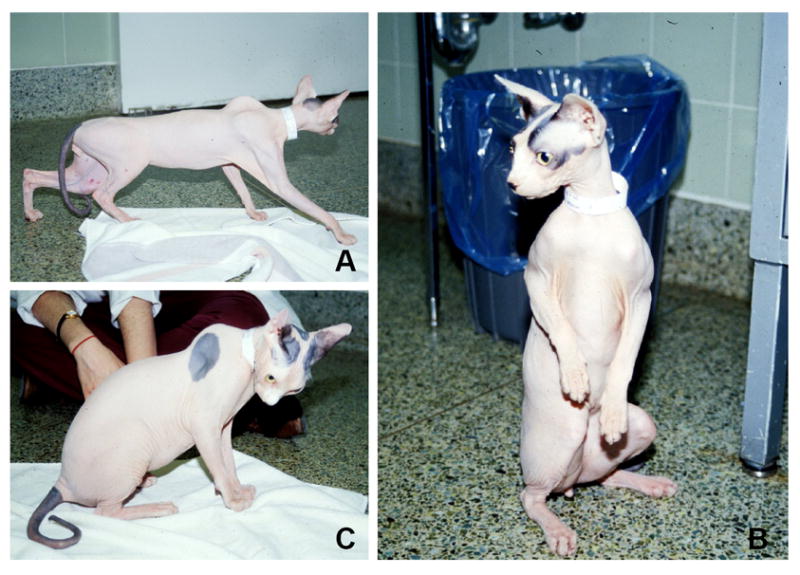
(A) dorsal protrusion of the scapulae, (B) “chipmunk” or “squirreling” posture, and (C) ventroflexion of the head and neck.
Case 2
An 11-month-old female Sphynx cat was presented to the VMTH for investigation of progressive weakness present since 10 weeks-of-age. The cat had required hand feeding for the first two months of its life. Physical findings were similar to Case 1 with decreased muscle mass, cervical ventroflexion, prominent scapulae and a crouching gait. Further diagnostics were done as for Case 1. Disease features remained static as reported by the owner, however, the cat was found dead approximately 10 months after examination.
Case 3
A male Devon Rex cat presented to the University Veterinary Centre Sydney with physical findings consistent with “hereditary myopathy of Devon Rex cats” [40]. Its physical signs were apparent at 6 weeks-of-age and were similar to those of the Sphinx cats. The cat could ambulate reasonably well, and could jump up onto small raised platforms. A barium swallow under fluoroscopy showed normal bolus formation in the pharynx, but slow transit of barium along the esophagus. Routine laboratory examinations were unremarkable, and the patient was followed over a six year period. During that time, there appeared to be a slow progression in the extent of the muscle weakness, with loss of skeletal muscle mass (body weight declining from 3.4 kg on initial presentation to 2.8 kg). The cat developed a number of upper and possible lower respiratory tract infections over the course of its life, and these were often very slow to respond to empiric antimicrobial therapy. Radiographs taken in the last year of life demonstrated pronounced bridging vertebral osteophytes between the twelfth thoracic and second lumbar vertebrae. After several years, it was decided that the cat had insufficient quality of life, and accordingly it was euthanized. Muscle specimens were collected immediately after euthanasia, snap frozen and transported to the Institute for Neuromuscular Research (INMR) Laboratory (KNN) for further studies. The main gross necropsy findings were megaesophagus and irregular thickening of the mitral valve. Histologically, there was chronic esophagitis without ulceration. Cardiac muscle was unremarkable in routine hematoxylin and eosin (H&E) stained sections.
2.2 Electrophysiology
Electrophysiological examination was performed under general anesthesia as previously described [42], and consisted of EMG, superficial peroneal motor (MNCV) and sensory (SNCV) nerve conduction studies and repetitive nerve stimulation. Electrophysiological data from affected cats were compared with laboratory and published normal values [43-45].
2.3 Histology, Histochemistry, and Immunofluorescence Staining
For Cases 1 and 2, biopsies were collected by an open procedure under general inhalational anesthesia from the lateral head of the triceps, vastus lateralis and cranial tibial muscles, and superficial peroneal nerve. The muscle biopsies were flash frozen in isopentane that was pre-cooled in liquid nitrogen and stored at -80°C until further processed by a standard panel of histochemical stains and reactions. Additional muscle specimens were immersion fixed in 5% glutaraldehyde in 0.1M phosphate buffer and stored in 0.1M phosphate buffer. Samples of peroneal nerve were flash frozen in pre-cooled isopentane, as well as immersion-fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer prior to processing for plastic sections. Glutaraldehyde-fixed nerve biopsy specimens were post-fixed in osmium tetroxide, and dehydrated in serial alcohol solutions and propylene oxide prior to embedding in araldite resin. Longitudinal sections of frozen nerve were stained with H&E, modified trichrome and acid phosphatase stains. Transverse resin embedded nerve sections (1 μm) were stained with toluidine blue-basic fuchsin for light microscopy. Tissues were collected at necropsy on the Devon Rex cat and handled similarly.
Unfixed 8 μm cryostat sections were processed by indirect immunofluorescence to assess the presence or absence of staining for dystrophin, dystrophin-associated glycoproteins and laminin-α2 using the following monoclonal antibodies (dilutions within parentheses): antibodies against the rod domain (1:20, NCL-DYS1) and carboxy terminus (1:20, NCL-DYS2) of dystrophin, utrophin (1:5, NCL-DRP2), spectrin (1:1000, NCL-SPEC2), α-sarcoglycan (1:50, NCL-a-SARC), β-sarcoglycan (1:50, NCL-b-SARC), γ-sarcoglycan (1:50, NCL-g-SARC), and β-dystroglycan (1:100, NCL-b-DG) were purchased from Novocastra (Newcastle-upon-Tyne, UK). A monoclonal antibody against laminin-α-2, 5H2 [46] (1:20,000, MAB1922) was purchased from Chemicon (Temecula, CA). Monoclonal antibodies that recognize natively glycosylated α-dystroglycan protein were a gift from Kevin Campbell (IIH6, University of Iowa) or purchased (1:100, VIA4-1, Upstate Biotechnology, Lake Placid, NY). Rabbit polyclonal antibodies against α-sarcoglycan (1:200) [47] and laminin-α2 [46] were as previously described. All dilutions of primary antibodies were made in 3% bovine serum albumin in phosphate-buffered saline, and incubations were for 1 h at 37°C or overnight at 4°C. Secondary antibodies were goat anti-mouse IgG-FITC (1:200, Jackson Imunolab) or goat anti-rabbit IgG-FITC (1:200) or goat anti-mouse IgG-Alexa 488 (1:200, Jackson Immunolab) and incubation was for 1 h at room temperature. In addition to biopsy specimens from the Sphynx and Devon Rex cats, matched muscle specimens from a healthy domestic short-haired cat and a healthy Devon Rex cat were included as controls.
Three anti-peptide antisera were made in rabbits against mouse α-DG polypeptides; DG1-EPSEAVDRWENQLEA, DG2-HIANKKPPLPKRVRR, and DG3-C-KIALVKKAFAFGDR. Amino acid sequences DG2 and DG3 are identical in mouse and cat, while the cat sequence for DG1 contains a 3 amino acid deletion (EAV) not present in the mouse sequence. Antisera were purified against the immunogenic peptides and characterized on purified mouse or rabbit α-DG protein to verify binding, as previously described [48].
