Abstract
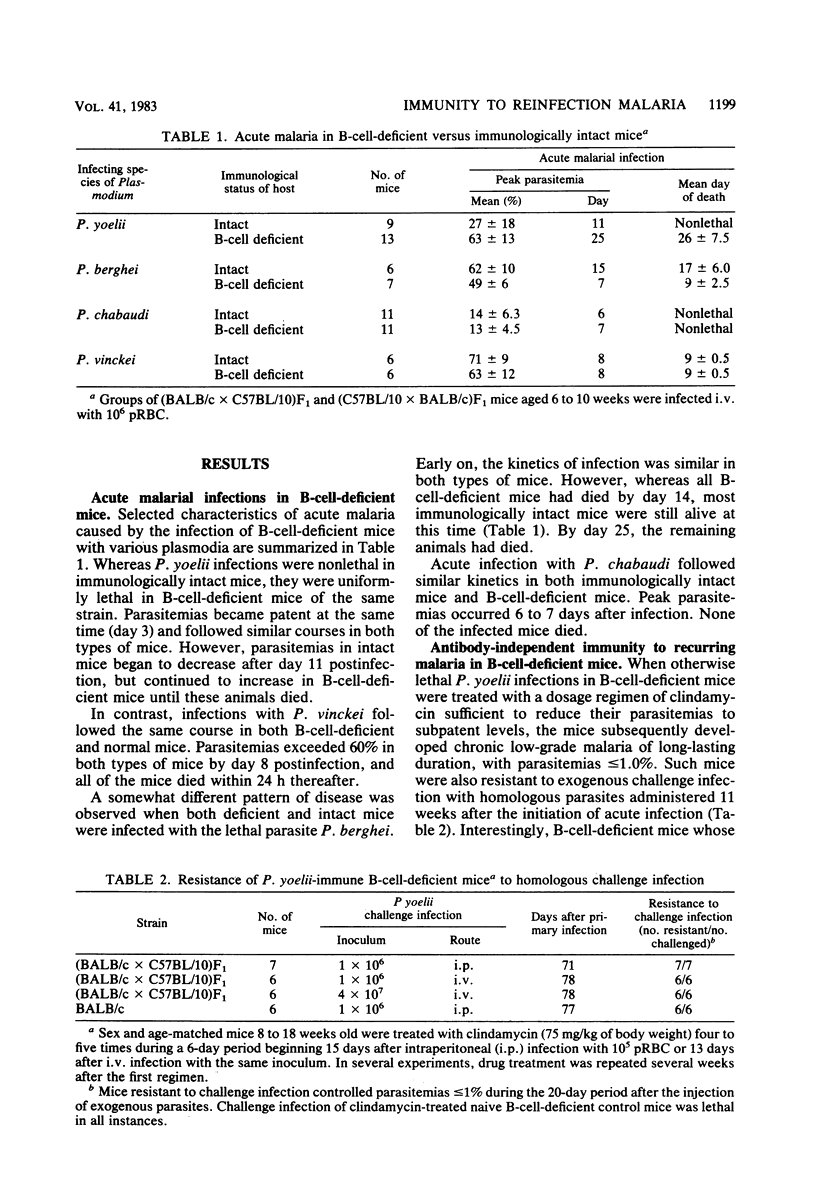
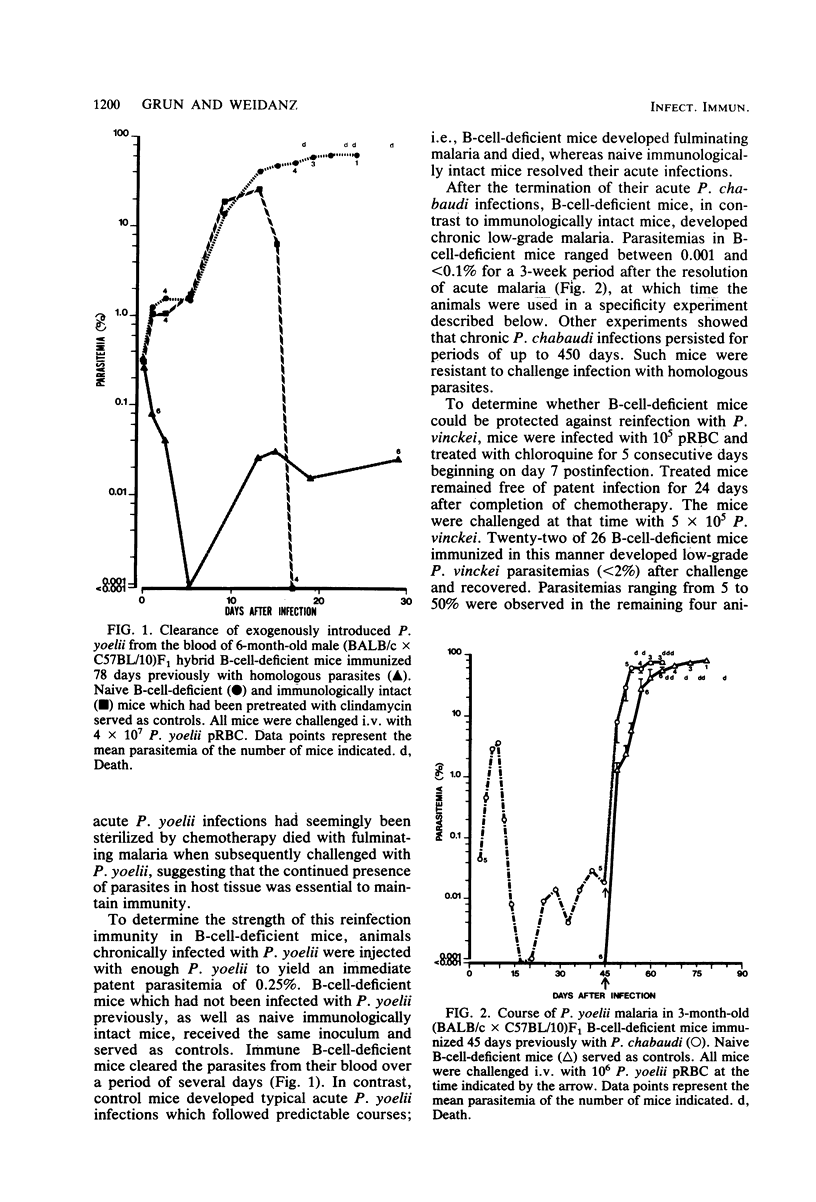
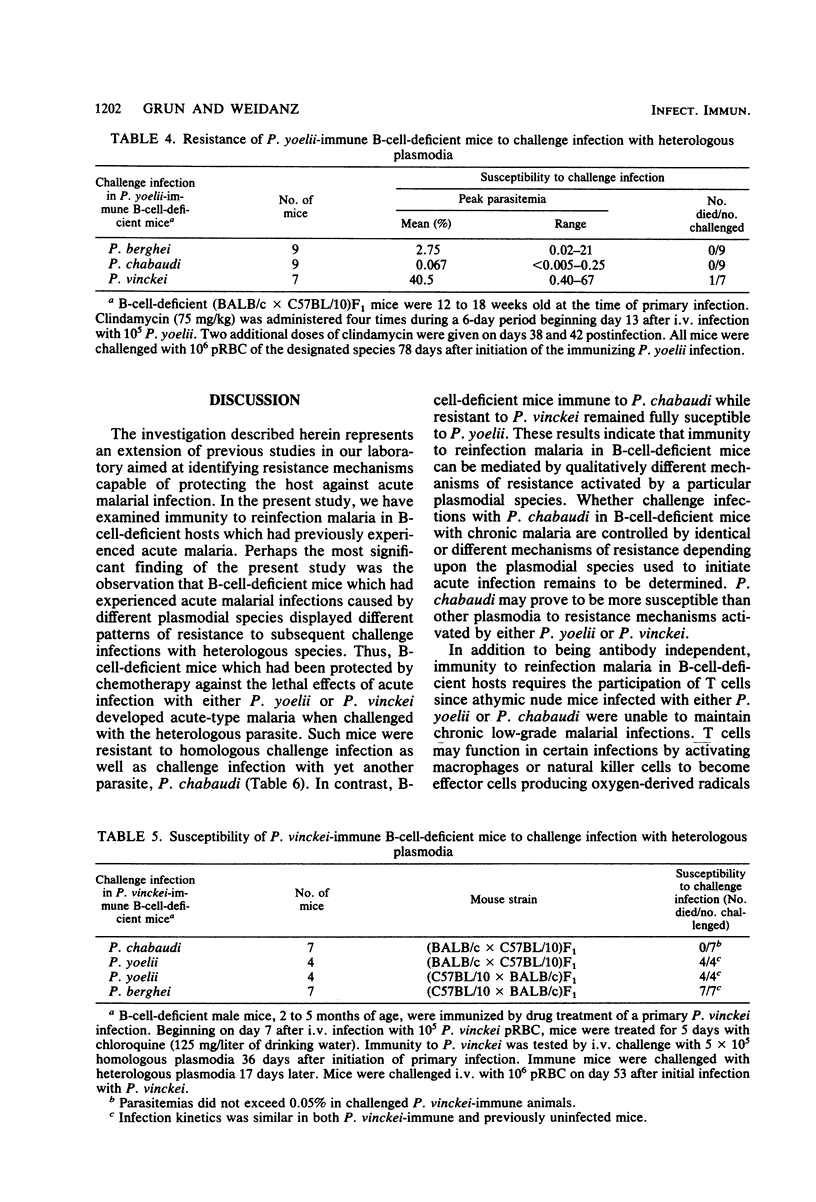
Immunity to "reinfection malaria" or "premunition" was studied in B-cell-deficient mice which had previously experienced acute malaria caused by the avirulent plasmodia Plasmodium yoelii or P. chabaudi or by the lethal P. vinckei. Such mice resisted challenge infection with large numbers of homologous parasites but differed in their capacity to resist challenge with heterologous species. Mice immune to P. yoelii resisted infection with P. chabaudi but developed acute-type, albeit nonlethal, infections when challenged with P. vinckei. Whereas mice immune to P. chabaudi resisted challenge with P. vinckei and vice versa, they developed fulminating malaria and died when infected with P. yoelii. The data suggest that immunity to reinfection malaria in B-cell-deficient mice, although antibody independent, is mediated by different mechanisms of resistance depending upon the plasmodial species used to initiate acute infection. Additional evidence supporting this concept was gained from preliminary experiments in which immunity to reinfection was measured by the ability of chronically infected mice to control endogenous parasites at low levels. B-cell-deficient mouse strains showed genotypic differences in their ability to develop immunity to reinfection with P. yoelii. In contrast, the same mouse strains uniformly developed immunity to