SUMMARY
Both the POLH-1 (pol eta) trans-lesion synthesis DNA polymerase and the GEI-17 SUMO E3 ligase are essential for the efficient replication of damaged chromosomes in C. elegans embryos. Here, we study how POLH-1 is regulated during a DNA damage response in these embryos. We report that DNA damage triggers the degradation of POLH-1, and that degradation is mediated by the Cul4-Ddb1-Cdt2 (CRL4-Cdt2) pathway that has previously been shown to degrade the replication factor Cdt1 during S phase. We also show that GEI-17 protects POLH-1 from CRL4-Cdt2 mediated destruction, until after it has performed its function in TLS, and this is likely via SUMOylation of POLH-1. These studies reveal that POLH-1 undergoes DNA damage-induced proteolysis, and that GEI-17 regulates the timing of this proteolysis. Implications for how this system may control the removal of POLH-1 from replication forks after TLS are discussed.
INTRODUCTION
In most eukaryotic cells, DNA replication and progression through the cell cycle are adversely affected by DNA damaging agents. When chromosomes are damaged, then replication is slowed, and this triggers activation of checkpoints that delay progression through the cell cycle. An interesting deviation from this paradigm is found during early embryogenesis in C. elegans. Cell division in this system is extraordinarily resistant to DNA damage-imposed delays (Holway et al., 2006), despite the presence of active checkpoint systems that respond to replication stress (Brauchle et al., 2003; Holway et al., 2006). DNA damage-induced checkpoint activation is avoided in early C. elegans embryos, at least in part, because they are remarkably proficient at replicating damaged chromosomes (Hartman et al., 1991; Jones and Hartman, 1996). Even massive doses of UV light (180 J/m2) do little to perturb replication, as revealed by studies using electron microscopy to examine the size and spacing of replication bubbles in UV-irradiated DNA (Jones and Hartman, 1996). The reason that early nematode embryos have evolved such proficiency at replicating damaged DNA is likely linked to the developmental sensitivity to cell cycle delays that has been observed. During embryogenesis the timing of cell division is fixed, and deviations from this timing program have been shown to cause missegregation of developmental regulators, patterning defects, and developmental failure (Encalada et al., 2000). Proficient replication of damaged chromosomes thus alleviates the embryo of developmental problems that might otherwise result from conditions that cause DNA damage. The C. elegans early embryo, therefore, represents an excellent model system to study mechanisms that promote the rapid replication of damaged DNA.
Our laboratory has studied DNA damage tolerance in early C. elegans embryos, and to date we have identified three genes which prevent DNA damage from slowing down the early cycles: rad-2/smk-1, gei-17, and polh-1 (Holway et al., 2005, 2006; Kim et al., 2007). The rad-2/smk-1 gene encodes a regulatory subunit of protein phosphatase 4, and has been recently described (Kim et al., 2007). When either polh-1 or gei-17 is inactivated by RNA interference (RNAi), very similar phenotypes are observed. In both cases, there is no observable affect on cell cycle progression or DNA replication in undamaged embryos. By contrast, when polh-1 or gei-17 RNAi embryos are exposed to the DNA damaging agent methyl methanesulphonate (MMS), then DNA replication is delayed, the replication checkpoint is activated, and cell cycle progression is blocked (Holway et al., 2006). The gei-17 gene encodes a SUMO E3 ligase (Holway et al., 2005), and polh-1 encodes the worm ortholog of the translesion synthesis (TLS) DNA polymerase pol eta (Holway et al., 2006). As detailed below, the biochemical activity of POLH-1 fits well with its role in promoting the rapid replication of damaged chromosomes. The function of GEI-17 in replicating damaged DNA was heretofore unknown.
POLH-1 belongs to the Y family of TLS DNA polymerases that allow lesion bypass during replication (reviewed in Prakash et al., 2005; Lehmann et al., 2007). Y-family polymerases contain a more open active site, and this allows these polymerases to accept damaged nucleotides into the active site, and to catalyze nucleotide insertion opposite DNA lesions. Consequently, TLS polymerases are called upon to promote the replication of damaged DNA, and to thereby allow S phase progression during a DNA damage response. One consequence to the cell for utilizing TLS polymerases during replication, however, is mutagenesis. TLS polymerases have low fidelity and thus make mistakes, even when replicating undamaged DNA. Despite this poor fidelity, the ability of TLS polymerases to promote replication of damaged chromosomes is clearly beneficial, as these enzymes are evolutionarily conserved throughout the kingdoms of life.
Because of their propensity for error-prone replication, access of TLS polymerases to the replication fork is tightly regulated. In eukaryotes, the major mechanism for regulating TLS polymerase activity involves mono-ubiquitylation of proliferating cell nuclear antigen (PCNA), by a complex composed of the Rad6 E2 ubiquitin conjugating enzyme and the Rad18 E3 ubiquitin ligase (reviewed in Prakash et al., 2005; Lehmann et al., 2007). More recent findings have shown that other pathways can also control access of TLS polymerases to sites of damage, in a manner independent of PCNA modification (Edmunds et al., 2008). Once it is recruited to the stalled replication fork, stable association of pol eta with the replisome depends on interaction with PCNA (Haracska et al., 2001). Pol eta binds to PCNA through its PCNA-interacting peptide, or PIP box (reviewed in Moldovan et al., 2007). PIP boxes are found in many proteins that engage PCNA, and have been shown to interact directly with the inter-domain connector loop of the trimeric PCNA. Mutation of the pol eta PIP box renders the protein non-functional for TLS in vivo, and compromises interaction with PCNA in vitro (Haracska et al., 2001; Parker et al., 2007). These data show that engagement of pol eta with PCNA via the PIP box allows pol eta-mediated TLS. The mechanism(s) involved in removing pol eta from the replication fork after TLS have not been elucidated.
Recent work has shown that PIP box – PCNA interaction can also target the PIP box-bearing protein for proteolysis (reviewed in Arias and Walter, 2007; O’Connell and Harper, 2007). For example, the replication initiation factor Cdt1 binds to DNA-bound PCNA through its PIP box and, by doing so, Cdt1 becomes a substrate for the Cul4-Ddb1-Cdt2 ubiquitin ligase (heretofore referred to as CRL4-Cdt2). CRL4-Cdt2-mediated ubiquitylation of Cdt1 then targets the protein for proteosome-mediated degradation. More recently, it has been shown that the CDK inhibitor p21 is also destroyed in a PIP box-dependent manner by CRL4-Cdt2 (Abbas et al., 2008; Kim et al., 2008; Nishitani et al., 2008). These studies on the proteolysis of PIP box-bearing proteins by CRL4-Cdt2 suggest that this pathway could regulate pol eta function during a DNA damage response.