2.4 Immunoblotting
Extraction of whole cell muscle proteins, immunoblotting, and quantification using scanning densitometry in the Sphynx cats were done as previously described [48-50]. For data shown in Fig. 5, identical amounts of whole cell muscle protein (80 μg, that had been extracted in 2% SDS with 4M urea and reducing agent) from the Sphynx cats and a normal cat were compared on 6% or 12% SDS-PAGE gels. Antibodies used were as described for immunostaining, and additionally DE-U-10, an antibody to desmin (Sigma; St. Louis, MO) and E62320 (Upstate Biotechnology; Lake Placid, NY), an antibody to dystrobrevin that recognizes all dystrobrevin splice forms, and DG3, a purified rabbit anti-peptide antibody to the C-terminal region of the α-DG polypeptide, were also used. Immunoblot analysis in the dystrophic Devon Rex cat was performed as previously described [51]. Proteins extracted from two 8 μm cryosections of skeletal muscle from the Devon Rex and a normal cat were compared on 3-8% NuPAGE gels (Invitrogen, Carlsbad, CA). Densitometry of bands was quantitated using ImageJ software. For data shown in Fig. 6, skeletal muscles were extracted in non-ionic detergent buffer (NP40), as before [48], and variable amounts of protein from control (C) and Sphynx (S) cats were compared on gradient SDS-PAGE gels. In addition, 40 μg of normal cat protein was compared to 40 μg of normal mouse protein from C57Bl/6 to show the differential gel migration of α-DG from these two species.
Figure 5. Protein expression of natively glycosylated α-dystroglycan is reduced in Sphynx and Devon Rex cats.
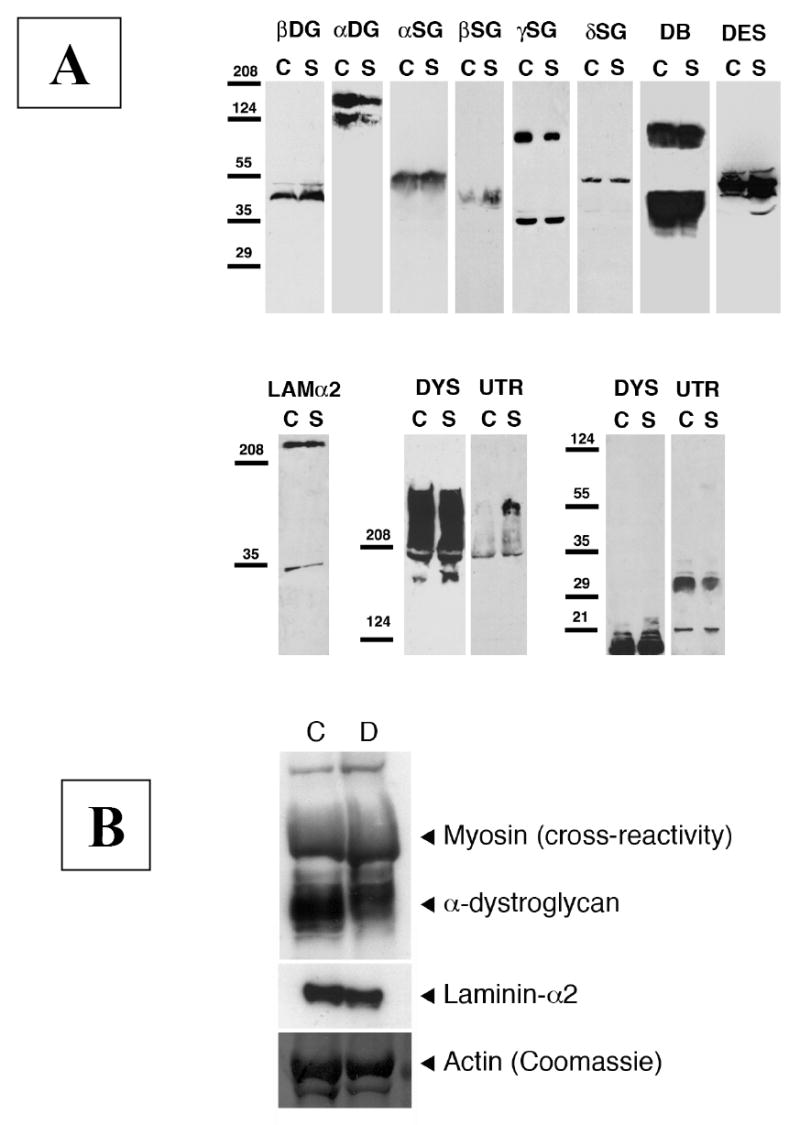
A. Muscle protein lysate from a Sphynx cat (S) and a normal (N) cat were compared by immunoblotting. Levels of dystrophin (DYS), laminin α2 (Lamα2), β dystroglycan (βDG), α-δ sarcoglycan (α-δSG), desmin (DES), and dystrobrevin (DB) were similar in both samples, while the amount of native α-dystroglycan (αDG, recognized by the IIH6 anti-carbohydrate antibody) was reduced and the level of utrophin (UTR) was increased. Some increase in β sarcoglycan may also have occurred, although this signal was very weak on immunoblots. Tentative molecular weights are 140 kDa and 120 kDa for α- DG, 43 kDa for β-DG, 50-55 kDa for desmin, 55 kDa for α- SG, 43 kDa for β-SG, 35 and 90 kDa for γ-SG, 50 kDa for δ-SG, 350 kDa for laminin-α2, 350-400 kDa for dystrophin and utrophin, and 80-100 kDa and 35-50 kDa for dystrobrevin. B. Muscle proteins from the affected Devon Rex (D) and control (C) cats were compared by immunoblot analysis. By densitometry, amounts of native α-DG (recognized by the VIA4-1 antibody) was reduced approximately 29% in the Devon Rex cat compared to control. This antibody also shows a high level of cross-reactivity with myosin heavy chain. By comparison, densitometry showed only a small decrease in levels of laminin-α2. Coomassie blue staining of actin demonstrates relative loading of skeletal muscle protein.
Figure 6. Relative expression of α-dystroglycan to β-dystroglycan and to total cell protein was reduced in Sphynx cat skeletal muscle.
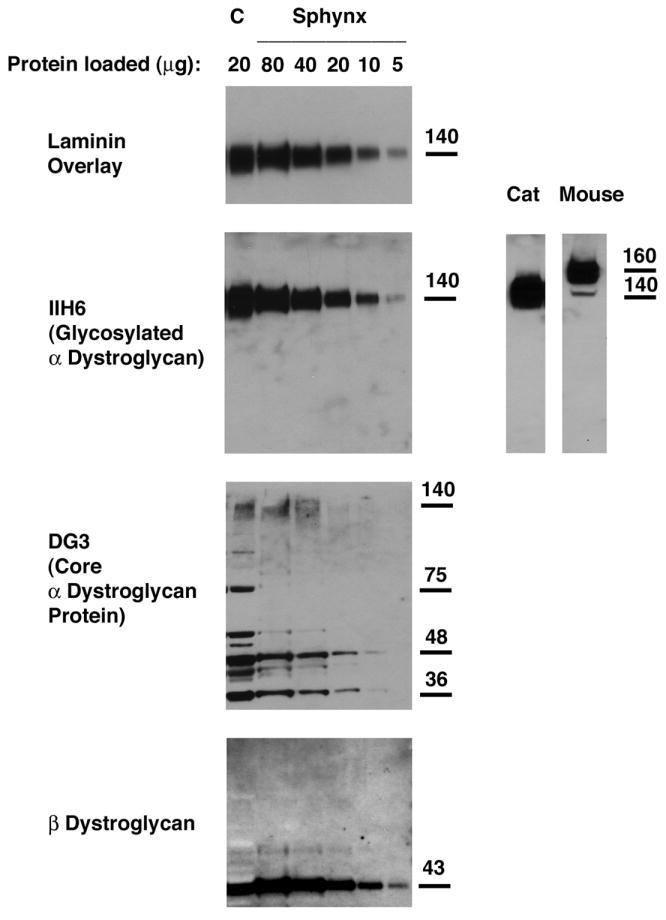
Different amounts of total muscle cell lysates were loaded to compare α- and β-dystroglycan expression by immunoblotting. An antibody that recognizes natively glycosylated α-dystroglycan (IIH6) and one that recognizes α-dystroglycan polypeptide (DG3) were used, and laminin-1 binding to α-dystroglycan was visualized by laminin overlay. Levels of α-dystroglycan, both by IIH6 and DG3 immunoblotting, were lower in Sphynx cat skeletal muscle than in normal controls (C), as was laminin binding to α dystroglycan, when comparing equivalent amounts of loaded protein (20ug). Increasing the amount of Sphynx protein lysate loaded increased IIH6 blotting and laminin binding to levels beyond wild type. α-dystroglycan in normal cat skeletal muscle migrates about 20kDa lower than α-dystroglycan in mouse skeletal muscle.