reinfection with P. chabaudi. These findings suggest that different genetic loci control resistance to reinfection malaria caused by different species of plasmodia. Finally, B-cell-deficient mice acutely infected with lethal plasmodia, P. vinckei or P. berghei, died at the same time or earlier than similarly infected immunologically intact mice, indicating that "early death" in virulent malarial infections is an antibody-independent phenomenon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Clark I. A. Specific and non-specific immunity to haemoprotozoa. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):216–222. doi: 10.4269/ajtmh.1977.26.216. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Immunity to malaria. Proc R Soc Lond B Biol Sci. 1979 Jan 15;203(1153):323–345. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Malaria infections in different strains of mice and their correlation with natural killer activity. Bull World Health Organ. 1979;57 (Suppl 1):231–238. [PMC free article] [PubMed] [Google Scholar]
- Ferris D. H., Beamer P. D., Stutz D. R. Observations on the response of dysgammaglobulinemic chickens to malarial infection. Avian Dis. 1973 Jan-Mar;17(1):12–23. [PubMed] [Google Scholar]
- Finley R. W., Mackey L. J., Lambert P. H. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J Immunol. 1982 Nov;129(5):2213–2218. [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Manning D. D. Recovery from anti-IG induced immunosuppression: implications for a model of Ig-secreting cell development. J Immunol. 1974 Aug;113(2):455–463. [PubMed] [Google Scholar]