In this work, we continue our analysis of the mechanisms for rapid replication of damaged chromosomes in early worm embryos, and we focus on the regulation of POLH-1 and the role of GEI-17 in this process. We report that POLH-1 is positively regulated by GEI-17 mediated SUMOylation, and negatively regulated by CRL4-Cdt2 mediated proteolysis, during the DNA damage response. Our results reveal a GEI-17/CRL4-Cdt2 based regulatory system that controls POLH-1 function, and that may be important for removing POLH-1 from the replication fork after TLS.
RESULTS
GEI-17 Protects POLH-1 from CRL4-Cdt2 Mediated Destruction During a DNA Damage Response
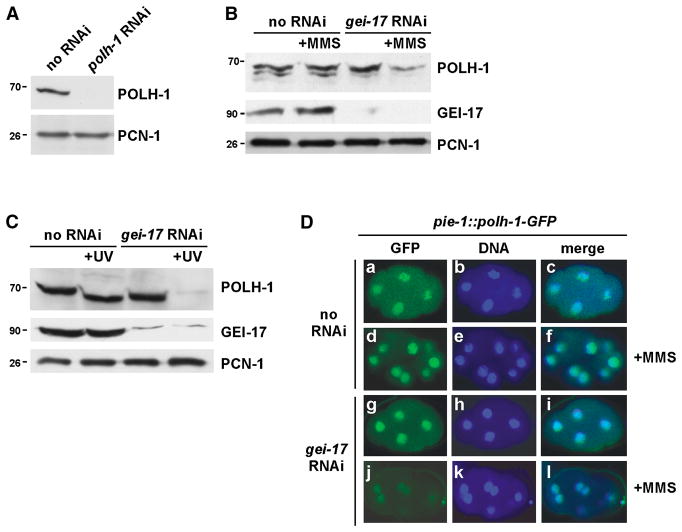
Inactivation of either gei-17 or polh-1 by RNAi produces similar DNA damage response phenotypes (Holway et al., 2006), which suggests that GEI-17 functions with POLH-1 to promote the efficient replication of damaged chromosomes. To initiate the current study, we prepared antibodies against POLH-1 and probed Western blots of early embryo extracts. These antibodies detected a ~68 kDa protein, the predicted size for POLH-1, that was eliminated by polh-1 RNAi (Figure 1A). This shows that our antibodies specifically recognize POLH-1. To pursue a role for GEI-17 in POLH-1 regulation, we examined POLH-1 in early embryos that had been treated with gei-17 RNAi, and optionally exposed to 0.005% MMS. There was no detectable difference in POLH-1 levels between wild type and gei-17 RNAi embryos in the control, undamaged samples. Surprisingly, however, we found that exposure to MMS caused a loss of POLH-1 from gei-17 RNAi embryos, but not wild type (Figure 1B). Similar results were obtained when UV light was used to damage the DNA (Figure 1C). These data show that GEI-17 controls POLH-1 protein levels in early embryos. To confirm these results using an alternative method, and to determine the subcellular localization of POLH-1, we produced a transgenic strain that expresses a POLH-1-GFP fusion protein off the pie-1 promoter. The pie-1 promoter allows transgene expression within the germ line (Strome et al., 2001), and thus early embryos produced by transgenic adults contain POLH-1-GFP. Expression of POHL-1-GFP did not appear to hamper the early embryonic DNA damage response, as the transgenic embryos showed a wild-type level of resistance to MMS (data not shown). We used confocal microscopy to localize POLH-1-GFP within the early embryo, and found that it was mostly in the nucleus (Figure 1D, panels a-c). Neither the nuclear localization, nor the intensity of the GFP signal within the nucleus, was noticeably changed by treatment with MMS (Figure 1D, panels d-f). We next used RNAi to inactivate gei-17 in these embryos, and we observed that while MMS did not alter the subcellular localization of POLH-1-GFP, it did reduce the intensity of the GFP signal within the nuclei (Figure 1D, panels g-l). These results are consistent with the data in Figures 1B and 1C and, based on them, we conclude that GEI-17 controls POLH-1 protein levels during the DNA damage response.
Figure 1. GEI-17 Controls POLH-1 Protein Levels During the DNA Damage Response.
(A) Early embryos were obtained by bleaching gravid N2 adults and extracts were prepared as described (Kim et al., 2007). The samples were fractionated on SDS-PAGE and then probed, by immunoblotting, with antibodies against POLH-1. The lysates were also probed with antibodies against PCN-1, to ensure equal loading of the samples. Prior to extract preparation the animals were either treated, or not, with polh-1 RNAi by feeding, as indicated.
(B) Early embryo extracts were prepared and then probed, by immunoblotting, with anti-POLH-1 antibodies, after SDS-PAGE. The lysates were also probed with anti-PCN-1 antibodies, to ensure equal loading of the samples, and with anti-GEI-17 antibodies, to monitor the effectiveness of the gei-17 RNAi. Prior to extract preparation, the animals were either treated, or not, with gei-17 RNAi and MMS, as indicated. MMS treatment was performed by inclusion of 0.005% MMS in the plate media and incubation of animals on these plates for 20 hours. We note that POLH-1 runs as doublet on this blot; this was not reproducibly observed, and we suspect that the lower band in the doublet represents a proteolytic fragment of POLH-1 that is occasionally produced during preparation of the extracts.
(C) Early embryo extracts were prepared after optional gei-17 RNAi, and then probed, by immunoblotting, with antibodies against POLH-1, GEI-17, or PCN-1, after SDS-PAGE. Prior to extract preparation the animals were either exposed, or not, to 100 J/m2 of UV light, as indicated. The samples were harvested 5 hours after UV irradiation.
(D) A POLH-1-GFP fusion protein was expressed in animals via stable transformation with a construct driving expression of POLH-1-GFP with the pie-1 promoter (“pie-1::polh-1-GFP”, see Experimental Procedures). These animals were treated with the indicated RNAi and early embryos were examined for GFP signals by fluorescence microscopy. The samples were also stained with Hoechst 33258, to visualize the DNA. “GFP” indicates GFP-based fluorescence, “DNA” indicates Hoechst 33258-based fluorescence, and “merge” shows both signals together in the same image. The exposure times during image capture was identical for all of the samples shown. Where indicated, animals were treated with MMS, as in Figure 1B.