2.5 Laminin overlay
Proteins were separated by 6% or 8% SDS-PAGE, followed by transfer to PVDF membranes. Laminin (LN) overlays were performed using mouse Engelbreth-Holm-Swarm (EHS) laminin (0.1 ug/ml), as previously described [39]. Similar results were also obtained using recombinant G1-G5 laminin α2 (0.1 ng/ml) (not shown in Fig. 7). Laminin was added in ligand binding buffer (LBB, 10 mM triethanolamine, 140 mM NaCl, 1 mM Ca2Cl, 1 mM MgCl2, pH 7.6) containing 3% BSA. After washing in the same buffer, binding was detected using a rabbit anti-laminin 1 antisera (1:10,000, Sigma-Aldrich, St. Louis, MO), followed by binding of HRP conjugated goat-anti rabbit secondary, all in binding buffer as above. Signals were detected using enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL).
Figure 7. Lectin precipitation profile of α dystroglycan did not differ between Sphynx and normal cat skeletal muscle.
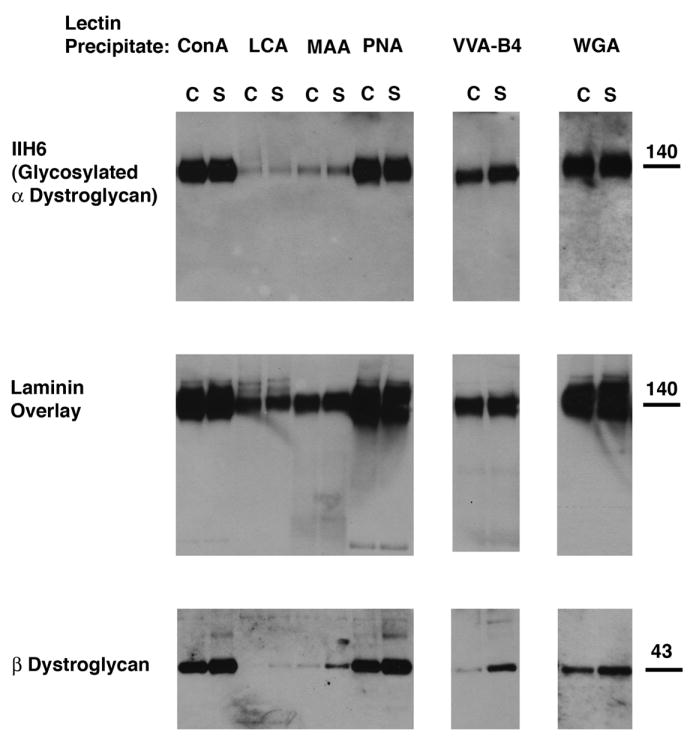
Skeletal muscle protein lysates were normalized to allow for precipitation of equivalent amounts of α-dystroglycan between control (C) and Sphynx (S) samples. Lysates were then subjected to lectin precipitation followed by immunoblotting for native (glycosylated) α-dystroglycan (IIH6) or β-dystroglycan. Laminin overlays were done on precipitated protein to visualize laminin binding to α-dystroglycan. ConA, PNA, and WGA precipitated α-dystroglycan well. VVA-B4 showed intermediate levels, while LCA and MAA showed poor precipitation. The profile of α-dystroglycan binding to lectins, and also of laminin binding of precipitated α-dystroglycan protein, was unchanged between Sphynx and normal cat muscle.
2.6 Lectin precipitation
Skeletal muscle proteins were solubilized in lysis buffer (TBS, pH7.5, 2 mM EDTA, 1% NP-40, protease inhibitors, 1mM PMSF) overnight at 4°C. Cell lysate was clarified by centrifugation at 14000 rpm for 10 min and the supernatant quantified for protein amount using a modified Bradford assay [48]. 150ug of control non-dystrophic cat lysate was compared to 300ug of Sphynx cat lysate. Lysates were incubated with 50ul of Triticum vulgaris (Wheat germ) agglutinin (WGA) agarose, Canavalia ensiformis (Concanavalin A) agglutinin (ConA) agarose, Maackia amurensis lectin (MAA) agarose, Arachis hypogaea (Peanut) agglutinin (PNA) agarose, Vicia Villosa Agglutinin Isolectin B4 agarose (VVA-B4), Lens culinaris (Lentil) agglutinin or (LCA) agarose overnight at 4C. Lectin agarose beads were separated by centrifugation at 1000g, washed 3-4 times in lysis buffer, and then centrifuged once at 14,000 rpm for 10 minutes. After removal of the final wash, beads were solubilized in 100ul of SDS lysis buffer with reducing agent, boiled, and analyzed by for α-DG or β-DG by immunoblot or for laminin binding by LN overlay.
2.7 Feline DAG1 Amplification and Sequencing
Using conserved sequence data for DAG1 from human (NM_004393.1), mouse (NM_010017) and canine (AJ012166.1), and the scaffold sequence for the cat (167915), primers were designed to the coding regions of only exons 2 and 3, since exon 1 is non-coding (Table 1). Total genomic DNA from Cases 2 and 3, an unrelated normal Devon Rex, an additional affected Devon Rex cat and a Devon Rex shown by test-mating to be a carrier of the Devon Rex dystrophy [40], as well as two unaffected random bred domestic short-haired cats, were used as PCR templates. To evaluate the exon 2 and 3 intron-exon junction, RNA from muscle was isolated using the Qiagen RNeasy kit (Qiagen; Valencia, CA) and cDNA was produced using SuperScript III Reverse Transcriptase (Invitrogen; Carlsbad, CA) according to the manufacturer’s protocol. The genomic and cDNA amplicons of DAG1 were each produced in 30 μl PCR reactions containing the following; 2 pmol of each forward and reverse primer, 2.0 mM dNTP, 1.75 mM MgCl2, 1X PCR buffer II and 0.375 U Amplitaq polymerase (Applied Biosystems; Foster City, CA). For each reaction, 25ng of template DNA or cDNA was amplified in a Perkin Elmer 9700 (Applied Biosystems; Foster City, CA) using the following PCR profile: 3 min denaturation at 94°C, 5 touchdown cycles of denaturation at 94°C for 30 s, annealing at 63°C for 20 s, and extension at 72°C for 90 s with the annealing temperature decreasing at 1°C/cycle. The touchdown cycles were followed with 30 cycles with a 58°C annealing temperature and a final extension of 10 min at 72°C. PCR amplicons were size separated on 1.8% agarose gels, fragments were excised and purified using the QIAquick Gel Extraction kit (Quiagen) according to manufacturers protocols. Purified template DNA was quantified and directly sequenced using the Big Dye terminator cycle sequencing kit V3.1 (Applied Biosystems). Sequencing products were purified with Centri-Sep Spin Columns (Princeton Separations; Adelphia, NJ) and size separated on an ABI 3730 DNA Analyzer. Sequences were visualized and interpreted using the Sequencher Software (Gene Codes Corp; Ann Arbor, MI) and sequence identity was confirmed using a basic local alignment search tool (BLAST) [52].
Table 1.