- Miller L. H., Carter R. A review. Innate resistance in malaria. Exp Parasitol. 1976 Aug;40(1):132–146. doi: 10.1016/0014-4894(76)90075-8. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Studies on immune responses to parasite antigens in mice. V. Different susceptibilities of hypothymic and intact mice to Babesia rodhaini. Int Arch Allergy Appl Immunol. 1977;53(4):385–388. doi: 10.1159/000231775. [DOI] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. Splenomegaly, enhanced phagocytosis, and anemia are thymus-dependent responses to malaria. Infect Immun. 1978 Jun;20(3):728–731. doi: 10.1128/iai.20.3.728-731.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- SERGENT E., SERGENT E. History of the concept of relative immunity or premunition correlated to latent infection. Indian J Malariol. 1956 Mar;10(1):53–80. [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zon A., Eling W., Jerusalem C. Histo- and immunopathology of a malaria (Plasmodium berghei) infection in mice. Isr J Med Sci. 1978 Jun;14(6):659–672. [PubMed] [Google Scholar]
- Viens P., Chevalier J. L., Sonea S., Yoeli M. The effect of reticulocytosis on Plasmodium vinckei infection in white mice. Action of phenylhydrazine and of repeated bleedings. Can J Microbiol. 1971 Feb;17(2):257–261. doi: 10.1139/m71-043. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]