The data in Figure 1 show that when GEI-17 is inactivated, then POLH-1 is lost from MMS-exposed embryos. SUMO ligases such as GEI-17 often act in opposition to ubiquitin ligases, to control the behavior of a common substrate (reviewed in Ulrich, 2005). One explanation for the observations in Figure 1, therefore, is that GEI-17 prevents ubiquitin-mediated proteolysis of POLH-1 during a DNA damage response. To test this possibility, we searched for sequence motifs within POLH-1 that might serve as degrons. POLH-1 contains a PIP box, and recent work has shown that PIP box-bearing proteins can be targeted for proteolysis by the CRL4-Cdt2 ubiquitin E3 ligase (reviewed in Moldovan et al., 2007; O’Connell et al., 2007). In POLH-1, the PIP box is located at 572-PKSLESFF-579, which conforms to the PIP box consensus motif of QXXL/I/MXXFF/Y. We created a mutant POLH-1, POLH-1-ΔPIP, which deletes the critical FF dipeptide from the PIP box. By co-immunoprecipitation of in vitro translated proteins, we found that while wild type POLH-1 could interact with PCN-1, the nematode ortholog of PCNA, the POLH-1-ΔPIP mutant could not (Supplementary Figure S1). This confirms that the POLH-1 PIP box can bind PCN-1. The POLH-1-ΔPIP mutant was fused to GFP, expressed in early embryos, and its level was then assessed after gei-17 RNAi and exposure to MMS. Wild type POLH-1-GFP was lost from MMS-exposed gei-17 RNAi embryos, as expected (Figure 2A). Importantly, however, ΔPIP POLH-1-GFP levels were not affected by depletion of gei-17 and exposure to MMS. Confocal imaging of the ΔPIP POLH-1-GFP mutant revealed that it localizes to the nucleus in early embryos in a manner indistinguishable from wild type POLH-1-GFP (data not shown). This result shows that deletion of the PIP box, a known degron, stabilizes the protein during a DNA damage response in gei-17 RNAi embryos.
Figure 2. POLH-1 is Destroyed in a PIP box and CRL4-Cdt2 Dependent Manner in MMS-exposed, GEI-17 Deficient Embryos.
(A) A PIP box deletion mutant (ΔPIP) was introduced into the pie-1::polh-1-GFP construct and stable transformants were obtained via particle bombardment (see Experimental Procedures). Early embryo extracts were prepared, and the WT POLH-1-GFP or ΔPIP POLH-1-GFP proteins were probed by immunoblotting, after SDS-PAGE, with anti-GFP antibodies. The lysates were also probed with antibodies against PCN-1, to ensure equal loading of the samples, and with anti-GEI-17 antibodies, to monitor the effectiveness of the gei-17 RNAi. The animals were exposed, or not, to MMS, as in Figure 1B.
(B) WT POLH-1-GFP worms were treated with gei-17/pcn-1 RNAi by soaking, as indicated, and then optionally exposed to MMS (as in Figure 1B). Early embryo extracts were prepared and the POLH-1-GFP protein was probed by immunoblotting, after SDS-PAGE, with anti-GFP antibodies. The lysates were also probed with antibodies against PCN-1 or GEI-17, to monitor the effectiveness of the gei-17/pcn-1 RNAi, as indicated. The intensity of the signals in the POLH-1-GFP blot were quantified using the histogram tool of Photoshop, and the vales obtained were 52, 51, 49.5, and 51, respectively, for the samples present in lanes 1–4 of the blot.
(C) Feeding RNAi, in various configurations, was performed on the WT POLH-1-GFP worm as indicated. The animals were optionally exposed to MMS (as in Figure 1B) and then embryo extracts were prepared. Extracts were fractionated on SDS-PAGE gels and then probed by immunoblotting for POLH-1-GFP (using anti-GFP antibodies) and GEI-17 and PCN-1, as indicated.
(D) Early embryo extracts were prepared and POLH-1, GEI-17, or PCN-1 proteins were then detected by immunoblotting, after SDS-PAGE. Prior to extract preparation, the animals were either treated, or not, with gei-17 RNAi by feeding and MMS (as in Figure 1B), as indicated.
The requirement for the PIP box in the loss of POLH-1 from gei-17 RNAi embryos suggested the involvement of the CRL4-Cdt2 pathway. In C. elegans, these components include pcn-1, cul-4 (Cul4), ddb-1 (DDB1), and cdt-2 (Cdt2). To determine if this pathway is responsible for the affects that we have observed, we co-depleted each of the components with gei-17, and then assessed POLH-1-GFP proteins levels after treatment with MMS. Co-depletion of pcn-1 stabilized POLH-1 in MMS-exposed gei-17 RNAi embryos (Figure 2B), as did co-depletion of cul-4, ddb-1, and cdt-2 (Figure 2C). The effects observed in the co-depletion experiments in Figures 2B and 2C were not due to dilution-based reduction in RNAi effectiveness, as co-depletion of gei-17 with any of 5 irrelevant RNAi feeding clones randomly chosen from the whole-genome RNAi feeding library (Kamath et al., 2003) resulted in destruction of POLH-1-GFP after MMS exposure (Supplementary Figure S2). Because antibodies against CUL-4, DDB-1, and CDT-2 are not available, we used RT-PCR and observed that the RNAi effectively knocked-down transcript levels for these three genes (Supplementary Figure S3). We next confirmed these results with the endogenous protein, by comparing the levels of native POLH-1 in gei-17 to gei-17/cdt-2 RNAi embryos. MMS caused the loss of POLH-1 from gei-17 RNAi embryos, as expected, but not from gei-17/cdt-2 RNAi embryos (Figure 2D). This shows that the endogenous POLH-1 behaves in the same way as the wild type POLH-1-GFP fusion protein examined in Figures 2B-2C. Based on the data in Figure 2, we conclude that the loss of POLH-1 in damaged gei-17 RNAi embryos is through CRL4-CDT-2 mediated proteolysis.