PCR primers for the analysis of feline DAG1.
| Target | Forward (5’->3’)
Reverse (5’->3’) |
Length
(bp) |
Real-time PCR Probe
(5’->3’) |
|---|---|---|---|
| DAG1 | GCAGGGACTGGGAGAACCA
ACAGCCTCGTGAAGGTCTGAA |
67 | CTTGAGGCGTCCATGCACTCAG
TGC |
| Exon 2 | TTGAACTGGACAGCACAGG
ACAGGTCCTAGAAGAACTGAGC |
663 | |
| 5’ Exon 3 | ATGGCATCTGCTCTCAGG
CCGTCAGAACAGTCACAGG |
595 | |
| Exon 3 | ACAGAGCCACACCCTGGA
GTGGTGGTTGAGGAGTCA |
999 | |
| Exon 3 | CTGGCTCCCTGAACCAGAA
AGCTTGCCCGGCCGCTTC |
546 | |
| 3’ Exon 3 | TATGTGGAGCCCACAGCAG
TTTGTCTCTCGACCTGCCC |
1507 | |
| mRNA | GGGCCTTTCGCTACTGCT
CCGTCAGAACAGTCACAGG |
554 | |
| GAPDH | GCCGTGGAATTTGCCGT
GCCATCAATGACCCCTTCAT |
81 | CTCAACTACATGGTCTACATGT
TCCAGTATGATTCCA |
2.8 DAG1 Transcript Quantification
Muscle biopsies from five domestic short-haired cats with no signs of neurological disease, and from two affected Sphynx cats (Cases 1 and 2) were used to quantify the DAG1 transcript. Total RNA extraction, cDNA preparation and real-time TaqMan PCR was done as previously described [53]. Real-time PCR primers and probe for DAG1 were designed based on feline sequence data, as described above (Table 1). A real-time PCR system for the feline housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was designed and validated as previously described [52]. PCR products were designed to be less than 150 bp in length, with either one of the primer pairs or internal probe (Table 1) placed over an exon-exon junction to allow discrimination between cDNA and gDNA. Transcript quantification was determined using the comparative CT method and reported as relative transcription, or the n-fold difference relative to the mean value for individual normal cerebral cortex samples [53]. Statistical significance was determined using a Mann-Whitney U test comparing the median values for control and affected cats.
3. Results
3.1 Animals
A complete blood count, serum chemistry analysis (including CK activity), urinalysis, brain MRI and cisternal CSF analysis were all unremarkable in Case 1. Cardiac auscultation revealed a grade 3/6 systolic murmur, maximal over the cardiac apex. Echocardiographic findings were consistent with hypertrophic cardiomyopathy. In Case 2, with the exception of a mature neutrophilia (23,990 /μl; reference range 2-9,000 /μl), decreased creatinine (49 μmol/L; reference range 110-220 μmol/L) and cholesterol (60mg/dl; reference range 89-258mg/dl), and mildly elevated CK activity (325 IU/L; reference range 73-260 IU/L) and globulins (5.6 g/dl; reference range 2.9-5.3 g/dl), routine laboratory evaluations, brain MRI and cisternal CSF analysis were all unremarkable. Ophthalmic examination revealed mild ulcerative keratitis and conjunctivitis in the right eye consistent with feline herpesvirus type 1 infection. Iris, lens and fundic examinations were unremarkable. In Case 3, routine laboratory evaluations including CK activity were within normal limits.
3.2 Electrophysiology
In Case 1, EMG showed spontaneous activity consisting of fibrillation potentials and complex repetitive discharges in multiple muscle groups. Waveform configuration and motor and sensory nerve conduction velocities were within normal limits for feline peroneal nerve, and cord dorsum potentials and late waves were present. Repetitive supramaximal nerve stimulation at 1-3 Hz revealed a decremental response that was not reversed by intravenous edrophonium. Electrophysiological findings in Case 2 were similar to Case 1 with fibrillation potentials, positive sharp waves and complex repetitive discharges in multiple muscle groups, and normal motor and sensory nerve conduction studies. A decremental response to repetitive nerve stimulation was not found, however. Electrophysiological studies were not performed on Case 3.
3.3 Histology, Histochemistry, and Immunofluorescence Staining
Biopsies from the quadriceps, triceps and cranial tibial muscles were evaluated from the Sphynx cats and controls (Fig 2), and from the dorsal cervical and triceps muscles of the Devon Rex cat (not shown). Pathological changes in the Sphynx cats were mildly myopathic (Fig. 2) with the most consistent findings including excessive variability in myofiber size, type 1 fiber predominance as demonstrated by the NADH-dehydrogenase reaction (Fig. 2C), and infrequent centrally located myofiber nuclei (not shown). Intramuscular nerve branches were unremarkable. No abnormalities were found in either frozen or resin embedded nerve biopsy sections (not shown). Pathologic changes in the muscle specimens from the Devon Rex cat also included excessive variability in myofiber size and internal nuclei; however, occasional necrotic fibers with histiocytic infiltration and several regenerating fibers were also noted. Interestingly, changes in the dorsal cervical muscle were more marked than in the triceps muscle (not shown).
Figure 2. Muscle biopsies from dystrophic Sphynx cats show only mild myopathic changes.
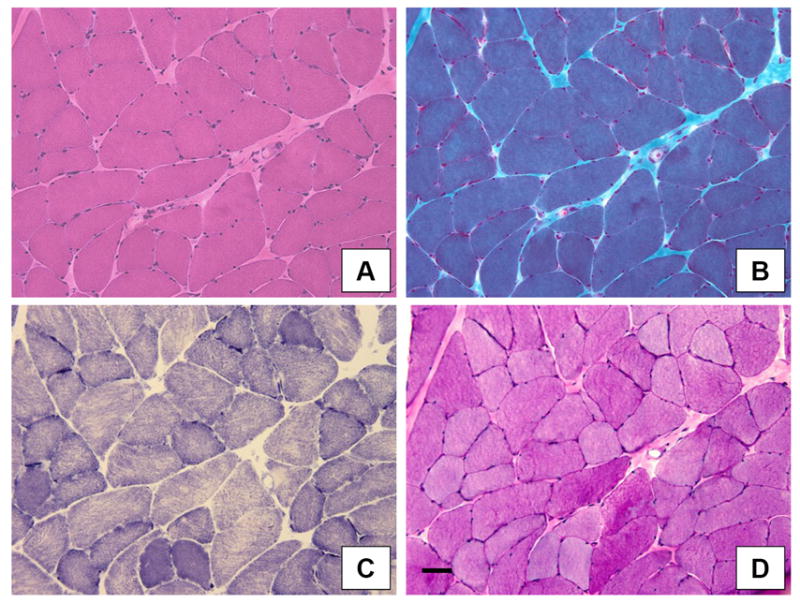
Pathological changes include variability in myofiber size (all figures) and a type 1 fiber predominance (C). A: H&E; B: Modified Gomori trichrome; C: NADH dehydrogenase; D: Periodic acid-Schiff. Bar = 50 μm for all figures.
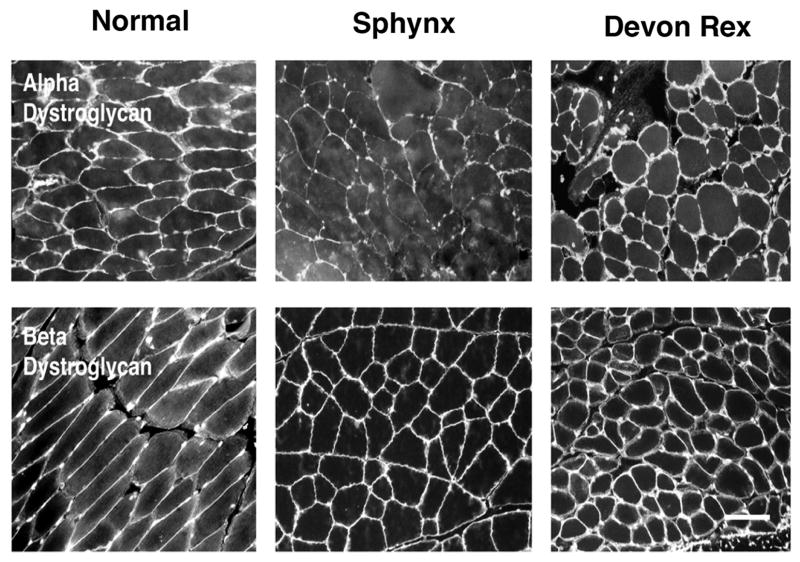
In the Sphynx cats, compared to sections from control (domestic short-haired and a normal Devon Rex cat) muscle, there was no difference in immunofluorescence staining for the rod and carboxy terminus of dystrophin, -α, β, and γ SG, and spectrin, while staining for laminin- α2 was decreased (Fig. 3). Compared to control muscle, staining for natively glycosylated α-DG, using the IIH6 monoclonal antibody, was decreased in the Sphynx cat, while staining for β-DG was similar to controls (Fig 4). Immunofluorescence of muscle sections from the affected Devon Rex cat and a control cat revealed no difference in staining for dystrophin, α-SG, laminin- α2, β-DG and glycosylated α-DG (not shown). Three anti-peptide antisera made against the α-DG protein (DG1, DG2 and DG3) did not stain muscle sections, but did work on immunoblots.