GEI-17 Directly SUMOylates POLH-1 to Protect it from CRL4-Cdt2
The data presented thus far show that GEI-17 protects POLH-1 from CDT-2 during a DNA damage response. We next considered the possibility that this protection is afforded via direct SUMOylation of POLH-1 by GEI-17. To address this, we first asked if recombinant GEI-17 could stimulate POLH-1 SUMOylation in a cell-free SUMOylation assay. POLH-1 was radio-labeled by in vitro transcription and translation in rabbit reticulocyte lysates containing [35S]-methionine. The lysates were then supplemented with components of the SUMOylation machinery (human SUMO 1 and SUMO 3, human SUMO E1 (SAE1/SAE2), and the C. elegans UBC-9 SUMO-conjugating enzyme), as previously described (Holway et al., 2005). After incubation, the reactions were fractionated by SDS-PAGE, and the reaction products visualized by Phosphorimager analysis of the gel. If POLH-1 is SUMOylated under these conditions, then we should observe the appearance of high molecular weight bands, corresponding to SUMOylated forms of POLH-1. In the sample that contained no SUMO components, POLH-1 ran as a single discrete band of ~68 kDa (Figure 3A, lane 1). By contrast, addition of SUMO E1, UBC-9, SUMO 1/3, and GEI-17 resulted in the appearance of high molecular weight POLH-1 bands on the gel, ranging in size from ~90 kDa to ~170 kDa (lane 4). These high molecular weight POLH-1 bands were dependent on the presence of both GEI-17 and SUMO 1/3 in the reaction; they did not appear when GEI-17 was excluded (lane 5), and, likewise, they failed to appear when SUMO 1/3 was omitted from the reaction (lane 3). These data demonstrate that recombinant GEI-17 stimulates SUMOylation of POLH-1 in vitro. We next determined if GEI-17 mediates POLH-1 SUMOylation in vivo. Wild type embryos were optionally exposed to MMS prior to preparation of extracts, and POLH-1 was then immunoprecipitated and analyzed by Western blotting. High molecular weight bands were observed on the POLH-1 blots that were specific for the MMS-exposed sample (Figure 3B, α-POLH-1 blot), and these high molecular weight bands were also recognized by anti-SUMO antibodies, showing that they correspond to SUMO-modified POLH-1 (Figure 3B, α-SUMO blot). To determine if POLH-1 SUMOylation is dependent on GEI-17, we repeated the experiment in gei-17/cdt-2 RNAi embryos. We used these doubly depleted embryos to ensure that POLH-1 would not be degraded upon exposure to MMS. As shown in Figure 3B, the SUMO-modified form of POLH-1 was absent in the MMS-exposed gei-17/cdt-2 RNAi embryos. These data show that POLH-1 is SUMOylated in vivo, in a manner dependent on DNA damage and GEI-17. Consistent with a stimulatory affect of DNA damage on POLH-1 SUMOylation, we also observed that GEI-17 preferentially associates with damaged chromatin in early embryos. For this, embryos were optionally exposed to MMS and, after incubation, chromatin fractions were prepared according to previously established conditions (Kim et al., 2007). The chromatin samples were then blotted for GEI-17 and, as a loading control, PCN-1. Quantification of the GEI-17 signals in the blot shown in Figure 3C revealed that 2.7-fold more GEI-17 was associated with chromatin in the MMS-treated sample, relative to the control. This preferential association of GEI-17 with damaged chromatin suggests that GEI-17 mediated SUMOylation of POLH-1 may take place at sites of damage.
Figure 3. GEI-17 Directly SUMOylates POLH-1 to Protect It from CRL4-Cdt2.
(A) In vitro SUMOylation assays were preformed as described previously (Holway et al., 2005; see also Experimental Procedures). POLH-1 was produced by transcription/translation in vitro using rabbit reticulocyte lysate and was radio-labeled by virtue of inclusion of 35S-methionine in the translation reaction. The reactions were then supplemented with recombinant forms of UBC-9 and SUMO activating enzyme (abbreviated as “E1”, this is a heterodimer of the SAE1 and SAE2 proteins), SUMO (SUMO 1 and 3), or GEI-17, as indicated. Reactions were then fractionated on SDS-PAGE and visualized via Phosphorimaging.
(B) Early embryo extracts were immunoprecipitated with anti-POLH-1 antibodies. These immunecomplexes were fractionated on SDS-PAGE and then probed, by immunoblotting, with either anti-POLH-1 or anti-SUMO antibodies, as indicated. Prior to embryo extract preparation, the animals were either treated, or not, with MMS, as in Figure 1B.
(C) Chromatin fractions from total embryo extracts were prepared as described (Kim et al., 2007) and blots of these fractions were probed by immunoblotting with anti-GEI-17 antibodies. The starting extract used to prepare the chromatin fractions (“total embryo extract”) was also examined. Prior to chromatin isolation, the animals were either treated, or not, with MMS, as in Figure 1B. The PCN-1 signal confirms that equivalent amounts of the chromatin fractions were loaded on the gel, and the anti-tubulin signal confirms that the chromatin fractions were not contaminated with cytoplasmic proteins.
(D) In vitro SUMOylation assays were performed as in Figure 3A. For these experiments, all assays contained the full complement of components (i.e. recombinant E1, UBC-9, SUMO 1/3, and GEI-17). Two different substrates, wild type POLH-1 and POLH-1 K85R/K260R double mutant, were included in the assays.
(E) The K85R/K260R double mutant was introduced into the pie-1::polh-1-GFP construct, and transformants were obtained via particle bombardment (see Experimental Procedures). These animals, and pie-1::polh-1-GFP animals, were then treated, or not, with MMS as in Figure 1B, and early embryo extracts were prepared. The POLH-1-GFP derivatives were then detected by immunoblotting, after SDS-PAGE, with anti-GFP antibodies. The lysates were also probed with antibodies against PCN-1, to ensure equal loading of the samples.
If SUMOylation is responsible for protecting POLH-1 from proteolysis during a DNA damage response, then a mutant POLH-1 that cannot be SUMOylated should be degraded in damage-exposed embryos. To address this, we searched the POLH-1 primary sequence for possible SUMO acceptor sites, and identified lysines 85 and 260 as candidates (Supplementary Figure S4). We produced three mutants, K85R, K260R, and the double K85/260R, and tested them for SUMOylation using the cell-free system. While both single mutants were less efficiently SUMOylated then wild type POLH-1, the double K85/260R mutant was even less efficiently SUMOylated (Supplementary Figure S4, Figure 3D). To determine the effect of these mutations on POLH-1 stability in vivo, we produced transgenic embryos expressing a K85/260R POLH-1-GFP. As shown in Figure 3E, the amount of wild type POLH-1-GFP present in MMS-exposed embryos was similar to that observed in undamaged embryos. By contrast, far less K85/260R POLH-1-GFP was observed in MMS-exposed embryos than in their undamaged counterparts, showing that K85/260R POLH-1-GFP is unstable during a DNA damage response. The results in Figure 3 strongly suggest that direct SUMOylation of POLH-1 by GEI-17 protects it from CRL4-Cdt2 mediated destruction. To determine if expression of K85/260R POLH-1-GFP affected the DNA damage response, we assessed sensitivity to MMS in transgenic embryos. We observed that embryos expressing K85/260R POLH-1-GFP were more sensitive to MMS than embryos expressing wild type POLH-1-GFP, as embryonic lethality for K85/260R POLH-1-GFP embryos was 71.2% with MMS, and 17.9% without MMS, whereas embryonic lethality for wild type POLH-1-GFP was 6.8% and 3.3%, respectively. This suggests that K85/260R POLH-1-GFP exerts a dominant-negative effect on the DNA damage response.
The Sole Purpose of GEI-17 Mediated SUMOylation of POLH-1 is to Prevent POLH-1 Degradation
The results obtained thus far show that one purpose of GEI-17 mediated SUMOylation of POLH-1 is to prevent CRL4-Cdt2 mediated destruction during a DNA damage response. If this represents the only function of GEI-17 in POLH-1 regulation, then co-inactivation of cdt-2 with gei-17 should suppress the gei-17 RNAi phenotypes that are otherwise observed during a DNA damage response. To test this hypothesis, we measured DNA synthesis in early embryos. Previous work has shown that gei-17 RNAi selectively perturbs the replication of damaged, but not undamaged, chromosomes in early embryos (Holway et al., 2006). If this replication phenotype was due solely to CDT-2 mediated loss of POLH-1, then co-inactivation of cdt-2 with gei-17 should suppress it. DNA synthesis was measured by uptake of [3H]-thymidine, according to published procedures (Hartman et al., 1991). As expected from previous work, DNA synthesis was reduced by MMS in gei-17 RNAi embryos, but not in wild type embryos (Figure 4). MMS did not, however, reduce DNA synthesis in embryos co-depleted of gei-17 and cdt-2, which shows that co-inactivation of cdt-2 suppresses the effect of loss of gei-17 on replication of damaged DNA. This is likely because loss of cdt-2 stabilizes POLH-1 in gei-17 RNAi embryos, but it was also formally possible that loss of cdt-2 activates a latent DNA damage response pathway that renders POLH-1 dispensable for the replication of damaged DNA. To test this, we measured DNA synthesis in polh-1 RNAi embryos, and in polh-1/cdt-2 RNAi embryos, and found that MMS reduced DNA synthesis in both cases (Figure 4). This shows that the replication of damaged DNA relies on POLH-1 even when cdt-2 has been inactivated, which rules out a POLH-1 independent pathway from contributing to the effects that we have observed. We also observed that inactivation of cdt-2 alone did not affect replication, either with or without MMS. These data show that the DNA replication defect in MMS-exposed gei-17 RNAi embryos can be suppressed by co-inactivation of cdt-2. In Supplementary Figure S5, we show that the delayed cell cycle progression phenotype that has been previously observed for MMS-exposed gei-17 RNAi embryos (Holway et al, 2006) is also reversed by co-inactivation of cdt-2. Based on these data, we conclude that the major function of GEI-17 during the early embryonic DNA damage response is to protect POLH-1 from the CRL4-Cdt2 pathway. Importantly, our data also show that GEI-17 is not directly required for POLH-1 to performs its function; rather, GEI-17 is required for POLH-1 to have the opportunity to perform its function, by staving off CRL4-Cdt2 mediated destruction.
Figure 4. The Sole Purpose of GEI-17 Mediated SUMOylation of POLH-1 is to Protect It From CRL4-Cdt2.
Animals were treated with the indicated RNAi and were optionally exposed to MMS, as in Figure 1B. Adults were then bleached and the embryos collected. 1000 embryos for each sample were then cultured in media containing 3H-thymidine. 30 minutes later samples were collected, lysates were prepared, and tritium incorporation was measured as described (Hartman et al., 1991). Samples were taken again at 60, 120, and 240 minutes, and tritium incorporation was again assessed. The amount of tritium incorporated by the wild type, undamaged sample at the 240 minute time point was set to 100, and all other values were adjusted accordingly.
CRL4-Cdt2 Mediated POLH-1 Proteolysis Occurs in Wild Type Embryos, and is Linked to the Number of Lesions Present in the Genome
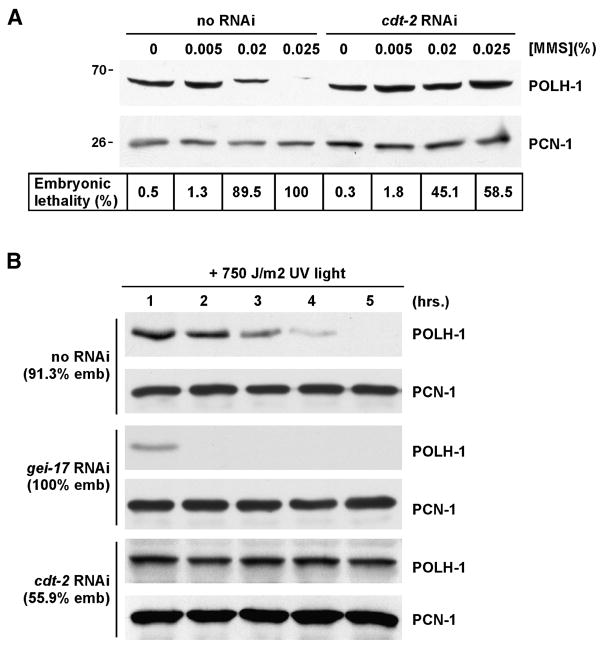
An important question that emerges from our studies concerns the role that CRL4-Cdt2 mediated destruction of POLH-1 plays in the DNA damage response. We have, thus far, only detected POLH-1 destruction when GEI-17 was inactivated. This may be because CRL4-Cdt2 only gains access to POLH-1 when GEI-17 is absent, perhaps because an un-SUMOylated POLH-1 is sensed as aberrant by the cell, and then degraded by CRL4-Cdt2 in response. Alternatively, proteolysis by CRL4-Cdt2 may be part of the normal cycle of POLH-1 function at sites of damage, and the concentration of MMS used in our experiments, 0.005%, does not produce enough lesions to noticeably affect the total pool of POLH-1 present in the cell. To distinguish between these possibilities, we exposed embryos to a range of MMS concentrations, and then monitored POLH-1 protein levels by Western blotting. As shown in Figure 5A, treatment with MMS reduced the amount of POLH-1 in early embryos, in a dose-dependent manner. Importantly, when this experiment was performed in cdt-2 RNAi embryos, then POLH-1 was not degraded after exposure to high concentrations of MMS (Figure 5A). This experiment shows that CDT-2 can act on POLH-1 even when GEI-17 is active, and that the dose of damaging agent determines the extent to which POLH-1 is degraded by the CRL4-Cdt2 pathway. Previous work from our laboratory has shown that concentrations of MMS greater than 0.005% cause lethality in wild type embryos (Holway et al., 2006). To see if this lethality was linked to POLH-1 degradation, we compared the MMS sensitivity of wild type to cdt-2 RNAi embryos at the different concentrations of MMS utilized in Figure 5A. Interestingly, cdt-2 RNAi embryos were more resistant to MMS than were wild type (Figure 5A), and this is likely due, at least in part, to the stabilization of POLH-1 that is conferred by CDT-2 inactivation. We note that while the high concentrations of MMS caused embryonic lethality, this lethality occurs during mid to late embryogenesis, as the dead embryos on the MMS plates all contained >200 nuclei (data not shown). The embryos taken for the Western blot in Figure 5A were early embryos, and live cell imaging confirmed that these embryos were alive and undergoing cell division at the time that they were taken for Western blot analysis.