Figure 3. Immunofluorescence staining of frozen muscle biopsy sections from a dystrophic Sphynx cat showed decreased staining for laminin α-2 and increased staining for utrophin compared to a control cat.
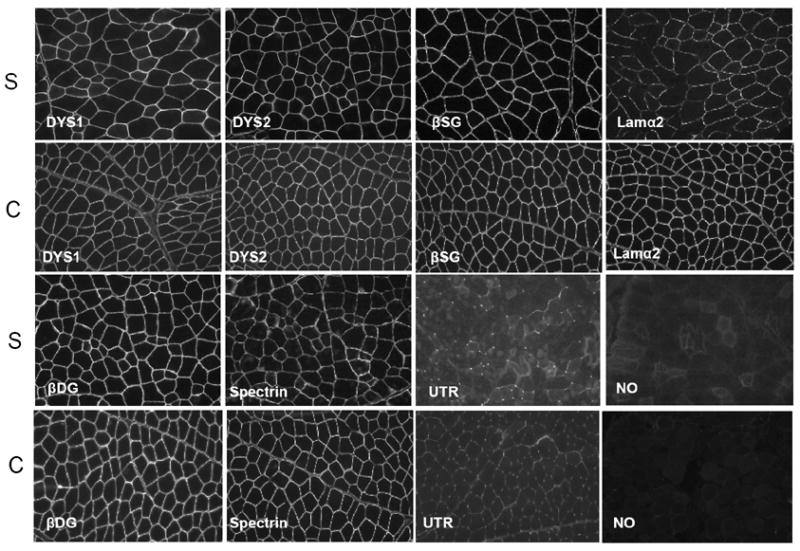
Staining intensity with monoclonal antibodies against the rod (DYS1) and carboxy terminus (DYS2) of dystrophin, β-sarcoglycan (β-SG), β-dystroglycan (β-DG), and spectrin in the Sphynx cat (S) was similar to control (C) muscle. Staining for laminin α-2 (Lama2) was decreased while staining for utrophin (UTR) was increased in the dystrophic Sphynx cat compared to control muscle. Bar = 50 μm.
Figure 4. Compared to staining in normal cats, staining for natively glycosylated α-dystroglycan was decreased.
Immunofluorescence staining of fresh frozen muscle biopsy sections from a dystrophic Sphynx cat, and control (mixed breed and normal Devon Rex) cats was compared with antibodies against natively glycosylated α-dystroglycan (IIH6 anti-carbohydrate antibody). Bar = 50 μm.
3.4. Immunoblotting and Laminin Overlays
Identical amounts of whole cell muscle protein from both affected Sphynx cats and a normal control were compared by immunoblotting (Fig 5A). Levels of dystrophin, laminin-α2, β-DG, α,β,and γ-SG, desmin and dystrobrevin were similar in both samples, while the amount of α-DG (recognized by the IIH6 antibody) was reduced. A comparison of 3 separate immunoblots by scanning densitometry showed α-DG levels reduced by 68%, on average, relative to control. Signal for IIH6 on immunoblots, however, was present in the same molecular weight range for Sphynx cat samples as for controls (with both centered on 160 kDa MW). The level of utrophin protein expression was increased, as can occur in response to regeneration in some dystrophies [54,55]. Some increase in β-SG may also have occurred, though this signal was very weak on immunoblots.
Muscle lysates from the affected Devon Rex cat and a control cat were also examined by immunoblot analysis, but in a different laboratory. An approximately 29% reduction in the level of expression of glycosylated α-DG was found in the affected Devon Rex compared to control (Fig. 5B). By comparison, densitometry showed only a small decrease in levels of laminin-α2 (9%) compared to control.
When different amounts of muscle protein lysate from the control (C) and Sphynx cats were compared to determine the extent of loss of α-dystroglycan expression (Fig. 6), reduced expression of natively glycoyslated α-DG (IIH6, reduced by 45%) and also of all dystroglycan protein (DG3), was found in the Sphynx, while β-DG levels were within 5% of each other. Anti-peptide sera (DG1 and DG2) made to different regions of the α-DG polypeptide also showed reductions in total signal in Sphynx muscle (not shown). LN binding was reduced by 31% when comparing equivalent amounts of total cell protein at 20μg. If Sphynx protein was increased in amount, such that IIH6 binding was equivalent to or surpassed wild type levels, LN binding showed no deficit. Thus, neither a reduction in α-DG glycosylation, which would be evident by a change in molecular weight, nor a deficit in LN binding occurs in dystrophic Sphynx muscle. Rather, there is a reduction in α-DG protein expression, while β-DG is expressed at normal levels. Cat α-DG (both normal and dystrophic) migrated at a molecular weight of 140kDa on 6% SDS-PAGE gels. This is about 20kDa below the level normally found in mouse and human. As the cat α-DG polypeptide is predicted to be only 3 amino acids shorter than that of mouse or human (which would account for only 353Da in molecular weight), the reduced migration suggests cat skeletal muscle α-DG is less glycosylated than mouse or human skeletal muscle α-DG.
3.5 Lectin Binding
To confirm that glycosylation of α-DG was not altered in Sphynx cat skeletal muscle, we performed lectin precipitations using skeletal muscle cell lysates from control (C) and Sphynx (S) cats (Fig. 7). Protein amounts in lectin precipitations were designed such that each lectin had access to an equivalent amount of α–DG protein. Lysates were precipitated with lectins that bind GlcNAc and sialic acid (WGA), N-linked mannose structures (ConA), α2,3-linked sialic acid (MAA), Galβ1,3GalNAcα- structures (PNA), α2,6-linked fucose (LCA), and GalNAc (VVA-B4). As before, WGA precipitated equal amounts of natively glycosylated α–DG protein, as evidenced with IIH6, when α–DG levels were normalized between samples. α-DG also precipitated well with ConA and PNA, at an intermediate level by VVA-B4, and very poorly with MAA and LCA. Importantly, there was no difference between control and Sphynx samples in the amount of α-DG precipitated by any lectin. Likewise, laminin binding paralleled antibody IIH6 blotting in all instances.
Cat α-DG appears to be underglycosylated with sialic acid, as it contains little MAA positive material (which binds α2,3 linked sialic acid, a glycan known to be present on α–DG in skeletal muscle), but it contains high levels of core 1 glycan (Galβ1,3GalNAcα), which is bound by PNA, and possibly also O-linked GalNAc, which is bound by VVA-B4. Thus, reduced amounts of sialic acid may explain the reduced molecular weight of cat α–DG in skeletal muscle. Comparing relative glycosylation with all lectins, however, there was no difference between Sphynx and normal samples that would suggest a glycosylation defect in α-DG.
3.6 Feline DAG1 Amplification and Sequencing
Case 2 (Sphynx), Case 3 (Devon Rex), an additional affected Devon Rex [40], two clinically normal Devon Rex cats and two normal domestic short-haired cats were chosen for sequence level evaluation for polymorphisms in the DAG1 (GenBank Accession no: EU024645). Feline DAG1 exon 2 was found to share a 9 bp/3 amino acid deletion in exon 2 with the dog. The feline gene has 90.3% sequence and 95.1% amino acid homology to humans. No polymorphisms were identified between the affected and unaffected cats for the entire 2680 translated bps of DAG1, although exon 1 (which is not translated) remains to be sequenced. Additionally, 554 bp of cDNA flanking the exon 2 and exon 3 junctions were evaluated for splicing variations and none were identified.
3.6 DAG1 Transcript Quantification
Relative transcription of the DAG1 gene in skeletal muscle biopsies was compared between five normal domestic short-haired cats, one normal Devon Rex and the two affected Sphynx cats. α-DG products were amplified from all animals. While levels of DAG1 expression in Sphynx skeletal muscle were reduced, on average, this did not result in a significant difference between the normal and affected samples (Fig. 8).
Figure 8. Expression of DAG1 mRNA in muscle biopsy specimens did not differ between dystrophic Sphynx cats and controls.
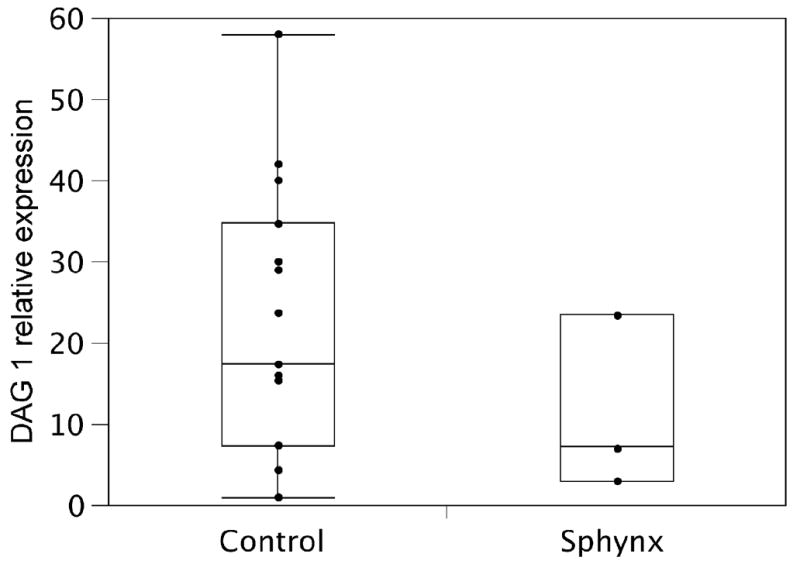
The top and bottom edge of the box plot represent the upper and lower quartile respectively. The line within the box represents the median. The tails extend to the farthest point that is within 1.5 interquartile ranges of the quartiles. DAG1 expression levels varied markedly between individual muscle samples, although DAG1 mRNA was present in all affected Sphynx muscle samples and no statistical difference in expression level was seen between control (5 control cats: 3 samples from each cat) and two Sphynx (3 samples from each Sphynx) cats (p=0.57).
4. Discussion
Counter to previous dystroglycanopathies, where dystroglycan glycosylation is clearly perturbed, we describe a potential new model for dystroglycanopathy where dystroglycan expression is reduced but its glycoslyation pattern appears unchanged. Reduced expression of natively glycosylated α-DG without altered expression of β-DG is consistent with findings in human muscular dystrophies where such an α-DG deficiency is the primary molecular correlate of disease (i.e. the dystroglycanopathies). Also consistent with a primary α-dystroglycan deficiency were our findings that dystrophin and other dystrophin-associated proteins were not altered in their expression (Figs. 3-5) and that the dystroglycan gene harbored no mutations or changes in expression at the level of transcription (Fig. 8). Most dystroglycanopathies, however, result in underglycosylation of α-DG, evidenced by reduced migration of the protein on SDS-PAGE gels using reagents that either identify the natively glycosylated protein (e.g IIH6) or the core polypeptide. Typically, laminin binding to α-DG is also reduced. In the Sphynx cats identified here, α-DG protein expression was reduced in skeletal muscle, but no decrease in molecular weight or LN binding capacity was observed that would suggest altered glycosylation. The lectin binding profile of α-DG from Sphynx skeletal muscle also showed no change relative to unaffected controls when α-DG protein levels were normalized between samples.
Although reduction of α- and β-DG protein has been associated with dystrophin deficiency in mice [56] and dogs [57], this is not due to the loss of dystroglycan glycosylation but to failure of dystroglycan to be properly anchored to the sarcolemmal membrane in the absence of dystrophin. The Sphynx and Devon Rex cats described here, by contrast, express normal amounts of dystrophin, yet native α-DG protein expression was reduced while β-DG was expressed at normal levels. Such a specific reduction in natively glycosylated α-DG would make the Sphynx and Devon Rex cats a new large animal model of dystroglycanopathy. While the gene defect involved has not yet been identified, we have shown that this disorder does not arise from mutations in the coding region of the cat dystroglycan (DAG1) gene. A potential change in DAG1 gene expression could not be excluded definitively because of the low number of affected Sphynx cats available. It is possible, therefore, that loss of α-DG protein may be due partially to reduced DAG1 gene expression. Given that α and β-DG are co-translated and co-spliced from the same two exons of the DAG1 gene, differential gene expression of α and β-DG at the level of mRNA seems unlikely. Differential expression of α and β-DG protein, on the other hand, could be caused by the preferential degradation of α-DG (for example by altered protease activity).
Alternatively, the Sphynx and Devon Rex cats studied here may bear a mutation in one of the genes known to affect the glycosylation of α-DG, which may in turn lead to its reduced expression in skeletal muscle. α-DG undergoes N-linked and extensive O-linked glycosylation and mutations in 6 glycosylation related genes (POMT1, POMT2, POMGNT1, FKTN, FKRP and LARGE) have been associated with a variety of clinical syndromes presenting with variably overlapping phenotypes [3,16,35,58,59]. As this list of genes is likely incomplete, this new model may also result from mutations in a novel, but as yet undescribed muscular dystrophy gene.
The phenotypic severity of dystroglycanopathy patients is extremely variable [35]. The Sphynx and Devon Rex cats described here had no clinically evident neurological abnormalities, similar to mutations in FKRP associated with LGMD2I and some cases of MDC1C [3]. While the spectrum of clinical severity and systems involvement associated with FKRP mutations is wide [30, 33, 35, 38], mutations that give rise to LGMD2I could be consistent with the presentation seen in dystrophic Sphynx and Devon Rex cats [34]. The serum CK concentrations in affected cats were normal to mildly elevated. A broad range of serum CK concentrations (4-50X normal) has recently been reported in human patients with dystroglycanopathy [35]. Further, a wide variation in the extent of α-DG glycosylation can occur in these patients, with absent α-DG in some and almost normal levels on immunoblotting in others (KNN, personal communication). Some natively glycosylated α-DG is usually present in such LGMD2I cases (e.g. [60]), in contrast to many of the more severe non-FKRP syndromes that are associated with profound loss of glycosylated α-DG [33,34]. Conversely these cats could represent a new type of dystroglycanopathy where glycosylation of a dystroglycan is not affected, but expression of the native glycoprotein is.
One of the Sphynx cats in this study had a decremental response following repetitive nerve stimulation, suggestive of defective neuromuscular transmission. α-DG can bind both laminins and agrin at the neuromuscular junction and is involved in consolidation and maintenance of acetylcholine receptor clusters [61]. Defects in neuromuscular structure were reported in mice with skeletal muscles lacking fukutin [62] or dystroglycan [63], suggesting that defects in neuromuscular transmission could arise in such disorders. Further, aberrant neuromuscular junctions and delayed terminal muscle fiber maturation have recently been described in human α-dystroglycanopathies [64]. Although cardiac tissue was not examined histologically except for the Devon Rex case, the presence of mild hypertrophic cardiomyopathy in one cat is consistent with reported human cases [65-69], although a definitive cause was not established.