Figure 5. DNA Damage Triggers CRL4-Cdt2 Mediated POLH-1 Destruction in Wild Type Embryos.
(A) Early embryo extracts were prepared and the POLH-1 protein was detected by immunoblotting, after SDS-PAGE, with anti-POLH-1 antibodies. The lysates were also probed with antibodies against PCN-1, to ensure equal loading of the samples. Prior to extract preparation, the animals were optionally depleted of cdt-2 by RNAi, and either exposed, or not, to 0.005%, 0.02%, and 0.025% MMS. Embryonic lethality was also determined for the different concentrations of MMS, and is displayed under the blot.
(B) Animals that were pre-treated with the indicated RNAi were exposed to 750 J/m2 of UV light. At the indicated time early embryos were collected, extracts were prepared, and POLH-1 and PCN-1 proteins were detected by Western blotting. Embryonic lethality (emb) under these conditions was also determined, and is displayed to the left of the blots.
The experiment in Figure 5A shows that CRL4-Cdt2 mediated turnover of POLH-1 occurs in wild type embryos during a DNA damage response, and that turnover correlates to the dose of damaging agent used in the experiment. The experiment also suggests that while POLH-1 is degraded during a damage response in wild type embryos, it is degraded less rapidly than in damage-exposed gei-17 RNAi embryos. To examine this directly, we pulsed wild type, gei-17 RNAi, and cdt-2 RNAi embryos with a high dose of UV light (750 J/m2), and then examined POLH-1 levels as a function of time after the induction of DNA damage. As shown in Figure 5B, in wild type animals, POLH-1 was gradually lost, with levels becoming undetectable at 5 hours post-irradiation. By contrast, in gei-17 RNAi embryos, POLH-1 was rapidly lost, with no detectable signal at 2 hours post-irradiation. As expected, POLH-1 levels did not fluctuate in cdt-2 RNAi embryos during the course of this experiment. As was the case with MMS, cdt-2 RNAi conferred resistance to this high dose of UV light, as embryonic lethality was reduced from 91.3% in the wild type to 55.9% after cdt-2 RNAi. Based on the data in Figure 5, we conclude that DNA damage triggers CDT-2 dependent destruction of POLH-1, and that GEI-17 controls the timing of this destruction.
DISCUSSION
In this study, we examined the role that GEI-17 plays in regulation of the TLS polymerase POLH-1. We found that inactivation of GEI-17 leads to the destruction of POLH-1 (Figure 1). Destruction is induced by low levels of DNA damaging agent, and is mediated by the CRL4-Cdt2 pathway (Figure 2). We also found that GEI-17 controls POLH-1 SUMOylation, both in vitro and in vivo, and that a mutant POLH-1 that cannot be SUMOylated is degraded in response to DNA damage (Figure 3). We went on to ask if preventing destruction represents the major function of GEI-17 mediated SUMOylation of POLH-1, and found that it does (Figure 4). Lastly, we observed that the CRL4-Cdt2 pathway destroys POLH-1 even when GEI-17 is present, when higher amounts of DNA damaging agent are utilized (Figure 5A), and that GEI-17 controls the timing of POLH-1 destruction during a DNA damage response (Figure 5B).
Our results lend insight into how the TLS polymerase POLH-1 is regulated in C. elegans early embryos. The data suggest that, upon association with sites of damage, POLH-1 is SUMOylated by GEI-17. If it is not, then the CRL4-Cdt2 pathway targets it for degradation, before it can perform its function (Figure 6A). Even with SUMOylation, however, POLH-1 is still degraded by the CRL4-Cdt2 pathway, but after it has performed its function (Figure 6B). The conclusion that non-SUMOylated POLH-1 is degraded prior to performing its function is supported by two findings. First, the replication of damaged chromosomes is as dependent on GEI-17 as it is on POLH-1, yet the requirement for GEI-17 is relieved by inactivation of CDT-2 (Figure 4). This strongly suggests that, when CDT-2 is active, POLH-1 function is totally dependent on GEI-17 and, therefore, that POLH-1 cannot perform its function unless it is first SUMOylated by GEI-17. Second, low levels of DNA damaging agent are sufficient to trigger POLH-1 degradation in gei-17 RNAi embryos, but higher levels are required in wild type embryos (Figures 1 and 5A). This suggests that more POLH-1 is degraded, on a per lesion basis, when it cannot be SUMOylated, and that this likely results from reiterated cycles of recruitment and degradation at a given lesion (Figure 6A). The conclusion that POLH-1 is degraded after performing its function (in wild type embryos) is based on the data in Figure 5. We observed a tight, inverse correlation between the concentration of MMS used in our experiment and POLH-1 protein levels, which suggests that as the number of MMS-induced lesions is increased, the more POLH-1 is degraded. This result strongly suggests that CRL4-Cdt2 dependent proteolysis of POLH-1 is a feature of many, if not all, normal cycles of TLS. We propose that at low lesion number (e.g., the number of lesions induced by 0.005% MMS), the degradation that occurs per TLS cycle does not affect the total supply of POLH-1 in the embryo (Figures 1A and 5A), and thus the rate of replication is not affected (Figure 4). As the number of lesions climbs, however, TLS-coupled POLH-1 degradation depletes the embryo of POLH-1 (Figure 5A), thereby inducing sensitivity to the DNA damaging agent. Inactivation of cdt-2 prevents POLH-1 degradation, and this allows the embryo to gain resistance to the damaging agent. This conclusion is supported by our finding that a high dose of UV light triggers gradual degradation of POLH-1 in wild type embryos, and that this same dose triggers much more rapid degradation in gei-17 RNAi embryos (Figure 5B). This result strongly suggests that GEI-17 controls the timing of POLH-1 degradation during a damage response, and is consistent with the hypothesis that GEI-17 protects POLH-1 from degradation during TLS, but not afterwards. We note that the two SUMO-acceptor sites that we have identified within POLH-1, K85 and K260, are likely to reside near active site of the enzyme, based on the crystal structures of yeast pol eta (Trinaco et al., 2001; Alt et al., 2007). This raises the possibility that POLH-1 SUMOylation may also affect its catalytic activity, although the finding that normal POLH-1 function is observed in vivo in gei-17/cdt-2 RNAi embryos argues against this possibility.
Figure 6. A Model for GEI-17/CRL4-Cdt2 Mediated Regulation of POLH-1 During the DNA Damage Response.