Sphynx cats are a hairless pedigreed breed of cat that are found throughout the world but are relatively rare compared to other breeds [70]. Hairless mutations in cats have been repeatedly identified over the past 70 years [71-75]. The current Sphynx breed has been expanded and developed using Devon Rex and other cat breeds, with the Devon Rex having had the most significant influence. Genetic evaluations of cat breeds have also confirmed the strong relationships between Devon Rex and Sphynx [75,76]. A congenital muscular dystrophy with an overlapping clinical presentation to the Sphynx has been described in the Devon Rex breed and shown to be inherited as an autosomal recessive trait [40]. Our results suggest that a deficiency of α-DG is present in affected cats of both breeds, which would be consistent with their very similar clinical presentation. Thus, inherited muscular dystrophies found in these two breeds of cats may indeed be conditions that are identical by descent, or may represent disease heterogeneity within and across the breeds.
In summary, our findings in the Sphynx and Devon Rex cats support a diagnosis of muscular dystrophy associated with deficiency of α-DG expression. This form of dystroglycanopathy has not yet been reported in human patients, and thus, this cat model of an expression defect adds to the expanding group of dystroglycanopathies. Phenotypic characteristics, immunohistochemistry, immunoblotting, ligand binding, dystroglycan gene sequencing and transcriptional analysis all suggest that this disorder results from underexpression of the α-DG protein, with many similarities to some of the milder human phenotypes associated with FKRP gene mutations. Further studies to elucidate the possible role of FKRP and other candidate genes in the Sphynx and Devon Rex dystrophy may provide a spontaneously occurring feline model for human dystroglycanopathies. The presence of a large animal model for this class of MD would have numerous advantages both for clinical and biochemical research, including the possibility of gene therapy.
Acknowledgments
This study was supported in part by funds from the Center for Companion Animal Health, University of California, Davis, CA (Dickinson, LeCouteur), the Muscular Dystrophy Association (Engvall, Shelton, Martin), the National Institutes of Health (Martin AR050202 and AR049722), the National Institutes of Health, National Center for Research Resources (Lyons RR016094) and the National Health and Medical Research Council of Australia (North 301946 and Lo 206527). The authors would like to thank Sybil Drummond, the owner of the affected Devon Rex cat; Michelle Berge and Christine Lane, the owners of the Sphynx cats; Drs. Mark Krockenberger and Graeme Allan; Ms FernYang and Professor Clive Harper for contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–17. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F. Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci. 2005;62:809–23. doi: 10.1007/s00018-004-4510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery AE. Population frequencies of inherited neuromuscular diseases – a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 5.Martin PT. Mechanisms of Disease: congenital muscular dystrophies-glycosylation takes center stage. Nat Clin Pract Neurol. 2006;2:222–30. doi: 10.1038/ncpneuro0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper BJ, Winand NJ, Stedman H, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy in dogs. Nature. 1988;334:154–56. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 7.Sharp NJ, Kornegay JN, Van Camp SD, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–21. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- 8.Howell JM, Fletcher S, Kakulas BA, O’Hara M, Lochmuller H, Karpati G. Use of the dog model for Duchenne muscular dystrophy in gene therapy trials. Neuromuscul Disord. 1997;7:325–28. doi: 10.1016/s0960-8966(97)00057-6. [DOI] [PubMed] [Google Scholar]
- 9.Gashen FP, Hoffman EP, Gorospe JR, Uhl EW, Senior DF, Cardinet GH, III, et al. Dystrophin deficiency causes lethal muscle hypertrophy in cats. J Neurol Sci. 1992;110:149–59. doi: 10.1016/0022-510x(92)90022-d. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien DP, Johnson GC, Liu LA, Guo LT, Engvall E, Powell HC, et al. Laminin α2 (merosin)-deficient muscular dystrophy and demyelinating neuropathy in two cats. J Neurol Sci. 2001;189:37–43. doi: 10.1016/s0022-510x(01)00559-7. [DOI] [PubMed] [Google Scholar]
- 11.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–80. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci USA. 1994;91:5572–76. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002:349–61. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y, et al. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci USA. 1997;94:13873–8. doi: 10.1073/pnas.94.25.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito F, Blank M, Schroder J, et al. Aberrant glycosylation of α-dystroglycan causes defective binding of laminin in the muscle of chicken muscular dystrophy. FEBS Lett. 2005;579:2359–2363. doi: 10.1016/j.febslet.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Mendell JR, Boue DR, Martin PT. The congenital muscular dystrophies: recent advances and molecular insights. Pediatr Dev Pathol. 2006;9:427–43. doi: 10.2350/06-07-0127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 18.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 19.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–23. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 21.Cohn RD, Henry MD, Michele DE, et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- 22.Russo K, Di Stasio E, Macchia G, Rosa G, Brancassio A, Petrucci TC. Characterization of the beta-dystroglycan-growth factor receptor 2 (Grb2) interaction. Biochem Biophys Res Commun. 2000;274:93–8. doi: 10.1006/bbrc.2000.3103. [DOI] [PubMed] [Google Scholar]
- 23.Chockalingam PS, Cholera R, Oak SA, Zheng Y, Jarrett HW, Thomason DB. Dystrophin-glycoprotein complex and Ras and Rho GTPase signaling are altered in muscle atrophy. Am J Physiol Cell Physiol. 2002;283:C500–11. doi: 10.1152/ajpcell.00529.2001. [DOI] [PubMed] [Google Scholar]
- 24.Langenback KJ, Rando TA. Inhibition of dystroglycan binding to laminin disrupts the PI3K/AKT pathway and survival signaling in muscle cells. Muscle nerve. 2002;26:644–653. doi: 10.1002/mus.10258. [DOI] [PubMed] [Google Scholar]
- 25.Spence HJ, Dhillon AS, James M, Winder SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5:484–89. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou YW, Thomnason DB, Gullberg D, Jarrett HW. Binding of laminin alpha 1-chain LG4-5 domain to alpha-dystroglycan causes tyrosine phosphorylation of syntrophin to initiate Rac1 signaling. Biochemistry. 2006;45:2042–52. doi: 10.1021/bi0519957. [DOI] [PubMed] [Google Scholar]
- 27.Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–43. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Reeuwijk J, Janssen M, van den Elzen C, et al. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42:907–12. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi K, Nakahori Y, Miyake M, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–92. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 30.Beltran-Valero de Bernabe D, Voit T, Longman C, et al. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J Med Genet. 2004;41:e61. doi: 10.1136/jmg.2003.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longman C, Brockington M, Torelli S, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 2003;12:2853–61. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 32.Manya H, Sakai K, Kobayashi K, Taniguchi K, Kawakita M, Toda T, Endo T. Loss-of-function of an N-acetylglucosaminyltransferase, POMNGnT1, in muscle-eye-brain disease. Biochem Biophys Res Commun. 2003;306:93–7. doi: 10.1016/s0006-291x(03)00924-0. [DOI] [PubMed] [Google Scholar]
- 33.Brockington M, Blake DJ, Prandini P, et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha 2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001;69:1198–209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brockington M, Yuva Y, Prandini P, et al. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001;10:2851–9. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey C, Clement E, Mein R, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130(Pt 10):2725–35. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K. Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology. 2001;57:115–21. doi: 10.1212/wnl.57.1.115. [DOI] [PubMed] [Google Scholar]
- 37.Kano H, Kobayashi K, Herrmann R, et al. Deficiency of alpha-dystroglycan in muscle-eye-brain disease. Biochem Biophys Res Commun. 2002;291:1283–6. doi: 10.1006/bbrc.2002.6608. [DOI] [PubMed] [Google Scholar]
- 38.Topaloglu H, Brockington M, Yuva Y, et al. FKRP gene mutations cause congenital muscular dystrophy, mental retardation, and cerebellar cysts. Neurology. 2003;60:988–92. doi: 10.1212/01.wnl.0000052996.14099.dc. [DOI] [PubMed] [Google Scholar]
- 39.Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 40.Malik R, Mepstead K, Yang F, Harper C. Hereditary myopathy of Devon Rex cats. J Small Anim Pract. 1993;34:539–46. [Google Scholar]
- 41.Robinson R. ‘Spasticity’ in the Devon Rex cat. Vet Rec. 1992;130:302. doi: 10.1136/vr.130.14.302-a. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson PJ, Anderson PJ, Williams DC, Powell HC, Shelton GD, Morris JG, LeCouteur RA. Assessment of the neurologic effects of dietary deficiencies of phenylalanine and tyrosine in cats. Am J Vet Res. 2004;65:671–80. doi: 10.2460/ajvr.2004.65.671. [DOI] [PubMed] [Google Scholar]
- 43.Redding RW, Ingram JT. Sensory nerve conduction velocity of cutaneous afferents of the radial, ulnar, peroneal, and tibial nerves of the cat: reference values. Am J Vet Res. 1984;45:1042–45. [PubMed] [Google Scholar]
- 44.Redding RW, Lee AH, Wilson SG. Spinal-evoked potentials and spinal conduction velocity of the cat: reference values. Am J Vet Res. 1984;45:2175–77. [PubMed] [Google Scholar]
- 45.Malik R, Ho S. Motor-nerve conduction parameters in the cat. J Small Anim Pract. 1989;30:396–400. [Google Scholar]
- 46.Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544–8. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu LA, Engvall E. Sarcoglycan isoforms in skeletal muscle. J Biol Chen. 1999;274:38171–6. doi: 10.1074/jbc.274.53.38171. [DOI] [PubMed] [Google Scholar]
- 48.Xia B, Hoyte K, Kammesheidt A, Deerinck T, Ellisman M, Martin PT. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev Biol. 2002;242:58–73. doi: 10.1006/dbio.2001.0530. [DOI] [PubMed] [Google Scholar]
- 49.Hoyte K, Jayasinha V, Xia B, Martin PT. Transgenic overexpression of dystroglycan does not inhibit muscular dystrophy in mdx mice. Am J Pathol. 2004;164:711–18. doi: 10.1016/S0002-9440(10)63158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci USA. 2002;99:5616–21. doi: 10.1073/pnas.082613599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper ST, Lo HP, North KN. Single section Western blot: improving the molecular diagnosis of the muscular dystrophies. Neurology. 2003;61:93–97. doi: 10.1212/01.wnl.0000069460.53438.38. [DOI] [PubMed] [Google Scholar]
- 52.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 53.Dickinson PJ, Roberts BN, Higgins RJ, Leutenegger CM, Bollen AW, Kass PH, LeCouteur RA. Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRα and c-Met in canine primary brain tumors. Veterinary and Comparative Oncology. 2006;4:132–40. doi: 10.1111/j.1476-5829.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 54.Hirst RC, McCullagh KJ, Davies KE. Utrophin upregulation in Duchenne muscular dystrophy. Acta Myol. 2005;24:209–16. [PubMed] [Google Scholar]
- 55.Gramolini AO, Karpati G, Jasmin BJ. Discordant expression of utrophin and its transcript in human and mouse skeletal muscles. J Neuropathol Exp Neurol. 1999;58:235–44. doi: 10.1097/00005072-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated protein in mdx mouse muscle. Nature. 1992;360:588–91. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- 57.Ervasti JM, Roberds SL, Anderson RD, Sharp NJ, Kornegay JN, Campbell KP. Alpha-dystroglycan deficiency correlates with elevated serum creatine kinase and decreased muscle contraction tension in golden retriever muscular dystrophy. FEBS Lett. 1994;350:173–76. doi: 10.1016/0014-5793(94)00748-9. [DOI] [PubMed] [Google Scholar]
- 58.D’Amico A, Tessa A, Bruno C, et al. Expanding the clinical spectrum of POMT1 phenotype. Neurology. 2006;66:1564–67. doi: 10.1212/01.wnl.0000216145.66476.36. [DOI] [PubMed] [Google Scholar]
- 59.Balci B, Uyanik G, Dincer P, et al. An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord. 2005;15:271–5. doi: 10.1016/j.nmd.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Brown SC, Torelli S, Brockington M, et al. Abnormalities in alpha-dystroglycan expression in MDC1C and LGMD2I muscular dystrophies. Am J Pathol. 2004;164:727–37. doi: 10.1016/s0002-9440(10)63160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobson C, Montanaro F, Lindenbaum M, Carbonetto S, Ferns M. Alpha-dystroglycan functions in acetylcholine receptor aggregation but is not a coreceptor for agrin-MuSK signaling. J Neurosci. 1998;18:6340–48. doi: 10.1523/JNEUROSCI.18-16-06340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saito F, Masaki T, Saito Y, et al. Defective peripheral nerve myelination and neuromuscular junction formation in fukutin-deficient chimeric mice. J Neurochem. 2007;101:1712–22. doi: 10.1111/j.1471-4159.2007.04462.x. [DOI] [PubMed] [Google Scholar]
- 63.Cote PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23:338–42. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- 64.Taniguchi M, Kurahashi H, Noguchi S, et al. Aberrant neuromuscular junctions and delayed terminal muscle fiber maturation in α-dystroglycanopathies. Hum Mol Genet. 2006;15:1279–1289. doi: 10.1093/hmg/ddl045. [DOI] [PubMed] [Google Scholar]
- 65.McNally EM. New approaches in the therapy of cardiomyopathy in muscular dystrophy. Ann Rev Med. 2007;58:75–88. doi: 10.1146/annurev.med.58.011706.144703. [DOI] [PubMed] [Google Scholar]
- 66.Valentine BA, Cummings JF, Cooper BJ. Development of Duchenne-type cardiomyopathy. Morphologic studies in a canine model. Am J Pathol. 1989;135:671–8. [PMC free article] [PubMed] [Google Scholar]
- 67.Nigro V, Okazaki Y, Belsito A, et al. Identification of the Syrian hamster cardiomyopathy gene. Hum Mol Genet. 1997;6:601–7. doi: 10.1093/hmg/6.4.601. [DOI] [PubMed] [Google Scholar]
- 68.Nakanishi T, Sakauchi M, Kaneda Y, Tomimatsu H, Saito K, Nakazawa M, Osawa M. Cardiac involvement in Fukuyama-type congenital muscular dystrophy. Pediatrics. 2006;117:e1187–92. doi: 10.1542/peds.2005-2469. [DOI] [PubMed] [Google Scholar]
- 69.Poppe M, Bourke J, Eagle M, et al. Cardiac and respiratory failure in limb-girdle muscular dystrophy 2I. Ann Neurol. 2004;56:738–41. doi: 10.1002/ana.20283. [DOI] [PubMed] [Google Scholar]
- 70.Cat Fanciers’Association. Cat Fanciers’ Association Registration Totals by Color and Breed-2003, and 1/1/58 to 12/31/03. Cat Fanciers’ Almanac. 2004;20:72–86. [Google Scholar]
- 71.Letard E. Hairless Siamese cats. J Hered. 1938;64:47–9. [Google Scholar]
- 72.Robinson R. The Canadian hairless or sphinx cat. J Hered. 1973;64:47–9. doi: 10.1093/oxfordjournals.jhered.a108339. [DOI] [PubMed] [Google Scholar]
- 73.Robinson R. A third hypotrichosis in the domestic cat. Genetica. 1981;55:39–40. [Google Scholar]
- 74.Hendy-Ibbs PM. Hairless cats in Great Britain. J Hered. 1984;75:506–7. [PubMed] [Google Scholar]
- 75.Lipinski MJ, Froenicke L, Baysac KC, Billings NC, Leutenegger CM, Levy AM, et al. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics. 2007 Nov;28 doi: 10.1016/j.ygeno.2007.10.009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menotti-Raymond M, David VA, Pflueger SM, Lindblad-Toh K, Wade CM, O’Brien SJ, et al. Patterns of molecular genetic variation among cat breeds. Genomics. 2007 Oct;24 doi: 10.1016/j.ygeno.2007.08.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]