(A) When GEI-17 is inactivated (GEI-17−), then POLH-1 is destroyed by CRL4-Cdt2 (CDT-2 in the figure) before it can perform its function in TLS. The model proposes that a single lesion (red square) can catalyze multiple rounds of POLH-1 destruction, as designated by the blue arrow.
(B) The model proposes that, in the wild type condition, GEI-17 SUMOylates POLH-1, and this prevents CRL4-Cdt2 mediated destruction until after POLH-1 has performed its role in TLS.
Our work has identified a key role for proteolysis in the regulation of POLH-1 at sites of damage in C. elegans embryos. Recent work on the budding yeast ortholog of pol eta, Rad30, has shown that ubiquitin-mediated proteolysis plays a role in maintaining the amount of Rad30 that is present in unstressed cells (Skonecza et al., 2007). In addition, it has been observed that several human TLS polymerases, including pol eta, are monoubiquitylated (Bienko et al., 2005; Hoeller et al., 2007). As monoubiquitylation is not thought be associated with proteolysis, it appears that the ubiquitin system can impact pol eta regulation in multiple ways. An important question that emerges from our work concerns the purpose that CRL4-Cdt2 mediated destruction of POLH-1 serves in the regulation of TLS. One possibility is that POLH-1 degradation is part of the mechanism that allows exchange of POLH-1 for the replicative polymerase after TLS. Current models for TLS suggest that post-TLS exchange occurs because pol eta, which exhibits low processivity in vitro (Washington et al., 1999; McCulloch et al., 2004, 2007), simply disassociates from the template after TLS, and this allows the replicative polymerase to regain access to the primed site. We propose that early embryos may employ a more active mechanism to remove POLH-1 from the template after TLS, and that this involves the CRL4-Cdt2 mediated proteolysis step that we have identified. Another important question surrounding our findings is the degree to which the GEI-17/CRL4-Cdt2 mediated regulation of POLH-1 that we have discovered in C. elegans is conserved in other organisms. The early embryonic cell cycles of C. elegans are unusual in that they proceed normally even when the chromosomes are heavily damaged, and this is likely connected to a developmental program that cannot tolerate cell cycle delay. It, therefore, may be that GEI-17/CRL4-Cdt2 system has evolved to accommodate the unusual coupling of TLS to rapid progression through the cell cycle that occurs in nematode embryos. It is equally possible, however, that GEI-17/CRL4-Cdt2 system is conserved in other organisms, and that it represents a core component of the mechanism that regulates pol eta activity. In support of this, we note that all of the components of the GEI-17/CRL4-Cdt2 system, including GEI-17, Cul4, Ddb1, Cdt2, and PCNA, are conserved in human cells. Moreover, the sequence determinants within POLH-1 that respond to this system, including the PIP box and the K85/260 residues, are also conserved in human pol eta. Given this conservation, it seems likely that the GEI-17/CRL4-Cdt2 system regulates human pol eta, and further work is required to determine if this is so.
EXPERIMENTAL PROCEDURES
C. elegans Strains and Culturing, RNAi, and MMS Sensitivity Assays
The N2 Bristol strain was used as wild type and culturing was as described (Brenner, 1974). RNAi by feeding was performed for polh-1, gei-17, cul-4, ddb-1, and cdt-2 (T01C3.1) as described previously (Timmons and Fire, 1998). gei-17/pcn-1 RNAi by soaking was performed as described previously (Maeda et al., 2001). MMS sensitivity assays were performed as described previously (Holway et al., 2005, 2006).
Mutant Construction, POLH-1-GFP Fusion Proteins, and Transgenic Worms
POLH-1-ΔPIP: The ΔPIP mutant was created by deleting the C-terminal 9 codons from the POLH-1 cDNA. This mutant removes the conserved and functionally critical FF dipeptide from the POLH-1 PIP box (Moldovan et al., 2007). POLH-1-K85/K260: The K85R/K260R mutant was generated by substituting both lysines with arginine, using a QuickChange II Site-Directed Mutagenesis Kit (Stratagene). For generating a strain expressing the POLH-1-GFP fusion protein, POLH-1 coding sequences were subcloned into the pJH4.52 vector (a gift from G. Seydoux, Johns Hopkins University, Baltimore, MD). The resulting construct placed POLH-1-GFP coding sequences downstream of the pie-1 promoter, which allows expression of transgenes in the C. elegans germ line (Strome et al., 2001). The pie-1::polh-1-GFP strain was then generated via a microparticle bombardment-mediated transformation. The ΔPIP-POLH-1GFP strain was generated in a similar manner, and both the wild type and ΔPIP POLH-1 transformants stably expressed the GFP fusion protein through multiple generations. To express the K85/260R POLH-1-GFP mutant in early embryos, we generated a construct containing K85/260R POLH-1 fused to GFP in the pJH4.52 vector. We again used particle bombardment for transformation, but unlike the POLH-1-GFP and POLH-1-ΔPIP-GFP constructs, we were unable to generate stable transformants expressing K85/260R POLH-1-GFP, as the construct was lost from the animals after three generations. To get around this, for the experiment in Figure 4E, we introduced the K85/260R POLH-1-GFP construct into young adults via particle bombardment, and hand-picked their GFP-expressing offspring. These animals were then optionally exposed to MMS, and their early embryos, which were all GFP-positive, were collected and examined for K85/260R POLH-1-GFP expression by Western blotting.
In Vitro SUMOylation Assay
The cDNAs encoding wild type POLH-1 and POLH-1-K85R/K260R proteins were transcribed and translated (TNT reaction) in the presence of 35S-methionine according to the manufacturer’s instruction (Promega). The SUMOylation kit was purchased from LAE Biotech International and in vitro SUMOylation reactions were performed according to the manufacturer’s instruction with the following exceptions. 20 μl reactions, containing 5 μl POLH-1 or POLH-1-R85/R260 proteins prepared by TNT reaction, 0.68 μg GST-tagged GEI-17 (Holway et al., 2005), 0.36 μg his-tagged UBC-9, 1 μg SUMO 1, 1 μg SUMO 3, and 0.15 μg E1, were assembled. The reaction were incubated 1 hour at 37 °C, then stopped by adding 20 μl of 2x Laemmli sample buffer (Sigma), boiled and separated by SDS-PAGE. 35S-labeled POLH-1 was then visualized on a Phosphorimager.
Antibodies, Immunoprecipitation, and Immunoblotting
Antibodies against C. elegans GEI-17 and POLH-1 were generated by immunizing rabbit with GST tagged GEI-17 protein and the peptide NAQKPKKPKSLESFFKKKKP, respectively (Bethyl Laboratories). The PCN-1 antibody has been described (Kim et el., 2007). Antibodies against GFP (ab290) and SUMO-1 (D-11) were purchased from Abcam and Santa Cruz Biotechnology, respectively. Preparation of total embryo extracts, chromatin protein fractions, immunoprecipitation, and immunoblotting were all performed as described previously (Kim et al., 2007).
Image Acquisition
Embryos were fixed and stained by Hoechst 33258 as described previously (Holway et el., 2006). The images of embryos in Figure 1D were obtained by a confocal system (LSM510 META; Carl Zeiss MicroImaging, Inc.) attached to a laser scanning microscope (Axiovert 100M; Carl Zeiss MicroImaging, Inc.). Exposure times for all images shown in the composite in Figure 1D were identical.
DNA Synthesis Assays
1000 embryos per sample were obtained by bleaching gravid hermaphrodites. Embryos were then cultured in media containing 3H-thymidine. Sample preparation and tritium incorporation measurements were accomplished as described previously (Hartman et al., 1991).
Supplementary Material
Acknowledgments
We thank Bodo Stern, Andrew Murray, Qing Liu, Craig Hunter, Nicole Francis, and Johannes Walter for comments on earlier drafts of this manuscript. This work was funded by a grant (R01GM067735) to W.M.M. from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–2506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt A, Lammens K, Chiocchini C, Lammens A, Pieck JC, Kuch D, Hopfner KP, Carell T. Bypass of DNA lesions generated during anticancer treatment with cisplatin by DNA polymerase η. Science. 2007;318:967–970. doi: 10.1126/science.1148242. [DOI] [PubMed] [Google Scholar]
- Arias EE, Walter JC. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 2007;21:497–518. doi: 10.1101/gad.1508907. [DOI] [PubMed] [Google Scholar]
- Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehman A, et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science. 2005;310:1821–1824. doi: 10.1126/science.1120615. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Baumer K, Gonczy P. Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr Biol. 2003;13:819–827. doi: 10.1016/s0960-9822(03)00295-1. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds CE, Simpson LJ, Sale JE. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol Cell. 2008;30:519–529. doi: 10.1016/j.molcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Encalada SE, Martin PR, Phillips JB, Lyczak R, Hamill DR, Swan KA, Bowerman B. DNA replication defects delay cell division and disrupt cell polarity in early Caenorhabditis elegans embryos. Dev Biol. 2000;228:225–238. doi: 10.1006/dbio.2000.9965. [DOI] [PubMed] [Google Scholar]
- Haracska L, Kondratick CM, Unk I, Prakash S, Prakash L. Interaction with PCNA is essential for yeast DNA polymerase eta function. Mol Cell. 2001;8:407–415. doi: 10.1016/s1097-2765(01)00319-7. [DOI] [PubMed] [Google Scholar]
- Hartman P, Reddy J, Svendsen BA. Does trans-lesion synthesis explain the UV-radiation resistance of DNA synthesis in C. elegans embryos? Mutat. Res. 1991;255:163–173. doi: 10.1016/0921-8777(91)90050-y. [DOI] [PubMed] [Google Scholar]
- Hoeller D, Hecker CM, Wagner S, Rogov V, Dotsch V, Dikic I. E3-dependent monoubiquitination of ubiquitin-binding proteins. Mol Cell. 2007;26:891–898. doi: 10.1016/j.molcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Holway AH, Hung C, Michael WM. Systematic, RNA-interference-mediated identification of mus-101 modifier genes in Caenorhabditis elegans. Genetics. 2005;169:1451–1460. doi: 10.1534/genetics.104.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holway AH, Kim SH, La Volpe A, Michael WM. Checkpoint silencing during the DNA damage response in Caenorhabditis elegans embryos. J Cell Biol. 2006;172:999–1008. doi: 10.1083/jcb.200512136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Hartman PS. Replication in UV-irradiated Caenorhabditis elegans embryos. Photochem Photobiol. 1996;63:187–192. doi: 10.1111/j.1751-1097.1996.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kim SH, Holway AH, Wolff S, Dillin A, Michael WM. SMK-1/PPH-4.1-mediated silencing of the CHK-1 response to DNA damage in early C. elegans embryos. J Cell Biol. 2007;179:41–52. doi: 10.1083/jcb.200705182. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair (Amst) 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11:171–6. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- McCulloch SD, Kokoska RJ, Masutani C, Iwai S, Hanaoka F, Kunkel TA. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature. 2004;428:97–100. doi: 10.1038/nature02352. [DOI] [PubMed] [Google Scholar]
- McCulloch SD, Wood A, Garg P, Burgers PM, Kunkel TA. Effects of accessory proteins on the bypass of a cis-syn thymine-thymine dimer by Saccharomyces cerevisiae DNA polymerase eta. Biochemistry. 2007;46:8888–8896. doi: 10.1021/bi700234t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T. CDK Inhibitor p21 Is Degraded by a Proliferating Cell Nuclear Antigen-coupled Cul4-DDB1Cdt2 Pathway during S Phase and after UV Irradiation. J Biol Chem. 2008;283:29045–29052. doi: 10.1074/jbc.M806045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell BC, Harper JW. Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr Opin Cell Biol. 2007;19:206–214. doi: 10.1016/j.ceb.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Parker JL, Bielen AB, Dikic I, Ulrich HD. Contributions of ubiquitin- and PCNA-binding domains to the activity of Polymerase eta in Saccharomyces cerevisiae. Nucleic Acids Res. 2007;35:881–889. doi: 10.1093/nar/gkl1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- Skonecza A, McIntyre J, Skoneczny M, Policinska Z, Sledziewska-Gojska E. Polymerase eta is a short-lived, proteosomally degraded protein that is temporarily stabilized following UV irradiation in Saccharomyces cerevisiae. J Mol Biol. 2007;366:1074–1086. doi: 10.1016/j.jmb.2006.11.093. [DOI] [PubMed] [Google Scholar]
- Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, Saxton W. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol Biol Cell. 2001;12:1751–1764. doi: 10.1091/mbc.12.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Trincao J, Johnson RE, Escalante CR, Prakash S, Prakash L, Aggarwal AK. Structure of the catalytic core of S. cerevisiae DNA polymerase η: Implications for translesion DNA synthesis. Mol Cell. 2001;8:417–426. doi: 10.1016/s1097-2765(01)00306-9. [DOI] [PubMed] [Google Scholar]
- Ulrich HD. Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends Cell Biol. 2005;15:525–532. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Washington MT, Johnson RE, Prakash S, Prakash L. Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase eta. J Biol Chem. 1999;274:36835–36838. doi: 10.1074/jbc.274.52.36835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.